Abstract
Chronic lymphocytic leukemia (CLL) with deletions of the p53 locus on chromosome 17 and/or refractory to fludarabine chemoimmunotherapy remains a major clinical problem with few therapeutic options. Currently, these types of CLL are treated with approaches that do not target the p53 pathway, such as small molecules and monoclonal antibodies (mAb). We have previously postulated anti-CCR7 mAb therapy as a novel CLL treatment. In the present study, we evaluated the in vitro efficacy of anti-CCR7 mAb as a single agent in CLL patients with high-risk cytogenetics and/or refractory to fludarabine, by measuring CCR7 surface expression and complement-dependent cytotoxicity. Our results demonstrate that CCR7 is highly expressed in challenging and heavily treated CLL patients. In addition, the complement-mediated mechanism of action of this mAb effectively eradicates CLL cells while sparing subsets of T cells in these patients. Moreover, this mAb outperformed the activity of alemtuzumab, the mAb with the highest efficacy in these groups. Finally, in vitro activity was also demonstrated in patients with a disease refractory to both fludarabine and alemtuzumab, and patients harboring 11q22 deletion. Our results propose that anti-CCR7 mAb is an effective and promising future treatment in high-risk CLL.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1670-z) contains supplementary material, which is available to authorized users.
Keywords: CCR7, mAb, p53, Fludarabine, CLL
Introduction
Chronic lymphocytic leukemia (CLL) is a clinically heterogeneous disease with a highly variable clinical course; some patients survive for decades, whereas others develop aggressive disease. In the last years, chemoimmunotherapy (CIT), the combination of monoclonal antibodies (mAb) with chemotherapy, has arisen as a more effective therapeutical approach in CLL. In particular, the standard therapy with the combination of FCR (fludarabine, cyclophosphamide, rituximab) has been shown to prolong both progression-free survival (PFS) and overall survival (OS) in CLL [1] and to decrease the likelihood of refractory disease [2].
However, there is an increasing number of patients for whom FCR or conventional CIT may not be the most appropriate therapy, including patients with 17p13 deletion (17p-), p53 mutation and/or fludarabine-refractory CLL (FR-CLL) [3–5]. Patients belonging to these subgroups show poor response to FCR, have short PFS and OS, and a few treatment options [1, 6, 7]. 17p-, which is found in 34–50 % of FR-CLL patients [2, 8, 9], renders patients resistant to chemotherapy with fludarabine or alkylating agent-based regimens, possibly because both agents require p53-dependent pathways to induce cell death [10]. For this reason, therapies not targeting the p53 pathway, such as mAb, have emerged as an effective and novel venue that shows better responses in patients with therapy-refractory disease or with the 17p- [11]. Few years ago, we proposed the CC chemokine receptor 7 (CCR7) as an interesting therapeutic target in CLL [12, 13]. We described the two major mechanisms of action of a murine antihuman CCR7 mAb: neutralization of CCR7-mediated in vitro migration of CLL cells and a potent complement-dependent cytotoxicity (CDC) against CLL cells [14]. Recently, we have shown that this anti-CCR7 mAb is highly effective in preclinical models of human mantle cell lymphoma (MCL) [15]. In the present work, we describe the efficacy of this anti-CCR7 mAb in killing leukemic cells from patients with 17p- and/or FR disease and compare its activity with anti-CD52 antibody alemtuzumab, the mAb with the highest efficacy in both subgroups of patients [11]. Our results postulate anti-CCR7 immunotherapy as an attractive option for patients with p53 abnormalities and/or with refractory disease and also for patients not considered appropriate candidates for alemtuzumab.
Materials and methods
Samples, reagents and flow cytometry (FCM)
All patients included in this study had been previously diagnosed with CLL according to standard immunophenotypic, morphological and clinical criteria [16]. The cytogenetics of each patient was determined by FISH (fluorescence in situ hybridization) according to the standard procedures. All CLL patients had been without therapy for at least 2 months prior to donating samples. Peripheral blood samples were obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of the Hospital Universitario de La Princesa. The characteristics of the 23 studied samples are summarized in Table 1 and Supplementary Table 1.
Table 1.
Characteristics of the studied patient samples
| Patient number | Rai stage | del 17p (FISH) | FR | FAR | Other cytogenetics (FISH) | Previous treatment | CDC | % B cell lysis after treatment with 5 μg/ml either anti-CCR7 or alemtuzumab | % B cell lysis after treatment with 0.5 μg/ml either anti-CCR7 or alemtuzumab |
|---|---|---|---|---|---|---|---|---|---|
| 1 | I | y | n | del 13q | t (1) | y | 97.80 vs 92.45 | 93.64 vs 83.16 | |
| 2 | 0 | y | n | ut | y | 94.27 vs 89.70 | 94.45 vs 81.64 | ||
| 3 | III | n | n | t (1) | n | nd | nd | ||
| 4 | IV | n | y | t (2) | y | 98.66 vs 92.24 | 93.35 vs 83.26 | ||
| 5 | IV | n | y | t (5) | y | 97.96 vs 96.62 | 72.36 vs 77.40 | ||
| 6 | O | y | n | ut | y | 96.94 vs 76.10 | 97.08 vs 17.16 | ||
| 7 | I | y | n | t (1) | y | 96.84 vs 16.69 | 91.27 vs 0 | ||
| 8 | III | n | n | t (1) | n | nd | nd | ||
| 9 | IV | y | n | t (1) | y | 87.82 vs 74.95 | 87.94 vs 5.18 | ||
| 10 | IV | y | y | y | de l 11q | t (5) | y | 99.06 vs 66.42 | 99.06 vs 12.83 |
| 11 | IV | y | y | t (4) | y | 97.50 vs 92.76 | 90.84 vs 69.04 | ||
| 12 | IV | n | y | del 11q | t (2) | y | 91.06 vs 39.24 | 82.13 vs 30.62 | |
| 13 | IV | y | y | del 11q, del 13q | t (4) | y | 83.31 vs 53.87 | 63.18 vs 27.73 | |
| 14 | IV | y | y | y | del 11q | t (3) | y | 94.55 vs 48.94 | 94.54 vs 33.37 |
| 15 | 0 | n | n | t (1) | n | nd | nd | ||
| 16 | IV | n | y | del 11q | t (2) | y | 99.14 vs 96.98 | 86.72 vs 74.01 | |
| 17 | I | y | n | del 13q | t (1) | y | 98.97 vs 83.29 | 98.52 vs 39.12 | |
| 18 | I | n | n | ut | n | nd | nd | ||
| 19 | IV | y | y | t (4) | y | 82.04 vs 63.29 | 80.02 vs 18.45 | ||
| 20 | 0 | n | n | ut | n | nd | nd | ||
| 21 | I | n | n | ut | n | nd | nd | ||
| 22 | 0 | n | n | ut | n | nd | nd | ||
| 23 | 0 | na | n | ut | n | nd | nd |
The table presents the cytogenetic abnormalities and refractory status of the studied patients, the CDC activity of anti-CCR7 mAb and alemtuzumab (anti-CD52)
FR fludarabine-refractory CLL, FAR CCL refractory to fludarabine and alemtuzumab, FISH fluorescence in situ hybridization, CDC complement-dependent cytotoxicity, y yes, n no, na not available, nd not determined, ut untreated, t treated (number of lines)
An initial immunophenotypical characterization of fresh whole blood cells was performed by 8-color FCM. Then, peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation (Ficoll-Paque Plus, Amersham Biosciences, Piscataway, NJ). PBMCs were incubated in RPMI 1640 media supplemented with 10 % heat-inactivated bovine serum, 2 mM l-glutamine and 100 U/mL penicillin/100 μg/mL streptomycin at 37 °C in an atmosphere of 5 % CO2.
