Abstract
The CD40 receptor is a member of the tumour necrosis factor receptor family and is widely expressed on various cell types. The antitumour activity of CD40 agonist antibody has been observed in B-cell-derived malignancies, but its activity on ovarian cancer remains unclear. However, in this paper, we first confirmed that the anti-CD40 agonist antibody could inhibit the growth of ovarian cancer cells and induce apoptosis. This study investigated the expression of CD40 by ovarian carcinoma tissues and cell lines, at the same time, we evaluated the effect of a recombinant soluble human CD40L (rshCD40L) and an anti-CD40 agonist antibody on cell growth and apoptosis. Flow cytometry and immunohistochemistry assay demonstrated that CD40 was expressed on ovarian carcinoma cell lines and primary ovarian carcinoma cells derived from ascites, as well as on ovarian carcinoma tissues. The growth inhibition of rshCD40L and the anti-CD40 agonist antibody on ovarian carcinoma cells was examined by MTT assay, and the proportion of apoptotic tumour cells was analysed by flow cytometry and Hoechst staining. Our study showed that CD40 was expressed on all ovarian carcinoma cell lines and was examined in 86.2% (162/188) of ovarian cancer tissue samples, but not in normal ovarian tissues (n = 20). Treatment with rshCD40L or anti-CD40 agonist antibody significantly inhibited ovarian carcinoma cell growth and induced apoptosis. Theses results suggest that CD40 is expressed on ovarian carcinoma cells, moreover, that rshCD40L and anti-CD40 agonist antibody have therapeutic potential to inhibit human ovarian cancer growth.
Keywords: Ovarian carcinoma, CD40L, CD40, Agonist antibody
Introduction
Ovarian carcinoma is a leading cause of cancer-related death among women. In 2009, more than 27,000 new cases were diagnosed in china, and nearly 14,800 deaths were attributable to ovarian cancer. Although patients with advanced disease respond initially to surgery followed by chemotherapy, 60–80% will relapse and ultimately die from complications associated with disease progression. Overall, the 5-year survival rate is less than 30% [1–3]. On this basis, new therapeutic approaches to conquer ovary carcinoma are vital for women’s health.
The 48-kDa type I trans-membrane glycoprotein CD40 is a member of the tumour necrosis factor (TNF) receptor family. It is expressed on a variety of normal cells, including B cells, dendritic cells, macrophages, stromal cells, epithelial cells, and endothelial cells [4, 5]. Activation of CD40 in resting B cells provides costimulatory signals for proliferation of B cells and isotype switching from IgM to IgG and IgE production [6]. Ligation of CD40 on the surface of antigen-presenting cells (APC) enhances the maturation of APCs and the expression of MHC and adhesion costimulatory molecules (including ICAM-1, CD23, CD62E, CD80, CD86, and CD106) and stimulates the production of various cytokines (GM-CSF, IL-1, IL-4, IL-6, IL-8, IL-10, IL-12, RANTES, TNFα). Ligation of CD40 on APCs can also enhance the cytolytic function of NK and NKT cells via cytokine induction [7–9]. Agonists of CD40 have been shown to replace the need for helper T cells in the generation of an effective CD8 T cell response [10].
In addition to its role in enhancing immune function, CD40 is also expressed by a large number of melanomas and carcinomas of the ovary, lung, colon, breast, prostate, kidney, and head and neck, as well as all of B-cell malignancies [11–16]. In contrast to the proliferative effects of CD40L to CD40 binding on normal B lymphocytes, recent data suggest that CD40L to CD40 binding on certain tumour cells can induce cell cycle arrest, apoptosis, and increase cell surface expression of MHC and costimulatory molecules that can enhance tumour cell recognition [17–19]. Thus, CD40 agonists may provide a dual and inherently synergistic therapy for cancer by facilitating the release of tumour antigens, increasing tumour recognition by immune cells, and enhancing antigen-presenting cell activity [20]. Indeed, several groups have demonstrated the effectiveness of CD40 activation for inducing antitumour immune responses using either anti-CD40 activating antibodies, soluble CD40 ligand proteins, or an adenovirus expressing a CD40 ligand [21–23]. These effects of CD40 activation on malignant cells suggest that CD40 agonists may be effective for cancer therapy. But there has been no study about the effectiveness of anti-CD40 agonist antibody on ovarian carcinoma, our goal was to confirm whether the anti-CD40 agonist antibody could inhibit the growth of ovarian cancer and induce apoptosis.
In this study, we examined the expression of CD40 and CD40L in both established human ovarian carcinoma cell lines and freshly obtained ovarian carcinoma cells isolated from ascites fluid of ovarian cancer patients. We also examined the expression of CD40 in ovarian cancer tissues and the effects of rhsCD40L or anti-CD40 agonist antibody on ovarian carcinoma cell growth and apoptosis. We found that CD40 was highly expressed in most ovarian carcinoma cells, but there was no detectable CD40L expression. Expression of CD40 was detected in 86.2% (162/188) of ovarian cancer tissues, but not in normal ovarian tissues (n = 20). The rhsCD40L and anti-CD40 agonist antibody treatment both significantly inhibited tumour cell growth and enhanced tumour cell apoptosis. These data suggested that the anti-CD40 agonist antibody could inhibit the growth of ovarian cancer cells and induce apoptosis as the CD40L. It indicated that the CD40 is an attractive target for ovarian carcinoma therapy, and the rhCD40L and anti-CD40 agonist antibody are potential candidates for clinical treatment in patients with ovarian carcinoma.
