Abstract
CD4+FoxP3+ regulatory T cells (Tregs) represent a major cellular mediator of cancer immune evasion. The expression of tumor necrosis factor receptor type II (TNFR2) on Tregs is reported to identify the maximally suppressive Treg population in both mice and human. We therefore investigated the phenotype and function of TNFR2+ Tregs present in the peripheral blood (PB) of 43 lung cancer patients. Further, the association of TNFR2 expression on Tregs with clinicopathological factors was analyzed. The results showed that in the PB of lung cancer patients, Tregs expressed markedly higher levels of TNFR2 than conventional T cells (Tconvs). Expression of TNFR2 appeared to correlate better than CD25+ and CD127− with FoxP3 expression. PB TNFR2+ Tregs in lung cancer patients were more proliferative and expressed higher levels of the immunosuppressive molecule CTLA-4, and consequently more potently suppressed IFNγ production by cocultured CD8 CTLs. More importantly, higher TNFR2 expression levels on Tregs were associated with lymphatic invasion, distant metastasis and more advanced clinical stage of lung cancer patients. Therefore, our study suggests that TNFR2+ Tregs play a role in promoting tumor progressive metastasis and expression of TNFR2 by PB Tregs may prove to be a useful prognostic marker in lung cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1751-z) contains supplementary material, which is available to authorized users.
Keywords: CD4+FoxP3+ regulatory T cells, Tumor necrosis factor receptor type II (TNFR2), Lung cancer, Clinical pathology, Immunosuppression
Introduction
There is compelling evidence that the immunosuppressive effects of CD4+FoxP3+ regulatory T cells (Tregs) represent a major cellular mechanism underlying immune evasion of lung cancer [1, 2]. Consequently, targeting Tregs has become a strategy to promote antitumor immune responses and to enhance the efficacy of current cancer immunotherapy [3, 4]. Higher levels of Tregs were found in the peripheral blood (PB) of lung cancer patients, as compared with healthy controls [5, 6]. Elevation in the number of Tregs is proposed to have diagnostic value and usually indicates a poor outcome of treatment [7]. Tregs are known to consist of heterogeneous populations, with differential suppressive capacities and trafficking properties [8, 9]. Thus, further characterization of tumor-associated Tregs is required to utilize Tregs as a prognostic indicator as well as to devise more effective antitumor immunotherapy.
Recently, it has been shown that the expression of TNFR2 identified a subpopulation of Tregs with the maximally suppressive function. The highly inhibitory TNFR2+ Tregs were found in normal or tumor-bearing mice [10, 11] and in patients with various diseases such as diabetes [12], malaria [13], ovarian cancer [14] and acute myeloid leukemia (AML) [15, 16]. In a mouse cancer model, tumor-infiltrating Tregs consisted mainly of TNFR2+ Tregs [11], suggesting that TNFR2 expression may be a characteristic of tumor-associated Tregs. Recently, it has repeatedly been shown that depletion of TNFR2+ Tregs was associated with antitumor effect of therapeutics. For example, tumor eradication after cyclophosphamide depended on concurrent depletion of TNFR2+ Tregs in a mouse tumor model [17]. Treatment with panobinostat and azacitidine decreased the levels of TNFR2+ Tregs in PB and bone marrow of AML patients, and this effect was associated with their clinical benefits [16]. Moreover, lenalidomide enhanced the immune effector function in acute myeloid leukemia patients, which was also attributable to its effect on decreasing the number of TNFR2+ Tregs [15]. These clinical data further support the idea that TNFR2-expressing Tregs are tumor-associated suppressors, and expression levels of TNFR2 on Tregs may have prognostic implications in cancer patients, including those with lung cancer.
In this study, we for the first time characterized TNFR2+ Tregs in PB of 43 lung cancer patients. Although the number of total PB Tregs increased as compared with that of healthy donors, there was no difference in total Tregs in patients with various stages of lung cancer. In contrast, the level of TNFR2 expression on Tregs was correlated with disease stages and metastasis, suggesting that TNFR2 expression by PB Tregs has diagnostic implications in lung cancer patients.
Materials and methods
Healthy donors and patients
Peripheral blood samples were obtained from 43 first-time-admitted lung cancer patients in Tianjin Medical University Cancer Institute & Hospital (Tianjin, China) and 18 healthy donors, after receiving written informed consent. This was approved by the Ethics Committee of the Tianjin Medical University Cancer Institute & Hospital. None of these patients had been treated with surgery, radiotherapy, chemotherapy or other medical interventions before collection of blood for this study. Characteristics of study subjects are summarized in Table 1.
Table 1.