CCR7, CD52 and CD20 expression was determined on B and T cells by FCM using CD5-FITC (fluorescein isothiocyanate), CD19-APC (allophycocyanin), CD3-APC-H7 (allophycocyanin-H7) (all from BD Biosciences, San Jose, CA), CCR7-PE (phycoerythrin) (R&D Systems, MN), CD52-PE (BD Biosciences) and CD20-PE (Biolegend, San Diego, CA). In all cases, an appropriate PE-isotype control (IC) was included and a minimum of 5000 neoplastic B cells was acquired. Immunofluorescence staining was analyzed on a FACSCanto II flow cytometer using Infinicyt v.1.3.0 (Cytognos, Salamanca, Spain) and Diva v.2.4 (BD Biosciences) softwares. Results are presented as the percentage of CCR7-positive cells and the median fluorescence intensity of CCR7, CD52 and CD20 expression relative to the IC (RMFI).
For the cytotoxicity assays, the therapeutic antibody alemtuzumab was provided by the pharmacy at our center. Purified mouse antihuman CCR7 mAb (150,503 clone, IgG2a isotype) and the respective IC were obtained from R&D Systems and were resuspended in sterile water. The DNA dye 7-aminoactinomycin-D (7-AAD), used in cell viability assays, was purchased from BD Biosciences.
Complement-dependent cytototoxicity (CDC)
CDC assays were performed as described previously [14]. Briefly, 2 × 105 PBMC target cells were plated in a 96-well round-bottom plate together with the indicated concentrations of purified anti-CCR7, alemtuzumab (anti-CD52) or IC antibodies. After 30 min at 37 °C, the cells were washed and complete RPMI 1640 medium containing 25 % rabbit complement (Serotec, Oxford, UK) with or without prior heat inactivation (56 °C, 30 min) was added. After 1.5 h, the cells were stained with anti-CD19-FITC, anti-CD3-PE and anti-CD5-APC mAb to discriminate between CLL cells and T cell populations. 7-AAD was used as a viability exclusion dye. The percentage of specific lysis (% SL) with heat-inactivated complement was used to calculate the specific lysis with the formula: % SL = 100 × (% dead cells with activated complement − % dead cells with inactivated complement)/(100 − % dead cells with inactivated complement).
Statistical analyses
The Statistical Package for the Social Sciences (SPSS) software (version 15.0, SPSS Inc., Chicago, IL) was used for the statistical analysis. To compare means between two independent groups, t test was used. Three or more groups were compared by one-way analysis of variance (ANOVA). When variances were not homogeneous according to Levene’s test, a Mann–Whitney U test was used. The differences were considered significant at a p value <0.05.
Results
CCR7 is highly expressed on B cells from patients with 17p- and/or with fludarabine-refractory disease
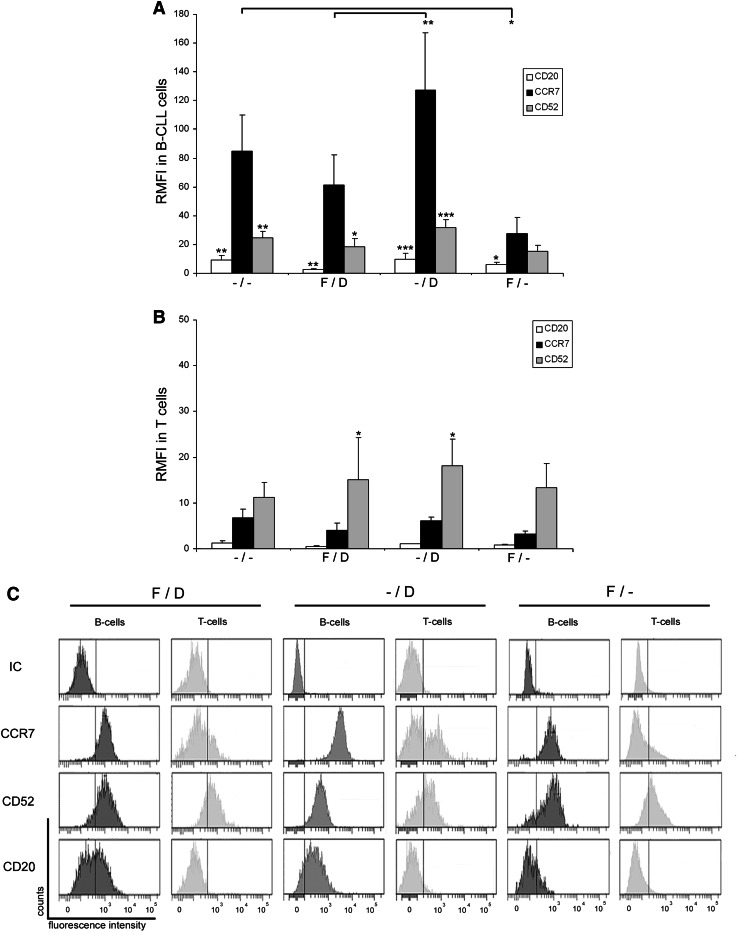
In order to determine whether patients with 17p- and/or FR-CLL can profit from a potential novel therapy based on an anti-CCR7 mAb, we first analyzed CCR7 surface expression and compared it with CD52 and CD20 expressions, the target proteins for alemtuzumab and rituximab, respectively. Twenty CLL patients (Table 1) were recruited and distributed in three experimental groups and a control group (Fig. 1): patients with FR-CLL and harboring 17p- (F/D), patients not previously treated with fludarabine and with 17p- (–/D) and patients with FR-CLL and both copies of p53 (F/–). As a control group, untreated patients with normal cytogenetics (–/–) were included. Interestingly, CCR7 was highly expressed on CLL cells and no differences were observed between the studied groups, except in the F/– patients who had 3.1-fold lower CCR7 expression than the control group (p < 0.05, Fig. 1a). This reduction was also observed in the case of CD52 (1.6-fold lower than controls) and CD20 (1.5-fold lower than controls). Nonetheless, in this F/– group, CCR7 was 1.7-fold higher than CD52 and 4.7-fold higher than CD20. As in controls, patients with 17p- (F/D and –/D groups) expressed significantly higher CCR7 levels than the levels determined for CD52 and CD20. Moreover, patients with 17p- and not treated with fludarabine (–/D group) displayed slightly higher levels of CCR7 and CD52 than patients from the control group (Fig. 1a).
