Abstract
ALT-803, a novel IL-15/IL-15 receptor alpha complex, and the tyrosine kinase inhibitor, sunitinib, were examined for their single and combined effects on the growth of subcutaneous B16BL6 melanoma and on lymph node and lung metastasis. The study was conducted in immunocompetent C57BL/6 mice drinking water (Water mice) and in mice that chronically consumed alcohol (Alcohol mice), which are deficient in CD8+ T cells. Sunitinib inhibited melanoma growth and was more effective in Alcohol mice. ALT-803 did not alter tumor growth or survival in Water or Alcohol mice. Combined ALT-803 and sunitinib inhibited melanoma growth and increased survival, and these effects were greater than sunitinib alone in Water mice. ALT-803 and alcohol independently suppressed lymph node and lung metastasis, whereas sunitinib alone or in combination with ALT-803 increased lymph node and lung metastasis in Water and Alcohol mice. Initially, ALT-803 increased IFN-γ-producing CD8+CD44hi memory T cells and CD8+CD44hiCD62Llo effector memory T cells and sunitinib decreased immunosuppressive MDSC and T regulatory cells (Treg). However, the impact of these treatments diminished with time. Subcutaneous tumors from Water mice showed increased numbers of CD8+ T cells, CD8+CD44hi T cells, NK cells, and MDSC cells and decreased Treg cells after ALT-803 treatment.
Keywords: IL-15, Sunitinib, Melanoma, Metastasis, Immunotherapy
Introduction
The incidence of melanoma has been rapidly rising for several years and shows no sign of abating. While melanoma is curable if detected early, it has a propensity to metastasize and then is virtually incurable. However, recent advances in immunotherapy offer new hope for increased survival of patients with distant metastasis.
IL-15 is one of the most promising cytokines for use in cancer immunotherapy because of its low toxicity and efficacy in promoting proliferation and survival of memory CD8+ T cells as well as NK cells and for not inducing T cell activation-induced cell death [1]. ALT-803 is an IL-15 superagonist consisting of IL-15 complexed to the IL-15 receptor alpha, developed by Altor BioScience, Miramar, FL. It has significant antitumor activity against several murine tumors including 5T33P and MOPC-315P multiple myeloma [2], and non-muscle-invasive bladder cancer (NMIBC) [3]. ALT-803 enhanced proliferation of IFN-γ-producing CD8+CD44hi memory T cells in murine myeloma, and this was responsible for its antitumor activity [2]. CD8+CD44hi memory T cells and IFN-γ also are key factors that influence metastasis and host survival of melanoma [4–9]. Established s.c. B16F10 melanoma tumors were approximately fourfold smaller in mice administered two daily doses of IL-15 complexed to IL-15 receptor alpha than the tumors in untreated mice when measured 3 days later [10]. The initial antitumor effect was attributed to promotion of proliferation and effector capacity of resident CD8+ T cells [10]. Dubois et al. [11] also showed effectiveness of an IL-15/IL15 receptor alpha complex in mice bearing i.v. B16 melanoma and from in vitro culture experiments attributed the antitumor effect to CD8+CD44hi memory T cells. Thus, we hypothesized that ALT-803 would effectively inhibit s.c. growth and increase survival of mice inoculated with the more invasive and metastatic B16BL6 melanoma. This study is of additional significance since ALT-803 is currently being evaluated in a phase I clinical trial in patients with various surgically incurable advanced solid tumors including melanoma (ClinicalTrials.gov Identifier: NCT01946789).
In melanoma, MDSC are increased and correlate with tumor progression, metastasis, resistance to therapy, and decreased survival [12–14]. Several therapeutic approaches to targeting MDSC have been proposed to block tumor-induced immune tolerance and improve cancer immunotherapy [15–17]. Moreover, inhibiting MDSC as well as regulatory T cells (Treg) in a variety of different cancers, including melanoma, can enhance immune-based therapies [18–23]. Sunitinib is an orally effective, small molecule that inhibits multiple receptor tyrosine kinases [24] and can suppress MDSC [17] and Treg [17]. Conversely, IL-15 is a potent inducer of Treg [25]. Thus, an additional objective of the present investigation was to examine the potential additive effect of sunitinib and ALT-803 to inhibit growth and metastasis of melanoma and increase host survival in the B16BL6 murine model.
Alcohol abuse is a worldwide problem, and a recent meta-analysis confirms a positive association between alcohol consumption and melanoma risk [26]. Chronic alcohol consumption compromises the immune system, and this could impact the effectiveness of immunotherapy. We found previously that chronic alcohol intake decreases lymphocytes and in particular CD8+ T cells in the spleen of non-melanoma-inoculated mice and stimulates homeostatic proliferation resulting in an increase in CD8+CD44hi memory T cells and IFN-γ-producing CD8+ T cells [27]. Although these cells are higher in chronic alcohol drinking mice (hereafter referred to as Alcohol mice) than in mice drinking water (hereafter referred to as Water mice) in the steady state, CD8+CD44hi memory T cells fail to proliferate and IFN-γ-producing CD8+ T cells as well as antigen-specific CD8+ T cells decrease rapidly after s.c B16BL6 melanoma inoculation [17, 28]. This could be due to the fact that Alcohol mice exhibit an IL-15 deficiency, which can impair CD8+ T cell and CD8+CD44hi memory T cell proliferation and survival [29]. MDSC are elevated in the peripheral blood within 1 week after inoculation of B16BL6 melanoma in Alcohol mice, and this also could suppress T cell responses [28]. Thus, the goals of the present investigation were to (1) determine the potential additive effect of sunitinib and ALT-803 to inhibit growth and metastasis of B16BL6 melanoma and increase host survival in Water and Alcohol mice, (2) assess the relationship among these effects and the ability of the treatments to inhibit MDSC and Treg, and to increase CD8+ T memory cells and their ability to produce IFN-γ and (3) determine uptake and retention of leukocytes in s.c. tumors.
