Abstract
Purpose
NPC-1C is a chimeric immunoglobulin IgG1 developed from antigen tested in the Hollinshead tumor vaccine trials that recognizes an immunogenic MUC5AC-related tumor-associated antigen. In this article, we describe the pre-clinical characterization of this antibody that is currently being tested in human clinical trials.
Experimental design
The specificity of NPC-1C for pancreatic and colorectal cancer cell lines was tested by flow cytometry assays and immunohistochemical staining. Antibody-dependent cell cytotoxicity was measured using a tumor cell line lysis assay. Anti-tumor efficacy and biodistribution were assessed in nude mice bearing human pancreatic tumor xenografts.
Results
Human tumor cell binding measured by flow cytometry ranged from 52 to 94 % of cells stained positive with NPC-1C in three colorectal and one pancreatic cell lines, while IHC demonstrated staining of 43 % of colon cancers and 48 % of pancreatic cancer tissues, with little or no cross-reactivity of NPC-1C with normal colon or pancreas tissues. In vitro NPC-1C-mediated tumor cell killing occurred in a median of 44.5 % of four colorectal and three pancreatic tumor cell lines. In vivo anti-tumor efficacy in a human pancreatic CFPAC-1 tumor xenograft model was demonstrated with a twofold to threefold reduction in tumor growth in the NPC-1C-treated mice compared to saline and human IgG controls. Pharmacodynamic studies indicate NPC-1C localizes in antigen-positive tumors and has minimal uptake in normal mouse tissues.
Conclusions
NPC-1C, a chimeric monoclonal antibody that reacts with a MUC5AC-related antigen expressed by pancreatic and colorectal tumor tissues, has promising preclinical activity in pancreatic and colorectal adenocarcinoma.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1420-z) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic cancer, Colorectal cancer, Monoclonal antibody, NPC-1C, MUC5AC-related, Tumor vaccine
Introduction
Pancreatic cancer, the fourth leading cause of cancer-related death in the US, and colorectal cancer, the second leading cause of death across genders, are both in need of new therapeutic options such as immunotherapy because the current therapies for metastatic disease have modest efficacy and significant toxicity [1]. Although cancer vaccines are under development for these malignancies, these rely on induction of an effective immune response that may be difficult to accomplish in the immunosuppressive environment of advanced malignancy. Passive transfer of monoclonal antibodies against tumor antigens offers an opportunity to target immune effectors directly against the tumor.
Pancreatic and colon cancer tumor antigens that have been the target of various immunotherapy strategies include mesothelin (pancreatic) and CEA and MUC-1 (both) [2, 3]. Modest clinical efficacy of strategies to target these antigens with immunotherapy suggests that there is a need to identify a broader array of epitopes. An ideal target would be one for which there is demonstrated immune reactivity with clinical efficacy. In a phase I study, Hollinshead and colleagues immunized colorectal cancer patients with semi-purified, membrane-bound, tumor-associated antigens (TAA) derived from surgically resected, pooled allogeneic colon cancer specimens [4]. These TAA were isolated from the cancer cell membranes by repeated washings, followed by sonication to isolate membrane-bound proteins, Sephadex G-200 chromatography for further purification, and further refinement by discontinuous gradient gel electrophoresis. Sera of vaccinated patients contained antibodies that could recognize colon TAA, indicating that the TAA preparation is immunogenic and that the pooled specimens contained antibody targets [5]. In order to develop a therapeutic antibody that could bind to colon cancer TAA, we immunized mice with the Hollinshead TAA, generated hybridomas from splenic B cells, screened the resulting monoclonal antibodies for tumor cell binding by flow cytometry, selected IgG-secreting clones, further characterized the secreted antibodies by IHC and ELISA, and then cloned the IgG genes in order to engineer a recombinant chimeric antibody called NPC-1C. The target of NPC-1C has subsequently been found to be an aberrantly glycosylated mucin, MUC5AC-related, which is preferentially expressed in pancreatic and colorectal cancers [6]. Our goal was to assess the in vitro and in vivo activity of NPC-1C in preclinical models in preparation for assessing its safety and efficacy in clinical trials.
Methods
Cell lines
Human tumor cell lines AsPC-1 (Pancreatic), CFPAC-1 (Pancreatic), CFPAC-1 (Pancreatic), PANC-1 (Pancreatic), SW620 (Colorectal), LS174T (Colorectal), Colo-205 (Colorectal), SW480 (Colorectal), SW1463 (Colorectal), GEO (Colorectal), OVCAR-3 (Ovarian), MCF-7 (Breast), and SK-MEL (Melanoma) were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cell lines were grown in RPMI or DMEM with 10 % HI-FBS and maintained as recommended by ATCC. Cell lines were banked and used for no more than 3 months in continuous culture with weekly sub-culturing, after which a new vial of cells from the bank was thawed for use in experiments.