Fig. 1.
CCR7 surface expression in 17p-deleted and/or fludarabine-refractory CLL patients. a Relative median fluorescence intensity (RMFI) in CLL cells. CLL cells from peripheral blood samples (n = 20) were analyzed by FCM to determine CCR7, CD52 and CD20 surface density measured as median relative to a corresponding isotype control. Patients were distributed in four groups depending on their cytogenetic profile and fludarabine-refractory (FR) status: –/–, patients with normal cytogenetics and no previous treatment (n = 5); F/D, patients with 17p- and FR-CLL (n = 5); –/D, patients with 17p- and fludarabine-naïve CLL (n = 6); F/–, patients without 17p- and FR-CLL (n = 4). Bars represent mean ± standard mean error. b RMFI in T cells from CLL patient samples. CCR7, CD52 and CD20 were analyzed in T cells from the same blood samples as shown in a. c Frequency histograms showing CCR7, CD52 and CD20 in a representative patient of each group. The pattern and intensity of each surface marker is shown in both B and T cells. All statistical analyses in each group are referred to CCR7. *p < 0.05; **p < 0.01; ***p < 0.001
When considering CCR7 mAb as a potential novel therapy, one has to take into account potential side effects, especially in T cells. When we analyzed the T cells from the same patient groups, we observed that CCR7 levels in controls and –/D patients were similar (Fig. 1b). As in the case of CLL cells, CCR7 was diminished in T cells from patients treated with fludarabine when compared to controls (1.7-fold lower in F/D and 2.2-fold lower in F/–). In contrast, CD52 expression was similar in all the groups (Fig. 1b). In controls, CCR7 levels were similar to CD52 levels. In contrast, T cells in the rest of the groups displayed a statistically significant higher proportion of CD52 than CCR7. In all the groups, CCR7 displayed a bimodal distribution in T cells due to the presence of CCR7-positive T naïve and regulatory and CCR7-negative T effector subsets (Fig. 1c). These results point out that CCR7 expression is high in tumor cells from patients that are in need of novel treatments (Fig. 1c), particularly in the 17p- carriers with or without previous exposition to fludarabine. In the case of patients without the 17p deletion and FR, CCR7 surface levels were lower than controls but high enough to fulfill the requirements for mAb immunotherapy.
Anti-CCR7 mAb mediates a strong and specific in vitro CDC of CLL cells from patients with 17p- and/or with fludarabine-refractory disease
We have previously demonstrated that murine anti-CCR7 mAb exerts a strong CDC as main effector mechanism against CLL cells [14]. In the present study, once we proved that the malignant CLL cells from high-risk patients express high levels of CCR7, we performed CDC assays with fresh cells from the same patients (Table 1). The CDC assays were performed using different antibodies doses (5–0.008 μg/ml). Anti-CD52 was chosen as reference because it is the mAb with the highest efficacy in 17p- and/or FR-CLL patients [9, 11].
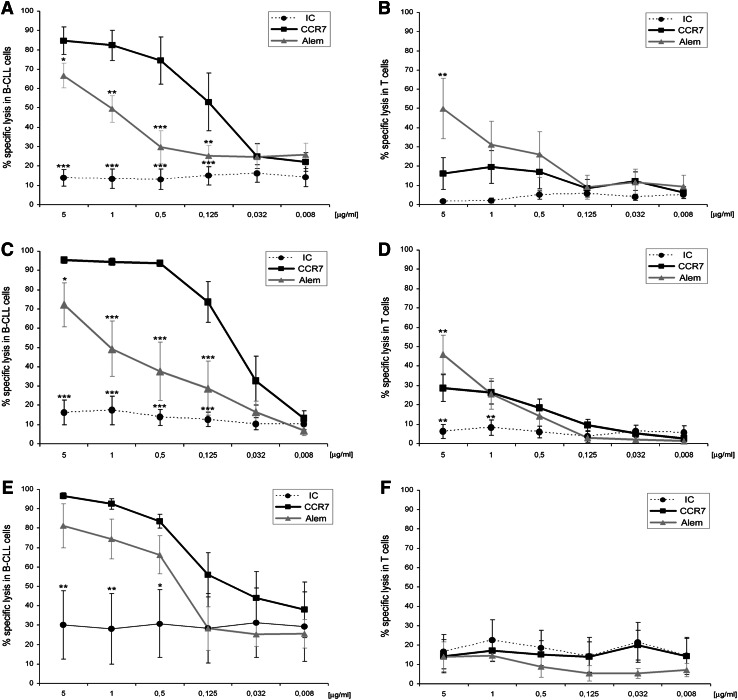
In the patients with 17p- and FR-CLL (F/D), anti-CCR7 mAb has a potent CDC activity revealing itself as an interesting therapeutic option. As shown in dose–response experiments (Fig. 2a), a high proportion of CLL cells pre-incubated with the anti-CCR7 mAb was killed after 1.5 h of treatment with rabbit complement, whereas no significant CDC was observed when the irrelevant IC was used. Even concentrations as low as 0.125 μg/ml of anti-CCR7 mAb were sufficient to mediate CDC of CLL cells (mean at 5 μg/m1 was 84.7 % and at 0.125 μg/ml 53.1 %). Similarly, the anti-CD52 mAb also mediated a significant CDC (Fig. 2a); however, its activity was lower than CCR7. Further, in T cells from these challenging patients, the CDC mediated by anti-CD52 mAb was higher than that by anti-CCR7 (Fig. 2b) at doses ranging from 5 (p < 0.01) to 0.5 μg/ml, which is a relevant point regarding potential toxicities of anti-CCR7 therapy.
Fig. 2.