Materials and methods
Animals, alcohol administration, and diet
Female C57BL/6 mice aged 6–7 weeks were purchased from Charles River Laboratories (Wilmington, MA) and housed in specific pathogen-free housing conditions within the vivarium at Washington State University. Mice were acclimated to the vivarium for at least 1 week before incorporating them into experiments. Mice in experiments were single housed in filter topped, polycarbonate cages and distributed to different treatment groups as described below. The starting body weight among the experimental groups was not different. Experimental protocols were approved by the Institutional Animal Care and Use Committee. The vivarium facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Mice received autoclaved double distilled, deionized water or 0.2 µm filtered 20 % w/v alcohol consisting of double distilled, deionized water and Everclear (Luxco, St. Louis, MO) as their sole drinking source throughout the experimental period. All mice received Purina 5001 Laboratory chow ad libitum. The alcohol model has been previously validated [30].
Primary tumor inoculation, measurement and assessment of metastasis
B16BL6 melanoma was originally obtained from Dr. Menashe Bar-Eli, Department of Cancer Biology, The University of Texas MD Anderson Cancer Center, Houston, TX. It was cultured in Dulbecco’s modified Eagle’s medium (Hyclone) supplemented with 10 % heat-inactivated fetal bovine serum (Sigma-Aldrich) in a humidified incubator with 5 % CO2 at 37 °C. Cells were harvested with 0.25 % trypsin-EDTA (Sigma Aldrich) when they reached 50–70 % confluence. Cells were then resuspended in sterile calcium-free, magnesium-free phosphate-buffered saline and used for injection within 5 in vitro passages. For assessment of treatment effects on primary tumors, mice were inoculated s.c. into the dorsal back near the right hip with 2 × 105 melanoma cells. Mice receiving alcohol were inoculated 12 weeks after alcohol consumption was initiated. Tumors became palpable approximately 7 days after inoculation and were then measured with calipers every 2 days thereafter. Tumor volume was calculated using the equation, V = π/6 × 1.58 × (a × b)3/2, where V is tumor volume, a is tumor length, and b is tumor width [31]. Metastasis to the right axillary lymph nodes and the lung as well as other potential organs was examined at necropsy. Lungs were fixed in Bouin’s solution (Ricca Chem. Co.) for later enumeration of superficial tumor colony number using a dissecting microscope.
Drugs and treatment groups
The independent and combined effects of sunitinib and ALT-803 (a gift from Dr. Hing C. Wong, Altor BioScience Corp., Miramar, FL) to reduce s.c. melanoma growth, inhibit metastasis, and increase host survival were evaluated in the B16BL6 melanoma model. ALT-803 was diluted in 0.9 % sterile saline. Sunitinib malate, >99 % pure (LC Laboratories), was dissolved in 10 % DMSO. Experimental groups consisted of Water mice and/or Alcohol mice as follows: (1) a Control group that received the vehicles used to solubilize the drugs, (2) a sunitinib only treatment group, (3) an ALT-803 only treatment group and (4), and a Combination group treated with sunitinib and ALT-803.
Unless otherwise indicated, mice treated with ALT-803 received 5 weekly i.v. injections of 0.2 mg/kg ALT-803 starting at Day 7 after tumor inoculation. This dosage is equivalent to that used in another study that showed in vivo antitumor activity in murine multiple myeloma models [2]. Unless otherwise indicated, mice treated with sunitinib received daily i.p. injections of 40 mg/kg starting at Day 5 after tumor inoculation for 38 days. The continuous dosing schedule of sunitinib as well as the daily dose of 40 mg/kg is known to inhibit MDSC as well as Treg [18, 19, 23].
Assessment of metastasis after i.v. melanoma inoculation
A separate assessment of metastasis was conducted in Water mice inoculated with 2 × 105 B16BL6 melanoma cells into the lateral tail vein according to a previously established protocol [32]. Mice were treated with sunitinib and/or ALT-803 on day 2 after tumor injection. Daily i.p. sunitinib injections were continued until mice were euthanized on Day 21. ALT-803 was injected again on Day 11 and Day 18 after tumor injection. The lungs were removed, fixed in Bouin’s solution, and visible lung tumor colonies were enumerated using a dissecting microscope.
Immunophenotyping in splenocytes and PBL
Immunophenotyping was conducted on the splenocytes and PBL obtained from the above-treated mice bearing s.c. melanoma tumors at various time points in a separate experiment. Splenocytes and PBL were isolated according to previously published procedures [28, 33]. Intracellular staining of splenocytes for IFN-γ was performed in splenocytes as previously described [27]. Staining for FOXP3 in CD4+CD25+ T cells in splenocytes was performed as previously described [28]. Samples were analyzed on a Becton–Dickinson FACScan flow cytometer, and data were analyzed using CellQuest Pro, version 5.1.1 software. For this experiment, Water and Alcohol mice were divided into four groups (Control, sunitinib, ALT-803, and Combination) each containing 9 mice per group and inoculated with B16BL6 melanoma as described above. The ALT-803 group received ALT-803 on day 9, 16, 23, and 30 after tumor inoculation. Sunitinib treatment began on day 7 after tumor inoculation and continued through day 32.
Treatment protocol and immunophenotyping of leukocytes in s.c. B16BL6 melanoma tumors
A separate cohort of Water mice were divided into three groups: Control, ALT-803, and Combination. The Control group received the vehicles used to solubilize the drugs. The ALT-803 group received ALT-803 (1.5 mg/kg i.v.) on day 7 and day 14 after tumor inoculation. The Combination group received sunitinib (40 mg/kg i.p) on day 7 through day 20 as well as ALT-803 (1.5 mg/kg i.v.) on day 7 and day 14. On day 21, mice were euthanized, the tumors were weighed, and the leukocytes isolated according to previously published procedures [28, 33] and then analyzed by flow cytometry as indicated above.
Antibodies used for immunophenotyping
PE-, FITC-, PerCP- and PerCP/Cyanine 5.5 (Cy5.5)-conjugated anti-mouse antibodies (clones) were purchased from the following suppliers. BioLegend: CD4 (RM4-5)-FITC, CD4 (RM4-5)-PerCP, CD8a (53-6.7)-Per/Cy5, CD3 (145-2C11)-PerCP, F4/80 (GM8)-PE, CD25 (PC61)-FITC, CD11b (M1/70)-PerCP/Cy5.5, Ly-6G/Ly-6C [Gr-1](RB6-8C5)-FITC, and IFN-γ (XMG1.2)-PE; BD Biosciences: CD44 (IM7)-FITC, CD62L (MEL-14)-PE; and eBioscience: FOXP3 (150D/E4)-PE.