Correlation between clinicopathological characteristics and the frequency of peripheral Tregs, the proportion of TNFR2+ cells and the expression of TNFR2 (MFI) by Tregs and Tconvs
| Cases | Fox3+T cells (%) | P value | TNFR2+ in Tregs (%) | P value | TNFR2+ in Tconvs (%) | P value | TNFR2 MFI of Tregs | P value | TNFR2 MFI of Tconvs | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | |||||||||||
| ≤ 60 | 22 | 6.34 ± 2.26 | 0.559 | 89.07 ± 5.79 | 0.422 | 48.19 ± 14.79 | 0.402 | 2689 ± 421 | 0.101 | 977 ± 206 | 0.063 |
| > 60 | 21 | 6.56 ± 1.78 | 90.51 ± 5.30 | 51.81 ± 12.64 | 2929 ± 463 | 1182 ± 332 | |||||
| Gender | |||||||||||
| Male | 14 | 6.60 ± 2.34 | 0.928 | 89.54 ± 5.94 | 0.736 | 47.81 ± 16.05 | 0.406 | 2722 ± 361 | 0.372 | 959 ± 189 | 0.060 |
| Female | 29 | 6.38 ± 1.90 | 89.93 ± 5.46 | 52.58 ± 12.32 | 2847 ± 486 | 1141 ± 318 | |||||
| Histology | |||||||||||
| Adenocarcinoma | 22 | 6.59 ± 2.23 | 0.553 | 90.38 ± 5.78 | 0.653 | 53.53 ± 17.80 | 0.928 | 2867 ± 468 | 0.647 | 1070 ± 275 | 0.874 |
| Squamous carcinoma | 13 | 6.64 ± 2.01 | 88.59 ± 6.29 | 52.96 ± 17.82 | 2791 ± 535 | 1137 ± 370 | |||||
| Other | 8 | 5.73 ± 1.14 | 90.14 ± 3.55 | 50.84 ± 12.19 | 2664 ± 163 | 1025 ± 211 | |||||
| Lymphatic invasion | |||||||||||
| Absent | 29 | 6.42 ± 2.02 | 0.826 | 88.25 ± 5.79 | 0.007 | 48.71 ± 12.24 | 0.058 | 2676 ± 386 | 0.019 | 998 ± 209 | 0.031 |
| Present | 14 | 6.51 ± 2.09 | 92.92 ± 3.22 | 55.82 ± 15.55 | 3077 ± 461 | 1256 ± 368 | |||||
| Distant metastasis | |||||||||||
| Absent | 31 | 6.39 ± 1.97 | 0.765 | 88.07 ± 5.51 | 0.0006 | 49.16 ± 14.25 | 0.189 | 2683 ± 421 | 0.002 | 1037 ± 271 | 0.122 |
| Present | 12 | 6.59 ± 2.10 | 94.17 ± 2.24 | 55.81 ± 10.99 | 3124 ± 366 | 1199 ± 327 | |||||
| Clinical stage | |||||||||||
| I + II | 27 | 6.23 ± 1.84 | 0.466 | 87.17 ± 5.25 | <0.0001 | 46.44 ± 12.93 | 0.008 | 2629 ± 377 | 0.0008 | 974 ± 214 | 0.003 |
| III + IV | 16 | 6.83 ± 2.31 | 94.00 ± 2.45 | 58.68 ± 11.43 | 3106 ± 407 | 1265 ± 324 | |||||
PBMCs were obtained from 43 lung cancer patients and prepared for FACS analysis. Lung cancer patients were grouped by age, gender, histology, with or without lymphatic invasion and distant metastasis, and clinical stage. Then, the proportion of total FoxP3+ Tregs, the proportion of TNFR2-expressing Tregs and Tconvs, MFI of TNFR2 expression by Tregs and Tconvs were analyzed. Bold values indicated statistical significance
Isolation of mononuclear cells
Peripheral venous blood was drawn and collected into tubes containing EDTA-K2. The blood was centrifuged through Lymphoprep™ (Axis-shield, Oslo, Norway), and PBMCs were collected at the interface and then washed with PBS.
Flow cytometry and antibodies
After blocking FcR, PB cells were incubated with appropriately diluted antibodies for phenotyping. Acquisition was performed by FACSCanto II equipped with FACSDiva version 6.1.3 (BD Biosciences, San Diego, CA, USA). Data analysis was conducted using FlowJo software (version 7.6.2, Tree Star, Ashland, OR, USA). The antibodies used for surface staining in this study included CD4-APC-Cy7, CD25-FITC, TNFR2-PE (all from BD Biosciences) and CD127-PE-Cy7 (BioLegend, San Diego, CA, USA). Antibodies used for intracellular staining included FoxP3-APC, CTLA-4-PE-Cy5, Ki-67-PerCP-Cy5.5 (BD Biosciences).
Cell purification and in vitro cell culture
CD4+ T cells and CD4-depleted cells were purified from freshly isolated human PBMCs using human CD4 microbeads and LS column (Miltenyi Biotec, Auburn, CA, USA). CD4+CD25+CD127−TNFR2+, CD4+CD25+CD127−TNFR2− T cells were purified from CD4+ T cells by FACS. CD8+ T cells were FACS-purified from CD4-depleted PBMCs. Flow sorting was performed using FACSAria II (BD Biosciences). The purity of FACS-sorted T cells was >93 %.
For in vitro Treg suppression assays, CD8+ T cells were cultured alone or cocultured with autologous CD4+CD25+CD127−TNFR2+, CD4+CD25+CD127−TNFR2− T cells at a ratio of 1:1 and seeded in a U-bottom 96-well plate in RPMI 1640 medium containing 10 % FBS, 2 mM glutamine, 100 U/mL penicillin, 100 ug/mL streptomycin and 5958 mg/L HEPES (Bioroc, Tianjin, China). The cells were stimulated with Dynabeads® Human T-Activator CD3/CD28 (Life Technologies AS, Norway) for 72 h. The supernatant was collected, and IFNγ levels were determined by ELISA kit (Dakewei, Beijing, China), according to the manufacturer’s instruction.
