Abstract
Despite temozolomide (TMZ) treatment, the prognosis for patients with glioblastoma multiforme is still dismal. As dose escalation of TMZ is limited by systemic toxicity, intratumoral delivery emerges as an attractive treatment modality, which may sustain cytotoxic drug concentrations intratumorally and induce immunogenic cell death. Both clinical and experimental gliomas have responded to immunotherapy, but the benefit of simultaneous chemo- and immunotherapy is inadequately studied. Here, we monitored survival of GL261-bearing C57BL/6 mice following a 3-day treatment with either intratumoral TMZ (micro-osmotic pump, 4.2 mg/kg/day) or systemic TMZ (i.p. injections, 50 mg/kg/day) alone, or combined with immunization using GM-CSF secreting GL261 cells. Peripheral and intratumoral leukocytes were analyzed by flow cytometry and immunohistochemistry. Intratumoral TMZ induced higher survival rate than systemic TMZ (45 vs. 8 %). When T cells were depleted following intratumoral TMZ, the therapeutic effect was completely abrogated (0 % survival). Intratumoral TMZ synergistically increased survival rate of immunized mice (from 25 to 83 %), while systemic TMZ failed (0 %). While systemic TMZ induced a transient leukopenia, intratumoral TMZ and immunotherapy sustained the proliferation of CD8+ T cells and decreased the number of intratumoral immunosuppressive cells. In conclusion, intratumoral TMZ alone or in combination with immunotherapy could cure glioma-bearing mice, due to attenuation of local immunosuppression and increase in potential effector immune cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1449-z) contains supplementary material, which is available to authorized users.
Keywords: GL261, Glioma, Immunotherapy, Intratumoral chemotherapy, T cell depletion, Temozolomide (TMZ)
Introduction
Patients with glioblastoma multiforme (GBM), the most common primary malignant brain tumor, have a dismal prognosis despite progress with conventional therapies including surgery, concomitant radio- and chemotherapy using the alkylating agent temozolomide (TMZ) and adjuvant TMZ. The addition of TMZ has prolonged median overall survival with 2.5 months, and the longtime follow-up shows an increase in 5-year survival to 9.8 versus 1.9 % with radiotherapy alone [1, 2]. This regimen has given an overall gradual modest survival improvement [3]. However, not all patients receive concomitant therapy due to toxicity or low performance status; thus, shorter overall survival has been reported by others [4, 5].
Dose escalation of TMZ is limited by adverse effects such as leukopenia [6, 7]. In order to sustain high cytotoxic drug levels intratumorally, while reducing systemical toxicity, intratumoral delivery could be an alternative approach. Intratumoral delivery of drugs has been administered to patients with malignant brain tumors in different clinical settings [8, 9]. Wafer implants of the chemotherapeutic drug, BCNU/carmustine, have been approved and licensed for the treatment of malignant gliomas with proof of efficacy, but with conflicting reports of adverse effects such as cerebral edema, infection and seizures [10–13]. Intratumoral delivery of TMZ has not been clinically tested, but promising results have been presented in experimental brain tumor models. In the 9L gliosarcoma rat model, locally delivered biodegradable or biocompatible polymers containing TMZ induced long-term survivors without neurological or systemic toxicities, while oral TMZ failed [14, 15]. When combined with radiotherapy, intratumoral TMZ further extended the survival [14]. TMZ administered by i.c. micro-infusion to human glioma xenografts in athymic rats induced cure without evidence of toxicity [16].
Due to the infiltrative nature of GBM, with diffuse dissemination of tumor cells beyond the tumor mass, and general resistance to therapy, immunotherapy may represent a promising therapeutic approach. Immunotherapeutic strategies have shown efficacy in experimental gliomas [17–19] and have been tested against human malignant gliomas, with partial clinical responses [20–22]. Moreover, there is accumulating evidence that high tumor infiltration of activated lymphocytes correlates with prognosis and survival in patients with GBM [23].
Granulocyte macrophage-colony stimulating factor (GM-CSF) is a hematopoietic cytokine that stimulates the recruitment and differentiation of myeloid progenitor cells into granulocytes, monocytes/macrophages. GM-CSF endorses antigen presentation of DCs and macrophages by inducing co-stimulatory molecules such as CD80 and MHC class II expression [24–26]. Although high levels of GM-CSF may expand immature myeloid derived suppressor cells (MDSCs) with an immunosuppressive capacity [27, 28], we have earlier demonstrated that immunization using GM-CSF-transduced tumor cells (GL-GM) increased survival of GL261 tumor-bearing mice compared with immunization using GL261 wt-tumor cells, in a T cell-dependent manner. Despite an initial increase in MDSCs following immunization, the number later declined, which coincided with an increase in differentiated macrophages and DCs [19, 29].
Repeated cycles of chemotherapeutic drugs may induce lymphopenia, which may counteract a T cell-dependent immunotherapy. However, synergistic effects have been reported when certain chemotherapeutics are combined with immunotherapy [20, 30, 31]. Several mechanisms have been proposed; the induction of immunogenic cell death of tumor cells, homeostatic proliferation of lymphocytes, reduction in intratumoral or peripheral immunosuppressive cells and upregulation of T cell attractant chemokines, thus favoring T cell recruitment [32–39]. Systemic TMZ in combination with recombinant interferon-β or dendritic cell vaccination have shown efficacy [40, 41]; however, intratumoral TMZ combined with active immunotherapy has not been evaluated.
In this study, we investigated the therapeutic efficacy of intratumoral or systemic TMZ as monotherapies or combined with immunizations using GL-GM cells in the GL261 model. Also, we assessed a potential T cell-dependent mechanism underlying the effect of intratumoral TMZ.
Materials and methods
Cell line
The GL261 mouse glioma cells of C57BL/6 origin [42, 43], kindly provided by Dr. G Safrany, Hungary, were previously transduced to produce GM-CSF (GL-GM) and cultured as previously described [19].
Cell viability following TMZ exposure
Temozolomide (Temodal®, Schering Plough, Stockholm, Sweden) was dissolved in DMSO (Sigma-Aldrich, Stockholm, Sweden) and further diluted in 0.9 % NaCl (Braun AG, Melsungen, Germany).
On day 1–3, 5 × 104 GL261 cells were daily exposed to 500, 100, 50, 10 and 0 μM of TMZ. On day 6, the number of viable cells was assessed by trypan blue staining (GIBCO-Life technologies, Sweden). The cell viability was determined by calculating the percentage of viable TMZ-treated cells/viable nontreated cells (mean and SEM out of 3 experiments).