Materials and methods
Cell lines
The established human ovarian carcinoma cell lines SKOV3, 8910, COC1, 2780, and OVCAR3, and the breast carcinoma cell line MCF-7 were purchased from the American Type Cell Collection (ATCC, Manassas, VA, USA). The human ovarian carcinoma cell lines OV112, OV126, and OV128 were isolated from the poorly differentiated ovarian cancer ascites of patients. The epithelial origin of human ovarian carcinoma cell lines was verified by morphological studies, as well as immunostaining with monoclonal antibodies (mAbs) directed against the epithelial marker epithelial cell adhesion molecule (EpCAM). All cell lines were grown in DMEM (Gibco, Grand Island, NY) supplemented with 10% foetal calf serum (Sigma, St. Louis, MO) at 37°C under a 5% CO2 atmosphere.
Tissue Microarrays (TMAs)
The ovarian cancer TMA consisted of 188 human ovarian tumour tissues and 20 normal ovarian tissues (Shanxi Chaoying Biotechnology Co., LCD). The paraffin-embedded ovarian cancer tissues were from 94 patients with a mean age of 50 years (range, 21–76). The control paraffin-embedded normal ovarian tissues were from ten patients with mean age of 23 years (range, 15–40). Tumour stage and grade were defined according to International Union Against Cancer and World Health Organization classifications.
Reagents
The recombinant soluble human CD40 ligand (rshCD40L) was obtained from Amgen (Thousand Oaks, CA). The antibodies used were obtained from the following sources: the non-conjugated mouse anti-human CD40-antibody was generated by our laboratory; fluorescein isothiocyanate (FITC)-conjugated murine anti-human CD40L-antibody was obtained from Serotec (Oxford, UK); PE-conjugated anti-human EPCAM antibody was obtained from Dako (clone VU-1D9; StemCells, Grenoble, France); FITC-conjugated sheep anti-mouse IgG was from Sigma (Poole, UK).
Flow cytometric detection of CD40 and CD40L expression
Flow cytometry was used to assess the expression of cell surface markers on tumour cell lines, established human ovarian carcinoma cell lines (OVCAR3, 2780, 8910, COC1, and SKOV-3), and three early passage ovarian tumour cells (OV112, OV126 and OV128) isolated from the poorly differentiated ovarian cancer ascites of three ovarian carcinoma patients. All donors gave their written informed consent before collection in accordance with the Declaration of Helsinki, and the present study was approved by the local Ethics Committee of Huaxi Hospital, Sichuan University. Briefly, 1 × 106 tumour cells were washed with phosphate-buffered saline (PBS) and resuspended in 100 μl PBS. The mouse anti-human CD40 antibody or isotype control antibody was then added to the cells, and the suspension was incubated for 30 min at room temperature. After 30 min, the cells were washed twice with PBS buffer and resuspended in 100 μl PBS. The FITC-conjugated sheep anti-mouse IgG was then added to the cells, and the PE-conjugated anti-human EPCAM antibody was also added to the freshly isolated human ovarian carcinoma cell lines. Then, the suspensions were incubated for another 30 min at room temperature. For assessing the expression of CD40L on tumour cells, the FITC-human CD40L-antibody or FITC-isotype control antibody was used. Expression of CD40 or CD40L was then analysed by flow cytometry (Becton–Dickinson).
Immunohistochemistry
Sections of tissue blocks were deparaffinized in xylol then rehydrated in serial dilutions of ethanol. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 15 min at room temperature. Antigenic sites were revealed by heating sections in a culinary autoclave with 10 mM EDTA citrate buffer, pH 6.0, for 3 min after pressurization. After incubation with goat non-immune serum for 15 min at 37°C, sections were incubated with mouse anti-human CD40 antibody at 1/100 dilution for 2 h at 37°C. Sections were then incubated with secondary biotinylated goat anti-mouse IgG antibody at 37°C for 20 min, followed by streptavidin–biotin-horseradish peroxidase complex at 37°C for 20 min. Peroxidase activity was revealed by 3,3′-diaminobenzidine (Sigma), and cells were counterstained with haematoxylin. Negative controls were processed with Tris buffer instead of the primary antibody. Saturation and intensity of immunostained cells were evaluated over eight visual fields at a power of 400× under a light microscope (Olympus Optical, Tokyo, Japan). For statistical analysis, total staining of CD40 was scored as the product of the staining intensity (on a scale of 0–3: negative = 0, weak = 1, moderate = 2, and strong = 3) × the percentage of cells stained (positively recorded on an ordered categorical scale: 0—zero, 1 = 1–25%, 2 = 26–50%, and 3 = 51–100%), resulting in a scale of 0–9. The evaluation was performed by two independent investigators.
Cell viability analysis by MTT assay
The MTT assay was used to measure cell growth as described previously. Briefly, cells were plated at 2–5 × 103 cells/well in 96-well plates and allowed to adhere overnight. The following day, cells were treated with recombinant soluble human CD40L (rshCD40L, 1 μg/ml) (Immunex, Seattle, USA), an agonist CD40-antibody (1 μg/ml), or vehicle control and incubated for a further 48 h. To determine cell growth, 20 μl of 10 mg/ml MTT (3-(4,4-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Sigma) was added to each well, and the plates were incubated at 37°C under 5% CO2 for 4 h. The supernatant was removed, and the formed formazan crystals were dissolved in 200 μl dimethyl sulphoxide. The absorbance was then measured at 450 nm on a Becton–Dickinson microplate reader. Growth inhibition was calculated by expressing the differences in optical densities between treatment wells and control wells as a percentage of the control.