Statistical analysis
Nonparametric t test of paired or independent samples was used to determine the statistical significance. P < 0.05 was considered to be significant. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
TNFR2 is preferentially expressed by PB Tregs in lung cancer patients
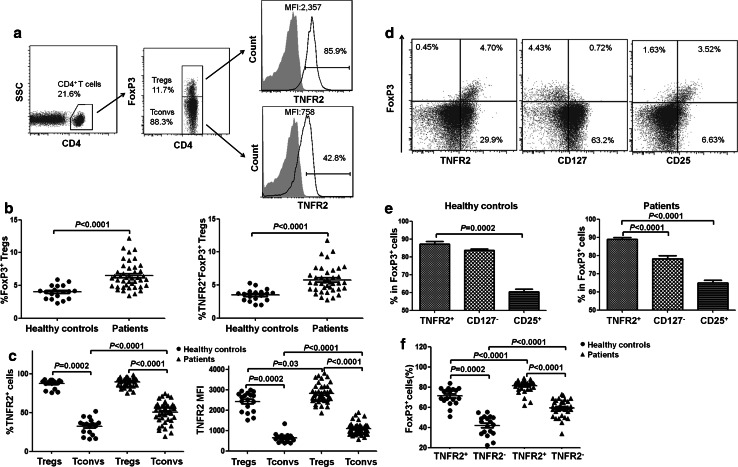
Consistent with previous reports [5, 6], the proportion of total FoxP3+ Tregs in the PB CD4 cells (6.50 ± 2.01 %) of lung cancer patients was markedly higher than that of healthy donors (3.98 ± 1.01 %, P < 0.0001, Fig. 1b, with gating strategy shown in Fig. 1a). Furthermore, the percentage of TNFR2+ Tregs in PB of patients (5.78 ± 2.02 %) was also significantly higher than in healthy controls (3.51 ± 0.91 %, P < 0.0001, Fig. 1b). We were also able to confirm a previous report [18] and observed that the proportion of TNFR2+ cells in FoxP3+ Tregs (87.37 ± 5.60 %) was ~2.6-fold higher than that in FoxP3− conventional CD4 T cells (Tconvs, 33.69 ± 12.64 %, P = 0.0002) in healthy donors (Fig. 1c, with gating strategy shown in Fig. 1a). Tconvs were defined by surface expression of CD4 and lack of intracellular expression of FoxP3. Similarly, the proportion of TNFR2+ cells in Tregs (89.80 ± 5.56 %) was also markedly higher than that in Tconvs from lung cancer patients (51.25 ± 13.92 %, P < 0.0001, Fig. 1c), although the proportion of TNFR2 expressed by Tconvs from patients was also significantly higher than that from healthy donors (P < 0.0001, Fig. 1c).
Fig. 1.
TNFR2 expression by periphery blood (PB) of healthy donors and lung cancer patients. PBMCs from indicated sources were stained for CD4, CD25, CD127, TNFR2 and intracellular FoxP3, and analyzed with FACS. a Representative flow plots of TNFR2 expression by Tregs and Tconvs in a lung cancer patient. b Proportion of FoxP3+ Tregs and TNFR2+ FoxP3+ Tregs in total CD4+ T cells from healthy donors and lung cancer patients. c The proportion of TNFR2+ cells and MFI of TNFR2 expression by Tregs and Tconvs from healthy donors and patients. d The relationship between FoxP3 expression and TNFR2, CD25, CD127 expression by total CD4+ cells present in the PBMCs of patients. Representative flow plots are shown. e The proportion of TNFR2+ cells, or CD127− cells, or CD25+ cells in the FoxP3+ Tregs from healthy donors and from lung cancer patients. f Proportion of FoxP3+ cells in CD4+CD25+CD127− cells, with or without TNFR2 expression, from healthy donors and lung cancer patients. The number in the FACS plots is the proportion of cells in the indicated gating or quadrants. Data shown in b, c, e and f were summarized from 18 healthy donors and 43 lung cancer patients. P value showed the comparison between indicated groups
The level of TNFR2 expression on a per cell basis, e.g., the mean fluorescence intensity (MFI), was also examined. MFI of TNFR2 expression by FoxP3+ Tregs (2427 ± 475) was 3.7-fold greater than that by FoxP3− Tconvs (649 ± 221, P = 0.0002) from healthy donors (Fig. 1c). In lung cancer patients, MFI of TNFR2 expression by FoxP3+ Tregs (2806 ± 449) was also ~2.6-fold higher than that by FoxP3− Tconvs (1082 ± 293, P < 0.0001, Fig. 1c). Further, MFI of TNFR2 expression by both Tregs and Tconvs was markedly higher in patients than in healthy donors (P = 0.03 and P < 0.0001, respectively, Fig. 1c). Thus, TNFR2 is preferentially, although not exclusively, expressed by PB CD4+ Tregs in lung cancer patients, and this receptor tends to be up-regulated on both Tregs and Tconvs of patients.
TNFR2 in combination with CD25 was reported to serve as surface markers to identify more functional human Tregs present in the PB of healthy donors, as compared with Tregs identified by CD25hi [18]. Further, CD25hiCD127lo were frequently used as surface markers of human Tregs [19]. Thus, we further analyzed the relationship between intracellular expression of FoxP3 and surface expression of TNFR2, CD25 or CD127 in PB CD4 cells derived from lung cancer patients. As shown in Fig. 1d, FoxP3 expression was largely restricted to TNFR2-expressing cells. Furthermore, FoxP3+ cells comprised more TNFR2+ cells as compared with CD25+ or CD127− cells in both healthy controls and lung cancer patients (Fig. 1e). Thus, TNFR2+ was superior to CD25+ and CD127− in correlating with FoxP3 expression.