Statistical analyses
Repeated measures ANOVA was used to determine differences in tumor growth over time with treatment. Kaplan–Meier survival analysis and the Gehan–Breslow–Wilcoxon test were used for determining overall survival. Two-way ANOVA was used to determine differences between Water and Alcohol mice. Differences in lung metastasis between treatment groups were analyzed by one-way ANOVA or one-way ANOVA on ranks as appropriate. Two-way ANOVA on ranks was used to determine differences between Water and Alcohol mice since the data were not uniformly distributed. Fisher’s exact test was used to determine statistical significance of axillary lymph node metastases. Immunophenotyping data in organs were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group. For leukocytes in the tumor, normally distributed data were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group. Nonparametric data were analyzed by Kruskal–Wallis one-way ANOVA on ranks followed by the Tukey test or Dunn’s method (NK cells) for pairwise multiple comparisons. Statistical significance was set at 95 % confidence limits. GraphPad Prism 5 and SigmaPlot 12.5 software were used for analyses.
Results
Sunitinib alone and in combination with ALT-803 plays a major role in inhibiting primary s.c. tumor growth and increasing host survival
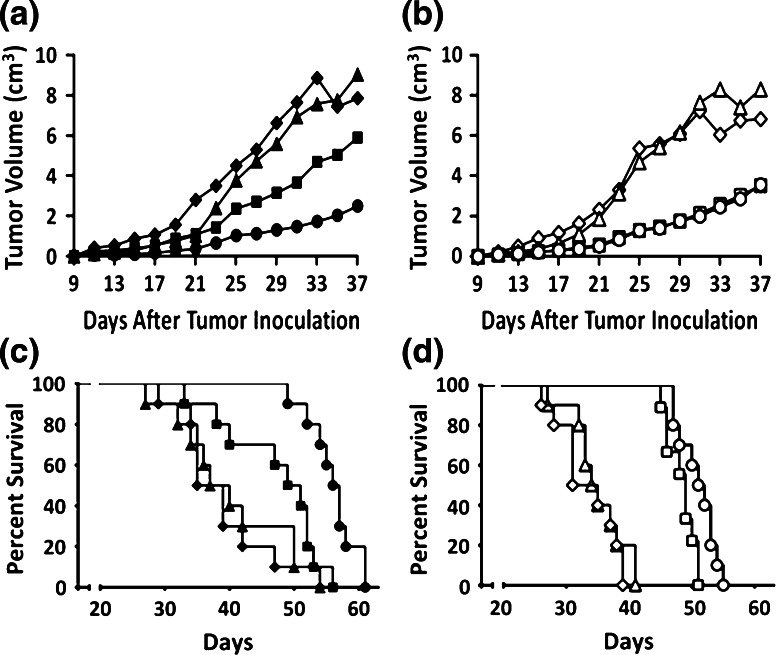
The effects of sunitinib, ALT-803, and Combination treatments on tumor growth and survival are shown in Fig. 1. Tumors became palpable approximately 7 days after melanoma inoculation. Sunitinib significantly decreased the rate of tumor growth in Water mice (p < 0.05) (Fig. 1a) and Alcohol mice (p < 0.05) (Fig. 1b) compared to Controls, and the decrease in growth rate was greater in the Alcohol mice as compared to the Water mice treated with sunitinib (p < 0.05). ALT-803 treatment delayed tumor growth during the first 21 days after tumor inoculation in the Water mice (Fig. 1a) as compared to the Control group but not thereafter. Although ALT-803 also delayed tumor growth during the first 21 days after tumor inoculation in Alcohol mice (Fig. 1b), the effect was overshadowed by the pronounced effect that alcohol had on tumor growth during this time period. Overall, the combination treatment decreased the tumor growth rate when compared to Control and ALT-803 groups in Water and Alcohol mice (p < 0.05). In Water mice, the Combination group showed a trend toward decreased tumor growth compared to the sunitinib group (p = 0.05). In Alcohol mice, the overall tumor growth rate after Day 21 in the Combination group was identical to the sunitinib group indicating a lack of interaction between ALT-803 and sunitinib on tumor growth in these mice.
Fig. 1.
Effect of treatment on primary s.c. B16BL6 melanoma tumor growth and survival. a Mean tumor volume as a function of treatment and time after tumor implantation in Water mice. b Mean tumor volume ± SEM as a function of treatment and time after tumor implantation in Alcohol mice. c Kaplan–Meier survival plot as a function of treatment in Water mice. d Kaplan–Meier survival plot as a function of treatment in Alcohol mice. Closed symbols Water mice, Open symbols Alcohol mice. Diamonds Control, Squares sunitinib, Triangles ALT-803, Circles Combination. Each group contained 10 mice. Repeated measures ANOVA was used to determine differences in tumor growth over time with treatment. The Gehan–Breslow–Wilcoxon test was used for determining overall survival in the Kaplan–Meier survival analysis
Median survival increased 43 % in the sunitinib group (p < 0.05) and 66 % in the Combination group (p < 0.05) compared to Control in the Water mice (Fig. 1c). ALT-803, which did not alter tumor growth overall likewise had no effect on survival in the Water mice. Survival in the Combination group was significantly different from the sunitinib group (p < 0.05), indicating a beneficial interaction between sunitinib and ALT-803 to increase survival.
In Alcohol mice, sunitinib increased median survival by 53 % (p < 0.05) and Combination treatment increased survival by 63 % (p < 0.05, Fig. 1d). ALT-803 alone did not alter survival compared to Control in the Alcohol mice; however, survival in the Combination group was statistically different (p < 0.05) from the sunitinib group indicating a modest effect attributable to ALT-803.
Sunitinib increases and alcohol and ALT-803 decrease lymph node and lung metastasis in primary tumor-bearing mice
Metastasis to the right inguinal and axillary lymph nodes and to the lung was evaluated at necropsy in mice inoculated s.c. with B16BL6 melanoma. All mice exhibited metastasis to the right inguinal lymph node, which is the draining node closest to the primary tumor inoculation site.