Apoptosis analysis by Hoechst staining
The human ovarian carcinoma cell lines and breast carcinoma cell line (MCF-7) were treated for 48 h with control medium, recombinant soluble human CD40 ligand (rshCD40L, 1 μg/ml), or anti-CD40 agonist antibody (1 μg/ml). Hoechst staining was performed according to the manufacturer’s instructions (Invitrogen). The cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan). Apoptotic nuclei will be surrounded by small pinpoint dots of fluorescence, either aggregated in clusters or rows, and the morphology may range from spherical bodies to filamentous-like forms. The negative apoptotic cells will show only diffuse nuclear staining.
Apoptosis analysis by flow cytometry
The ovarian carcinoma cell lines and breast carcinoma cell line MCF-7 were treated with control medium, recombinant soluble human CD40 ligand (rshCD40L, 1 μg/ml), or anti-CD40 agonist antibody (1 μg/ml). After 48 h, flow cytometric analysis was performed to detect sub-G1cells/apoptotic cells. Briefly, cells were suspended in 1 ml hypotonic fluorochrome solution containing 50 μg/ml propidium iodide in 0.1% sodium citrate plus 0.1% Triton X-100 and analysed by a flow cytometer. Apoptotic cells were defined as cells with DNA content less than that of G1 phase cells in the cell cycle distribution.
Statistical analysis
Data were expressed as the mean ± SD from at least three independent experiments. Differences between treatment groups in growth inhibition and apoptosis assays were analysed using analysis of variance (ANOVA). One-way analysis of variance was also used to analyse the staining CD40 differences between groups. A P < 0.05 was considered statistically significant. All statistical analyses were calculated by the SPSS 16.0 software package.
Results
CD40 and CD40L expression in ovarian tumour cell lines
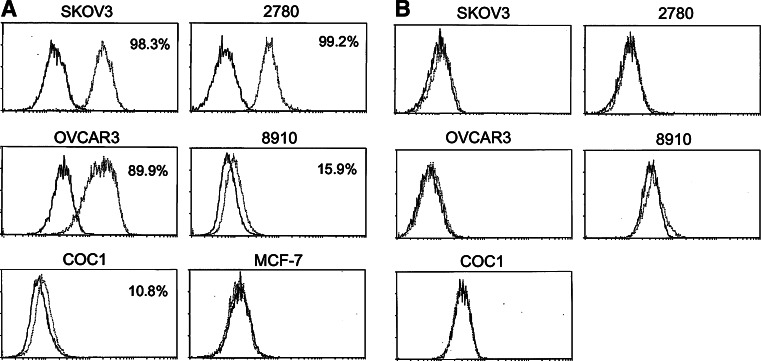
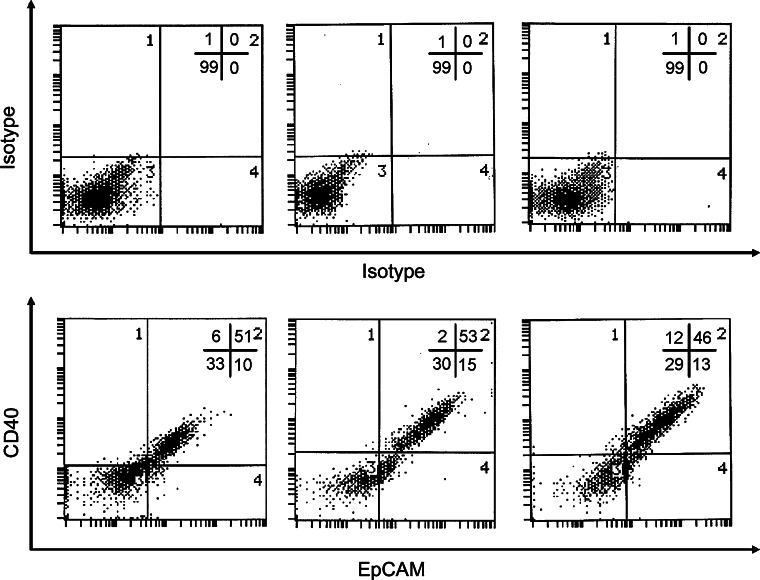
The expression of CD40 and CD40L in ovarian carcinoma cell lines was determined by flow cytometry. Five long-established lines (OVCAR3, 2780, 8910, COC1, and SKOV-3) and three early passage ovarian tumour cells isolated from poorly differentiated ovarian cancer ascites (OV112, OV126 and OV128) were analysed. The breast carcinoma cell line MCF-7 did not express CD40 and was used as a control. The five established ovarian carcinoma cell lines displayed variable levels of CD40 expression (Fig. 1a). Three of them highly expressed CD40, with approximately 90% of cells graded as CD40 positive (Fig. 1a). In contrast, CD40L expression was not detected in any of the cell lines examined (Fig. 1b). High expression of CD40 was also examined in ovarian carcinoma cells dissociated freshly from poorly differentiated ovarian cancer ascites. Because single cell suspension from ascitic fluid of ovarian cancer patients may contain mixed cell populations consisting of epithelial cells and stromal cells, the epithelial marker EpCAM was used to identify ovarian carcinoma cells. Double-staining revealed that 70–85% of EpCAM-positive ovarian carcinoma cells expressed CD40 (EpCAM/CD40 double-positive cells) (Fig. 2). The results showed that the poorly differentiated ovarian cancer cells displayed high levels of CD40 surface expression.
Fig. 1.
Expression of CD40 and CD40L on ovarian carcinoma cell lines. Cell surface expression of CD40 and CD40L on human ovarian carcinoma cell lines and breast carcinoma cell line (MCF-7) were studied separately using anti-CD40 (a) or anti-CD40L monoclonal antibodies (b) (open curve). Background staining is indicated (filled curve). The proportions of cells expressing the cell surface marker are indicated in each histogram
Fig. 2.