Although intracellular FoxP3 is the most specific marker of Tregs, it cannot be used for the viable isolation of human Tregs for functional study. To date, CD4+CD25hiCD127lo/− remains the most frequently used surrogate surface markers for the identification and flow sorting of human Treg cells [20]. Nevertheless, CD4+CD25+CD127− T cells from lung cancer patients contained a substantial portion of FoxP3− cells (26.52 ± 10.16 %). Thus, we wondered whether addition of TNFR2 could improve the purity of FoxP3-expressing Tregs. As shown in Fig. 1f, both in healthy controls and in lung cancer patients, the proportion of FoxP3+ cells present in CD4+CD25+CD127−TNFR2+ cells (72.16 ± 6.65 % in healthy controls, 82.77 ± 8.39 % in lung cancer patients) was markedly higher than that in CD4+CD25+CD127−TNFR2− cells (44.17 ± 11.38 % in healthy controls, 60.17 ± 13.38 % in lung cancer patients. P = 0.0002 and P < 0.0001, respectively). These data clearly indicated that TNFR2 expression could further enhance the proportion of FoxP3+ Tregs present in CD4+CD25+CD127− cells. Interestingly, TNFR2 appears to be a better indicator in lung cancer patients than in healthy donors in the identification of FoxP3+ Tregs by combination with CD4+CD25+CD127− (Fig. 1e, f).
Phenotypic characteristics of TNFR2+ Tregs in PBMCs of lung cancer patients
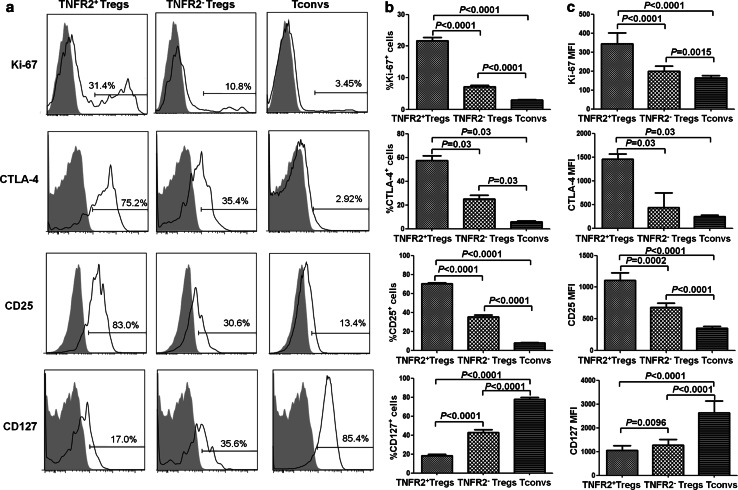
TNFR2 is a co-stimulatory molecule and has the capacity to promote the proliferative response of lymphocytes to TCR stimulation [21, 22]. TNFR2 expressed by mouse Tregs correlated with the expression of Ki-67, a nuclear antigen present only in replicating cells [23], indicative of the highly proliferative nature of Tregs [17, 24]. We therefore wondered whether TNFR2 expressed by Tregs from lung cancer patients also reflected their proliferation profile. Indeed, PB Tregs from lung cancer patients expressed a markedly higher level of Ki-67 in TNFR2+ Tregs (21.68 ± 7.13 %) as compared with TNFR2− Tregs (7.09 ± 3.74 %, P < 0.0001, Fig. 2a, b). As shown in Fig. 2c, the MFI of Ki-67 expression by TNFR2+ Tregs (376 ± 170) was also significantly higher than that expressed by TNFR2− Tregs (210 ± 108, P < 0.0001). However, TNFR2+ Tconvs also expressed a relatively higher level of Ki-67 (5.52 ± 2.61 %) than TNFR2− Tconvs (1.79 ± 1.01 %, P < 0.0001) in lung cancer patients (Supplementary Fig. 1), suggesting that TNFR2 expression was associated with proliferation of PB CD4 cells, regardless of their FoxP3 expression. Nevertheless, Treg cells in patients expressed markedly higher levels of TNFR2 (Fig. 1c, P < 0.0001) and Ki-67 (Fig. 2, P < 0.0001) than Tconvs. Presumably, in lung cancer patients, Tregs are more proliferative and have a higher turnover rate than Tconvs.
Fig. 2.
Phenotypic characteristics of TNFR2+ Tregs in lung cancer patients. PBMCs of lung cancer patients were stained for CD4, CD25,CD127 and TNFR2 and then fixed and stained intracellularly for FoxP3, Ki-67 and CTLA-4. Surface expression of CD25 and CD127 and intracellular expression of CTLA-4 and Ki-67 by indicated subsets of CD4+ cells were analyzed by FACS. a The typical FACS plots. Solid line histogram: Ab staining; gray filled histogram: isotype control IgG staining. Numbers in the plots indicate the proportion of positive cells. b The summary of proportion of Ki-67+, CLTA-4+, CD25+ and CD127+ cells (N = 43 except for CTLA-4 which was summarized from 6 patients). c The summary of MFI of Ki-67, CLTA-4, CD25 and CD127 expression (N = 43 except for CTLA-4 which was summarized from six patients) on indicated subsets. P value shown is comparison between indicated groups
Expression of characteristic molecules by Tregs has implications in their phenotype as well as their suppressive function. We therefore further examined the expression of typical Treg molecules by both TNFR2+ and TNFR2− Tregs in the PB of lung cancer patients. Highly suppressive Tregs expressed elevated levels of an immunosuppressive molecule CTLA-4 which was critical for cell–cell contact-dependent immunosuppressive function of Tregs [25]. As shown in Fig. 2a, b, TNFR2+ Tregs expressed markedly higher levels of CTLA-4 (57.50 ± 9.96 %) than TNFR2− Tregs (25.25 ± 7.11 %) and Tconvs (5.76 ± 2.46 %). CD25, the alpha chain of IL-2 receptor, is a marker of activated T cells. The expression of CD25 on TNFR2+ Tregs was more than twofold higher as compared with that expressed by TNFR2− Tregs. In contrast, the expression of CD127 was the lowest by TNFR2+ Tregs, indicative of co-expression of TNFR2 and CD25+/CD127− as Treg markers. Further, the MFI of CTLA-4, CD25 and CD127 expression on TNFR2+ Tregs, TNFR2− Tregs and Tconvs was analyzed. As shown in Fig. 2c, the MFI of CTLA-4, CD25 expression on TNFR2+ Tregs was the highest and the MFI of CD127 expression on TNFR2+ Tregs was the lowest among the indicated subsets. Thus, in the PB of lung cancer patients, TNFR2+ Tregs possessed a more activated and more suppressive phenotype than their TNFR2 counterparts.