Anti-CCR7 mAb mediates strong CDC of CLL cells sparing T cells in 17p- and/or fludarabine-refractory CLL. PBMC from the patients were incubated with anti-CCR7, anti-CD52 (alemtuzumab, Alem) or an isotype control (IC) at the indicated concentrations and then exposed to rabbit complement for 1.5 h. Cell lysis was determined in the different populations by staining CD19, CD3 and CD5 and analyzing 7-AAD (7-aminoactinomycin-D) incorporation by FCM. In all cases, the percentage of specific lysis was calculated according to the formula shown in “Materials and methods”. a, b CLL and T cells from patients with 17p- and fludarabine-refractory (FR) CLL (n = 5). C, d CLL and T cells from patients with 17p- and fludarabine-naïve CLL (n = 6). e and f CLL and T cells from patients without 17p deletion and FR-CLL (n = 4). All statistical analyses are referred to CCR7. Each dot, square or triangle represents mean ± standard mean error. *p < 0.05; **p < 0.01; ***p < 0.001
Among all the groups, the best responses are observed in CLL patients with 17p- but not treated before with fludarabine (–/D). These patients also showed the highest levels of CCR7 (Fig. 1a), and the specific lysis with the mAb at 5–0.5 μg/ml reached almost 100 % in CLL cells (Fig. 2c). Remarkably, anti-CCR7 mAb outperformed the rate of specific lysis mediated by anti-CD52 even at 0.032 μg/ml in these patients. As was the case with the patients from group F/D, anti-CCR7 mAb spared the T cells (Fig. 2d). The specific lysis of T cells at the highest concentration (5 μg/ml) was 28.6 %, whereas with anti-CD52 it was 1.6-fold higher (p < 0.01).
Despite the lower CCR7 levels in the patients with FR-CLL and no deletion of p53 genes (F/–), anti-CCR7 mAb had also activity in this group (Fig. 2e) where the basal death caused by an irrelevant antibody was noticeable high in probable relationship with a high susceptibility to unspecific insults of these heavily treated cells. Further, neither antibody had activity in T cell from these patients (Fig. 2f), which is surprising since anti-CD52 is expressed at similar levels in all the patient groups (Fig. 1b–c).
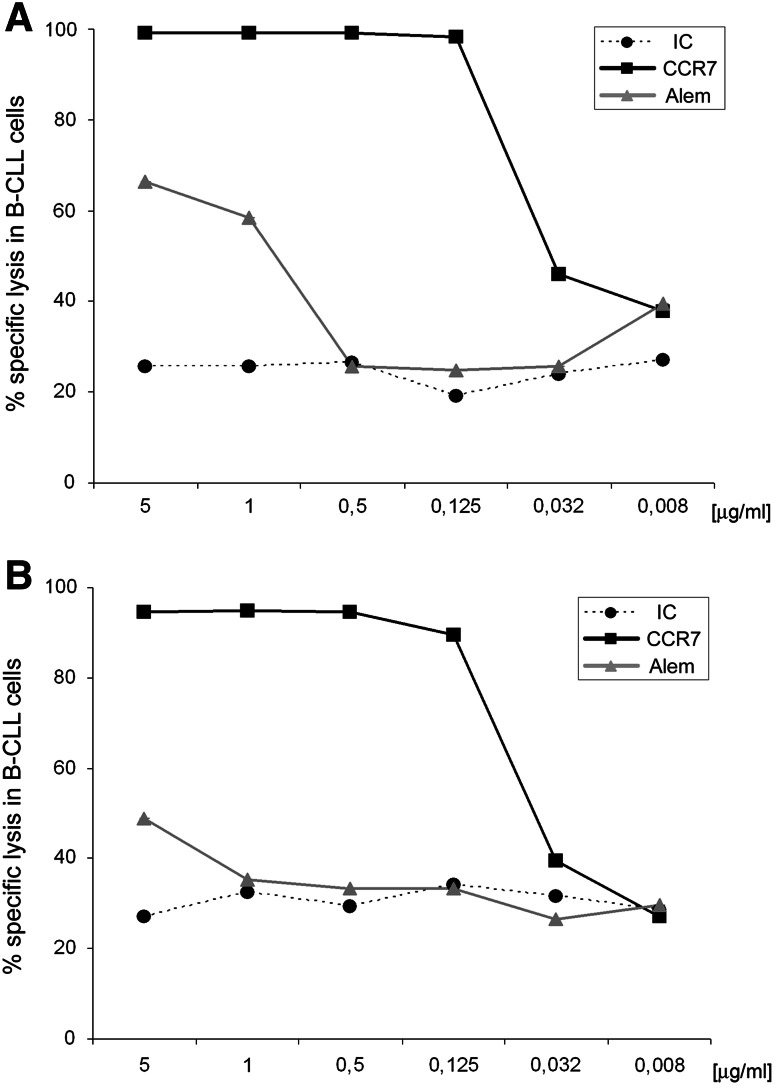
CCR7-mediated CDC is highly effective against CLL cells from patients refractory to both fludarabine and alemtuzumab (FAR)
CLL that is refractory to both fludarabine and alemtuzumab (FAR) is associated with a very poor prognosis [7]. In our cohort, patients 10 and 14 (Table 1) were considered FAR and both presented an adverse cytogenetic profile with 17p- and 11q-. Therefore, the potential of anti-CCR7 mAb to mediate CDC was analyzed separately in these patients (Fig. 3). As expected, in patient 10 (Fig. 3a), the specific lysis with anti-CD52 mAb was similar to the IC except in the case of saturating doses where anti-CD52 activity was 2.6-fold higher at 5 μg/ml (IC 25.6 %, anti-CD52 66.4 %) and 2.2-fold higher at 1 μg/ml (IC 25.7 %, anti-CD52 58.3 %). In contrast, anti-CCR7 activity was notably higher than both IC and anti-CD52 at almost all doses. Similar results were seen in the case of patient 14 (Fig. 3b) where the specific lysis with anti-CD52 mAb was similar to IC except at 5 μg/ml (IC 27.03 %, anti-CD52 48.9 %). Again, anti-CCR7 activity was notably higher than anti-CD52 in the majority of the doses tested.
Fig. 3.