Generation of NPC-1C antibody
A colon cancer TAA vaccine preparation was used as the immunogenic material to generate monoclonal antibodies in mice. Preparation of the vaccine from pooled allogeneic colon cancer tissues has been described previously [4, 5]. The TAA preparation (approximately 100 μg per mouse) was admixed with Complete Freund’s adjuvant (CFA) and injected subcutaneously in the flanks of normal female BALB/c mice. This was followed by three booster injections (approximately 50 μg per mouse) in incomplete Freund’s adjuvant (IFA), separated by 2–3 weeks. Three weeks after the final immunization, mouse serum was tested by ELISA for antibody responses against the immunizing antigen. Mice with potent serum ELISA responses (OD450 values >0.3 at serum dilution of 1:10,000 or more) were used to generate immortalized hybridoma cells by fusing the mouse splenic B cells with the SP2/0-Ag14 myeloma cell line. All hybridoma cultures were maintained in RPMI containing 10 % HI-FBS, 2 mM glutamine, and 1× penicillin–streptomycin solution. The cell cultures were expanded and re-tested by ELISA, and monoclonal hybridoma cell lines were generated by well-known limiting dilution culture techniques. Single-cell clones were selected after approximately 2–3 weeks based on robust reactivity to the immunizing Hollinshead antigen by ELISA and isotypes that included mouse immunoglobulin (IgG). Those clones were expanded and cell banks were prepared and stored under liquid nitrogen.
Hybridoma cell culture medium was tested for the expression of mouse IgG that was reactive with the immunizing TAA preparation by ELISA and also by testing against colon cancer cell line extracts by ELISA (ex. HT-29, COLO-205, LS174T). Colon cancer cell extracts were made using cell cultures that were 70–90 % confluent. The adherent cells were scraped from a culture flask, washed once with PBS, then 0.1 % Triton X-100 was added and cells were gently lysed to make the extraction of cell membrane-associated proteins. Cell extracts were stored at −70 °C. Following the initial screening by ELISA methods, specific cell clones were further screened by testing the cultured cell medium containing mouse IgG by flow cytometry using several live, non-permeabilized human colorectal and pancreatic tumor lines, including AsPC-1, CFPAC-1, and LS174T. By FACS, a number of positive cell clones were selected by their ability to bind to the cell surface of live cells expressing native tumor antigens. These clones were then expanded in culture flasks so that larger quantities of the mouse IgG expressed by the cells could be purified using standard protocols involving protein-A resins. The purified mouse IgG preparations, including mAb NPC-1, were subjected to further analyses.
NPC-1 antibody was isotyped by testing culture medium collected from cells using isotyping strips (Amersham-Pharmacia) and by an ELISA-based isotyping kit (BD-Pharmingen). Results from both tests showed the mouse NPC-1 to express an IgG1 heavy chain and a kappa light chain.
The nucleotide sequence of the genes encoding NPC-1 heavy chain (HC) and light chain (LC) was determined. RNA extracted from 1 × 107 hybridoma cells was prepared (RNeasy kit, Qiagen), and cDNA was synthesized using reverse transcriptase. The polymerase chain reaction (PCR) was used to amplify the specific HC and LC variable regions using primers specific for mouse IgG1/Kappa genes that hybridized to CH1 and CL domains. The amplified DNA fragments were then cloned into a TOPO vector and subjected to dideoxynucleotide chain-terminating DNA sequencing reactions. After determining the sequences of the variable regions, new primers were designed and used to perform 5′ RACE and 3′ RACE experiments in order to generate the full-length sequences of the HC and LC of murine NPC-1. These sequences were found to be unique by BLAST database search. The DNA sequences encoding the heavy chain and light chain proteins were determined twice using RNA extractions made at different passage numbers of the hybridoma cells. Both sequence determinations resulted in the identical DNA sequences, indicating that the sequences were reproducible and authentic.
For each HC and LC, molecular engineering was used to fuse the mouse variable regions (including leader sequence, CDR sequences, and framework sequences) in-frame with human HC and LC IgG1 constant regions. The resulting chimeric antibody was renamed NPC-1C. A mammalian expression vector contains the murine dihydrofolate reductase (dhfr) gene (pBF-dhfr vector purchased from Biofactura, Rockville, MD) and utilizes the hCMV promoter/enhancer region to efficiently transcribe the inserted target IgG genes and the dhfr gene as a selectable marker (pBF-dhfr). This vector provides a high level of antibody production when expressed in dhfr gene-deleted Chinese Hamster Ovary (CHO) cells. The NPC-1C gene constructs were individually synthesized using codon sequences optimized for CHO cells and containing the correct restriction enzyme sites at the 5′ and 3′ ends for direct cloning into the pBF-dhfr mammalian expression vector. A bi-cistronic plasmid containing both HC and LC genes was constructed such that approximately equivalent levels of each gene product would be synthesized. The plasmids were grown in LB-ampicillin (1 L) and purified by CsCl ultracentrifugation (2×) and transfected into CHO-DG44 cells (Invitrogen) using Lipofectamine 2000 (Invitrogen). Stable NPC-1C expressing CHO cell lines were developed by an amplification procedure by increasing the methotrexate concentrations up to 20 μM in the CHO cell culture medium. Clones expressing high levels of functional antibody were selected and expanded.
Following several weeks of amplification using culture medium containing methotrexate, assaying for IgG1 production, and cloning the highest producing cell clones, the 4B7 clone was selected as the production cell clone. The 4B7 clone was adapted to serum-free growth conditions in Opti-CHO (Invitrogen) and banked for manufacturing the GMP-grade NPC-1C drug product.