TNFR2+ Tregs suppress IFNγ production by CD8+ T cells
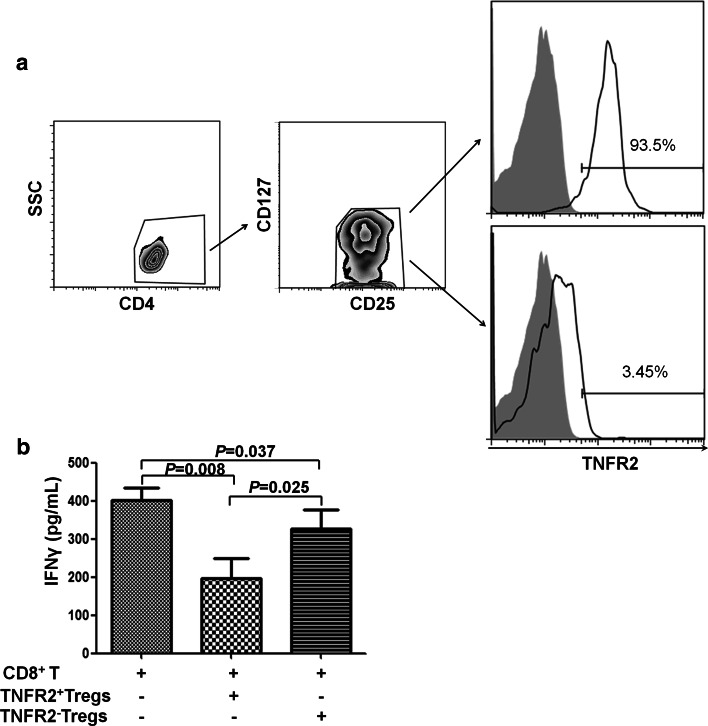
CD8+ cytotoxic T lymphocytes (CTLs) and production of IFNγ by CD8+ CTLs play an important role in cancer immune surveillance and antitumor immunity [26–28]. Therefore, the suppressive potential of TNFR2+ and TNFR2− Tregs from lung cancer patients on IFNγ production by cocultured autologous CD8+ T cells was examined. To this end, Tregs (CD4+CD25+CD127−) were further sorted based on their TNFR2 expression (Fig. 3a), and autologous CD8+ T cells were sorted by FACS. As shown in Fig. 3b, stimulation with CD3/CD28 dynabeads resulted in the production of IFNγ by purified CD8+ T cells (401.9 ± 72.91 pg/mL). When cocultured with TNFR2+ Tregs, IFNγ level was decreased by 50 % to 196.8 ± 116.1 pg/mL (P = 0.008). In contrast, in the cocultures of TNFR2− Tregs and CD8+ T cells, supernatant IFNγ level was 327.4 ± 112.2 pg/mL, which was lower than CD8 cell culture alone (P = 0.037, 18 % inhibition) but markedly higher than cocultures of TNFR2+ Tregs (P = 0.025). This result was unlikely attributable to the IFNγ produced by TNFR2+ Tregs or TNFR2− Tregs, since there was no detectable IFNγ in the supernatant of cultures containing TNFR2+ Tregs alone or TNFR2− Tregs alone (data not shown). Thus, TNFR2 is able to identify the highly immunosuppressive subset of Tregs in lung cancer patients.
Fig. 3.