Alcohol profoundly decreased the percentage of mice with right axillary lymph node involvement compared to Water Control (p < 0.05, Fig. 2a). The percentage of mice with visible metastasis in the right axillary lymph node was elevated in mice in the sunitinib and Combination groups in both Water and Alcohol mice compared to their respective Controls. A significantly lower percentage of mice with metastasis was observed in the Water ALT-803 group than in the Water Combination group (p < 0.05). The percentage of mice with right axillary lymph node metastasis treated with ALT-803 was identical to the Control in the Alcohol mice and the percentage of mice exhibiting metastasis in both these groups was significantly lower than sunitinib and Combination groups in Alcohol mice (p < 0.05).
Fig. 2.
Effect of treatment on metastasis of B16BL6 melanoma. a Percentage of mice at necropsy that exhibit right axillary lymph node metastasis after s.c. melanoma inoculation. Black bars Water mice, White bars Alcohol mice. b Tumor lung colony number at necropsy in Water mice after s.c. melanoma inoculation. c Tumor lung colony number in Alcohol mice after s.c. melanoma inoculation. d Tumor lung colony number after i.v. melanoma inoculation. b–d Closed symbols Water mice, Open symbols Alcohol mice. Diamonds Control, Squares sunitinib, Triangles ALT-803, Circles, Combination, Bar median number of lung colony tumors. Each group contained 10 mice. Fisher’s exact test was used to determine statistical significance of axillary lymph node and lung metastases
The number of lung metastases determined at necropsy was lower (p < 0.05) in the ALT-803 groups compared to their respective Water (Fig. 2b) and Alcohol (Fig. 2c) Control, sunitinib, and Combination groups. The sunitinib and Combination groups exhibited higher levels of lung tumor colonies compared with respective Water and Alcohol Controls (p < 0.05). Metastasis was significantly decreased in the Alcohol Control relative to the Water Control group (p < 0.05). Metastasis in the sunitinib groups also was lower in Alcohol mice compared to Water mice (p < 0.05). While alcohol had a mitigating effect on metastasis in mice treated with sunitinib alone, this was not the case in the Combination group, where lung metastasis was significantly higher than the sunitinib group in the Alcohol mice (p < 0.05).
Sunitinib and ALT-803 similarly inhibit lung metastasis after i.v. tumor inoculation
Injection of tumor cells i.v. bypasses the early steps in metastasis associated with interactions between the primary tumor and the microenvironment. Thus, this method of introduction of tumor cells examines more directly the effects of treatment on tumor colony development within the lung, which is a major metastatic site of melanoma. The effect of sunitinib and ALT-803 on metastasis after i.v. injection of melanoma was examined in Water mice. The results in Fig. 2d show that the lung colony number is decreased ~40–50 % in all three treatment groups compared to Control (p < 0.05).
ALT-803 alone and in combination treatment increase total CD8+ T cells, CD8+CD44hi memory T cells, and especially CD8+CD44hiCD62Llo effector memory T cells
Based on the results of other tumor systems, the major effect of ALT-803 on the immune response in melanoma-bearing mice was expected to be an increase in the number of CD8+ T cells and CD8+CD44hi memory T cells. Herein, we also examined the effect of ALT-803 on the CD8+CD44hiCD62Llo effector memory T cells, which are antigen-experienced T cells that upon activation secrete large amounts of IFN-γ and are able to rapidly lyse antigen-expressing tumor cells [34–36].
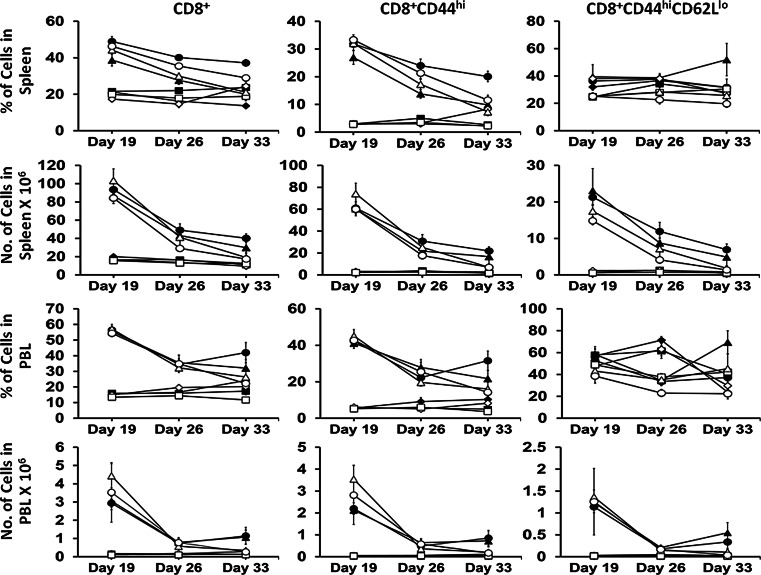
As expected, ALT-803 alone and in combination with sunitinib greatly increased (p < 0.05) CD8+ T cells, CD8+CD44hi memory T cells, and CD8+CD44hiCD62Llo effector memory T cells in the spleen and PBL in both Water and Alcohol mice on Day 19. The degree of increase on Day 19 was greater in the Water as compared to the Alcohol mice (p < 0.05) in the spleen but was not different in the PBL (Fig. 3). Sunitinib alone did not affect the percentage or number of CD8+ T cells or CD8+CD44hiCD62Llo effector memory T cells in the spleen and PBL as compared to respective Control groups in Water and Alcohol mice. The response to ALT-803 was not sustained over time, and the numbers of these cells were decreased on Day 26 and Day 33 in the ALT-803 and Combination of Water and Alcohol mice. In the Alcohol mice, the numbers of these cells in the ALT-803 and Combination groups on Day 33 decreased to Control values while in the Water mice they were still somewhat elevated (p < 0.05) (Fig. 3). The response of CD8+CD44hi memory T cells to ALT-803 in the Alcohol mice on Day 19 was significantly higher than in Water mice as compared to the Control group in the spleen and PBL; however, the decrease in these cells on Day 26 was also more precipitous (p < 0.05). Thus, ALT-803 was initially effective in increasing CD8+ T cells, CD8+CD44hi memory T cells, and CD8+CD44hiCD62Llo effector memory T cells; however, continued treatment was not effective in sustaining the levels of these cells over time.