Anti-CCR7 mAb complement-mediated activity is highly effective against CLL cells from patients refractory to both fludarabine and alemtuzumab (FAR). PBMC from patients 10 and 14 (Table 1) were incubated with anti-CCR7, anti-CD52 (alemtuzumab, Alem) or an isotype control (IC) at the indicated concentrations and then exposed to rabbit complement for 1.5 h. Cell lysis was determined in the different populations by staining CD19, CD3 and CD5 and analyzing 7-AAD (7-aminoactinomycin-D) incorporation by FCM. In all cases, the percentage of specific lysis was calculated according to the formula shown in “Materials and methods”. a Specific lysis of CLL cells from patient 10. b Specific lysis of CLL cells from patient 14. In both patients, results from one experiment are shown
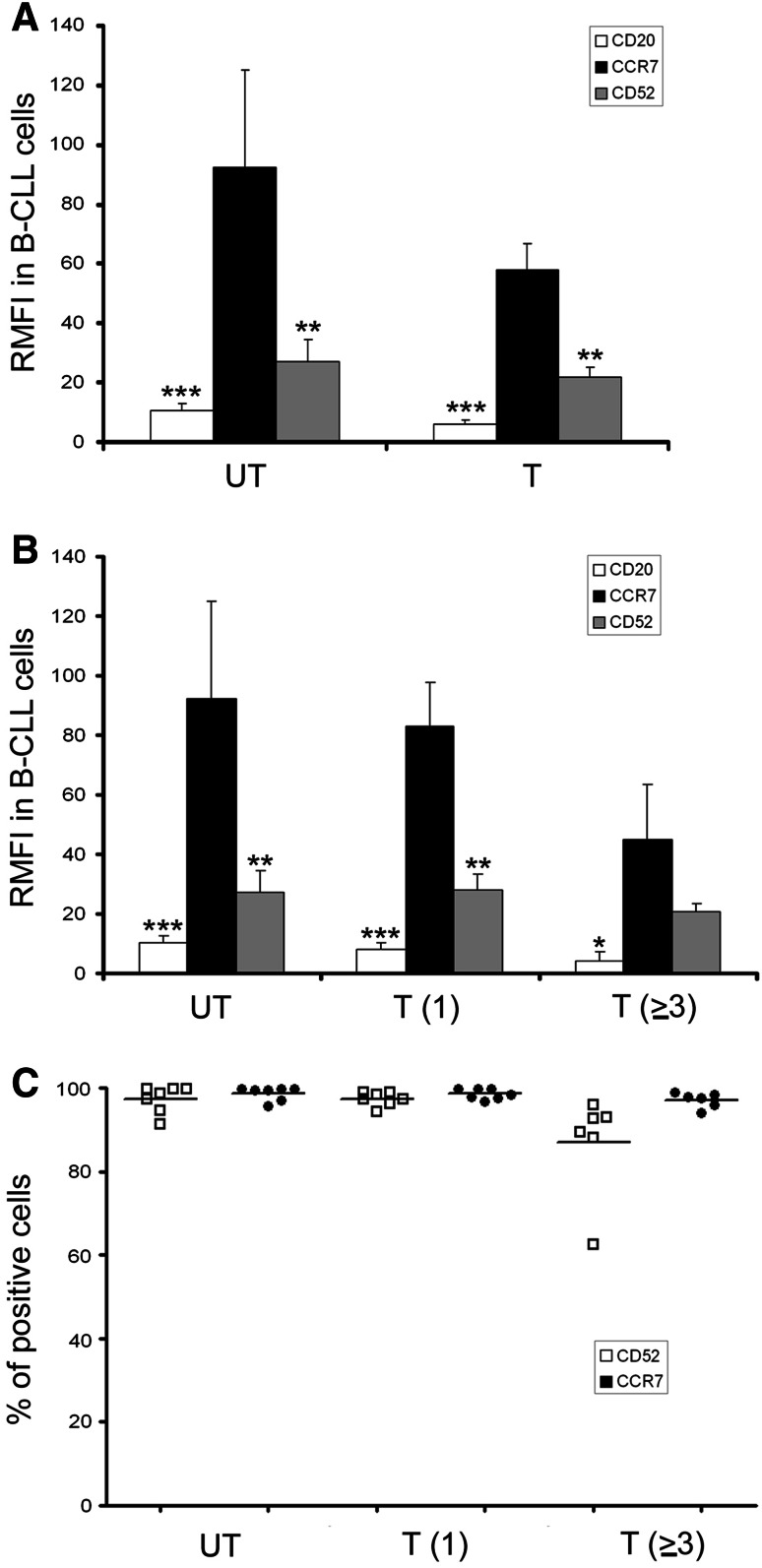
CCR7 surface levels are high in both untreated and treated CLL patients
As shown in this work, cells from high-risk CLL express high CCR7 surface levels. As these patients receive a high number of different treatments, our next question was whether CCR7 remains high in heavily treated patients. For this reason, we determined CCR7 expression in untreated CLL patients, in previously treated patients with one line of therapy and in patients who had received two, three or more different therapies. As seen in Fig. 4a, CCR7 levels were high in both untreated (UT) and treated (T) patients. Moreover, CCR7 expression was high in the majority of patients regardless prior treatments (Fig. 4). In both UT and T, CCR7 levels were significantly higher than CD20 and CD52 levels (p < 0.01), whereas no significant differences were observed in CCR7 between these groups. Interestingly, CCR7 expression remained high even after several treatment regimens (Fig. 4b). CCR7 levels were similar between UT patients and patients who had received one line (92.2 ± 32.7 vs 83.2 ± 14.7) or two lines (data not shown) of treatment. Patients who had received additional treatments (three, four or five), showed a reduction in CCR7 levels (92.2 ± 32.7 in UT vs 45.2 ± 13.7 in T ≥ 3); however, this lost of fluorescence intensity was also observed in the case of CD20 (10.5 ± 2.3 in UT vs 4.3 ± 1.7 in T ≥ 3) and CD52 levels (27.2 ± 7.4 in UT vs 20.8 ± 4.6 in T ≥ 3) (Fig. 4a–b). In addition, our results demonstrated that the proportion of pathological CCR7 + CLL cells was highly homogenous after all the different lines of treatment regardless the therapeutic repertoire used. When we determined the percentage of CLL cells positive for CCR7 and CD52, we observed no differences in untreated patients and patients receiving only one treatment (Fig. 4c). In the case of patients treated with three or more lines of treatment, the percentage of CCR7-positive cells remained close to 100 % in almost all patients, whereas the percentage of CD52-positive cells decreased in a number of patients to around 85 % (Fig. 4c).
Fig. 4.