TNFR2+ Tregs potently inhibit the production of IFNγ by CD8+ T cells. CD4+ T and CD4− depleted cells were purified from freshly isolated PBMCs of lung cancer patients using human CD4 microbeads. CD4+CD25+CD127−TNFR2+ and CD4+CD25+CD127−TNFR2− T cells were flow-sorted from CD4+ T cells. FACS-sorted CD8+ T cells (5 × 104 cells/well), used as responder cells, were cultured alone or cocultured with indicated CD4+ T subsets at a ratio of 1:1. The cells were stimulated with CD3/CD28 dynabeads for 72 h. IFNγ level in the supernatant was determined by ELISA. a The purity of TNFR2+ and TNFR2− Tregs. Data shown are representative FACS plots. Number in the histograms is the proportion of TNFR2+ cells. b IFNγ levels in the supernatant. Data shown are summary from six lung cancer patients. P value shown is comparison between indicated groups
TNFR2 expression on Tregs is highly associated with clinicopathological characteristics of lung cancer patients
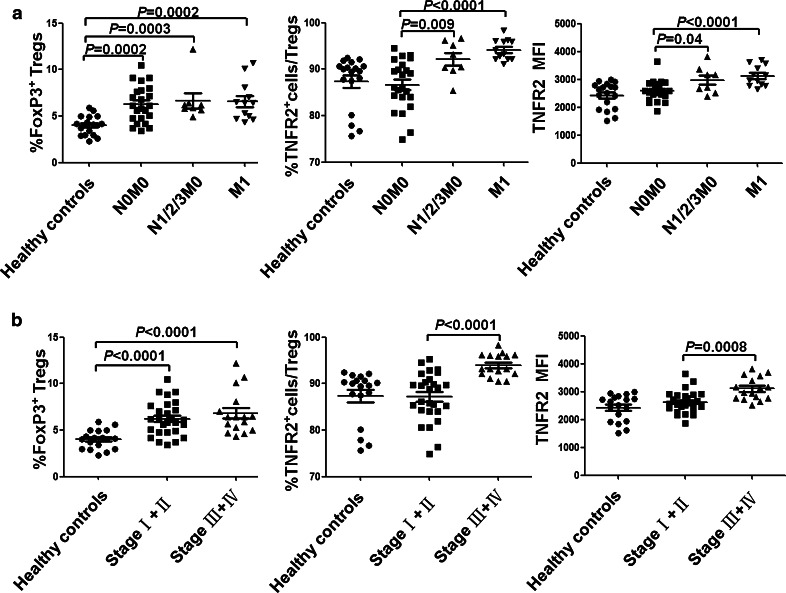
Due to their superior suppressive function, TNFR2 expression on Tregs may identify the most disease-relevant Tregs in lung cancer and may be more indicative of greater cancer malignancy, development, metastasis and clinical outcomes. To test this possibility, we analyzed the percentage of TNFR2+ cells within the FoxP3+ Tregs in PBMCs from 43 patients and 18 healthy controls. As shown in Fig. 4 and Table 1, although the proportion of total Tregs, as defined by FoxP3 expression, in PB CD4 cells of lung cancer patients (6.50 ± 2.01 %) was higher than healthy controls (3.98 ± 1.01 %, P < 0.0001), there was no correlation between proportion of total FoxP3+ Tregs and these clinicopathological characteristics. However, the proportion of TNFR2+ subset in FoxP3+ Tregs was highly correlated with lymphatic invasion (P = 0.007), distant metastasis (P = 0.0006) and clinical stage (stage I + II vs. stage III + IV, P < 0.0001), whereas the proportion of TNFR2+ cells in Tconvs was only correlated with clinical stage (stage I + II vs. stage III + IV, P = 0.008). There was no association of the proportion of TNFR2+ cells in Tregs with age (P = 0.422), gender (P = 0.736) and histology (P = 0.653). Further, MFI of TNFR2 expression by FoxP3+ Tregs was also highly correlated with lymphatic invasion (P = 0.019), distant metastasis (P = 0.002) and advanced clinical stage (stage I + II vs. stage III + IV, P = 0.0008). Nevertheless, MFI of TNFR2 expression by FoxP3− Tconvs was also correlated with lymphatic invasion (P = 0.031) and clinical stage (stage I + II vs. stage III + IV, P = 0.003). Therefore, these data indicate that the expression levels of TNFR2, including both proportion of TNFR2-expressing cells and MFI of TNFR2 expression, by Tregs were highly associated with clinical pathology and thus may be useful as a prognostic biomarker.
Fig. 4.
Correlation of TNFR2 expression with metastasis and clinical stage of lung cancer patients. PBMCs were freshly isolated by Lymphoprep™ centrifugation and prepared for FACS analysis. a Comparison of the proportion of total Tregs, the proportion of TNFR2-expressing Tregs and MFI of TNFR2 expression by Tregs derived from healthy controls (N = 18), or patients without lymphatic metastasis and distant metastasis (N0M0, N = 23), or patients with lymphatic metastasis but without distant metastasis (N1/2/3M0, N = 8), or patients with distant metastasis (M1, N = 12). b Comparison of the proportion of total FoxP3+ Tregs, the proportion of TNFR2-expressing Tregs and MFI of TNFR2 expression by Tregs derived from healthy controls (N = 18), or stage I + II lung cancer patients (N = 27), or stage III + IV lung cancer patients (N = 16). P value shown in the figure is the comparison of indicated groups
Discussion
Previous studies have reported that higher numbers of Tregs were present in lung cancer patients than in healthy donors, and were associated with tumor progression [6, 29]. In contrast, other studies failed to reveal the correlation between the levels of Tregs and the stages of lung cancer [1, 7]. We were able to confirm that the levels of FoxP3+ Treg cells were higher in lung cancer patients as compared with healthy controls (Fig. 1b). However, we did not find a correlation between the levels of total FoxP3+ Tregs with clinicopathological characteristics of the patients, which was in agreement with a number of previous reports [1, 7]. Interestingly, compared with non-metastatic patients, the expression level of TNFR2 on Tregs was considerably increased in patients with tumor-invaded lymph nodes or with distant organ metastasis, while there was no difference in TNFR2 expression on Tregs between lung cancer patients without metastasis and healthy controls. Therefore, TNFR2 expression on Tregs was increased only in patients with more advanced stages of lung tumors and thus could be served as a prognostic biomarker.
The correlation of TNFR2 expression on Tregs with tumor development and metastasis is likely due to the immune evasion caused by highly suppressive TNFR2+ Tregs. However, we cannot exclude the possibility that more advanced tumor may enhance TNFR2 expression on Tregs. For example, patients with lung cancer and other solid tumors usually have higher levels of serum TNF [30, 31], and TNF is known to preferentially up-regulate TNFR2 expression on Tregs [32]. It is also possible that TNFR2+ Tregs and tumor form positive feedback loops to reciprocally promote each other, resulting in the increasingly immunosuppressive environment as well as rapidly growing tumor. Therefore, targeting TNF–TNFR2 may represent a novel and better strategy to enhance antitumor immunity by eliminating Treg activity than existing methods [3, 33, 34]. This idea is supported by the observations that therapeutic elimination of TNFR2+ Tregs contributed to their antitumor effect [15, 16].