Expression of EpCAM and CD40 by isolated ovarian carcinoma primary cells. The ovarian carcinoma cell lines (OV112, OV126, and OV128) were dissociated from ascitic fluid of three ovarian cancer patients. Cell surface expression of EpCAM and CD40 on isolated ovarian carcinoma primary cells was studied by double-staining using anti-EpCAM and anti-CD40 monoclonal antibodies (lower panel). Background staining is indicated (upper panel). Results are expressed in dot blot form. The proportions of cells expressing the cell surface marker in each quadrant are indicated
CD40 expression in ovarian carcinoma tissues
Expression of CD40 in paraffin-embedded ovarian tissues was investigated by immunohistochemistry. In total, 208 paraffin wax-embedded sections of normal ovarian tissues and ovarian carcinomas of a variety of histological subtypes were analysed. Expression of CD40 was detected in 162 of 188 (86.2%) of ovarian carcinoma cases, but not in any of the 20 normal ovarian tissue samples. The expression difference between ovarian carcinoma tissues and normal ovarian tissues was significant (P < 0.001) (Fig. 3, Table 1). Figure 3 shows four examples representing the spectrum of normal ovarian tissues, well-differentiated ovarian carcinoma tissues, moderately differentiated ovarian carcinoma tissues, and poorly differentiated ovarian carcinoma tissues (Fig. 3). Well-differentiated and moderately differentiated ovarian carcinoma tissues exhibited lower CD40 expression, whereas a higher CD40 expression was seen in poorly differentiated ovarian carcinoma (Fig. 3). The staining score of CD40 was 4.04 ± 2.815 in the poorly differentiated ovarian carcinoma tissue group but was only 2.64 ± 2.632 in the well-differentiated group, indicating that CD40 expression was significantly correlated with histologic grade (P = 0.007) (Table 2). However, CD40 expression was not correlated with FIGO stage and TNM stage, and CD40 expression was also not correlated with age (Table 2).
Fig. 3.
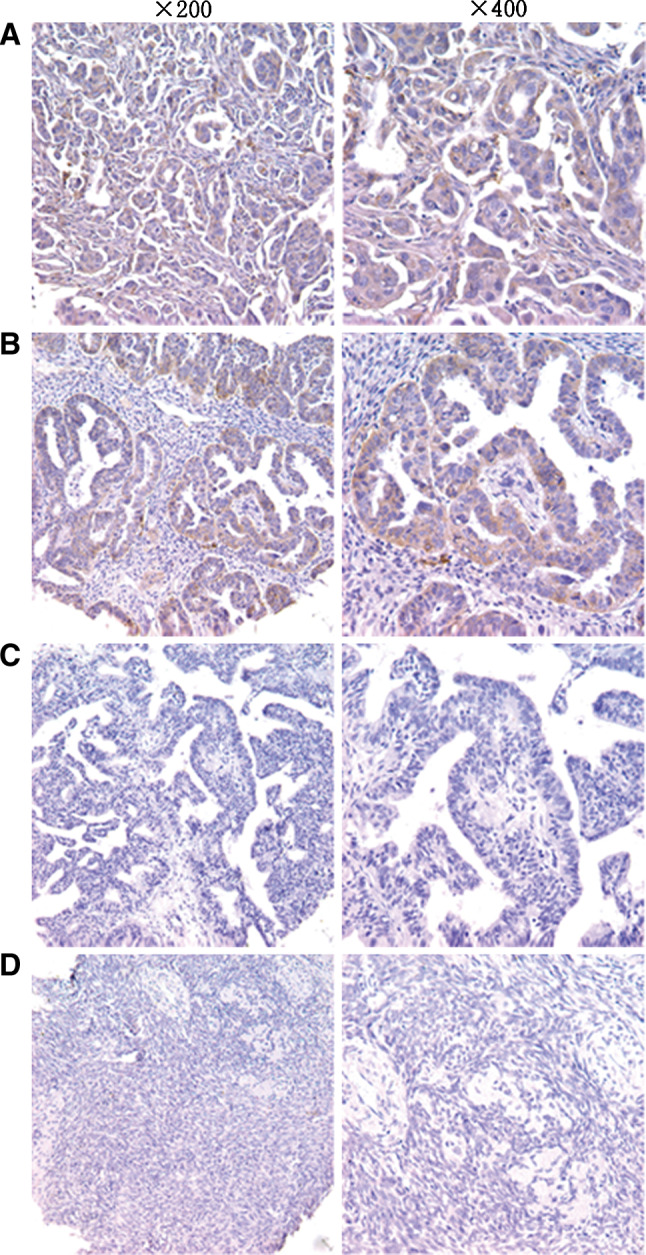
CD40 is expressed in different ovarian tissues. Paraffin wax-embedded ovarian carcinoma tissues were stained by immunohistochemistry using a CD40-antibody. The CD40-positive cells are brown-stained. Staining of four representative tissues is shown: a poorly differentiated ovarian carcinoma tissues, b moderately differentiated ovarian carcinoma tissue, c well-differentiated ovarian carcinoma tissue, and d normal ovarian tissue
Table 1.
The expression of CD40 in different ovarian tissues by immunohistochemistry
| Histological type | Positive (n) | Negative (n) | Total (n) | P |
|---|---|---|---|---|
| Normal ovarian tissues | 0 | 20 | 20 | 0.000 |
| Ovarian carcinoma tissues | 162 | 26 | 188 |
Table 2.