Measurements of MHC class I, MHC class II, calreticulin and IP-10
On day 1–3, 5 × 104 GL261 cells were exposed to 100 μM of TMZ. On day 4, the cells were stained (30 min, 4 °C) using mouse PE-conjugated anti-mouse-H-2Db (KH95, MHC class I) and anti-mouse-I-Ab (AF6-120.1, MHC class II) antibodies. On day 5, cells were stained for calreticulin using purified mouse anti-calreticulin antibody with the secondary antibody anti-mouse IgG-FITC (BD Pharmingen, Stockholm, Sweden). The expression was measured by flow cytometry (Accuri, UK) and analyzed using CFlowPlus software (Accuri). The percentages of positive cells were calculated by subtraction of isotype control staining or secondary staining (mean and SEM out of triplicate samples). On day 4, 5 and 6, the supernatants of the TMZ-treated cell cultures were collected and IP-10 concentration was analyzed using an IP-10 mouse ELISA kit (Quantikine, IP-10/CRG-2/CXCL10, R&D systems, UK).
Survival study
All procedures were performed according to the practices of the Swedish Board of Animal Research and approved by the Committee of Animal Ethics in Lund-Malmö.
On day 0, syngeneic female C57BL/6 mice (Scanbur/Charles River, Germany), 8–10 weeks old, were inoculated i.c. with 5 × 103 GL261 cells as previously described [19]. Mice were observed daily and immediately euthanized when neurological symptoms appeared, and brains were examined for macroscopically visible tumors.
Surviving mice were either killed 100 days following tumor challenge or rechallenged with a second tumor at the contralateral side with no further treatment and observed for another 70 days (n = 11). Mice receiving their first tumor served as controls (n = 4). At the end of the experiment, all brains were examined for macroscopically visible tumors or freeze-sectioned and stained for remnant tumor cells.
TMZ administration
In order to determine the optimal systemic TMZ dose, different doses of TMZ were administered on day 1, 2 and 3 following tumor inoculation (2.5 mg/kg, 12.5 mg/kg or 25 mg/kg mouse, n = 5–10/group, for experimental setup, see Supplementary Fig. S1a). In the 25 mg/kg TMZ treatment group, 1 mouse (10 %) survived, whereas all other mice developed lethal tumors (Supplementary Fig. S1b). As no signs of toxicity were detected, we increased the TMZ dose to 50 mg/kg.
For systemic TMZ delivery (TMZ IP), tumor-inoculated mice (n = 10) were injected i.p. on day 7–9 with 50 mg/kg of TMZ diluted in 0.9 % NaCl.
For intratumoral delivery (TMZ IC), a 3-day active micro-osmotic pump (Alzet® model 1003D, 100 μl, pumping rate 1 μl/h, Nova SCB AB, Sollentuna, Sweden) was filled with 2.5 mg/ml of TMZ (Temodal®, Merck Sharp & Dohme, Sollentuna, Sweden), coupled to a brain infusion kit (Alzet® brain infusion kit 3, Nova SCB AB) and incubated over night in 37 °C. On day 7, pumps filled with TMZ (4.2 mg/kg/day) were implanted into a subcutaneous pocket on the back of the tumor-inoculated mice (n = 22). A brain cannula (connected to the pump via a catheter tube placed subcutaneously along the neck) was inserted through the skull intratumorally and fixed using cyanoacrylate adhesive (Alzet® LOCTITE gel, Nova SCB). The pump was removed when no longer active. No loss of body weight or other signs of toxicity were observed during the treatment.
T cell depletion
The mouse anti-CD4 (GK1.5) and anti-CD8 (53–6.7) depleting antibodies (eBioscience, San Diego, USA) were titrated by analyzing blood from antibody-treated mice as previously described [29]. 100 μg depleted 98–99 % of all circulating T cells.
I.p. injections with 100 + 100 μg of mouse anti-CD4/CD8 antibodies started 3 days before tumor inoculation, followed by injections twice/week during 5 weeks. The survival of T cell-depleted mice (n = 8) treated with intratumoral TMZ (TMZ IC + CD4/CD8-abs) was compared with the survival of nondepleted TMZ IC-treated mice (n = 9).
Combined immunization and TMZ treatment
Initially, GL261-bearing mice were treated with systemic TMZ on day 1, 2 and 3 (2.5, 12.5 and 25 mg/kg) and then immunized with GL-GM cells on day 6, 20 and 34 (for experimental setup, see Supplementary Fig. S1c). As this treatment strategy was ineffective (Supplementary Fig. S1d), another setting was explored.
On day 5, 19 and 33 following tumor inoculation, all mice (n = 30) were immunized i.p. with 2 × 106 irradiated (40 Gy) GL-GM cells/0.2 ml. Mice were then divided into three treatment groups: (1) GL-GM (receiving immunization only, n = 12), (2) GL-GM + TMZ IP (treated with systemic TMZ on day 7–9, n = 6) and (3) GL-GM + TMZ IC (treated with intratumoral TMZ, on day 7–9, n = 12). For experimental setup see Fig. 2e.
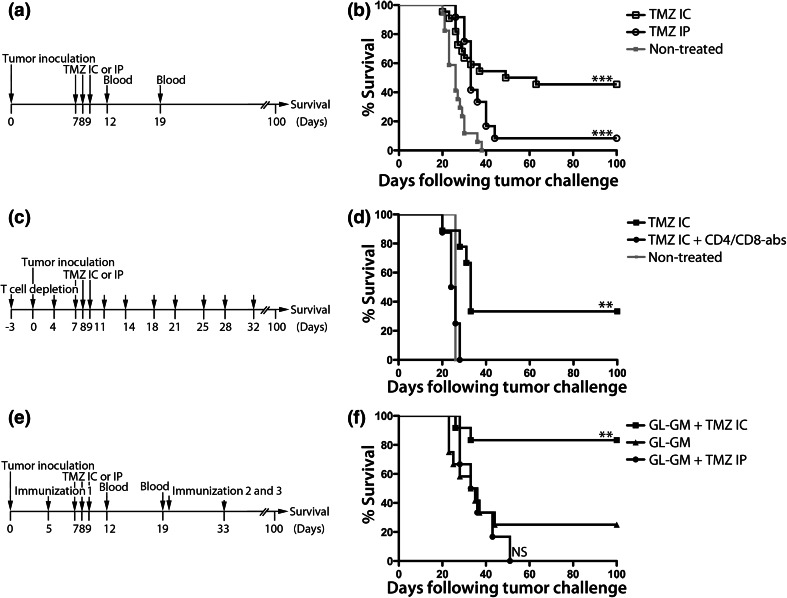
Fig. 2.