Fig. 3.
Effect of treatment on CD8+ T cells, CD8+CD44hi memory T cells, and CD8+CD44hiCD61Llo effector memory T cells in the spleen and PBL of s.c. B16BL6 melanoma-bearing Water and Alcohol mice at various time periods after tumor inoculation. Values are mean for N = 9 mice per group. Closed symbols Water mice, Open symbols Alcohol mice. Diamonds Control, Squares sunitinib, Triangles ALT-803, Circles Combination. Data were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group
ALT-803 increases IFN-γ-expressing CD8+ T cells and CD8+CD44hi memory T cells
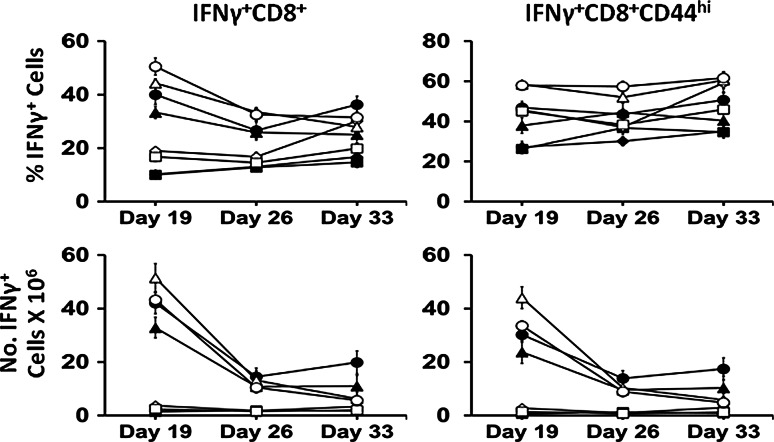
The major antitumor effect of CD8+ T cells and particularly CD8+CD44hi memory T cells is associated with their ability to produce IFN-γ [6–9], and the goal herein was to achieve this via administration of ALT-803. IFN-γ-producing CD8+CD44hi memory T cells are responsible for the greater part of the antitumor activity against many other types of tumors [2, 37]. The numbers of IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells in the spleen were dramatically elevated in the ALT-803 and Combination groups on Day 19 as compared to Control of both Water and Alcohol mice (p < 0.05, Fig. 4). In the Water mice these cells remained elevated in the ALT-803 and Combination groups compared to Control through Day 33 (p < 0.05) even though they decreased with time. This decrease was more pronounced in the Alcohol mice, where on Day 33, the numbers were not different from the Control group. The percentage and number of IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells were higher on Day 19 in the ALT-803 Alcohol mice as compared to Water mice (p < 0.05). In addition, the numbers of these cells in the Alcohol mice were higher in the ALT-803 group compared to the Combination group on Day 19 (p < 0.05). Sunitinib had no effect on the number of IFN-γ-producing CD8+ T cells or CD8+CD44hi memory T cells in the spleen. While ALT-803 was initially effective in facilitating these IFN-γ producing cells, the effect was not long lasting.
Fig. 4.
Effect of treatment on IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells in the spleen of s.c. B16BL6 melanoma-bearing Water and Alcohol mice at various days after tumor inoculation. Values are mean ± SEM for N = 9 mice per group. Closed symbols Water mice, Open symbols Alcohol mice. Diamonds Control, Squares sunitinib, Triangles ALT-803, Circles Combination. Data were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group
Sunitinib alone and in combination decreases MDSC, but the effect is short lived
MDSC have strong immunosuppressive activities. They can influence tumor progression and survival as well as play an important role in the development of immune tolerance and interfere with immunotherapy in melanoma as well as other cancers [12, 14, 15, 17]. There is strong evidence that sunitinib suppresses MDSC [17, 24]. Since chronic alcohol consumption can increase MDSC in the PBL [28], we investigated whether sunitinib could mollify this effect in Alcohol mice.
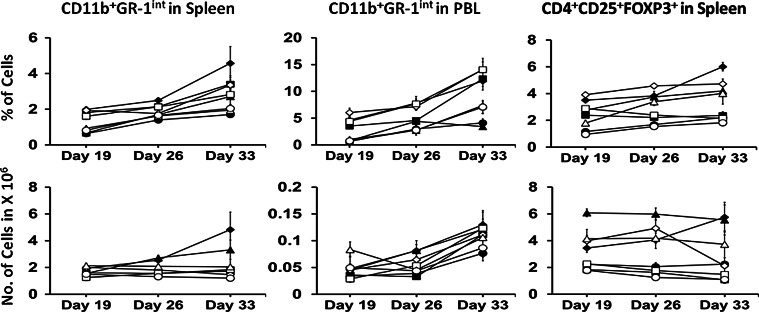
The percentage of CD11b+GR-1int MDSC increased in the spleen and PBL of Control groups with time in both Water and Alcohol mice (p < 0.05, Fig. 5). The numbers of MDSC (CD11b+GR-1int cells) in the spleen of Alcohol mice did not change over time. However, in Water mice the numbers of these cells increased in the Control and ALT-803 groups over time (p < 0.05). Sunitinib and Combination treatment held steady the numbers of MDSC in the spleen of Water mice over time, and they were lower than Control and ALT-803 groups on Day 33 (p < 0.05). The numbers of MDSC in PBL of Water mice on Day 26 were lower in the sunitinib and Combination groups as compared to Control and ALT-803 groups (p < 0.05). However, by Day 33, MDSC levels increased in Control and sunitinib groups as compared to Day 19 (p < 0.05) and increased in the ALT-803 group as compared to Day 26 (p < 0.05). In Alcohol mice, sunitinib did not effectively suppress MDSC in the PBL, and the numbers increased over time in Control and sunitinib groups as compared to Day 19 (p < 0.05) and on Day 33 were higher in the ALT-803 group as compared to Day 26 (p < 0.05). In both the Water and Alcohol Combination treatment groups, the numbers of MDSC in the PBL did not change significantly over time. Overall, the results indicate that sunitinib initially inhibited expansion of MDSC in melanoma-bearing Water mice; however, the effect in PBL was not sustainable.
Fig. 5.