Immunostaining of CD40 in ovarian carcinoma TAM: correlation with clinicopathological parameters
| Factors | Number | Staining score | P value |
|---|---|---|---|
| Age | |||
| ≤40 years | 37 | 3.32 ± 2.63 | 0.63 |
| 41–59 years | 115 | 3.29 ± 2.85 | |
| ≥60 years | 36 | 2.81 ± 2.84 | |
| Differentiation | |||
| Well | 21 | 2.81 ± 2.63 | 0.007 |
| Moderate | 76 | 2.64 ± 2.63 | |
| Poor | 70 | 4.04 ± 2.81 | |
| T stage | |||
| T1 | 84 | 3.19 ± 2.76 | 0.38 |
| T2 | 34 | 3.59 ± 3.12 | |
| T3 | 54 | 2.63 ± 2.43 | |
| T4 | 15 | 3.43 ± 3.40 | |
| N (lymph node metastasis) | |||
| N0 | 15 | 2.80 ± 3.21 | 0.85 |
| N1 | 127 | 3.16 ± 2.75 | |
| N2 | 46 | 3.26 ± 2.86 | |
| Clinical stage | |||
| I | 88 | 3.34 ± 2.80 | 0.59 |
| II | 26 | 3.23 ± 2.98 | |
| III | 54 | 3.09 ± 2.74 | |
| IV | 16 | 2.31 ± 2.49 | |
CD40 agonist treatment inhibited ovarian tumour cell growth
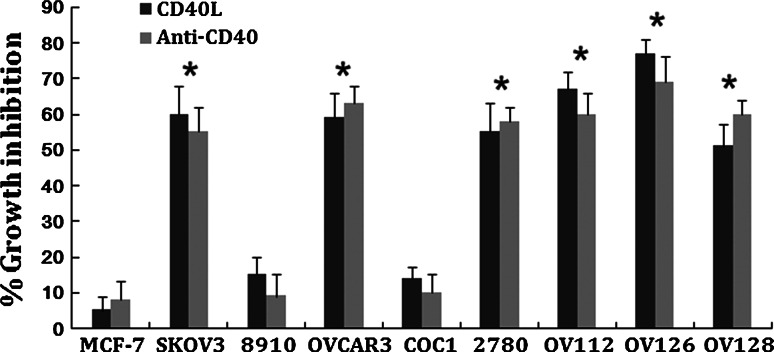
The expression of CD40 on ovarian carcinomas prompted us to examine the effects of activation of this pathway on cell growth. For this purpose, we used rshCD40L and an anti-CD40 agonist antibody. Ovarian carcinoma cell lines were treated with 1 μg/ml rshCD40L or 1 μg/ml anti-CD40 agonist antibody, and cell growth was determined 48 h later using MTT assays. Treatment of CD40-positive tumour cells with rshCD40L or anti-CD40 agonist antibody significantly inhibited proliferation (Fig. 4). A significant reduction in growth of OVCAR3, 2780, SKOV-3, OV112, OV126, and OV128 lines was also observed compared to the CD40 negative tumour cell line MCF-7 (Fig. 4, P < 0.05). Growth inhibition ranged from 40 to 75% after treatment with rshCD40L or anti-CD40 agonist antibody (Fig. 4). The results showed that the anti-CD40 agonist antibody and rshCD40L could significantly inhibit the growth of ovarian cancer cells.
Fig. 4.
CD40 agonist treatment inhibited ovarian tumour cell growth. The ovarian carcinoma cell lines and breast carcinoma cell line (MCF-7) were treated for 48 h with 1 μg/ml recombinant soluble human CD40 ligand (rshCD40L) or 1 μg/ml anti-CD40 agonist antibody, and cell growth was assessed by MTT assay. Data are expressed as per cent growth inhibition. Data are from three independent experiments (mean + standard deviation). *P < 0.05 versus MCF-7
CD40 agonist treatment induced apoptosis
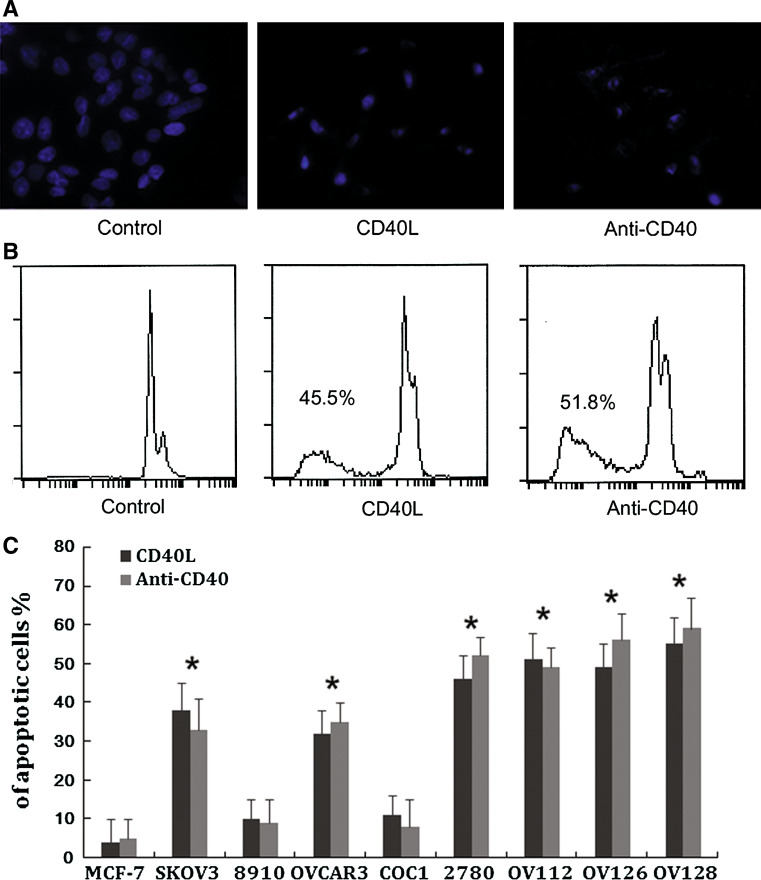
The pro-apoptotic effects of rshCD40L or anti-CD40 agonist antibody on ovarian carcinoma cells were quantified by Hoechst staining and flow cytometry. An increase in the percentage of apoptotic cells was observed after treatment with 1 μg/ml rshCD40L or 1 μg/ml anti-CD40 agonist antibody for 24 h (Fig. 5). The apoptosis rate of the CD40-expressing cell lines OVCAR3, 2780, SKOV-3, OV112, OV126, and OV128 ranged from 33.30 to 59.63% (Fig. 5) which was significantly higher than the apoptotic rate observed for the CD40 negative tumour cell line MCF-7 following the same treatment (P < 0.05). The study indicated that the anti-CD40 agonist antibody and rshCD40L could induce apoptosis of ovarian cancer cells.