Tumor binding assays
Flow cytometry was performed to assess tumor cell binding by median fluorescence intensity compared to an isotype control in human colorectal tumor cell lines (LS174T, Colo-205, SW480) and pancreatic tumor cells (CFPAC-1). A purified pooled human IgG isotype (Pierce) or a purified human IgG1 isotype (Axxora, Farmingdale, NY, USA) was included as a negative control. Tumor cells were harvested by brief exposure to trypsin, or by scraping, washing twice with cold PBS, then resuspension in cold PBS containing 0.1 % bovine serum albumin (BSA) at 3 × 106 cells/mL. NPC-1C or isotype control antibodies were incubated with 0.1 mL of cells for 45 min on ice. Cells were washed once with cold PBS and resuspended in 0.1 mL of PBS containing 0.1 % BSA. A 1:100 dilution of FITC-labeled rabbit anti-human IgG secondary (Pierce) was then incubated with the cells for 45 min on ice in the dark. Cells were washed twice with PBS and resuspended in 0.1 mL and immediately analyzed using a flow cytometry instrument (FACSort, Becton–Dickinson). Cells were exposed to minimal light after the FITC-labeled reagent was incubated with the cells. Data were analyzed using Cellquest software. Both isotype controls reacted similarly when tested against the human tumor cell lines by FACS with generally less than 3 % binding to live cells. In human tissue staining studies, biotin-labeled NPC-1C was tested along with a human IgG1 isotype negative control (Axxora, Farmingdale, NY, USA), by immunohistochemistry using Accumax (Biocarta, San Diego, CA, USA) and CHTN (Cooperative Human Tissue Network, Charlottesville, VA, USA) arrays. Staining intensity was characterized on a scale of +4 (highest intensity and widespread throughout the tissue section) to 1+ (lowest intensity and prevalence in tissue section). Non-specific or lack of staining was characterized as “weak” or “negative” staining.
ADCC assays
On the day prior to conducting an ADCC assay, PBMC from healthy human donors were activated with 100 U/mL of human IL-2 mixed in RPMI/10 % HI-FBS overnight (16–18 h). On the day of the ADCC assay, tumor target cells were radiolabeled with 111-Indium (aqueous) for 30 min and then washed 3 times with PBS. The activated PBMC were mixed with 20 μg/mL NPC-1C and 111-Indium-labeled target cells at various ratios of effector to target cells (50:1, 25:1, and 12.5:1) in 96-well U-bottom microtiter plates. Plates were centrifuged at 200 rpm for 2 min to initiate cell contact. Control wells were included that had isotype antibodies, no antibodies (spontaneous release), and wells on a separate plate that contained radiolabeled target cells mixed with 0.1 % Triton X-100 to lyse all cells (maximum release). After incubation at 37 °C for 4-h, the supernatant was collected and counted for radioactive Indium release into the culture medium. Cytotoxicity was calculated according to the equation: % Specific lysis = [(experimental cpm release−spontaneous cpm release)/(maximum cpm release−spontaneous cpm release)] × 100.
Animal anti-tumor efficacy studies
All studies involving animals were handled in accordance with the NIH Policy on Human Care and Use of Laboratory Animals and protocols were approved by the local IACUCs. In an anti-tumor efficacy model, nude/nude mice on BALB/c background (Charles River Laboratories, Durham NC) were implanted subcutaneously in the hind flank with a suspension of 3 × 106 cells of the human pancreatic cancer cell line AsPC-1 or the human colorectal cancer cell line LS174T (8–9 mice per group). Following harvest from flasks, the cells were washed and resuspended in cold PBS at 3 × 107/mL, kept on ice during transfer to the vivarium, and injected through a 15-gauge needle (0.1 mL per injection). Tumor masses were allowed to grow to approximately 20–50 mm3, which occurred by 4–6 days. The treatment phase included intraperitoneal injection of 200 μg (10 mg/kg) of research-grade NPC-1C or a negative control human IgG (purchased from Pierce, Rockford IL), followed on the next day with an intraperitoneal injection of IL-2-activated normal human PBMCs prepared as described above (approximately 2 × 107 per mouse per injection). Initial experiments showed polyclonal human IgG to be equivalent to the specific human IgG1 isotype, and because the former was available in more ample supply, polyclonal IgG was chosen for use as the control reagent. Either two cycles, with antibody injections on days 5 and 8 (data shown in Supplemental Figure 1), or four cycles, with antibody injections on days 4, 7, 10 and 13 (data shown in Fig. 2), of treatment were administered in separate studies. Throughout the studies, the tumor growth was monitored twice weekly by measurement with a caliper. Tumor volume was calculated using the equation: Volume = (width2 × length)/2. If a tumor reached approximately 800 mm3, the mouse was killed according to institutional IACUC guidelines, and otherwise, the study was terminated at day 34 (data shown in Supplemental Figure 1) or day 39 (data shown in Fig. 2). The colorectal tumor line LS174T grows aggressively in nude mice and this anti-tumor study was terminated on day 18 due to many tumors in excess of 1,000 mm3, as per institutional guidelines. Statistical analysis was performed using ANOVA at various timepoints. P values of ≤0.05 were considered statistically significant and are indicated with an asterisk in Figs. 2 and 3.