CCR7 surface levels are high in heavily treated CLL patients. a Relative median fluorescence intensity in CLL cells from untreated or treated patients. PBMC samples from 23 CLL patients were co-stained with anti-CD19-FITC and anti-CD5-APC monoclonal antibodies, and either with a PE-conjugated isotype control mAb, a PE-conjugated antihuman CCR7 mAb, a PE-conjugated antihuman CD52 mAb, or a PE-conjugated antihuman CD20 mAb. Cells were analyzed by FCM, and CCR7, CD52 and CD20 surface density was measured. Relative median fluorescence intensity (RMFI) of each marker respect to the isotype control is represented. Bars represent mean ± mean standard error. UT, untreated patients (n = 7), T, treated patients (n = 16). Statistical analysis was done by comparing CCR7 with CD52 and CD20. *p < 0.05; **p < 0.01; ***p < 0.0001. b CCR7 levels are high in heavily treated patients. The same CLL patients as presented in a were classified according to the number of treatments received. CCR7, CD52 and CD20 surface density was analyzed, and relative median fluorescence intensity (RMFI) is shown. Bars represent mean ± mean standard error. UT, untreated patients (n = 7); T(1), patients treated once (n = 7); T(≥3), patients treated three or more times (n = 6). *p < 0.05; **p < 0.01; ***p < 0.0001. c The number of CCR7-positive CLL cells remains high in heavily treated patients. The same CLL patients analyzed in a were classified according to the number of treatments received, and the percentage and mean of CCR7-positive and CD52-positive cells were determined. UT, untreated patients (n = 7); T(1), patients treated once (n = 7); T(≥3), patients treated three or more times (n = 6)
Anti-CCR7 mAb mediates a slight CDC activity against normal B cells
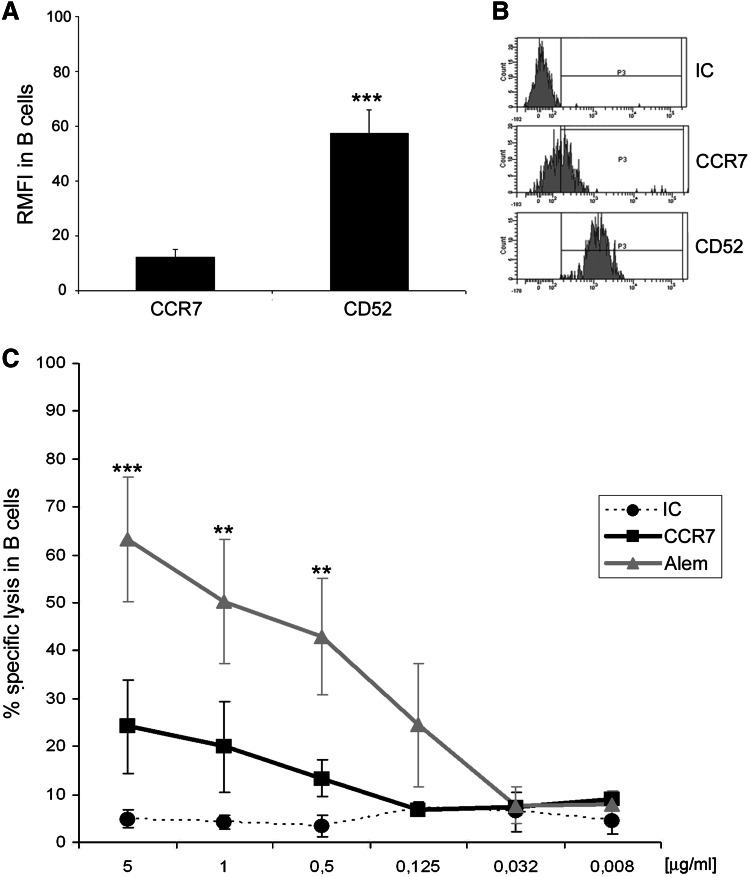
Finally, to complete the evaluation of the potential undesirable effects of anti-CCR7 therapy, we assessed whether normal B cells expressing CCR7 were eliminated by the mAb. As seen in Fig. 5a–b, CCR7 is expressed less in normal B cells compared to CLL cells (Fig. 1). In contrast, CD52 RMFI is 4.7-fold higher than CCR7 (12.12 ± 7.17 in CCR7 vs 57.4 ± 20.7 in CD52) explaining the high CDC activity of anti-CD52 antibodies observed in normal B cells (Fig. 5c). These results confirm our previously stated notion that CDC activity correlates with CCR7 surface density [14] and once more highlight a potential low toxicity associated with anti-CCR7 therapy.
Fig. 5.
Anti-CCR7 mediates a slight complement-mediated cytotoxicity in CD19 + cells from healthy donors. a Relative median fluorescence intensity (RMFI) in healthy B cells. B cells from healthy donors (n = 6) were analyzed by FCM to discriminate their corresponding CCR7 and CD52 surface density measured as median relative to an appropriate isotype control (IC). Bars represent mean ± standard mean error. The statistical analysis is referred to CCR7. ***p < 0.001. b Frequency histograms showing CCR7 and CD52 in a representative healthy donor. The pattern and intensity of each surface marker is shown in B cells from a representative healthy donor. c Percentage of specific lysis in healthy B cells. PBMC from healthy donors (n = 4) were incubated with different concentrations of anti-CCR7, alemtuzumab (anti-CD52, Alem) or the respective isotype control (IC) in the presence of 25 % of either active or heat-inactivated rabbit complement. Percentage of cell lysis as a result of CDC was determined according to the formula stated in “Materials and methods” by 7-AAD incorporation and FCM analysis in gated CD19 + cells. Each dot, square or triangle represents mean ± standard mean error. All statistical analyses are referred to CCR7. *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
CLL patients with a disease refractory to fludarabine CIT and/or 17p- remain a major clinical problem with only a few standard therapeutic options. Newly diagnosed patients with abnormalities in the p53 pathway or FR-CLL patients with acquired high-risk chromosomal abnormalities are expected to have resistance to therapies that depend on DNA damage or interference with its repair [9]. Thus, new therapies with p53-independent mechanisms such as mAb are needed for the treatment of these patients.