Fig. 5.
CD40 agonist treatment induced apoptosis. The ovarian carcinoma cell lines and breast carcinoma cell line (MCF-7) were treated for 48 h with 1 μg/ml recombinant soluble human CD40 ligand (rshCD40L) or 1 μg/ml anti-CD40 agonist antibody or control medium. a Hoechst staining was used to assess apoptosis under a fluorescence microscope. Apoptotic cells had nuclei surrounded by small pinpoint dots of fluorescence, either aggregated in clusters or rows, and the morphology ranged from spherical bodies to filamentous-like forms. Non-apoptotic cells showed only diffuse nuclear staining. b Flow cytometric detection was also used to assess apoptosis. Flow cytometric analysis revealed the proportion of sub-G1 cells (apoptotic cells) to be 3.0% (NS, left panel), 45.5% (rshCD40L, middle panel), and 51.8% (CD40 antibody, right panel). c Data are expressed as per cent growth inhibition. Data are from three independent experiments (mean ± standard deviation). *P < 0.05 versus MCF-7
Discussion
The CD40 is a co-stimulatory molecule well known for its contribution to the regulation of the immune responses [24], yet the biological role of CD40 in carcinoma cells is unclear. Several studies have documented CD40 expression in a range of cancer types [25–29]. Our results demonstrated that CD40 was present at high levels in paraffin-embedded ovarian carcinoma tissues, suggesting that CD40 may contribute to the pathogenesis of ovarian carcinoma. Cytofluorometry analysis also revealed that this molecule was highly expressed on ovarian carcinoma cell lines tested, including three well-established cell lines and three isolated from ascites. In contrast, CD40L was not endogenously expressed. We were able to demonstrate that CD40 was functional in these cell lines, as ligation by anti-CD40 agonist antibody resulted in growth inhibition and apoptosis as the rshCD40L. Furthermore, the sensitivity to the growth-inhibitory or pro-apoptotic signals mediated by CD40L or anti-CD40 agonist antibody binding was correlated to the level of CD40 expression.
In this paper, we studied the expression of CD40 in ovarian cancer from cell lines, primary cells, tumour tissues. In ovarian cancer cell lines, the expression of CD40 was up to 90% in three cell lines. And for the three primary ovarian cancer cells, CD40 expression level was as high as 80% in all three primary cells. Expression of CD40 in 208 paraffin wax-embedded sections of normal ovarian tissues and ovarian carcinomas of a variety of histological subtypes were investigated by immunohistochemistry. Our result indicated that the rate of CD40 expression was 86.2% in ovarian cancer tissues, but CD40 expression was not detected or was expressed at very low level by normal ovarian tissues. In summary, the study indicated that the 80% ovarian cancer cells showed a high expression level of CD40. And the results of anti-CD40 agonist antibody treatment illustrated that the effectiveness of anti-CD40 agonist antibody therapy depended on the expression level of CD40 on the surface of cancer cells. So these data suggest that the CD40 is an attractive target for ovarian carcinoma therapy and that the anti-CD40 agonist antibody and CD40L are potential candidates for clinical treatment in patients with ovarian carcinoma.
On another way, our results indicated the CD40 expression was significantly correlated with histologic grade of ovarian carcinoma tissues. In the poorly differentiated ovarian carcinomas, CD40 was overexpressed compared to the well-differentiated ovarian carcinomas. The staining score of CD40 was 4.04 ± 2.815 in the poorly differentiated ovarian carcinoma tissue group but was only 2.64 ± 2.632 in the well-differentiated group. Several studies have shown that the major impacts on the prognosis of ovarian cancer are the tumour differentiation, clinical stage and residual tumour size. The tumour differentiation directly effects on survival rate and survival time of patients. The 5-year survival rate with well-differentiated tumour is 60%, and with poorly differentiated tumour is 7%. Therefore, poorly differentiated ovarian cancer is in a relatively high degree of malignancy and is a class of relatively poor prognosis. Taken together, these results indicated that the CD40 agonist antibody and CD40L therapy should have a higher efficacy on poorly differentiated ovarian carcinoma. Moreover, the CD40 is a potential target for ovarian carcinoma therapy, specifically for poorly differentiated ovarian carcinoma.