Survival following intratumoral versus systemic TMZ, following T cell depletion or combined with immunotherapy. Experimental setups and Kaplan–Meier survival curves of GL261-bearing mice receiving. a, b TMZ at day 7–9 administered either i.p. (TMZ IP, 50 mg/kg/day) or i.c. using a micro-osmotic pump/brain infusion kit (TMZ IC, 4.2 mg/kg/day) or no treatment (nontreated), n = 51. c, d TMZ IC alone or TMZ IC with injections of 100 + 100 μg CD4+ and CD8+ T cell depletion antibodies starting 3 days prior to tumor inoculation, twice a week for 5 weeks (TMZ IC + CD4/CD8-abs), n = 17. e, f TMZ IC or IP and/or i.p. immunization with irradiated 2 × 106 GL-GM cells at day 5, 19 and 33, n = 30. Nontreated versus TMZ IC ***p < 0.0001. Nontreated versus TMZ IP ***p = 0.0004. TMZ IC + CD4/CD8-abs versus TMZ IC **p = 0.0014. GL-GM versus nontreated *p = 0.0119. GL-GM + TMZ IC versus nontreated ***p < 0.0001. GL-GM + TMZ IP versus nontreated *p = 0.0137. GL-GM + TMZ IC versus TMZ IP ***p = 0.0005. GL-GM + TMZ IC versus TMZ IC *p = 0.0421. GL-GM + TMZ IC versus GL-GM **p = 0.0048. GL-GM + TMZ IC versus GL-GM + TMZ IP ***p = 0.0009. Log rank test. Symptom-free survival was monitored for 100 days
Blood leukocyte analysis
Non-immunized and GL-GM immunized tumor-bearing mice (n = 6/group) were administered TMZ IP or TMZ IC. Blood was collected from vena saphena on day 12 and 19 and mixed with heparin (LEO Pharma AB, Malmö, Sweden). 25 μl of untreated blood was added to each sample. Cells were Fc-blocked (anti-CD16/32, 2.4G), washed in PBS (with 1 % bovine serum albumin) and incubated (30 min, 4 °C) with mouse FITC-CD4 (GK1.5), FITC-CD8α (53–6.7), FITC-Ly6G (1A8), PE-CD4 (GK 1.5), PE-CD25 (PC61.5), PE-CD45 (30-F11), PE-CD11b (M1/70), PerCPCy5.5-CD45 (30-F11), PerCPCy5.5-Ly-6C (AL-21), APC-CD3 (145-2C11, BD Pharmingen) and FITC-F4/80 Antigen (AbD Serotec) antibodies. Cells were fixed with BD Cytofix/Cytoperm™ Fixation/Permeabilization kit and stained with a FITC anti-human Ki-67 antibody (BD Pharmingen) or fixed and stained using a FoxP3 Fixation/Permeabilization staining kit and an APC-FoxP3 antibody (FJK-16 s, eBioscience). At the final step, 200 μl of PBS (with 1 % bovine serum albumin) was added to each cell pellet, and fluorescence (the number of positive events) was measured on a flow cytometer (Accuri) with a volume gate set to 130 μl (corresponding to 16 μl of untreated blood). Data were analyzed using CFlowPlus software (Accuri).
Immunohistochemistry of tumor infiltrating T cells
Tumor-bearing mice (n = 3–7/group) were GL-GM immunized on day 12 and/or administered TMZ IC or IP on day 14–16 and killed on day 20. Brains were collected, snap-frozen in liquid nitrogen-cooled isopentane (−55 °C, VWR International AB, Lund, Sweden), cut into 6 μm-thick sections using a cryostat (Leica, Wetzlar, Germany), mounted on super-frost glass slides (VWR International AB) and stored in −80 °C. Prior to staining, the sections were thawed and fixed for 10 min in 4 % paraformaldehyde (VWR International AB). Sections were washed in PBS (GIBCO-Life technologies), blocked for 20 min with 5 % goat serum (Jackson ImmunoResearch Laboratories Inc. West Grove, USA) and stained with primary antibodies: biotin anti-mouse CD8α (53–6.7), rat anti-mouse CD4-PE (GK1.5) (5 μg/ml, BD Pharmingen), biotin rat anti-mouse NK-1.1 (PK136) (10 μg/ml, BD Pharmingen) and hamster anti-mouse TCR-γδ (5 μg/ml, BD Pharmingen) for 60 min in room temperature. Sections were washed, incubated with the secondary antibodies streptavidin Alexa Flour 594, donkey anti-rat Alexa Fluor 594, goat anti-hamster Alexa Flour 488 5 μg/ml, (Molecular Probes, Eugene, USA) and mounted with Pro-long Gold anti-fading reagent containing DAPI (Molecular Probes) for nuclear staining. As negative control, primary antibodies were omitted. Images were taken at 20x magnification using a fluorescent microscope (BX-53, Olympus LRI instrument AB, Lund, Sweden). Images covering a representative tumor area (1585,000 μm2) were merged using Multi-image alignment (Cellsens Dimension software, Olympus LRI instrument AB), and the ratio of the stained area within the representative area was calculated and expressed as percent stained area.
Tumor infiltrating immunosuppressive cells
Tumor-bearing mice (n = 6/group) were immunized on day 9 with GL-GM cells and administered either TMZ IP or TMZ IC on day 12–14. On day 20, the frontal part of the mouse brain containing the tumor was dissected out and transferred to PBS. The brain was mechanically dissociated and incubated with TrypLE Express (10 min, 37 °C, GIBCO-Life technologies) before incubated with DNase (20 μg/ml, Sigma-Aldrich) for 60 min. The cells were filtered through a cell strainer (70 μg/ml, BD Pharmingen) and stained and analyzed by flow cytometry for the percentage of CD11b+Gr1+ cells (of total cells) and FoxP3+CD25+CD4+ (of CD4+ cells) as mentioned above (see paragraph Blood leukocytes analysis). A gate based on blood leukocytes was set.
Statistics
Statistical differences between cells were determined using the two-way ANOVA (Fig. 1b), the Kaplan–Meier survival curves, using a log rank test (Fig. 2; Fig. S1), and the leukocyte populations, using the nonparametric Mann–Whitney U test (Tables 1, 2; Fig. 3; Fig. S2; Fig. S3). The analyses were performed using GraphPad Prism® version 5.0a software (GraphPad software Inc, San Diego, USA). p < 0.05 was regarded as statistically significant.