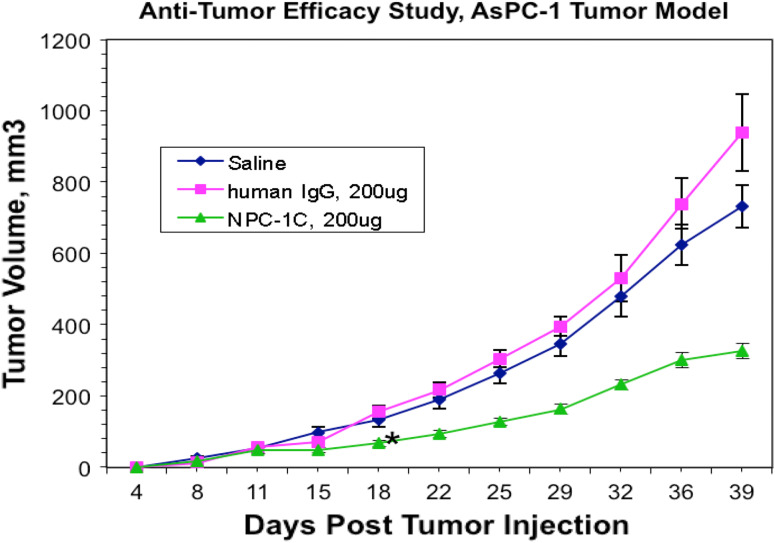
Fig. 2.
A comparison of model subcutaneous pancreatic (AsPC-1) tumor volume in mice over time with saline control, human IgG control, and 4 doses of NPC-1C (n = 8 per group). Tumor volume was assessed every 3–4 days in each mouse cohort, with reduction in tumor volume in the NPC-1C-treated cohort relative to the control groups. Mice received antibodies or saline on days 4, 7, 10, 13 followed by normal human PBMC on days 5, 8, 11, 14. All injections were intraperitoneal. Eight mice in each cohort, each data point represents the mean tumor volume for all mice in a cohort at a given time point. Asterisk indicates point at which p < 0.05 by ANOVA between groups. Experiment performed once. IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cells. The human IgG control was selected for use in the in vivo mouse studies because of the ease to procure and prepare this reagent for injection. The immunoreactivity as a negative control of the purified human IgG control (Pierce) against human AsPC-1 cells was shown in flow cytometry experiments to be similar to that of purified human IgG1-specific isotype (Axxora)
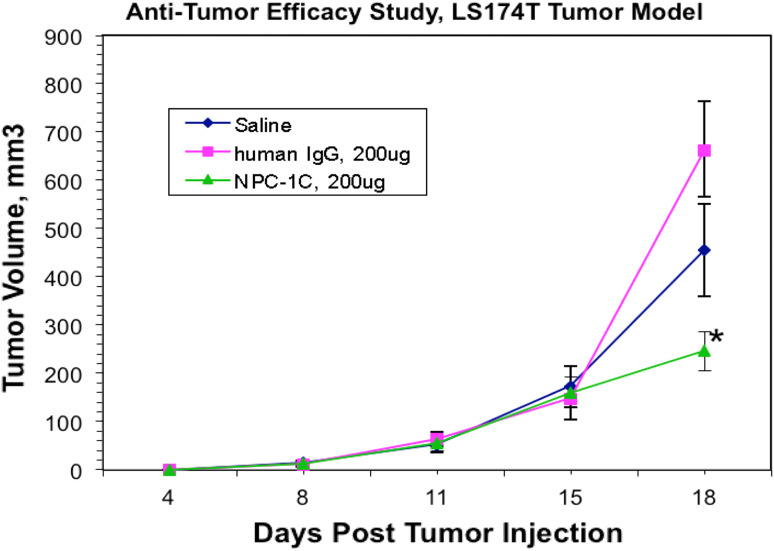
Fig. 3.
A comparison of model subcutaneous colorectal (LS174T) tumor volume in mice over time with saline control, human IgG control, and 4 doses of NPC-1C (n = 9 per group). Tumor volume was assessed every 3–4 days in each mouse cohort, with reduction in tumor volume in the NPC-1C-treated cohort relative to the control groups. Mice received antibodies or saline on days 4, 7, 10, 13 followed by normal human PBMC on days 5, 8, 11, 14. All injections were intraperitoneal. Nine mice in each cohort, each data point represents the mean of tumor volume for all mice in a cohort at a given time point. Asterisk indicates point at which p < 0.05 by ANOVA between groups. Experiment performed once. IgG immunoglobulin G, PBMC peripheral blood mononuclear cells
Biodistribution studies
The biodistribution of the NPC-1C antibody was evaluated in tumor-bearing mice using radiolabeled NPC-1C (by Comparative Biosciences, Sunnyvale, CA). The pre-clinical toxicology lot of NPC-1C was labeled on surface-exposed tyrosines with 125-Iodine, using the chloramine-T reaction method, to 0.2 mCi/mg, purified via gel filtration chromatography, tested by ELISA for antigen binding, and was shown to bind target antigen to acceptable levels. Nude/nude mice (BALB/c background) were injected subcutaneously in the hind flank with either human pancreatic tumors (4 × 106 cells per injection of CFPAC-1) or colorectal tumors (2 × 106 cells per injection of LS174T) to establish tumors. When tumors grew to approximately 50–150 mm3 volume (8 days for the CFPAC-1 tumors; 12 days for LS174T tumors), the mice were injected intravenously via tail vein with the radioiodinated NPC-1C (20 μCi/mouse in 0.1 mL volume). Mice were killed on study day 1 (24 h), study day 2, study day 4, and study day 6. There were 5 males and 5 females for each time point and for each tumor model. On necropsy days, mice were exsanguinated and major organs (lungs, intestine, liver, pancreas, spleen, kidneys, blood) including the subcutaneous tumor were collected. All tissues were weighed and counted for radioactivity. The raw data and individual tissue uptakes in % of injected 125I dose per gram of tissue (ID/g) and in ratio of tissue-count to blood-count (T:B ratio, cpm/g of tissue: cpm/g of blood) were calculated. Data were plotted for each tissue as the tissue localization index (relative to blood) as a function of time of necropsy. Where statistical analysis was judged to be useful in interpreting the results, the following methods were employed; P values of ≤0.05 were considered statistically significant. For data from the multiple (>2) dosing groups, Bartlett’s test for equal variances was used to determine homogeneity. Where variance was homogeneous, one-way analysis of variance (ANOVA) was used, followed by the Dunnett’s multiple comparisons post hoc test if the ANOVA was significant. For non-homogeneous variance, the Kruskal–Wallis (nonparametric) test was used, followed by Dunn’s multiple comparisons post hoc test if the Kruskal–Wallis was significant.