In the present work, we tested the activity of an anti-CCR7 antibody in highly challenging CLL patients. Our results indicate that this kind of mAb positions as an interesting therapeutic tool. Firstly, CCR7 outperformed the in vitro cytotoxicity of the humanized anti-CD52 mAb alemtuzumab, the mAb with the highest efficacy in patients with 17p- and FR-CLL [17–20]. Secondly, anti-CCR7 mAb revealed less toxicity in vitro than anti-CD52 mAb in non-tumor cells and postulates a less toxicity in vivo, in part due to the restricted expression of CCR7 in hematological populations [14]. Our results suggest that the treatment of challenging CLL with anti-CCR7 mAb, even at low concentrations, may result in an effective elimination of the tumor cells without lysing CCR7-negative T cells. In contrast, anti-CCR7 therapy eliminates CCR7 + T cells, including the natural regulatory T cells subset that is significantly increased in CLL patients [21] and contributes to the induction and maintenance of the tumor tolerance [22]. This way, one can expect that anti-CCR7 therapy may overcome the tumor tolerance mediated by this subset of cells. Moreover, CCR7 is expressed in the majority of peripheral blood B cells, including naïve, memory, switch memory and transitional B cells [23, 24]. However, specific lysis of normal B cells expressing CCR7 is limited probably as a result of the lower expression of CCR7 in these cells when compared with CLL cells. For this reason, targeting CCR7 could affect B cell homing during antigen-dependent and independent B cell differentiation; however, CCR7-deficient mice show splenic B cell responses upon bacterial challenge [25]. In addition, as B cell bone marrow precursors and plasma cells lack CCR7 [12], it is likely that CCR7 therapy would not affect B cell lymphopoiesis nor immunoglobulin secretory function. Finally, mice treated with anti-CCR7 mAb have not shown any evident unwanted effect caused by the treatment [15].
In addition to CLL patients with 17p-, there is a smaller group of patients with inactivating mutations of the p53 gene, which also have a poor response to conventional therapy and inferior survival [4]. Although we have no data regarding this population, similar results would be expected as in both cases the mechanism of action of anti-CCR7 mAb will be the same and p53-independent. Interestingly, our data show efficacy of the anti-CCR7 mAb in another important component of the p53 pathway, the ATM (ataxia telangiectasia mutated) protein whose gen is located at 11q22 [26]. In five patients with 11q- (Table 1, patients 10, 12, 13, 14 and 16), anti-CCR7 exerted a potent activity confirming that anti-CCR7 therapy may be extended to patients with other high-risk cytogenetics.
CLL that is FAR is associated with a very poor prognosis with a reported median OS of 8 months in this group [7, 11]. In these patients, the fully human anti-CD20 mAb ofatumumab (HuMax-CD20)(Arzerra) is effective as single agent although in the case of patients with 17p-, the reported efficacy is lower [27, 28]. Our preliminary data with this FAR group have also pointed out that anti-CCR7 mAb can be effective even in FAR patients with 17p-.
According to our present and previous data [14], the only factor affecting the magnitude of CDC with anti-CCR7 mAb is the expression levels of the receptor. As CCR7 surface density is notably high in CLL patients regardless the number of received treatments, CCR7 can be defined as an ideal surface target for immunotherapy in this group. However, CCR7 is slightly downregulated in patients previously treated with fludarabine, which is known to decrease membrane expression of some proteins such as CD20, CD55 and CD59 on CLL cells [29] by diminishing mRNA levels. Thereby, this mechanism of action could potentially also affect CCR7. Another drug that theoretically could affect CCR7 levels in heavily treated patients is lenalidomide, which enhances CD20 internalization without influencing transcription [30]. Whatever the mechanism inducing this downregulation is, it seems clear that CCR7 surface levels are high enough to induce a potent CDC activity in these patients.
In its current form, the anti-CCR7 IgG2a mAb used in this study neither is an activator of ADCC [14] (Supplementary Fig. 1) nor has direct pro-apoptotic effects in CLL cells (Supplementary Fig. 2). However, this mAb has a potential additional mechanism to induce CLL cell death by blocking the CCR7 ligands interaction, which increases CLL cell viability through PI3Kδ, ERK and JNK signaling [13, 31]. Nevertheless, current techniques are useful to obtain and optimize the desired characteristics of a therapeutic mAb [32, 33]. Once available, a human anti-CCR7 molecule may be used as a single agent or can be combined with most novel agents in development, particularly with molecules targeting the microenvironment such as CXCR4 antagonists [34] and the emerging BCR pathway inhibitors, such as the kinase inhibitors ibrutinib and idelalisib, that in recent works have demonstrated to improve PFS and OS in high-risk CLL [35–37]. Future studies in CLL combining these agents with an anti-CCR7 mAb are guaranteed as treatment combinations with mAb will be likely more pursued in order to avoid potential acquired resistances [35].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Klaus Schwamborn, PhD, for critical comments on experimental procedures. Funding for this work was provided by Grant Number PI09/01336-PI012/00494 from the Spanish Fondo de Investigaciones Sanitarias del Ministerio de Sanidad y Consumo to Cecilia Muñoz-Calleja.
Conflict of interest
The authors have no competing interests.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- APC
Allophycocyanin
- APC-H7
Allophycocyanin-H7
- ATM
Ataxia telangiectasia mutated protein
- BCR
B cell receptor
- CCR7
Chemokine (C-C motif) receptor 7
- CD
Cluster of differentiation
- CDC
Complement-dependent cytotoxicity
- CIT
Chemoimmunotherapy
- CLL
Chronic lymphocytic leukemia
- CXCR4
Chemokine (C-X-C motif) receptor 4
- ERK
Extracellular-regulated kinase
- FAR
Fludarabine and alemtuzumab refractory CLL
- FCM
Flow cytometry
- FCR
Fludarabine, cyclophosphamide and rituximab
- FISH
Fluorescence in situ hybridization
- FITC
Fluorescein isothiocyanate
- FR-CLL
Fludarabine-refractory CLL
- IC
Isotype control
- JNK
c-Jun N-terminal kinase
- mAb
Monoclonal antibody
- MCL
Mantle cell lymphoma
- OS
Overall survival
- PBMC
Peripheral blood mononuclear cells
- PE
Phycoerythrin
- PFS
Progression-free survival
- PI3Kδ
Phosphatidylinositol-3-OH-kinase delta
- RMFI
Relative median fluorescence intensity
- SLOs
Secondary lymphoid organs
- 17p-
17p13 deletion
- 11q-
11q22 deletion
- 7-AAD
7-Aminoactinomycin-D
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grunhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jager U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Buhler A, Winkler D, Zenz T, Bottcher S, Ritgen M, Mendila M, Kneba M, Dohner H, Stilgenbauer S. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Zenz T BR, Fink A et al (2010) Genetics of patients with F-refractory CLL or early relapse after FC or FCR: results from the CLL8 trial of the GCLLSG. Blood 116:2427 [Abstract]
