Abstract
Mucosa-associated invariant T (MAIT) cells are innate-like T lymphocytes that are unusually abundant in the human liver, a common site of colorectal carcinoma (CRC) metastasis. However, whether they contribute to immune surveillance against colorectal liver metastasis (CRLM) is essentially unexplored. In addition, whether MAIT cell functions can be impacted by chemotherapy is unclear. These are important questions given MAIT cells’ potent immunomodulatory and inflammatory properties. Herein, we examined the frequencies and functions of peripheral blood, healthy liver tissue, tumor-margin and tumor-infiltrating MAIT cells in 21 CRLM patients who received no chemotherapy, FOLFOX, or a combination of FOLFOX and Avastin before they underwent liver resection. We found that MAIT cells, defined as CD3ε+Vα7.2+CD161++ or CD3ε+MR1 tetramer+ cells, were present within both healthy and tumor-afflicted hepatic tissues. Paired and grouped analyses of samples revealed the physical proximity of MAIT cells to metastatic lesions to drastically influence their functional competence. Accordingly, unlike those residing in the healthy liver compartment, tumor-infiltrating MAIT cells failed to produce IFN-γ in response to a panel of TCR and cytokine receptor ligands, and tumor-margin MAIT cells were only partially active. Furthermore, chemotherapy did not account for intratumoral MAIT cell insufficiencies. Our findings demonstrate for the first time that CRLM-penetrating MAIT cells exhibit wide-ranging functional impairments, which are dictated by their physical location but not by preoperative chemotherapy. Therefore, we propose that MAIT cells may provide an attractive therapeutic target in CRC and that their ligands may be combined with chemotherapeutic agents to treat CRLM.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2050-7) contains supplementary material, which is available to authorized users.
Keywords: MAIT cells, Colon cancer, Liver metastasis, Tumor-infiltrating lymphocytes, Immune surveillance, Chemotherapy
Introduction
Colorectal carcinoma (CRC) is a major cause of morbidity and mortality worldwide. In the United States, CRC is considered the third leading cause of death from cancer among men and women and was also estimated to constitute the third most common type of cancer to occur in 2016 [1]. Although advances in screening and systemic therapeutic approaches over the past few decades have improved the 5-year overall survival rate for CRC, approximately one-third of patients still succumb to this malignancy within 5 years of diagnosis [2].
One of the most devastating clinical complications of CRC is metastasis to various organs, frequently to the liver, which may be present at diagnosis or detected subsequently. In fact, over 50% of CRC patients develop hepatic metastases during their lifetime [3, 4]. Surgical resection is currently the only curative option for colorectal liver metastasis (CRLM). Although preoperative chemotherapy may reduce the tumor burden to ‘downstage’ a fraction of otherwise ‘unresectable’ patients, only a small minority of CRLM patients meets the eligibility criteria for hepatic resection [4]. Therefore, immunotherapy, which is typically more tumor-specific and less toxic than chemotherapy, has brought renewed hope to many cancer patients, including those with CRC. Once optimized and successfully implemented, immunotherapeutic modalities may shrink a tumor mass and prepare the host for other treatments (e.g., surgery) or even prevent postoperative relapse.
The general goal of CRC immunotherapy is twofold, namely to augment antitumor responses of innate and adaptive nature, for instance via administration of tumor vaccines, adjuvants and cytokines and through enhancement of pro-inflammatory cytokine-producing cells, and to relieve tumor-mediated immunosuppression [5]. Several cell types belonging to innate and adaptive arms of immunity have been implicated in anti-CRC immune surveillance. These include intratumoral NK cells [6, 7], invariant natural killer T (NKT) cells [8], macrophages [9] and memory, T helper 1 (TH1)-polarized and cytotoxic CD8+ T cells [10, 11]. It is noteworthy, however, that markers used in many studies to identify T, NK and NKT cells or to distinguish them from each other (e.g., CD3, CD8, CD56, CD57, NKG2D) are shared by certain unconventional T cell types, including mucosa-associated invariant T (MAIT) cells that are the main subject of this work.
Many studies to date have focused on immunophenotyping of TILs as opposed to their functional attributes. Moreover, it is not well understood whether and/or how chemotherapy may alter the composition and functions of TILs within the CRC tumor landscape. Finally, TILs present in or extracted from the primary site of neoplastic transformation (i.e., the colorectal tissue) have been commonly investigated, whereas their functions within the metastatic lesions, especially in the liver (i.e., in CRLM), are far from clear. This is particularly important in light of the facts that: (i) the liver receives a large volume of blood from the gastrointestinal tract where CRC originates; (ii) the liver is a unique immunological site with tolerogenic characteristics [12]; and (iii) it also paradoxically accommodates many innate-like T cells with anticancer potentials. Most notably, MAIT cells, which can express cytotoxic effector molecules (e.g., granzymes) and exhibit a T helper (TH)1- or TH17-type inflammatory cytokine profile depending on their local environmental imprinting [13, 14] are highly enriched in the human liver. They comprise 30–50% of the total hepatic lymphocyte pool in humans [15, 16]; yet, their ability to penetrate hepatic metastases of CRC and to produce inflammatory cytokines in situ remains unexplored.