Results
NPC-1C binds to human colon and pancreatic cancer cells
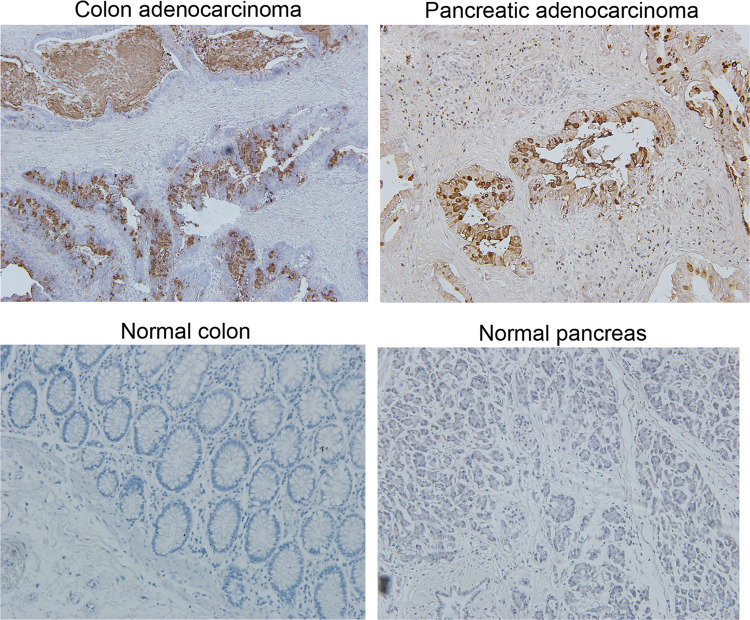
The ability of NPC-1C to bind to colorectal and pancreatic cancer cell lines was assessed by flow cytometry. The NPC-1C antibody was shown to bind to 50–90 % of cells within the panel of human colorectal and pancreatic tumor cell lines tested (Table 1). Importantly, the chimeric antibody retains the tumor cell binding activity and specificity that was observed with the murine antibody (Supplemental Table 1), indicating that neither the (1) chimerization process nor (2) recombinant expression of the NPC-1C antibody from CHO cells disrupted its antigen specificity. To confirm the flow cytometric studies and demonstrate localization of the NPC-1C within tumors, immunohistochemical staining of normal and malignant human tissues was performed. As demonstrated in Supplemental Table 2, 43 % of colorectal cancer specimens (21 of 48 total) and 48 % of the pancreatic cancer specimens (52 of 108 total) stained positively with NPC-1C. In most positive tissues, the staining pattern was observed to include the cytoplasm, the membrane, and the secretory product elaborated into the lumen by tumor cells. Representative micrographs are shown in Fig. 1 to illustrate the staining patterns observed in colorectal and pancreatic tumor tissues, as well as the lack of cross-reactivity to normal colon and pancreas tissues. Although an exhaustive analysis of other tumor types was not performed here, we did note that 24 % of uterine cancers also stained for NPC-1C. Only 1 of 4 normal colon and 0 of 3 normal pancreas specimens stained for NPC-1C. Microarray data corroborating these immunohistochemical findings are shown in Supplemental Table 3. In addition, 34 normal human tissue samples from various organs were tested for cross-reactivity with NPC-1C, with 2/3 colon, 3/3 small intestine, and 1/3 esophageal mucosa positive by IHC. Additionally, at higher concentrations of 50 μg/mL, 1/3 salivary mucosa was positive for cross-reactivity (data not shown).
Table 1.
Quantification of chimeric NPC-1C binding to various tumor cell lines by flow cytometry, values representing percentage of cells stained with median fluorescent intensity value in parentheses
| Tumor cell line | % Cells stained (mfi) | |
|---|---|---|
| Isotype control | NPC-1C | |
| LS174T colorectal | 3.85 (35) | 89.72 (103) |
| Colo-205 colorectal | 2.33 (34) | 94.67 (175) |
| SW480 colorectal | 3.38 (56) | 58.98 (118) |
| CFPAC-1 pancreatic | 1.79 (25) | 52.56 (59) |
Three colorectal and one pancreatic tumor cell line were assessed for binding by NPC-1C and an IgG1 isotype control by flow cytometry. Experiment performed once
Fig. 1.