The mechanism by which ligation to CD40 induces cell death in carcinoma has been studied previously. Although CD40 does not have its own intrinsic death domain, the adapter protein of the TRAF (TNF receptor-associated factor) family, mostly notably TRAF2 and TRAF6, appeared to mediate the activation of CD40 [30, 31]. The results from the latter study also suggested that the cytotoxic activity induced by CD40 ligation was related to induction of Fas and Fas ligand, TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL), and that the binding of CD40 could up-regulate the expression of Fas23 [32].
The antitumour activity of CD40 agonist antibody has so far been observed in preclinical models of B-cell derived malignancies [33, 34]. For example, SGN-40 is a humanized IgG1 immunoglobulin and a partial agonist of CD40 that induced apoptosis and antibody-dependent cellular cytotoxicity against a panel of malignant B cell lines and resulted in tumour regression in human multiple myeloma and lymphoma [35, 36]. But little is known about the activity of CD40 agonist antibody in epithelial tumours, and no study reported the activity of CD40 agonist antibody on ovarian carcinoma. However, our results for the first time demonstrated that a CD40 agonist antibody could decrease tumour growth and induce apoptosis through CD40 on ovarian carcinoma cells. In murine models of T cell-mediated immunity, the growth-inhibitory effects of CD40 agonist antibody have been shown to engage the same signalling pathways as CD40L [37]. In addition, CD40 agonist antibodies can overcome T cell tolerance in tumour-bearing mice, evoke effective cytotoxic T cell responses, contribute to antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity, and enhance the efficacy of anti-tumour vaccines [38–41].
In conclusion, we have demonstrated that ovarian carcinoma cell lines and clinical tumour specimens expressed high levels of CD40, and that expression was correlated with the degree of differentiation. We also verified that there was no CD40L expressed in ovarian carcinoma cell lines. The CD40 agonist antibody and CD40L therapy could inhibit growth and promote apoptosis of ovarian carcinoma cells. Taken together, the present data suggest that CD40 activation may be one of the major pathways inhibiting cell growth in ovarian carcinoma, and the CD40 agonist antibody and CD40L are potential candidates for clinical treatment in patients with ovarian carcinoma.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Yan Zhou and Jing He contributed equally to this paper.
References
- 1.Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G, Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, Nick AM, Stone RL, Lu C, Lutgendorf SK, Cole SW, Lokshin AE, Sood AK. Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res. 2009;15(8):2695–2702. doi: 10.1158/1078-0432.CCR-08-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, Deavers M, Malpica AL, Kavanagh JJ. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, Baalbergen A, Burger CW, Ansink AC. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009;116(3):372–380. doi: 10.1111/j.1471-0528.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 4.Chiodoni C, Iezzi M, Guiducci C, Sangaletti S, Alessandrini I, Ratti C, Tiboni F, Musiani P, Granger DN, Colombo MP. Triggering CD40 on endothelial cells contributes to tumor growth. J Exp Med. 2006;203(11):2441–2450. doi: 10.1084/jem.20060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisch T, Kropff B, Sinzger C, Mach M. Upregulation of CD40 expression on endothelial cells infected with human cytomegalovirus. J Virol. 2002;76(24):12803–12812. doi: 10.1128/JVI.76.24.12803-12812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120(4):885–891. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luczynski W, Kowalczuk O, Ilendo E, Stasiak-Barmuta A, Krawczuk-Rybak M. Upregulation of antigen-processing machinery components at mRNA level in acute lymphoblastic leukemia cells after CD40 stimulation. Ann Hematol. 2007;86(5):339–345. doi: 10.1007/s00277-007-0256-z. [DOI] [PubMed] [Google Scholar]
- 8.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38(2):400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burysek L, Syrovets T, Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J Biol Chem. 2002;277(36):33509–33517. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- 10.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164(2):725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 11.Villarroel Dorrego M, Speight PM, Barrett AW. CD40 in human oral epithelia. Oral Oncol. 2007;43(7):626–633. doi: 10.1016/j.oraloncology.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee JK, Seki N, Sayers TJ, Subleski J, Gruys EM, Murphy WJ, Wiltrout RH. Constitutive expression of functional CD40 on mouse renal cancer cells: induction of Fas and Fas-mediated killing by CD40L. Cell Immunol. 2005;235(2):145–152. doi: 10.1016/j.cellimm.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Bereznaya NM, Kirnasovskaya EA, Vinnichuk YD, Belova OB, Lukyanova NY. Expression of CD40 by the cells of benign and malignant breast tumors and antitumor action of autologous lymphocytes against chemoresistant and chemosensitive tumors. Exp Oncol. 2008;30(4):295–299. [PubMed] [Google Scholar]
- 14.Jang TJ. Expression of CD40 and Fas ligand in Bowen’s disease, squamous cell carcinoma and basal cell carcinoma. Yonsei Med J. 2002;43(3):304–308. doi: 10.3349/ymj.2002.43.3.304. [DOI] [PubMed] [Google Scholar]
- 15.Wu JQ, Zhao WH, Yin KS, Cheng YL. Adeno-associated virus-mediated CD40 ligand transfer into human lung cancer cells. Zhonghua Zhong Liu Za Zhi. 2007;29(4):253–257. [PubMed] [Google Scholar]