MAIT cells are best known for their antimicrobial properties [14]. They express a semi-invariant TCR that is typically composed of a TRAV1-2/TRAJ12/20/33 α chain [17, 18], which can pair with one of several β chains to recognize riboflavin-based antigens of bacterial origin [19] or other bacterial products [20]. Antigen recognition by MAIT cells occurs in an MHC-related protein 1 (MR1)-dependent fashion [19–21]. MR1 is a monomorphic MHC class I-like molecule that is remarkably conserved among mammalian species [22]. Of note, MAIT cell TCR ligands may not be limited to bacterial products, and MAIT cell activation can also be achieved in a TCR-independent fashion, for instance by a combination of IL-12 and IL-18 [23], potent cytokines known for their antitumor activities.
MAIT cell TCR transcripts have been previously detected and correlated with pro-inflammatory cytokine expression in kidney and brain cancer biopsies [24]. In addition, human MAIT cells express NKG2D [15], a C-type lectin-like receptor that plays an important role in tumor immune surveillance [25]. NKG2D ligands are highly expressed by CRC tumors, especially in their early stages, but are progressively lost, and their expression levels correlate with patient survival [26]. Nonetheless, direct evidence for MAIT cell-mediated responses to cancer in general and to CRC and its metastases in particular is either scarce or completely absent.
In this work, we have assessed both the frequencies and the functional competence, or lack thereof, of MAIT cells in peripheral blood, healthy liver tissue and hepatic metastases of patients with CRC. We have also explored whether preoperative chemotherapy with FOLFOX [leucovorin calcium (folinic acid)/5-fluorouracil/oxaliplatin] alone or in combination with the anti-angiogenic agent Avastin (bevacizumab) has any influence on the above parameters. The potential benefits of MAIT cell-based tumor immunotherapy will be discussed.
Materials and methods
Human subjects
Twenty-one consenting patients with CRC, 11 males and 10 females ranging in age from 40 to 80, were prospectively enrolled in this study at the London Health Sciences Centre following informed consent (Table 1). Inclusion criteria included an established diagnosis of CRLM, a curative intent for major liver resection, and technical feasibility of R0 resection. Patients younger than 18 were excluded. Additional exclusion criteria consisted of pregnancy, concomitant immunosuppressive therapy, immunodeficiency, a Do-Not-Resuscitate order, a poor performance status [an Eastern Cooperative Oncology Group (ECOG) grade of ≥2] [27], American Society of Anesthesiologists (ASA) fitness category of ≥4, disease progression despite chemotherapy, and extrahepatic (e.g., peritoneal, pulmonary, brain, bone marrow) metastasis detected prior to surgery or intraoperatively.
Table 1.
Demographic and clinical characteristics of CRC patients participating in this study
| Variable | Chemotherapy (n = 13) | No chemotherapy (n = 8) |
|---|---|---|
| Demographic attributes | ||
| Female gender, n (%) | 6 (46) | 4 (50) |
| Mean age, years (±SD) | 54 ± 9 | 74 ± 5 |
| Median age, years (range) | 55 (40–64) | 73 (71–80) |
| Male gender, n (%) | 7 (54) | 4 (50) |
| Mean age, years (±SD) | 59 ± 10 | 62 ± 13 |
| Median age, years (range) | 63 (46–70) | 62 (47–77) |
| Body mass index, Mean ± SD | 25 ± 5 | 27 ± 4 |
| Oncological attributes | ||
| Type of primary cancer, n (%) | ||
| Colon | 10 (77) | 6 (75) |
| Rectum | 3 (23) | 2 (25) |
| CEA, median level in ng/mL (range) | 28.3 (1–4220) | 10 (8–49) |
| Synchronous metastases, n (%) | 12 (92) | 4 (50) |
| Median number of lesions, n (range) | 3 (2–9) | 2 (1–3) |
| Chemotherapy | ||
| FOLFOX, n (%) | 8 (62) | N/A |
| FOLFOX + Avastin, n (%) | 5 (38) | N/A |
| Surgical attributes | ||
| ASAa fitness category, n (%) | ||
| <3 | 6 (46) | 1 (12) |
| 3 | 7 (54) | 7 (82) |
| ≥4 | 0 (0) | 0 (0) |
| Simultaneous resection, n (%) | 1 (8) | 2 (25) |
| Complicationsb, n (%) | ||
| Minor (≤grade III) | 4 (31) | 2 (25) |
| Major (grade IIIa) | 2 (15) | 0 (0) |
| Severe (≥grade IIIb) | 2 (15) | 0 (0) |
| Clinical outcomes | ||
| Median length of hospital stay (days) | 7 | 9 |
| 90-day mortality, n (%) | 0 (0) | 0 (0)c |
| Recurrenced, n (%) | 1 (8) | 1 (20) |
CEA carcinoembryonic antigen, N/A not applicable
a According to American Society of Anesthesiologists (ASA) physical status classification (https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system)
b According to the Clavien–Dindo classification
c Zero for five patients and data not available yet for the remaining three patients
d Local, regional and/or distal recurrence
As part of standard of care, a multidisciplinary ‘tumor board’ discussed each case and reached a consensus on the most suitable (i.e., surgical and/or chemotherapeutic) course of action. Eligible patients received no chemotherapy or at least one cycle of FOLFOX alone or in combination with Avastin (Table 1) before they underwent surgical liver resection between August 2015 and May 2017. All surgeries were performed at London Health Sciences Centre University Hospital or Victoria Hospital, both located in London, Ontario, Canada.