- 3.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 4.Zenz T, Eichhorst B, Busch R, Denzel T, Habe S, Winkler D, Buhler A, Edelmann J, Bergmann M, Hopfinger G, Hensel M, Hallek M, Dohner H, Stilgenbauer S. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29):4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, O’Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M, Lerner S, Kantarjian H. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(9):1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- 6.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Program. 2010;2010:481–488. doi: 10.1182/asheducation-2010.1.481. [DOI] [PubMed] [Google Scholar]
- 7.Tam CS, O’Brien S, Lerner S, Khouri I, Ferrajoli A, Faderl S, Browning M, Tsimberidou AM, Kantarjian H, Wierda WG. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma. 2007;48(10):1931–1939. doi: 10.1080/10428190701573257. [DOI] [PubMed] [Google Scholar]
- 8.Stilgenbauer S, Zenz T, Winkler D, Buhler A, Schlenk RF, Groner S, Busch R, Hensel M, Duhrsen U, Finke J, Dreger P, Jager U, Lengfelder E, Hohloch K, Soling U, Schlag R, Kneba M, Hallek M, Dohner H. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27(24):3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- 9.Schnaiter A, Stilgenbauer S. Refractory chronic lymphocytic leukemia–new therapeutic strategies. Oncotarget. 2010;1(7):472–482. doi: 10.18632/oncotarget.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenz T, Habe S, Denzel T, Mohr J, Winkler D, Buhler A, Sarno A, Groner S, Mertens D, Busch R, Hallek M, Dohner H, Stilgenbauer S. Detailed analysis of p53 pathway defects in fludarabine-refractory chronic lymphocytic leukemia (CLL): dissecting the contribution of 17p deletion, TP53 mutation, p53-p21 dysfunction, and miR34a in a prospective clinical trial. Blood. 2009;114(13):2589–2597. doi: 10.1182/blood-2009-05-224071. [DOI] [PubMed] [Google Scholar]
- 11.Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Program. 2011;2011:110–118. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-Perez M, Sala-Valdes M, De Soria VG, Fernandez-Ranada JM, Fernandez-Ruiz E, Munoz C. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol. 2004;76(2):462–471. doi: 10.1189/jlb.1203652. [DOI] [PubMed] [Google Scholar]
- 13.Cuesta-Mateos C, Lopez-Giral S, Alfonso-Perez M, de Soria VG, Loscertales J, Guasch-Vidal S, Beltran AE, Zapata JM, Munoz-Calleja C. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol. 2010;38(9):756–764. doi: 10.1016/j.exphem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso-Perez M, Lopez-Giral S, Quintana NE, Loscertales J, Martin-Jimenez P, Munoz C. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79(6):1157–1165. doi: 10.1189/jlb.1105623. [DOI] [PubMed] [Google Scholar]
- 15.Somovilla-Crespo B, Alfonso-Perez M, Cuesta-Mateos C, Carballo-de Dios C, Beltran AE, Terron F, Perez-Villar JJ, Gamallo-Amat C, Perez-Chacon G, Fernandez-Ruiz E, Zapata JM, Munoz-Calleja C. Anti-CCR7 therapy exerts a potent anti-tumor activity in a xenograft model of human mantle cell lymphoma. J Hematol Oncol. 2013;6(1):89. doi: 10.1186/1756-8722-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, Moran M, Lucas M, Lin T, Hackbarth ML, Proffitt JH, Lucas D, Grever MR, Byrd JC. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103(9):3278–3281. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 18.Fiegl M, Erdel M, Tinhofer I, Brychtova Y, Panovska A, Doubek M, Eigenberger K, Fonatsch C, Hopfinger G, Muhlberger H, Zabernigg A, Falkner F, Gastl G, Mayer J, Greil R. Clinical outcome of pretreated B-cell chronic lymphocytic leukemia following alemtuzumab therapy: a retrospective study on various cytogenetic risk categories. Ann Oncol. 2010;21(12):2410–2419. doi: 10.1093/annonc/mdq236. [DOI] [PubMed] [Google Scholar]
- 19.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, Albitar M, Brettman L, Santabarbara P, Wacker B, Rai KR. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.V99.10.3554. [DOI] [PubMed] [Google Scholar]
- 20.Stilgenbauer S, Dohner H. Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med. 2002;347(6):452–453. doi: 10.1056/NEJM200208083470619. [DOI] [PubMed] [Google Scholar]
- 21.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL. Reduced frequencies and suppressive function of CD4 + CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106(6):2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 22.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 23.Corcione A, Ferlito F, Gattorno M, Gregorio A, Pistorio A, Gastaldi R, Gambini C, Martini A, Traggiai E, Pistoia V. Phenotypic and functional characterization of switch memory B cells from patients with oligoarticular juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11(5):R150. doi: 10.1186/ar2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne D, Drinkwater S, Baretto R, Duddridge M, Browning MJ. Expression of chemokine receptors CXCR4, CXCR5 and CCR7 on B and T lymphocytes from patients with primary antibody deficiency. Clin Exp Immunol. 2009;156(2):254–262. doi: 10.1111/j.1365-2249.2009.03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopken UE, Achtman AH, Kruger K, Lipp M. Distinct and overlapping roles of CXCR5 and CCR7 in B-1 cell homing and early immunity against bacterial pathogens. J Leukoc Biol. 2004;76(3):709–718. doi: 10.1189/jlb.1203643. [DOI] [PubMed] [Google Scholar]
- 26.Austen B, Powell JE, Alvi A, Edwards I, Hooper L, Starczynski J, Taylor AM, Fegan C, Moss P, Stankovic T. Mutations in the ATM gene lead to impaired overall and treatment-free survival that is independent of IGVH mutation status in patients with B-CLL. Blood. 2005;106(9):3175–3182. doi: 10.1182/blood-2004-11-4516. [DOI] [PubMed] [Google Scholar]
- 27.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJ, Padmanabhan S, Piotrowska M, Kozak T, Chan G, Davis R, Losic N, Wilms J, Russell CA, Osterborg A. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118(19):5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Gaetano N, Xiao Y, Erba E, Bassan R, Rambaldi A, Golay J, Introna M. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114(4):800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 30.Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, Ramanunni A, Smith LL, Blum W, Andritsos L, Wang DS, Lehman A, Chen CS, Johnson AJ, Marcucci G, Lee RJ, Lee LJ, Tridandapani S, Muthusamy N, Byrd JC. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood. 2008;112(13):5180–5189. doi: 10.1182/blood-2008-01-133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ticchioni M, Essafi M, Jeandel PY, Davi F, Cassuto JP, Deckert M, Bernard A. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26(50):7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 32.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10(2):101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 33.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, Sondermann P, Jager C, Strein P, Fertig G, Friess T, Schull C, Bauer S, Dal Porto J, Del Nagro C, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umana P. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 35.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O’Brien SM. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Izumi R, Hamdy A, Chang BY, Graef T, Clow F, Buggy JJ, James DF, Byrd JC. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.