Immunohistochemical staining of colon and pancreatic tissue with NPC-1C (top). Normal human pancreas and colon tissues were shown not to stain with NPC-1C in the same study (bottom)
We also confirmed that the NPC-1C binds to MUC5AC-related as evidenced by mass spectrometry, sandwich ELISA, and competitive ELISA. Mass spectrometry was used for NPC-1C antigen identification in several NPC-1C affinity-purified antigen preparations from different human colorectal and pancreatic tumor cell lines (LS-174T, CFPAC-1, and HT-29). The results of six mass spectrometry experiments demonstrated repeatedly the presence of MUC5AC-derived peptides in the NPC-1 immunopurified preparations (data not shown). As shown in Supplemental Table 4A,4B, sandwich ELISA in which either NPC-1C as capture antibody, anti-MUC5AC as detection antibody or anti-MUC5AC as capture antibody, NPC-1C as detection antibody, and NPC-1C specific antigen can be detected in both formats and in a dose-dependent manner. Perlecan 1 and 2 represent control extracellular matrix proteins. Supplemental Figure 3 demonstrates specificity of NPC-1C binding to pancreatic AsPC-1 cell line compared to ovarian TOV-21G cancer cells by flow cytometry. Furthermore, NPC-1C binds to 57–64 % MUC5AC expressed on CFPAC-1 cells, but does not react to the MUC5AC on A549 cells (lung adenocarcinoma epithelial cell line) in flow cytometry, in which double staining of commercial anti-MUC5AC antibodies (MAB2-11, 45M1) and NPC-1C antibody was performed (Supplemental Figure 4). Competitive ELISA was performed to confirm specificity of NPC-1C binding to pancreatic MUC5AC (CFPAC-1) and colorectal MUC5AC (LS174T) compared to A549 (lung adenocarcinoma) and confirms the tumor specificity of the NPC-1C antigen to colorectal and pancreatic adenocarcinoma in contrast to the commercially available antibodies against MUC5AC. In competitive assay, the plate was coated with affinity-purified NPC-1 antigen from CFPAC-1 cells. Cell extract from CFPAC-1, LS174T, or A549 (1 mg/mL serially diluted) was added to the diluted NPC-1C antibody (0.5 μg/mL). The antigens presented in CFPAC-1 cells and LS174T cells compete with NPC-1C affinity-purified antigen to bind to NPC-1C significantly, not the antigen from A549 cells (Supplemental Figure 5).
NPC-1C induces ADCC
Since the chimerized NPC-1C antibody contained human IgG1 constant regions, it was expected to mediate antibody-dependent cellular cytotoxicity (ADCC) activity against several antigen-positive colorectal and pancreatic tumor cell targets in vitro [7]. As demonstrated in Supplemental Table 5, NPC-1C induced ADCC of the colorectal and pancreatic cancer cells lines but not melanoma or prostate cancer cell lines. These in vitro results demonstrate that the NPC-1C antibody is capable of binding to the specific MUC5AC-related target antigen and directing antibody-dependent cell cytotoxic activity in the presence of normal human PBMCs.
In vivo anti-tumor efficacy studies
The NPC-1C antibody was tested for anti-tumor efficacy using the human AsPC-1 pancreas tumor xenograft model in nude mice. As shown in Supplemental Figure 1, tumor growth inhibition was observed during the antibody treatment phase of the study, and the difference between the NPC-1C-treated mice and the control groups was statistically significant beginning on day 13 and continuing for the remainder of the study with P = 0.0072 by one-way ANOVA (n = 9 per group).
This anti-tumor efficacy study was repeated in a separate study using the same AsPC-1 pancreas tumor model and the 200 μg (10 mg/kg) dose of NPC-1C antibody. However, in the second study, four cycles of treatment were administered instead of two cycles. All other parameters were kept the same as the previous study. The data are shown in Fig. 2 which demonstrates very similar growth inhibition in response to treatment with NPC-1C. Tumor inhibition was evident during the treatment phase of the study, and the difference between the NPC-1C-treated mice and the human IgG control mice was statistically significant beginning on day 18 and continuing for the remainder of the study with P = 0.0044 by one-way ANOVA (n = 8 per group).
Since it was observed that the LS174T colorectal tumor cell line was a target in vitro in the ADCC assay, this tumor model was used as a second xenograft model. The LS174T cells were implanted subcutaneously in nude mice and the same treatment regimen was administered to these mice. In this aggressively growing tumor model, we observed a twofold to threefold reduction in tumor growth in NPC-1C-treated mice compared to the two control groups of mice following the treatment cycles (Fig. 3). The anti-tumor effect upon treatment with NPC-1C was significant on the last day of measuring tumors with p = 0.0145 by one-way ANOVA. However, many of the tumors in the control groups were greater than 1,000 mm3 requiring the study to be terminated.