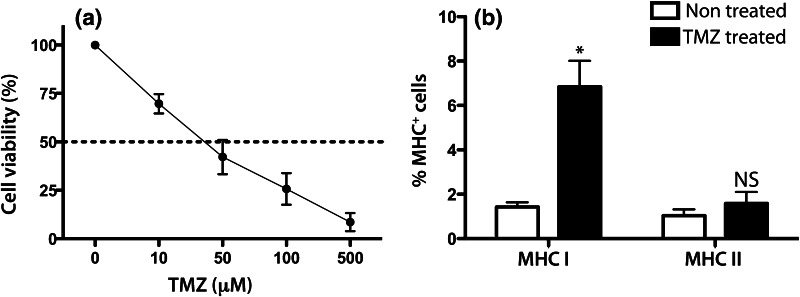
Fig. 1.
Effect of TMZ on cell viability and MHC expression of the GL261 mouse glioma cell line following TMZ exposure in vitro. a GL261 cells were daily exposed to different doses of TMZ for 72 h. 3 days following TMZ, the number of viable cells was counted using trypan blue. The percentage of treated viable cells/untreated viable cells was calculated. The viability of the untreated cells was regarded as 100 %. The mean values and SEM out of 3 experiments are shown. b GL261 cells were daily exposed to 100 μM of TMZ for 72 h. The following day cells were stained for MHC class I and II and analyzed with flow cytometry. The percentage of positive cells from 1 out of 3 experiments is presented here (mean values and SEM out of triplicates) MHC class I expression: TMZ treated versus nontreated, *p = 0.0441, two-way ANOVA
Table 1.
Surface and intracellular characterization of leukocytes day 12 in blood of TMZ IP- versus TMZ IC-treated mice (median values, n = 6/group)
| Cell type | Treatment | ||
|---|---|---|---|
| TMZ IP | TMZ IC | p a | |
| CD45 count | 4,521 | 21,918 | 0.0043** |
| F4/80 count | 887 | 1,747 | 0.0087** |
| Ly6G count | 467 | 1,728 | 0.0152* |
| CD11bGr1 count | 1,315 | 3,158 | 0.0152* |
| CD4CD3 count | 5,335 | 7,961 | 0.1320 NS |
| CD8CD3 count | 2,157 | 4,452 | 0.0260* |
| Ki67/CD8 % | 3.65 | 4.30 | 0.0411* |
| FoxP3CD25/CD4 % | 6.90 | 5.35 | 0.1320 NS |
aStatistical differences using nonparametric Mann–Whitney U test between: TMZ IP versus TMZ IC. All p-values in bold represent significant differences between treatments
Table 2.
Surface and intracellular characterization of leukocytes day 12 in blood of GL-GM immunized mice and/or TMZ IP- or TMZ IC-treated mice (median values, n = 6/group)
| Cell type | Treatment | |||
|---|---|---|---|---|
| GL-GM + TMZ IP | GL-GM + TMZ IC | GL-GM | p a,b,c | |
| CD45 count | 10,124 | 29,124 | 15,552 | 0.0022** ,a |
| F4/80 count | 777 | 2,197 | 1,922 | 0.0022** ,a |
| Ly6G count | 674 | 2,205 | 950 |
0.0043** ,a 0.0022** ,b 0.0260* ,c |
| CD11bGr1 count | 1,189 | 3,138 | 2,531 |
0.0022** ,a 0.0411* ,b |
| CD4CD3 count | 4,865 | 7,114 | 9,563 |
0.0411* ,a 0.0260* ,b |
| CD8CD3 count | 2,069 | 3,809 | 4,073 |
0.0087** ,a 0.0260* ,b |
| Ki67/CD8 % | 5.96 | 12.3 | 9.15 | 0.0022** ,a |
| FoxP3CD25/CD4 % | 9.15 | 6.65 | 5.55 |
0.0649 NSa 0.0411* ,b 0.0411* ,c |
Statistical differences using nonparametric Mann–Whitney U test between: a GL-GM + TMZ IP versus GL-GM + TMZ IC; b GL-GM + TMZ IP versus GL-GM; c GL-GM + TMZ IC versus GL-GM. All p-values in bold represent significant differences between treatments
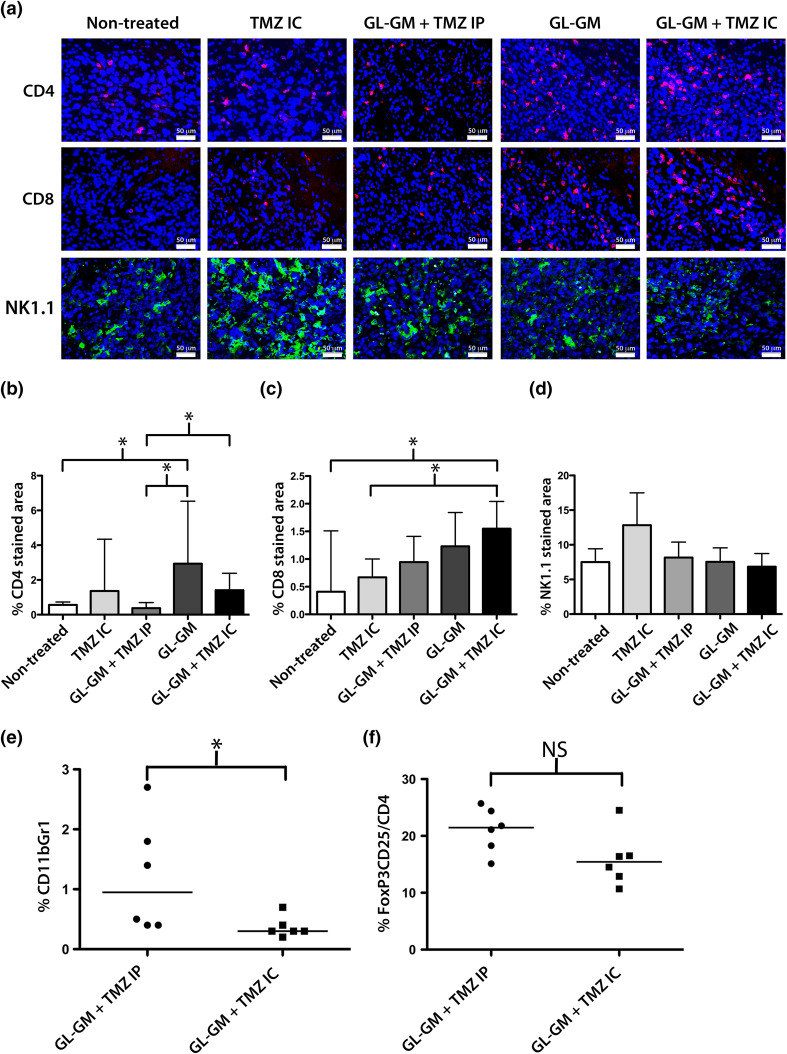
Fig. 3.