- 16.Zhou Z, Shi Q, Wang J, Pan J, Zhang X (2000) Anti-CD40 McAb induces CD40+ malignant B cell proliferation arrest and apoptosis. Zhonghua Xue Ye Xue Za Zhi 21(5):237–239. doi:10.1016/j.oraloncology.2006.12.008 [PubMed]
- 17.Dzojic H, Loskog A, Totterman TH, Essand M. Adenovirus-mediated CD40 ligand therapy induces tumor cell apoptosis and systemic immunity in the TRAMP-C2 mouse prostate cancer model. Prostate. 2006;66(8):831–838. doi: 10.1002/pros.20344. [DOI] [PubMed] [Google Scholar]
- 18.Villarroel Dorrego M, Whawell SA, Speight PM, Barrett AW. Transfection of CD40 in a human oral squamous cell carcinoma keratinocyte line upregulates immune potency and costimulatory molecules. Br J Dermatol. 2006;154(2):231–238. doi: 10.1111/j.1365-2133.2005.07006.x. [DOI] [PubMed] [Google Scholar]
- 19.Mayr C, Kofler DM, Buning H, Bund D, Hallek M, Wendtner CM. Transduction of CLL cells by CD40 ligand enhances an antigen-specific immune recognition by autologous T cells. Blood. 2005;106(9):3223–3226. doi: 10.1182/blood-2005-04-1742. [DOI] [PubMed] [Google Scholar]
- 20.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167(5):2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 21.von Euler H, Sadeghi A, Carlsson B, Rivera P, Loskog A, Segall T, Korsgren O, Totterman TH. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J Immunother. 2008;31(4):377–384. doi: 10.1097/CJI.0b013e31816a812d. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Xi H, Ma QR, Chen C, Zhang F, Zhang XG, Gu ZJ. Therapeutic effect of agonistic CD40 monoclonal antibody combined with CTL on hu-SCID mouse B lymphoma model. Zhonghua Zhong Liu Za Zhi. 2007;29(3):181–185. [PubMed] [Google Scholar]
- 23.Wu Y, Wang L, He X, Xu H, Zhou L, Zhao F, Zhang Y. Expression of CD40 and growth-inhibitory activity of CD40 ligand in colon cancer ex vivo. Cell Immunol. 2008;253(1–2):102–109. doi: 10.1016/j.cellimm.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Chen WC, Pang XQ, Zhang XG. Expression of CD40 in human gastric cancer tissue and its prognostic correlation. Zhonghua Yi Xue Za Zhi. 2009;89(30):2124–2128. [PubMed] [Google Scholar]
- 26.Bereznaya NM, Chekhun VF. Expression of CD40 and CD40L on tumor cells: the role of their interaction and new approach to immunotherapy. Exp Oncol. 2007;29(1):2–12. [PubMed] [Google Scholar]
- 27.Posner MR, Cavacini LA, Upton MP, Tillman KC, Gornstein ER, Norris CM., Jr Surface membrane-expressed CD40 is present on tumor cells from squamous cell cancer of the head and neck in vitro and in vivo and regulates cell growth in tumor cell lines. Clin Cancer Res. 1999;5(8):2261–2270. [PubMed] [Google Scholar]
- 28.Melichar B, Patenia R, Gallardo S, Melicharova K, Hu W, Freedman RS. Expression of CD40 and growth-inhibitory activity of CD40 ligand in ovarian cancer cell lines. Gynecol Oncol. 2007;104(3):707–713. doi: 10.1016/j.ygyno.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 29.Hollmann A, Aloyz R, Baker K, Dirnhofer S, Owens T, Sladek R, Tzankov A. Vav-1 expression correlates with NFkappaB activation and CD40-mediated cell death in diffuse large B-cell lymphoma cell lines. Hematol Oncol. 2010;28(3):142–150. doi: 10.1002/hon.935. [DOI] [PubMed] [Google Scholar]
- 30.Fotin-Mleczek M, Henkler F, Hausser A, Glauner H, Samel D, Graness A, Scheurich P, Mauri D, Wajant H. Tumor necrosis factor receptor-associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-kappaB activation. J Biol Chem. 2004;279(1):677–685. doi: 10.1074/jbc.M310969200. [DOI] [PubMed] [Google Scholar]
- 31.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–151. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 32.Jiang E, He X, Chen X, Sun G, Wu H, Wei Y, Zhao X. Expression of CD40 in ovarian cancer and adenovirus-mediated CD40 ligand therapy on ovarian cancer in vitro. Tumori. 2008;94(3):356–361. doi: 10.1177/030089160809400312. [DOI] [PubMed] [Google Scholar]
- 33.Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, Mixan BJ, Lee WP, Lin Z, Valdez P, Wahl AF, Grewal IS. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65(18):8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 34.Kelley SK, Gelzleichter T, Xie D, Lee WP, Darbonne WC, Qureshi F, Kissler K, Oflazoglu E, Grewal IS. Preclinical pharmacokinetics, pharmacodynamics, and activity of a humanized anti-CD40 antibody (SGN-40) in rodents and non-human primates. Br J Pharmacol. 2006;148(8):1116–1123. doi: 10.1038/sj.bjp.0706828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai YT, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, Hideshima T, Chauhan D, Hamasaki M, Ishitsuka K, Richardson P, Treon SP, Munshi NC, Anderson KC. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64(8):2846–2852. doi: 10.1158/0008-5472.CAN-03-3630. [DOI] [PubMed] [Google Scholar]
- 36.Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, Harrop K, Drachman JG, Whiting N. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95(5):845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronald PG, Timothy P, Susan HC et al. (2011) The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice. Cancer Immunol Immunother. doi:10.1007/s00262-011-1014-6 [DOI] [PMC free article] [PubMed]
- 38.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168(6):2720–2728. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 39.Davis ID, Chen Q, Morris L, et al. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 40.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T cell response that eradicates lymphoma and bypasses T cell help. Nat Med. 1999;5:548–553. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 41.Mackey MF, Gunn JR, Ting PP, et al. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–2574. [PubMed] [Google Scholar]