Effect of treatment on Gr-1int cells in the spleen and PBL and CD4+FOXP3+ T cells in the spleen of s.c. B16BL6 melanoma-bearing Water and Alcohol mice at various days after tumor inoculation. Values are mean ± SEM for N = 9 mice per group. Closed symbols Water mice, Open symbols Alcohol mice. Diamonds Control, Squares sunitinib, Triangles ALT-803, Circles Combination. Data were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group
Sunitinib prevents Treg cell expansion and their increase in response to ALT-803
Treg also are associated with inhibition of host antitumor immunity, and depletion of these cells favors immune-mediated tumor rejection. Since IL-15 is a potent inducer of Treg [25], we hypothesized that sunitinib, which is purported to decrease Treg [17], would restrict the induction of these cells after ALT-803 therapy. The numbers of Treg in the ALT-803 group were higher than Control, sunitinib, and Combination groups in Water and Alcohol mice on Day 19, Day 26, and Day 33 (p < 0.05), with the exception that Control was not different from ALT-803 in Alcohol mice on Day 33 (Fig. 5). Treg were lower in the sunitinib and Combination groups in Water and Alcohol mice compared to Control on Day 19 and Day 26 (p < 0.05), and they did not increase over time. Treg clearly increased in Water mice over time (p < 0.05). The overall findings were that ALT-803 resulted in elevated Treg and that sunitinib counteracted this effect.
ALT-803 increases CD8+ T cells, CD8+CD44hi memory T cells, and MDSC and decreases Treg cells in primary melanoma tumors in Water mice
At necropsy, the mean tumor weight was 2.96 g ± 0.99 in the Control group, 0.76 g ± 0.19 in the ALT-803 group, and 0.36 g ± 0.06 in the Combination group. Tumor weights of three groups were different from each other (p < 0.05) indicating that ALT-803 decreased growth and that the combination treatment was more effective than ALT-803 alone. The uptake and retention of leukocytes in the tumor is shown in Fig. 6. CD8+ T cells and primarily those exhibiting the CD8+CD44hi memory phenotype were the major type of leukocyte comprising almost 90 % of the total cells. ALT-803 treatment increased the numbers of these cells per 100 mg tumor by threefold compared to Control; however, combined treatment with sunitinib dampened this effect. NK cells increased 11- to 13-fold in the ALT-803 and Combination groups. Treg decreased about sevenfold in the ALT-803 group and fourfold in the Combination group. MDSC increased about ninefold in the ALT-803 group and only threefold in the Combination group indicating an inhibitory effect of sunitinib on MDSC accumulation in the tumor along with ALT-803 treatment.
Fig. 6.
Leukocytes in s.c. B16BL6 melanoma tumors. a Percentage of leukocytes in s.c. B16BL6 melanoma tumors expressed on a log scale. b Number of leukocytes per 100 mg of B16BL6 melanoma tumors expressed on a log scale. The percentage and numbers of leukocytes were determined in Water mice on day 21 after s.c. B16BL6 melanoma tumor inoculation. The Control group (Black bars) received vehicles as indicated in the Materials and Methods. The ALT-803 group (White bars) received l.5 mg/kg i.v. into the lateral tail vein on day 7 and 14 after tumor inoculation. The Combination group (Gray bars) received 40 mg/kg sunitinib i.p. from day 7 through day 20 after tumor inoculation and 1.5 mg/kg ALT-803 i.v. on day 7 and day 14 after tumor inoculation. Mice were euthanized on day 21 and the s.c. tumors analyzed for content of various leukocytes as indicated in the figure. Values are mean ± SEM for N = 6 mice per group. *p < 0.05. Normally distributed data were analyzed by one-way ANOVA followed by the Holm–Sidak method for multiple comparisons analysis between two analyses within a treatment group. Nonparametric data were analyzed by Kruskal–Wallis one-way ANOVA on ranks followed by Dunn’s method for pairwise multiple comparisons (NK cells)
Discussion
There are few effective treatment options for patients with melanoma. Recent clinical and preclinical studies suggest that immune-enhancing agents provide a promising approach to treatment; however, their effectiveness can be limited by development of resistance. IL-15 and particularly IL-15 combined with its receptor alpha chain including ALT-803 show great potential in the immunotherapy of cancer. The tyrosine kinase inhibitor, sunitinib, which inhibits immunosuppressive MDSC and Treg cells, can synergize with cancer immunotherapy [38]. The objective of this study was not only to examine the effectiveness of ALT-803 to inhibit primary B16BL6 melanoma growth and metastasis, and to increase survival, but also to determine the ability of sunitinib to enhance the affect of ALT-803 in mice that are immunocompetent and those that are immunodeficient due to chronic alcohol consumption.
Sunitinib decreased tumor growth throughout the experiment in both Water and Alcohol mice; however, the effect was more pronounced in the Alcohol mice (Fig. 1). ALT-803 inhibited tumor growth in Water and Alcohol mice during the first 21 days after tumor implantation but was ineffective thereafter (Fig. 1). ALT-803 in combination with sunitinib more effectively inhibited tumor growth throughout the experiment in Water mice, and these mice had the greatest survival. In Alcohol mice, ALT-803 in combination with sunitinib became ineffective in controlling growth after 21 days as compared to sunitinib treatment alone, and this was also reflected in only a modest increase in survival compared to sunitinib treatment alone. The initial growth inhibitory effect of ALT-803 and Combination therapy in Water and Alcohol mice correlated strongly with high levels of CD8+ T cells and their memory phenotype in the spleen and PBL (Fig. 3) and with IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells in the spleen (Fig. 4). The numbers of these cells were higher in the Alcohol mice than in the Water mice on Day 19 (p < 0.05); however, the decrease observed in Alcohol and Water mice on Day 26 was more precipitous in Alcohol mice. Tumor levels of CD8+ T cells, CD8+CD44hi T cells, and NK cells also were greatly increased in primary tumors when examined in Water mice on day 21 (Fig. 6).