Tumor infiltrating cells of GL-GM immunized and/or TMZ-treated mice. Tumor-bearing mice (n = 3–7/group) were either nontreated, immunized with GL-GM cells on a–d day 12 or e, f day 9, and/or administered TMZ on a–d day 14–16 or e, f day 12–14 either i.p. (TMZ IP, 50 mg/kg/day) or i.c. using a micro-osmotic pump/brain infusion kit (TMZ IC, 4.2 mg/kg/day). Brain samples were collected on day 20 following tumor challenge. a–d Frozen brain sections were stained for CD4+ (red, above), CD8α+ (red, middle), or NK-1.1.+ (green, below) cells and analyzed by immunohistochemistry. a Images of representative data from one animal of each treatment: nontreated, TMZ IC, GL-GM + TMZ IP, GL-GM and GL-GM + TMZ IC. Images were taken at 20x magnification. DAPI (blue) was used as nuclear staining. Quantitative analysis of the percent of b CD4, c CD8 and d NK-1-1. Stained area of a representative tumor area (1,585,000 μm2). The medians and range for each group are shown here. Statistical differences of CD4 stained area between: Nontreated versus GL-GM *p = 0.0286; GL-GM + TMZ IP versus GL-GM *p = 0.0286; GL-GM + TMZ IP versus GL-GM + TMZ IC *p = 0.0424 or CD8 stained area between: Nontreated versus GL-GM + TMZ IC *p = 0.0424 and TMZ IC versus GL-GM + TMZ IC *p = 0.0242, nonparametric Mann–Whitney test. e, f The percentage of e MDSCs (CD11b+Gr1+) and f Tregs (FoxP3+CD25+) out of CD4+ T cells in brain samples were determined by flow cytometry. Each value and the median for each group (GL-GM + TMZ IP or GL-GM + TMZ IC) are presented here. Statistical differences between: GL-GM + TMZ IP versus GL-GM + TMZ IC: MDSCs *p = 0.0295, nonparametric Mann–Whitney U test (color figure online)
Results
TMZ induces cell death and immunogenic modulation of GL261 cells
The cell viability of the GL261 cells was decreased with increasing doses of TMZ in vitro with an IC50 of 35 μM of TMZ (Fig. 1a).
We have previously reported an up-regulation of MHC class I and II expression on GL261 following interferon-γ exposure and irradiation [19]. Since TMZ might affect the immunogenicity of the cells, we examined the modulation of MHC I and II expression following TMZ exposure.
MHC class I was significantly up-regulated on TMZ-treated as compared with nontreated cells (6.8 ± 1.2 vs. 1.4 ± 0.2 %) while MHC class II expression was not significantly different (1.6 ± 0.5 vs. 1.0 ± 0.2 %, Fig. 1b).
Others have reported that certain chemotherapeutics may induce immunogenic cell death via surface exposure of calreticulin, or induce the production of T cell attractant chemokines such as IP-10 [39, 44]. However, we could not detect any calreticulin surface expression on the GL261 cells following TMZ exposure or any increase in the IP-10 production as nontreated GL261 cells had a higher production of IP-10 than TMZ-treated cells (data not shown).
Intratumoral TMZ induces a higher survival rate than systemic administration and the effect is T cell dependent
Next, the efficacy of intratumoral or systemic TMZ administration was investigated by monitoring survival in the GL261 model (Fig. 2a).
All nontreated mice developed lethal tumors. Mice treated with intratumoral TMZ (TMZ IC) had a survival rate of 45 %, while only 8 % of systemically TMZ-treated mice (TMZ IP) survived (Fig. 2b).
In order to elucidate whether the therapeutic effect of intratumoral TMZ was dependent on T cells, TMZ-treated mice were depleted of CD4+ and CD8+ T cells (Fig. 2c).
All T cell-depleted TMZ-treated mice developed lethal tumors, whereas 33 % of the nondepleted TMZ-treated mice survived (Fig. 2d).
Increased survival following immunotherapy combined with intratumoral TMZ
Next, we investigated whether TMZ administered intratumorally or systemically would increase the survival of mice immunized with GL-GM cells (Fig. 2e).
25 % of the GL-GM immunized mice survived tumor challenge. The addition of intratumoral TMZ (GL-GM + TMZ IC) increased survival rate to 83 %. However, mice immunized with GL-GM followed by systemic TMZ (GL-GM + TMZ IP) all died (Fig. 2f).
Systemic but not intratumoral TMZ depletes blood leukocytes
Several chemotherapeutics have been reported to exert some of their effects by activating anti-tumor immune reactivity. Here, we evaluated the immunological effects of TMZ by assessing different leukocyte populations in blood of tumor-bearing mice administered with either systemic or intratumoral TMZ (day 7–9, Fig. 2a). On day 12, there were a significantly lower number of total leukocytes (CD45+) in TMZ IP-treated mice, compared with TMZ IC-treated mice (Table 1, Supplementary Fig. S2a). More specifically, there were significantly lower numbers of macrophages (F4/80+), granulocytes (Ly6G+) and MDSCs (CD11b+Gr+) found in TMZ IP-treated compared with TMZ IC-treated mice (Table 1, Supplementary Fig. S2b–d).
The numbers of CD4+CD3+ T cells were not significantly lower in TMZ IP-treated mice compared with TMZ IC-treated mice (Table 1, Supplementary Fig. S2e). However, the number of CD8+CD3+ T cells were significantly lower in TMZ IP-treated mice compared with TMZ IC-treated mice (Table 1, Supplementary Fig. S2f). Also, the percentage of proliferating CD8+ T cells was significantly lower in TMZ IP-treated mice compared with TMZ IC-treated mice (Table 1, Supplementary Fig. S2g).
On day 19, the numbers of total leukocytes, macrophages, granulocytes and MDSCs in TMZ IP-treated mice were reconstituted to equivalent levels as in TMZ IC-treated mice, while the percentages of both proliferating CD8+ and CD4+ T cells were lower in TMZ IP-treated than in TMZ IC-treated mice (data not shown).
It has been reported that a low dose of TMZ selectively can reduce suppressive immune cell populations by depleting FoxP3+ regulatory T cells (Tregs) [36]. We therefore studied the Treg/CD4 T cell ratio (CD4+CD25+FoxP3+/CD4+) in blood following TMZ treatment. However, the Treg/CD4 ratio in TMZ IP-treated and TMZ IC-treated mice was not significantly different (Table 1, Supplementary Fig. S2h).