Our data also showed that Tconvs from lung cancer patients also expressed markedly higher levels of TNFR2, as compared with their counterparts from healthy donors (Fig. 1c). Furthermore, expression of TNFR2 on Tconvs from patients with advanced cancer (stage III + IV) was markedly higher than that from less advanced patients (stage I + II) (P = 0.007, Supplementary Fig. 2). It has been shown that Tconvs with enhanced TNFR2 expression were more proliferative and more resistant to Treg-mediated inhibition and produced higher levels of cytokines [24, 35]. Indeed, we also found that TNFR2-expressing Tconvs were more proliferative, as indicated by their higher levels of Ki-67 expression (Supplementary Fig. 1). Nevertheless, Tregs from patients expressed markedly higher levels of TNFR2 than Tconvs (Fig. 1c). Moreover, in the advanced cancer patients, TNFR2 expression on Tregs (94 %, MFI: 3106) remained markedly higher than that on Tconvs (58 %, MFI: 1265, P < 0.0001, Supplementary Fig. 3). These data suggest that Tregs still preferentially utilize TNF–TNFR2 co-stimulation for activation in tumor patients, and those tumor-associated Tregs were likely to inhibit activation of Tconvs, as previously shown in a study based on a mouse tumor model [11]. The ratio of TNFR2+ Tregs to TNFR2+ Tconvs was not increased in the advanced cancer patients (data now shown), which was likely caused by the high basal levels of TNFR2 expression on Tregs (Fig. 4) and the expression of this protein on Tregs might have reached a plateau in patients with advanced cancer.
TNFR2 is one of the two receptors that transduce biological function of TNF [36]. In contrast to the broadly expressed and cytotoxicity mediating effect of TNFR1, TNFR2 is primarily expressed by lymphocytes and acts as a co-stimulatory molecule to enhance T cell responses to TCR stimulation [21, 22]. It has been shown that TNFR2 is critical for the maintenance of FoxP3 expression by Tregs [37], which is attributable to the requirement of TNFR2 or its major signaling component IKKα for the in vivo function of Tregs [37–39]. Presumably, activation of NF-κB by TNFR2 signaling [40, 41] contributes to the stability of FoxP3 expression [42]. Meanwhile, NF-κB pathway can be activated by TNF–TNFR2 interaction [40, 41]. This may explain why TNFR2, as compared with CD25+ or CD127low/−, was highly correlated with FoxP3 expression by Tregs in both healthy donors and lung cancer patients (Fig. 1d, e). Overall, co-expression of surface markers CD25 and TNFR2 and lack of CD127 appeared to be superb markers to define functionally suppressive Tregs in lung cancer patients.
In our study, IFNγ production from autologous CD8 T cells was inhibited by 50 % when TNFR2+ Tregs were added at a 1:1 ratio (Fig. 3). It was previously shown that TNFR2+ Tregs at a 1:1 ratio almost completely inhibited the capacity of CD4 responders to produce IFNγ [18]. This discrepancy may be attributable to the different stimulus used in the in vitro Treg function assays. Dynabeads® Human T-Activator CD3/CD28 used in our study could provide more potent TCR stimulation to responder T cells, which might result in an enhanced resistance to Tregs, as compared with APCs (CD4-depleted and irradiated PBMCs) plus soluble anti-CD3 Ab used by others [18]. In addition, difference in responder cells, such as CD4 cells from healthy donors [18] and CD8 cells from cancer patients in our study, may also contribute to the discrepancy.
Taken together, our study provides novel evidence that the expression of TNFR2 by peripheral Tregs, not total Tregs by themselves, was correlated with clinicopathological progression and metastasis of lung cancer patients. With highly replicating and highly suppressive properties, these TNFR2+ Tregs present in the circulation of lung cancer patients may prove to be a useful prognostic marker of advanced lung cancer patients, especially those with metastatic diseases. Consequently, therapeutically targeting of TNFR2+ Tregs may improve the efficacy of current immunotherapy in the treatment of lung cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from Natural Science Foundation of China (No. 81171983 and No. 81401888) and Tianjin Natural Science Foundation (No. 12JCYBJC16100). We honestly thank Dr. Joost J. Oppenheim for his critical review and comments that greatly improved the manuscript.
Abbreviations
- AML
Acute myeloid leukemia
- CTLs
Cytotoxic T lymphocytes
- CTLA-4
Cytotoxic T lymphocyte-associated antigen 4
- ELISA
Enzyme-linked immunosorbent assay
- FoxP3
Forkhead box P3
- IFN
Interferon
- IgG
Immunoglobulin G
- MFI
Mean fluorescence intensity
- PB
Peripheral blood
- PBMCs
Peripheral blood mononuclear cells
- Tconvs
Conventional T cells
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- TNFR1
Tumor necrosis factor receptor type I
- TNFR2
Tumor necrosis factor receptor type II
- Tregs
CD4+FoxP3+ regulatory T cells
Compliance with ethical standards
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Footnotes
Fan Yan and Ruijuan Du have contributed equally to this work.
Contributor Information
Xin Chen, Phone: 853-88224513, Email: xchen@umac.mo.
Hui Li, Phone: 86-22-23520860, Phone: 86-22-23340123, Email: lihui@tjmuch.com.