The increase in tumor growth in the ALT-803 treated Water and Alcohol mice on day 21 correlated with increased MDSC and Treg (Fig. 5), decreased CD8+ T cells, CD8+CD44hi memory T cells, and CD8+CD44hiCD62Llo effector memory T cells (Fig. 3) in the spleen and PBL and decreased IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells in the spleen (Fig. 4). While Treg were greatly elevated in the spleen of ALT-803-treated mice compared to Control (Fig. 5), they were greatly decreased in the primary tumors from Water mice on day 21 (Fig. 6). In contrast, MDSC were greatly elevated in the tumor by ALT-803 treatment. All together these data strongly suggest that B16BL6 melanoma cells developed resistance to ALT-803 and Combination therapy associated with elevated MDSC and decreased memory T cells. IL-15 internalization is necessary for proliferation and survival of responding cells after trans-presentation of IL-15 complexed with IL-15Rα followed by recycling of the receptor to the cell surface [39]. Thus, it is possible that the ability of the ALT-803 complex to be cleaved over time is impaired in melanoma-bearing mice. Another possibility is that various other immunosuppressive factors that foster resistance of CD8+ T cells to IL-15 lead to the eventual decrease in number and function of these cells as tumor growth continues.
Sunitinib decreased tumor growth and increased survival in the Water and Alcohol mice independent of increased CD8+ T cells, CD8+CD44hi memory T cells, CD8+CD44hiCD62Llo effector memory T cells, and IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells since the numbers of these cells remained unchanged over time in this treatment group compared to Controls (Fig. 3). The decrease in the number of these cells associated with continued combination treatment was generally greater in the Alcohol mice. In addition, the numbers of IFN-γ-producing CD8+ T cells and CD8+CD44hi memory T cells were lower in the Alcohol mice on Day 33 compared to Water mice, suggesting that maintenance of survival and/or proliferation of these cells in response to ALT-803 was inhibited by alcohol. This study further confirms our previous findings that while chronic alcohol consumption itself facilitates an enhanced immune response, the microenvironment associated with an ever-present alcohol/melanoma interaction is strongly immunosuppressive.
It is likely that the antiangiogenesis activity of sunitinib contributed to the overall antitumor response in this treatment group, since it is known to inhibit B16 melanoma neovascularization [40]. However, sunitinib in another study did not inhibit s.c. growth of B16 melanoma tumors in immunodeficient mice [41] indicating that an intact immune system is essential for its ability to inhibit melanoma growth. Thus, additional studies are required to determine the mechanism associated with the effect of sunitinib on melanoma growth and survival.
Combination therapy with ALT-803 and sunitinib in Water mice was the most effective therapy in reducing primary tumor growth and increasing survival adding support for the original hypothesis that sunitinib by virtue of its suppressive effect on MDSC and Treg facilitated CD8+ T cell-mediated antitumor immune cells. Further supporting this hypothesis is the fact that the lowest numbers of MDSC in the spleen and PBL were observed in the Combination group (Fig. 5) and in the tumors from Water mice (Fig. 6).
ALT-803 and alcohol alone and in combination had a strong inhibitory effect on spontaneous metastasis of s.c-inoculated B16BL6 melanoma. The effect of alcohol on metastasis was previously shown to be IFN-γ dependent [5]. While the mechanism underlying the antimetastatic effect of ALT-803 is not known, it is likely that the effect is related to increased levels of IFN-γ.
Sunitinib increased metastasis and overcame the antimetastatic effect of ALT-803. Similar findings have been described for sunitinib treatment in B16 melanoma and other murine and human tumor lines implanted orthotopically [42, 43]. The increase in metastasis after s.c. tumor implantation might be related to increased local tumor cell invasion [42], adaptation of the tumor cell microenvironment triggered by changes in the tumor vasculature [43], augmented intratumoral heterogeneity [44], or ensuing hypoxia induced by sunitinib antiangiogenesis therapy creating an immunosuppressive microenvironment, which fosters metastasis [45]. Oxygen deprivation clearly increases B16F10 melanoma metastasis to the lung [46, 47]. Timing and duration of treatment is critical to increasing survival in response to sunitinib treatment after i.v. tumor inoculation [42]. Survival of mice inoculated i.v. with B16 or human MeWo melanoma was decreased if sunitinib was given prior to tumor inoculation or for a short 7-day treatment after tumor injection. Moreover, sustained treatment such as that used in the present study increased survival. While we did not examine survival after i.v. inoculation of melanoma, we did observe increased survival in mice continuously administered sunitinib i.p., and we observed decreased metastasis in mice inoculated i.v. and treated continuously with sunitinib. Ebos et al. [48] examined the pre- and postsurgical effects of antiangiogenic therapy found in established breast, kidney, and melanoma models and found that sunitinib did not predict postsurgical recurrence of metastatic disease. While there is strong evidence that antiangiogenic therapy increases metastasis in animal models of cancer, the risk associated with human cancer patients is still unresolved.
Immunotherapy is a major focus and a promising area for treatment of melanoma. While the present study indicates a beneficial effect of ALT-803 to augment the immune response in s.c. B16BL6 melanoma and of sunitinib to enhance the efficacy of ALT-803, we witnessed a common problem that often arises with immunotherapy—the development of resistance to treatment over time. Moreover, the development of resistance appeared to be greater in the immune-compromised, chronic alcohol drinking mice, which could reflect additional complexity to successfully treating human alcoholic cancer patients. Further studies are warranted to assess the role of ALT-803 in combination with other immune-enhancing therapies for the treatment of melanoma as well as the role of preexisting immune deficiency in the efficacy of these therapeutic approaches.
Acknowledgements
The authors greatly appreciate Dr. Hing C. Wong and Altor BioScience for the generous gift of ALT-803. They also thank and appreciate the guidance provided by Dr. Sterling McPherson regarding statistical analysis. The authors also acknowledge the technical contributions of Dung Luong to the collection of data for the manuscript. This work was supported by the following grants: National Institutes of Health (NIH) Grants K05AA017149 to Gary G. Meadows, and R21AA022098 to Hui Zhang and Gary G. Meadows; Research Assistantships for Diverse Scholars Program, Washington State University, to Alexander A. Little; and National Science Foundation (NSF) Graduate Research Fellowship DGE-1347973 to Kari A. Gaither.