Immunotherapy-induced T cell proliferation persists in blood following intratumoral TMZ
Next, we investigated the effect of systemic or intratumoral TMZ (day 7–9) on the number of leukocytes in blood of GL-GM immunized mice (day 5, Fig. 2e). In conformity with TMZ IP-treated mice, on day 12, there was a significantly lower number of total leukocytes, macrophages, granulocytes and MDSCs in GL-GM + TMZ IP-treated mice when compared with both GL-GM immunized and GL-GM + TMZ IC-treated mice (Table 2, Supplementary Fig. S3a–d). The amount of granulocytes on day 12 was higher in GL-GM + TMZ IC-treated mice compared with GL-GM immunized mice (Table 2, Supplementary Fig. S3c).
The number of CD4+ and CD8+ T cells in GL-GM + TMZ IC-treated mice was sustained at equal levels as in GL-GM immunized mice (Table 2, Supplementary Fig. S3e, f). However, both CD4+ and CD8+ T cell numbers were lower in GL-GM + TMZ IP-treated mice compared with GL-GM immunized and GL-GM + TMZ IC-treated mice (Table 2, Supplementary Fig. S3e, f). Furthermore, there was a higher percentage of proliferating CD8+ cells (and CD4+ T cells, data not shown) in GL-GM + TMZ IC-treated than in GL-GM + TMZ IP-treated mice (Table 2, Supplementary Fig. S3g).
One week later, the numbers of macrophages had recovered in GL-GM + TMZ IP-treated mice, while the total amount of leukocytes, granulocytes and MDSCs were still lower than in GL-GM immunized mice (data not shown). Although no longer as pronounced, the percentages of proliferating CD8+ T cells were still higher in the GL-GM + TMZ IC-treated and GL-GM immunized mice compared with GL-GM + TMZ IP-treated mice (data not shown).
On day 12, the Treg/CD4 ratio in both GL-GM + TMZ IC-treated and GL-GM + TMZ IP-treated mice was higher compared with GL-GM immunized mice (Table 2, Supplementary Fig. S3h), but not on day 19 (data not shown). Although not significantly different, GL-GM + TMZ IP-treated had a slightly higher percentage of Tregs on day 12 compared with GL-GM + TMZ IC-treated mice (Table 2, Supplementary Fig. S3h).
Increased intratumoral CD8+cell infiltration following immunotherapy and intratumoral TMZ
The previous T cell depletion results indicated that the effect of intratumoral TMZ was T cell-mediated. Therefore, the amount of tumor infiltrating CD4+ and CD8+ cells T cells was assessed. We also analyzed the infiltration of NK-1.1+ and TCRγδ+ cells as these cells may contribute to the effect of the therapy. In order to study the effects shortly after TMZ delivery, immunization started day 12 and intratumoral TMZ treatment was postponed to day 14–16. Representable images of sections from tumors (day 20) and quantitative results of the percent of CD4, CD8 or NK-1.1 stained tumor area are shown in Fig. 3a–d.
Only occasional infiltrating CD4+ and CD8+ cells were detectable in nontreated tumors (panel 1 Fig. 3a and Fig. 3b,c). The infiltration of CD4+ or CD8+ cells in TMZ IC-treated tumors was not significantly different from nontreated tumors (panel 2, Figs. 3a–c). GL-GM + TMZ IP-treated tumors (panel 3, Fig. 3a) showed a less dense infiltration of CD4+ cells compared with tumors of GL-GM (panel 4, Fig 3a) or GL-GM + TMZ IC-treated mice (panel 5, Fig 3a, * Fig. 3b). GL-GM + TMZ IC-treated tumors had a significantly higher infiltration of CD8+ cells compared with nontreated or TMZ IC-treated tumors (*, Fig. 3c).
There was a high infiltration of NK-1.1+ cells in all treatment groups (Fig. 3a). TMZ IC tumors had the highest infiltration of NK1.1+ cells; however, this was not significantly different compared with any other treatment groups (Fig. 3d). NK-1.1 + cells were double labeled with either CD4 or CD8 in order to exclude that also NK cells were depleted following CD4 and CD8 antibody injections. The vast majority of the infiltrating NK-1.1+ cells were negative for CD4 or CD8 (data not shown). TCRγδ+ cells were also detected in tumors of all treatment groups; however, there was no significant difference between any of the treatment groups (data not shown).
Lower percentage of intratumoral MDSCs following immunotherapy and intratumoral TMZ
Next, we investigated the immunosuppressive cell populations infiltrating the tumors (day 20) following GL-GM immunization (day 9) and TMZ IP or IC (day 12–14).
The percentage of MDSCs was significantly lower in tumors treated with GL-GM + TMZ IC compared with GL-GM + TMZ IP (*, Fig. 3e). Although not significant, there was a trend toward a lower percentage of Tregs in tumors treated with GL-GM + TMZ IC than with GL-GM + TMZ IP (Fig. 3f).
Immunotherapy and intratumoral TMZ induce long-term memory
Finally, we wanted to assess whether intratumoral chemotherapy alone or combined with immunotherapy induced a memory response. Mice that had rejected their first tumor (n = 11) were rechallenged day 105 with a second tumor with no further treatment, and compared with mice receiving their first tumor (n = 4).
Despite no additional treatment, one mouse immunized with GL-GM cells and five mice treated with GL-GM + TMZ IC eradicated their second tumors (100 % survival), demonstrating an evoked long-term memory response (Table 3). Only one out of five mice receiving TMZ IC treatment without immunization developed a lethal tumor after rechallenge, indicating that intratumoral TMZ by itself induced a weaker immunological memory response (80 % survival, Table 3).
Table 3.
Immunization and intratumoral TMZ induced a long-term memory
| Treatment | n | Survival rate (%) |
|---|---|---|
| GL-GMa | 1 | 100 |
| GL-GM + TMZ ICa | 5 | 100 |
| TMZ ICa | 5 | 80 |
| Nontreatedb | 4 | 0 |
aMice surviving more than 100 days after tumor challenge were rechallenged with a second i.c. tumor with no further treatment
bNontreated mice receiving their first tumor served as controls
Discussion
In the present study, intratumoral delivery of TMZ was more effective than systemic administration. Also, this is the first report demonstrating a synergy of intratumoral chemotherapy and active immunotherapy in the treatment of experimental gliomas.