Biodistribution
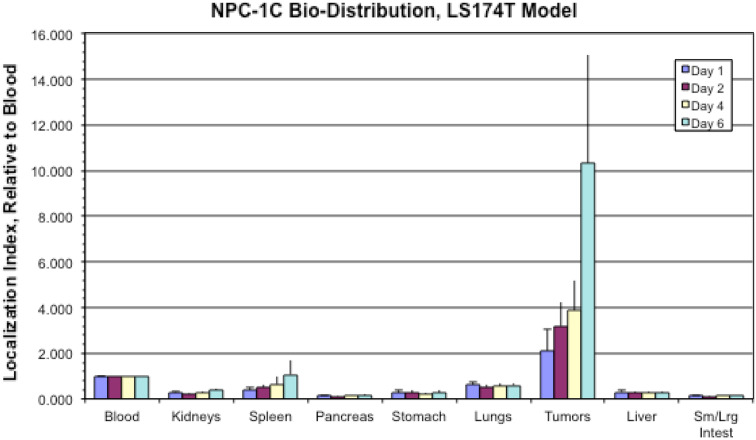
To evaluate in vivo biodistribution of the antibody, radiolabeled NPC-1C was injected into nude mice bearing established subcutaneous human pancreatic tumors (CFPAC-1) or colorectal tumors (LS174T). The data in Fig. 4 show that radiolabeled NPC-1C localized predominantly in the established tumor xenografts, and importantly, not in other non-target tissues examined. In the pancreatic CPFAC-1 tumor model (Supplemental Figure 2), NPC-1C uptake was statistically higher in tumors than in any other tissue type at all timepoints, except when compared to those in blood in females only on day 6. Interestingly, mice harboring the colorectal LS174T tumor (Fig. 4) demonstrated NPC-1C uptake that increased in both sexes reaching the highest levels on day 6. The uptake was statistically higher in tumors than in any other tissue type examined at any timepoint during the study. Overall, CFPAC-1 has decreased uptake compared to LS174T as would be expected given lower NPC-1C staining at baseline in the former. These studies indicate that NPC-1C localizes to the tumor site following IV administration. Representative micrographs indicative of immunohistochemical grading (0–4+) of NPC-1C positivity in colon tissue used in pre-clinical studies are shown in Supplemental Figure 6.
Fig. 4.
Comparative localization of NPC-1C into various tissues in mice, including model subcutaneous colorectal cancer (LS174T) indexed to blood at 1.000. Localization of the antibody to tumor increased with time, highest at day 6. Ten mice (5 male, 5 female) in each group were assessed over 6 days period. Experiment performed once. Sm small, Lrg large, Intest intestine
Discussion
Monoclonal antibodies play an integral role in the treatment of cancer, but for pancreas and colon cancer either there are no available agents (pancreas) or the available agents have modest activity (colorectal). Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) monoclonal antibodies that have single agent activity in kras wild-type colorectal cancer. However, responses are limited to those patients with wild-type kras and are of limited clinical benefit (1–2 months) [8, 9]. Bevacizumab is a monoclonal antibody that binds vascular endothelial growth factor (VEGF) and has a demonstrable 4-month overall survival advantage when used with combination chemotherapy. Unfortunately, bevacizumab has limited single agent activity and is associated with serious adverse events such as bowel perforation, hypertension, and thromboembolic phenomena [10]. Thus, development of novel targeted therapies for colorectal and pancreatic cancer is needed.
We developed an antibody-based strategy for the treatment of colon cancer that would induce ADCC specifically against antigen-positive tumor cells without toxicity to normal tissues. To generate such an antibody, we utilized a colon cancer vaccine containing human colon tumor tissue-derived antigens (the Hollinshead colon cancer vaccine) to generate antibodies in mice. These antigens were shown previously to illicit an immune response in humans with development of delayed-type hypersensitivity (DTH) reactions with increased endogenous vaccine-reactive antibody production. From mice vaccinated with these antigens, we developed hybridomas from which we eventually selected the candidate antibody NPC-1. Our preclinical data demonstrated that the engineered chimeric (mouse/human IgG1) NPC-1C could bind to colon cancer cell lines and tissues. In addition, we also observed that NPC-1C cross-reacted with many pancreatic cancer tissues, with little or no immunoreactivity to normal colon and pancreas tissues. NPC-1C was also shown to induce ADCC in vitro and slowed the progression of aggressive human tumor xenografts in vivo. There are several important aspects of the preclinical studies reported herein. We determined that NPC-1C was expressed in tumor tissue but minimally expressed in normal tissues. This should permit selectivity in human clinical trials, limiting off-target-related toxicity.
We have recently determined that the target antigen for NPC-1C is a glycoprotein variant of human MUC5AC. The MUC5AC gene has been reported to be expressed mainly in the surface epithelium of normal gastric mucosa (foveolar cells) and normal airway epithelium (bronchial submucosal cells). This is in contrast to MUC1, which is expressed on the apical epithelium of breast, respiratory, digestive and genitourinary organs, and MUC2 which is expressed in goblet cells of the colon and small intestine [11]. Considered an oncofetal protein, MUC5AC is also expressed in the fetal and pre-cancerous colonic mucosa, but to a lesser extent in normal adult colon [12]. The gene was also shown to be overexpressed in epithelial mucosal cells in inflammatory conditions such as chronic obstructive pulmonary disease and H. pylori infections of the GI tract [13, 14]. These conditions lead to excessive mucus secretion by respiratory and gastrointestinal epithelial mucosa associated with abundantly glycosylated MUC5AC glycoprotein. Overexpression of MUC5AC has also been shown to be associated with pancreatic and colorectal cancer [15, 16]. However, it is believed that unlike in normal tissues and the inflammatory conditions noted above where native MUC5AC is expressed or overexpressed, in pancreas and colon tumors, MUC5AC was reported to be expressed in an aberrantly glycosylated form. Thus, it appears that NPC-1C antibody can discriminate between the native MUC5AC and the aberrantly glycosylated variant MUC5AC. Studies are underway to identify the chemical nature of the epitope for NPC-1C and to reveal the difference in glycosylation pattern of native MUC5AC and the variant MUC5AC to which NPC-1C binds.