Abbreviations
- Cy5.5
Cyanine 5.5
- IL-15Rα
IL-15 receptor alpha
- Treg
Regulatory T cells
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
K. A. Gaither and A. A. Little have contributed equally in this paper.
G. G. Meadows and H. Zhang are co-corresponding authors in this paper.
Contributor Information
Gary G. Meadows, Phone: 509-334-4239, Email: meadows@wsu.edu
Hui Zhang, Phone: 509-368-6580, Email: hzhang@wsu.edu.
References
- 1.Wu J. IL-15 agonists: the cancer cure cytokine. J Mol Genet Med. 2013;7:85. doi: 10.4172/1747-0862.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu W, Jones M, Liu B, Zhu X, Johnson CB, Edwards AC, et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 2013;73(10):3075–3086. doi: 10.1158/0008-5472.CAN-12-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes-Giacoia E, Miyake M, Goodison S, Sriharan A, Zhang G, You L, et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One. 2014;9(6):e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakuta S, Tagawa Y, Shibata S, Nanno M, Iwakura Y. Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms. Immunology. 2002;105(1):92–100. doi: 10.1046/j.0019-2805.2001.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zhu Z, McKinley JM, Meadows GG. IFN-gamma is essential for the inhibition of B16BL6 melanoma lung metastasis in chronic alcohol drinking mice. Clin Exp Metastasis. 2011;28(3):301–307. doi: 10.1007/s10585-011-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120(6):2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 10.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68(8):2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 11.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 12.Umansky V, Sevko A, Gebhardt C, Utikal J. Myeloid-derived suppressor cells in malignant melanoma. J Dtsch Dermatol Ges. 2014;12(11):1021–1027. doi: 10.1111/ddg.12411. [DOI] [PubMed] [Google Scholar]
- 13.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 14.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, et al. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62(11):1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messmer MN, Netherby CS, Banik D, Abrams SI. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64(1):1–13. doi: 10.1007/s00262-014-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albeituni SH, Ding C, Yan J. Hampering immune suppressors: therapeutic targeting of myeloid-derived suppressor cells in cancer. Cancer J. 2013;19(6):490–501. doi: 10.1097/PPO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011;77(1):12–19. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9(4):470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 23.Kujawski M, Zhang C, Herrmann A, Reckamp K, Scuto A, Jensen M, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 2010;70(23):9599–9610. doi: 10.1158/0008-5472.CAN-10-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roskoski R., Jr Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356(2):323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- 25.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008;38(6):1621–1630. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer. 2014;112(3):580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78(5):1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Meadows GG. Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice. Cancer Immunol Immunother. 2010;59(8):1151–1159. doi: 10.1007/s00262-010-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Meadows GG. Exogenous IL-15 in combination with IL-15R alpha rescues natural killer cells from apoptosis induced by chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33(3):419–427. doi: 10.1111/j.1530-0277.2008.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Souza El-Guindy NB, Kovacs EJ, De Witte P, Spies C, Littleton JM, de Villiers WJ, et al. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010;34(9):1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman JP, Goldwasser R, Mark S, Schwartz J, Orion I. A mathematical model for tumor volume evaluation using two-dimensions. JAQM. 2009;4(4):455–462. [Google Scholar]
- 32.Meadows GG, Elstad CA, Blank SE, Gallucci RM, Pfister LJ. Alcohol consumption suppresses metastasis of B16-BL6 melanoma in mice. Clin Exp Metastasis. 1993;11(2):191–199. doi: 10.1007/BF00114977. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Zhu Z, Meadows GG. Chronic alcohol consumption decreases the percentage and number of NK cells in the peripheral lymph nodes and exacerbates B16BL6 melanoma metastasis into the draining lymph nodes. Cell Immunol. 2011;266(2):172–179. doi: 10.1016/j.cellimm.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton SE, Jameson SC. CD8 T cell memory: it takes all kinds. Front Immunol. 2012;3:353. doi: 10.3389/fimmu.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8 T cells into innate-like effector cells with antitumor activity. Oncoimmunology. 2013;2(11):e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwilas AR, Donahue RN, Tsang KY, Hodge JW. Immune consequences of tyrosine kinase inhibitors that synergize with cancer immunotherapy. Cancer Cell Microenviron. 2015;2(1):e677. doi: 10.14800/ccm.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamzalit F, Barbieux I, Plet A, Heim J, Nedellec S, Morisseau S, et al. IL-15.IL-15Ralpha complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci U S A. 2014;111(23):8565–8570. doi: 10.1073/pnas.1405514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Hedlund EM, Lim S, Chen F, Zhang Y, Sun B, et al. Antiangiogenic agents significantly improve survival in tumor-bearing mice by increasing tolerance to chemotherapy-induced toxicity. Proc Natl Acad Sci U S A. 2011;108(10):4117–4122. doi: 10.1073/pnas.1016220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passarella RJ, Zhou L, Phillips JG, Wu H, Hallahan DE, Diaz R. Recombinant peptides as biomarkers for tumor response to molecular targeted therapy. Clin Cancer Res. 2009;15(20):6421–6429. doi: 10.1158/1078-0432.CCR-09-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart GD, O’Mahony FC, Laird A, Eory L, Lubbock AL, Mackay A, et al. Sunitinib treatment exacerbates intratumoral heterogeneity in metastatic renal cancer. Clin Cancer Res. 2015;21(18):4212–4223. doi: 10.1158/1078-0432.CCR-15-0207. [DOI] [PubMed] [Google Scholar]
- 45.Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res. 2014;74(24):7185–7190. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 46.Mitrus I, Bryndza E, Kazura M, Smagur A, Sochanik A, Cichon T, et al. Properties of B16-F10 murine melanoma cells subjected to metabolic stress conditions. Acta Biochim Pol. 2012;59(3):363–366. [PubMed] [Google Scholar]
- 47.Almendros I, Montserrat JM, Torres M, Dalmases M, Cabanas ML, Campos-Rodriguez F, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186(3):303–307. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Ebos JM, Mastri M, Lee CR, Tracz A, Hudson JM, Attwood K, et al. Neoadjuvant antiangiogenic therapy reveals contrasts in primary and metastatic tumor efficacy. EMBO Mol Med. 2014;6(12):1561–1576. doi: 10.15252/emmm.201403989. [DOI] [PMC free article] [PubMed] [Google Scholar]