Although the GL261 cells were sensitive to TMZ in vitro, the therapeutic effect of systemic TMZ in vivo was minimal. Even when we started systemic TMZ treatment (2.5–25 mg/kg) on day 1, only 0–10 % mice survived. Others have reported a 20–40 % survival after 2.5–10 mg/kg/day of systemic TMZ on day 2–6 in the similar GL26 model [45]. To our knowledge, the beneficial effect on survival following systemic TMZ as monotherapy has only been modest in experimental brain tumor models, and in most studies, very high doses of TMZ were used [46].
The superior effect of intratumorally delivered TMZ reported here confirms previous results of other experimental brain tumor models [46], for example, the 9L gliosarcoma model [14] and human xenografts in athymic rats [16], although opposing data have been described in the F98 glioma model [47]. The effect of intratumoral chemotherapy is not restricted to TMZ as intratumoral carboplatin showed superior effect to systemic administration [47]. The variable efficacy of TMZ in glioma-bearing rodents may depend on several factors; differences in the dosing, timing, administration and formulation of TMZ and the immunogenicity and aggressiveness of the tumor model [46]. In this respect, prolonged treatment might lead to immunosuppression and thus reduce the effect [48].
As reported by others, toxicity after local TMZ delivery was absent at doses that could reject tumors [14, 16], tentatively due to a substantial dose reduction, implying that the dose-limiting factor for TMZ is systemic toxicity rather than local. In our experience, the toxicity of intratumoral TMZ seems to be less than for other chemotherapeutics such as cisplatin, which is promising from a clinical perspective (unpublished data).
Other investigators have suggested an immune-mediated mechanism of certain chemotherapeutics and ablative radiotherapy, via the induction of immunogenic cell death [32, 48]. Immunogenic cell death encompasses features including release of high-mobility-group-box-1, up-regulation of MHC, translocation of calreticulin to the plasma membrane or up-regulation of T cell attractant chemokines such as IP-10 [18, 33, 39, 49, 50]. The induction of high-mobility-group-box-1 release from TMZ-treated GL261 cells has been reported [51]. After TMZ exposure in vitro, we observed an up-regulation of MHC class I on the GL261 cells, which may facilitate the cytotoxic killing by CD8+ T cells in vivo. However, we could neither detect any calreticulin expression at the cell surface, nor verify an increase in IP-10 expression, as the IP-10 levels from nontreated cells were higher. It was recently suggested that chemotherapeutic drugs can act immunostimulatory even in the absence of classical immunogenic cell death [52].
After rechallenge, tumor rejection was seen in all immunized animals, but also in four TMZ IC-treated mice, suggesting an ability of TMZ to evoke a T cell-mediated memory response. The absence of a curative effect of TMZ following T cell depletion indeed emphasizes a T cell dependency. The effect could either be direct by increased T cell susceptibility in tumor cells as previously reported or indirect by effects on antigen presentation and immune activation. Nevertheless, we could not statistically distinguish the number of T cells infiltrating nontreated tumors from TMZ IC-treated tumors or GL-GM tumors from GL-GM + TMZ IC-treated tumors. We speculate that the T cells in the latter setting were more effective in tumor cell lysis. One study indeed reported that chemotherapy could make tumor cells more susceptible to cytotoxic T cells through an increased permeability for Granzyme B [53].
An explanation for a synergistic effect of systemic chemotherapy and immunotherapy has been proposed; chemotherapy reduces the pool of naïve T cells, leaving the remaining T cells with relatively increased availability of cytokines. Through a peripheral homeostatic proliferation of T cells, the expansion of specific anti-tumor T cells may be facilitated [54, 55]. In this context, immunization during the lymphocytic recovery period following chemotherapy has been shown to enhance anti-tumor immunity [56]. We therefore immunized glioma-bearing mice during the lymphocytic recovery phase following TMZ, and although features of homeostatic proliferation were detected, systemic TMZ followed by immunotherapy did not increase survival.
Furthermore, chemo- and immunotherapy may work in synergy by reducing the numbers or function of immunosuppressive cells, as shown in a study where gemcitabine enhanced anti-tumor activity by reducing the number of splenic MDSCs in tumor-bearing animals [34]. A low-dose metronomic regimen of the chemotherapeutic cyclophosphamide or TMZ was shown to specifically deplete Tregs either systemically or intratumorally, but with lack of effect on tumor growth [35–38]. In long-term treated melanoma patients, TMZ induced lymphopenia and a selective reduction of CD4+CD25+ cells [6]. Contrary to this, we observed a relative increase in peripheral Tregs following both intratumoral and systemic TMZ, and a nonselective reduction in peripheral T cells following systemic TMZ. While we cannot exclude a selective depletion of a subclass of more potent Tregs systemically [57], we conclude that the superior effect of intratumoral TMZ is due to sustained levels of cytotoxic T cells rather than depletion of circulating Tregs. Though, we observed a trend toward a lower Treg/CD4 T cell ratio and reduced numbers of MDSCs intratumorally after intratumoral than after systemic TMZ, implying that intratumoral TMZ reduces immunosuppressive cells locally.
The lower numbers of immunosuppressive cells following intratumoral TMZ may facilitate the survival of peripherally activated cytotoxic T cells with the capacity of eradicating remnant tumor cells. We speculate that local TMZ increases the intratumoral drug concentration, which elevates the accumulation of dead cells and exposure of tumor antigens presented on MHC class I and II for infiltrating T cells, although this needs to be confirmed in future experimental studies.
Intratumoral TMZ could be delivered in several ways for treatment of patients. TMZ could be implanted either directly into the tumor resection cavity following surgery using sustained release formulations, or by placing a catheter for convection enhanced delivery, either in combination with immunotherapy or with standard treatments. The use of Gliadel BCNU-loaded polymeric wafers in combination with radiotherapy for the treatment of newly diagnosed or recurrent GBM has proven to be effective and safe [58]. Patients that were implanted with Gliadel wafers following surgery and then administered TMZ and radiotherapy treatment had an overall survival of 20.7 months [13].
In conclusion, the therapeutic effect of intratumoral TMZ is mediated by T cells and acts in synergy with immunotherapy. Our results imply that toxicity of TMZ can be minimized by local delivery, while retaining intratumoral cytotoxic effects, which makes this concept clinically attractive. Given the poor prognosis for GBM patients undergoing current standard therapy, these results are of high relevance when designing more potent clinical trials, while minimizing drug toxicity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Hans and Märit Rausing Charitable Trust (to L.G. Salford), the Swedish Childhood Cancer Foundation, the Neuroblastoma-CNS Network (NBCNS) Foundation, the Skåne region funds ALF (all to P. Siesjö) and the Royal Physiographic Society in Lund (to A. Darabi). We would like to thank Hanna Fritzell and Jeremy Wales for reviewing the manuscript.