The feasibility of therapeutically targeting a mucin is supported by the fact that several other mucins are currently being investigated as targets for monoclonal antibody directed therapy. MUC1 (CA 15-3) is a poor prognostic marker identified initially in breast cancer but is also a marker for aggressive prostate, lung, and thyroid cancer [17, 18]. DMC209 is a monoclonal antibody against MUC1 that is being evaluated for use in clinical trials. MUC16 (CA-125) is an important marker in ovarian cancer, and multiple monoclonal antibodies and immunoconjugates are being evaluated for use in the treatment of this disease expressing CA125 [19]. Given the specificity of binding of NPC-1C to malignant colonic and pancreatic epithelium, a clinical trial is underway to study the dosing and efficacy of this antibody. Furthermore, with the success of radioimmunoconjugates such as [Y-90] ibritumomab and [I-131] tositumomab in relapsed/refractory follicular lymphoma and drug-antibody conjugates such as T-DM1 in refractory HER2+ breast cancer, therapeutic conjugation strategies with this antibody are being explored [20, 21].
In summary, we developed a novel antibody by immunizing mice with a colon cancer vaccine that was used previously in human clinical trials and shown to be safe and elicit anti-tumor activity in patients treated with the vaccine. The murine antibody (NPC-1) was chimerized to be a human IgG1 isotype (NPC-1C) by genetic engineering and expressed by a recombinant CHO cell line. The purified NPC-1C showed anti-tumor activity against both colon and pancreatic cancer cell lines in vitro and in xenografted tumors in vivo. The specificity of NPC-1C was demonstrated by flow cytometry and immunohistochemistry, which revealed NPC-1C to bind predominantly to tumor tissues with little or no cross-reactivity to normal colon and pancreas tissues. In vivo biodistribution studies in tumor-bearing mice showed localization and time-dependent accumulation of the antibody at the site of the established tumors. Based on these results, a Phase 1/2a clinical trial is ongoing in patients with advanced pancreatic and colorectal adenocarcinoma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Precision Biologics (Andrew Bristol, Olga Saric, Xue-Ping Wang, Alex Dubeykovskiy, Philip Arlen); T32 Training Grant (Sandip Patel).
Conflict of interest
No financial conflicts of interest related to this work to disclose.
References
- 1.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 2.Jimeno A, Hidalgo M. Molecular biomarkers: their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Mol Cancer Ther. 2006;5(4):787–796. doi: 10.1158/1535-7163.MCT-06-0005. [DOI] [PubMed] [Google Scholar]
- 3.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer science driving clinical progress. Nat Rev Cancer. 2005;5(6):459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 4.Hollinshead AC, McWright CG, Alford TC, Glew DH, Gold P, Herbeman RB. Separation of skin reactive intestinal cancer antigen from the carcinoembryonic antigen of gold. Science. 1972;177(52):887–889. doi: 10.1126/science.177.4052.887. [DOI] [PubMed] [Google Scholar]
- 5.Hollinshead A, Elias EG, Arlen M, Buda B, Mosley M, Scherrer J. Specific active immunotherapy in patients with adenocarcinoma of the colon utilizing tumor-associated antigens (TAA). A phase I clinical trial. Cancer. 1985;56(3):480–489. doi: 10.1002/1097-0142(19850801)56:3<480::AID-CNCR2820560312>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Luka J, Arlen PM, Bristol A (2011) Development of a serum biomarker assay that differentiates tumor-associated MUC5AC (NPC-1C ANTIGEN) from normal MUC5AC. J Biomed Biotechnol 2011. Article ID 934757. doi:10.1155/2011/934757 [DOI] [PMC free article] [PubMed]
- 7.Qi CF, Nieroda C, De Filippi R, Greiner JW, Correale P, Schlom J, Tsang KY. Macrophage colony-stimulating factor enhancement of antibody-dependent cellular cytotoxicity against human colon carcinoma cells. Immunol Lett. 1995;47(1):15–24. doi: 10.1016/0165-2478(95)00054-9. [DOI] [PubMed] [Google Scholar]
- 8.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61–69. doi: 10.1309/9R6673QEC06D86Y4. [DOI] [PubMed] [Google Scholar]
- 12.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006;61(5):448–454. doi: 10.1136/thx.2004.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim GE, Bae HI, Park HU, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialylated antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas A comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63:8614–8622. [PubMed] [Google Scholar]
- 16.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/A:1025815113599. [DOI] [PubMed] [Google Scholar]
- 17.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;2001(91):1973–1982. doi: 10.1002/1097-0142(20010601)91:11<1973::AID-CNCR1222>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Khodarev N, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res. 2007;67:4924–4932. doi: 10.1158/0008-5472.CAN-06-4512. [DOI] [PubMed] [Google Scholar]
- 20.Hagenbeek A. VIII. Radioimmunotherapy in malignant lymphoma: an underused tool? Ann Oncol. 2011;22(suppl 4):iv51–iv53. doi: 10.1093/annonc/mdr174. [DOI] [Google Scholar]
- 21.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.