References
- 1.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, Fietta AM. Foxp3 expressing CD4+CD25+and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75(1):95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 4.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 6.Erfani N, Mehrabadi SM, Ghayumi MA, Haghshenas MR, Mojtahedi Z, Ghaderi A, Amani D. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC) Lung Cancer. 2012;77(2):306–311. doi: 10.1016/j.lungcan.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa T, Suzuki H, Yamaura T, Muto S, Okabe N, Osugi J, Hoshino M, Higuchi M, Ise K, Gotoh M. Prognostic value of peripheral and local forkhead box P3 regulatory T cells in patients with non-small-cell lung cancer. Mol Clin Oncol. 2014;2(5):685–694. doi: 10.3892/mco.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, Whiteside TL. Phenotypic and functional characteristics of CD4+CD39+FOXP3+and CD4+CD39+FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42(7):1876–1885. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17+regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186(7):4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 10.Tsakiri N, Papadopoulos D, Denis MC, Mitsikostas DD, Kollias G. TNFR2 on non-haematopoietic cells is required for Foxp3+Treg-cell function and disease suppression in EAE. Eur J Immunol. 2012;42(2):403–412. doi: 10.1002/eji.201141659. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180(10):6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryba M, Rybarczyk-Kapturska K, Zorena K, Myśliwiec M, Myśliwska J. Lower frequency of CD62L(high) and higher frequency of TNFR2(+) Tregs are associated with inflammatory conditions in type 1 diabetic patients. Mediat Inflamm. 2011;2011:645643. doi: 10.1155/2011/645643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5(4):e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, Plebanski M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+Tregs within the tumor microenvironment. Clin Immunol. 2013;149(1):97–110. doi: 10.1016/j.clim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am J Hematol. 2014;89(8):795–802. doi: 10.1002/ajh.23746. [DOI] [PubMed] [Google Scholar]
- 16.Govindaraj C, Tan P, Walker P, Wei A, Spencer A, Plebanski M. Reducing TNF receptor 2+regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin Cancer Res. 2014;20(3):724–735. doi: 10.1158/1078-0432.CCR-13-1576. [DOI] [PubMed] [Google Scholar]
- 17.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, Lake RA. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58(8):1219–1228. doi: 10.1007/s00262-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40(4):1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Fazekas, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X. CD4(+)CD25(+)CD127 (low/-) T Cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35(6):1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176(2):1026–1035. doi: 10.4049/jimmunol.176.2.1026. [DOI] [PubMed] [Google Scholar]
- 22.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173(7):4500–4509. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 23.Soares A, Govender L, Hughes J, Mavakla W, de Kock M, Barnard C, Pienaar B, Janse van Rensburg E, Jacobs G, Khomba G, Stone L, Abel B, Scriba TJ, Hanekom WA. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362(1–2):43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3− conventional T cells to suppression by CD4+FoxP3+regulatory T cells. J Immunol. 2010;185(1):174–182. doi: 10.4049/jimmunol.0903548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nechushtan H, Pham D, Zhang Y, Morgensztern D, Yi KH, Shin SU, Federoff HJ, Bowers WJ, Tolba KA, Rosenblatt JD. Augmentation of anti-tumor responses of adoptively transferred CD8+T cells in the lymphopenic setting by HSV amplicon transduction. Cancer Immunol Immunother. 2008;57(5):663–675. doi: 10.1007/s00262-007-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15(2):148–154. doi: 10.1016/S0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 29.Wang WJ, Tao Z, Gu W, Sun LH. Variation of blood T lymphocyte subgroups in patients with non- small cell lung cancer. Asian Pac J Cancer Prev P. 2013;14(8):4671–4673. doi: 10.7314/APJCP.2013.14.8.4671. [DOI] [PubMed] [Google Scholar]
- 30.De Vita F, Orditura M, Auriemma A, Infusino S, Catalano G. Serum concentrations of proinflammatory cytokines in advanced non small cell lung cancer patients. J Exp Clin Cancer Res. 1998;17(4):413–417. [PubMed] [Google Scholar]
- 31.Ardizzoia A, Lissoni P, Brivio F, Tisi E, Perego MS, Grassi MG, Pittalis S, Crispino S, Barni S, Tancini G. Tumor necrosis factor in solid tumors: increased blood levels in the metastatic disease. J Biol Regul Homeost Agents. 1992;6(3):103–107. [PubMed] [Google Scholar]
- 32.Hamano R, Huang J, Yoshimura T, Oppenheim JJ, Chen X. TNF optimally activates regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40. Eur J Immunol. 2011;41(7):2010–2020. doi: 10.1002/eji.201041205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, Merghoub T, Houghton AN, Wolchok JD. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5(5):e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Govindaraj C, Scalzo-Inguanti K, Scholzen A, Li S, Plebanski M. TNFR2 Expression on CD25(hi)FOXP3(+) T Cells Induced upon TCR Stimulation of CD4 T Cells identifies maximal cytokine-producing effectors. Front Immunol. 2013;4:233. doi: 10.3389/fimmu.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors–structure and function. Immunol Res. 1992;11(2):81–90. doi: 10.1007/BF02918612. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190(3):1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186(12):6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Willette-Brown J, Wu X, Hu Y, Howard OM, Oppenheim JJ. IKKalpha is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. Faseb J. 2015;29(2):443–454. doi: 10.1096/fj.14-259564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauert H, Wicovsky A, Muller N, Siegmund D, Spindler V, Waschke J, Kneitz C, Wajant H. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 2010;285(10):7394–7404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279(31):32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 42.Barbarulo A, Grazioli P, Campese AF, Bellavia D, Di Mario G, Pelullo M, Ciuffetta A, Colantoni S, Vacca A, Frati L, Gulino A, Felli MP, Screpanti I. Notch3 and canonical NF-kappaB signaling pathways cooperatively regulate Foxp3 transcription. J Immunol. 2011;186(11):6199–6206. doi: 10.4049/jimmunol.1002136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.