Conflict of interest
All authors declare no conflicts of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Darefsky AS, King JT, Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of surveillance, epidemiology, and end results registries. Cancer. 2012;118(8):2163–2172. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro-Oncology. 2012;14(3):351–359. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marina O, Suh JH, Reddy CA, Barnett GH, Vogelbaum MA, Peereboom DM, Stevens GH, Elinzano H, Chao ST. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg. 2011;115(2):220–229. doi: 10.3171/2011.3.JNS10495. [DOI] [PubMed] [Google Scholar]
- 6.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB. Selective CD4 + lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allhenn D, Boushehri MA, Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int J Pharm. 2012;436(1–2):299–310. doi: 10.1016/j.ijpharm.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Buonerba C, Di Lorenzo G, Marinelli A, Federico P, Palmieri G, Imbimbo M, Conti P, Peluso G, De Placido S, Sampson JH. A comprehensive outlook on intracerebral therapy of malignant gliomas. Crit Rev Oncol Hematol. 2011;80(1):54–68. doi: 10.1016/j.critrevonc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33(4):441–449. doi: 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabel M, Giese A. Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin. 2008;24(11):3239–3257. doi: 10.1185/03007990802508180. [DOI] [PubMed] [Google Scholar]
- 12.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 13.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brem S, Tyler B, Li K, Pradilla G, Legnani F, Caplan J, Brem H. Local delivery of temozolomide by biodegradable polymers is superior to oral administration in a rodent glioma model. Cancer Chemother Pharmacol. 2007;60(5):643–650. doi: 10.1007/s00280-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 15.Scott AW, Tyler BM, Masi BC, Upadhyay UM, Patta YR, Grossman R, Basaldella L, Langer RS, Brem H, Cima MJ. Intracranial microcapsule drug delivery device for the treatment of an experimental gliosarcoma model. Biomaterials. 2011;32(10):2532–2539. doi: 10.1016/j.biomaterials.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Heimberger AB, Archer GE, McLendon RE, Hulette C, Friedman AH, Friedman HS, Bigner DD, Sampson JH. Temozolomide delivered by intracerebral microinfusion is safe and efficacious against malignant gliomas in rats. Clin Cancer Res. 2000;6(10):4148–4153. [PubMed] [Google Scholar]
- 17.Eberstal S, Badn W, Fritzell S, Esbjornsson M, Darabi A, Visse E, Siesjo P. Inhibition of cyclooxygenase-2 enhances immunotherapy against experimental brain tumors. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-011-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, Lan L, Dewyngaert JK, Zagzag D, McBride WH, Formenti SC. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 19.Smith KE, Janelidze S, Visse E, Badn W, Salford L, Siesjo P, Darabi A. Synergism between GM-CSF and IFNgamma: enhanced immunotherapy in mice with glioma. Int J Cancer. 2007;120(1):75–80. doi: 10.1002/ijc.22286. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-Oncology. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka R. Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med. 2008;14(5):228–235. doi: 10.1016/j.molmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 23.Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993;90(7):3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60(12):3239–3246. [PubMed] [Google Scholar]
- 27.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8 + T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162(10):5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 28.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 29.Smith KE, Fritzell S, Badn W, Eberstal S, Janelidze S, Visse E, Darabi A, Siesjo P. Cure of established GL261 mouse gliomas after combined immunotherapy with GM-CSF and IFNgamma is mediated by both CD8+ and CD4+ T-cells. Int J Cancer. 2009;124(3):630–637. doi: 10.1002/ijc.23986. [DOI] [PubMed] [Google Scholar]
- 30.Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K, Gilbert M, Hassenbusch SJ, Sawaya R, Schmittling B, Archer GE, Mitchell DA, Bigner DD, Sampson JH. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro-Oncology. 2008;10(1):98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wack C, Kirst A, Becker JC, Lutz WK, Brocker EB, Fischer WH. Chemoimmunotherapy for melanoma with dacarbazine and 2,4-dinitrochlorobenzene elicits a specific T cell-dependent immune response. Cancer Immunol Immunother. 2002;51(8):431–439. doi: 10.1007/s00262-002-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 33.Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of anthracyclines: moving towards more personalized medicine. Trends Mol Med. 2008;14(4):141–151. doi: 10.1016/j.molmed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 35.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206(5):1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58(10):1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, Demarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69(10):4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, Kato M, Prevost-Blondel A, Avril MF, Nardin A, Abastado JP. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71(22):6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 40.Park JA, Joe YA, Kim TG, Hong YK. Potentiation of antiglioma effect with combined temozolomide and interferon-beta. Oncol Rep. 2006;16(6):1253–1260. [PubMed] [Google Scholar]
- 41.Kim CH, Woo SJ, Park JS, Kim HS, Park MY, Park SD, Hong YK, Kim TG. Enhanced antitumour immunity by combined use of temozolomide and TAT-survivin pulsed dendritic cells in a murine glioma. Immunology. 2007;122(4):615–622. doi: 10.1111/j.1365-2567.2007.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seligman AM, Shear MJ. Studies in carcinogenesis. VIII. Experimental production of brain tumors in mice with methyl-cholanthrene. Am J Cancer. 1939;37:364–395. [Google Scholar]
- 43.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30(9):2394–2400. [PubMed] [Google Scholar]
- 44.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 45.Park SD, Kim CH, Kim CK, Park JA, Sohn HJ, Hong YK, Kim TG. Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine. 2007;25(17):3485–3491. doi: 10.1016/j.vaccine.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 46.Hirst TC, Vesterinen HM, Sena ES, Egan KJ, Macleod MR, Whittle IR. Systematic review and meta-analysis of temozolomide in animal models of glioma: was clinical efficacy predicted? Br J Cancer. 2013;108(1):64–71. doi: 10.1038/bjc.2012.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, Huo T, Barth RF, Gupta N, Weldon M, Grecula JC, Ross BD, Hoff BA, Chou TC, Rousseau J, Elleaume H. Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol. 2010 doi: 10.1007/s11060-010-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Expression of HLA class I antigens and restoration of antigen-specific ctl response in melanoma cells following 5-aza-2’-deoxycytidine treatment. Int J Cancer. 2001;94(2):243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 51.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, Gameiro SR. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013 doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13(2 Pt 1):644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 55.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62(14):3914–3919. [PubMed] [Google Scholar]
- 57.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brem H, Ewend MG, Piantadosi S, Greenhoot J, Burger PC, Sisti M. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol. 1995;26(2):111–123. doi: 10.1007/BF01060217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.