All work involving human samples was performed in conformity with standard ethical guidelines and using a protocol that was approved by the Western University Research Ethics Board for Health Sciences Research Involving Human Subjects (approval number: HSREB 106937) and by the Lawson Health Research Institute (approval number: R-15-360).
Whole blood and tissue sample collection and processing
Peripheral blood was collected into heparin-coated BD Vacutainers® (BD Biosciences, Mississauga, ON) from patients via a surgically placed central line.
After extrahepatic disease was ruled out during laparotomy and technical resectability was confirmed through intraoperative ultrasonography, tissue samples were harvested. The extent of liver resection, ≥3 segments according to the Brisbane 2000 nomenclature [28], was at the discretion of the operating surgeon aiming to achieve negative surgical margins as well as a liver remnant of sufficient volume to allow for normal hepatic function. Tumor-containing and healthy liver tissue specimens were placed in 15 mL of ice-cold RPMI 1640 medium supplemented with 10% heat-inactivated FBS, GlutaMAX™-I, 0.1 mM MEM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin and 10 mM HEPES, which will hereafter be referred to as complete medium. Samples were immediately transported on ice for further processing.
PBMCs were isolated by density gradient centrifugation after transferring diluted cells into SepMate™-50 tubes (Stemcell Technologies, Vancouver, BC) containing low-endotoxin (<0.12 EU/mL) Ficoll-Paque PLUS (GE Healthcare Life Sciences, Mississauga, ON).
Hepatic [non-parenchymal] mononuclear cells (HMNCs) and tumor-infiltrating MNCs were extracted from respective tissue specimens shortly after liver resection. Briefly, the specimens were cut into small pieces, pushed through a wire mesh filter and washed with 2% FBS in cold, sterile PBS. After discarding the supernatant, cell pellets were washed again, resuspended, laid over 33.75% low-endotoxin Percoll PLUS (GE Healthcare), and spun at 700×g with no brake for 12 min at room temperature. The pelleted MNCs were treated with ACK lysis buffer for 2 min to eliminate contaminating erythrocytes, washed with 2% FBS in cold PBS, and passed through 70-µm pores of a nylon mesh strainer to remove clumped cells and debris.
Ex vivo MNC stimulation
Isolated PBMCs, HMNCs and tumor-infiltrating MNCs were resuspended in complete medium and seeded at a density of 1 × 106 cells/250 μL/well of U-bottom microplates. Cells were left untreated or stimulated for 24 h with a combination of recombinant [human] interleukin (rIL)-12 (PeproTech, Rocky Hill, NJ) and rIL-18 (R&D Systems, Minneapolis, MN), both used at 5 ng/mL. Parallel cultures were stimulated with staphylococcal enterotoxin B (SEB) (100 ng/mL) or crude Klebsiella pneumoniae lysate, which were prepared as described below. In several experiments, we used 0.5 µg/mL of a mouse anti-human CD3ε (clone OKT3) from Bio X Cell (West Lebanon, NH) plus 5 ng/mL of rIL-12. During the final 5 h of cultures, 1 µM brefeldin A (Sigma-Aldrich, Oakville, ON) and 2 µM monensin (eBioscience, San Diego, CA) were present to retain IFN-γ and IL-17A among other soluble molecules inside the activated cells.
Recombinant SEB was provided by Dr. John McCormick (Western University). It was generated using an approved institutional biosafety protocol. In brief, SEB, from Staphylococcus aureus strain COL, was cloned, expressed in BL21 (DE3) competent Escherichia coli, and purified by nickel column chromatography [29].
A stock of Klebsiella pneumoniae lysate was prepared from a clinical isolate, Parkwood-18, which was a gift from Dr. Miguel Valvano (Queen’s University Belfast). After overnight culture at 37 °C in Luria broth, bacterial cells were washed in cold PBS before adjusting the OD600 to 6.5. Cell membrane rupture was achieved by exposing the bacteria to 30,000 PSI of pressure for 5 min. The resulting lysate was kept at −80°C until use.
Cytofluorimetric analyses
Freshly isolated or cultured cells were washed and stained for 30 min at 4°C with fluorochrome-labeled, mouse anti-human mAbs against CD3ε (clone HIT3a, eBioscience, San Diego, CA), CD161 (HP-3G10, eBioscience), CD212 (IL-12Rβ1) (2.4E6, BD Biosciences, San Jose, CA), CD218a (IL-18Rα)(H44, eBioscience) and/or TCR Vα7.2 (3C10, BioLegend, San Diego, CA) as indicated.
MAIT cells were defined as CD3+Vα7.2+CD161++ cells. In several experiments, MAIT cells were identified through cell surface staining with MR1 tetramer reagents [30, 31]. Biotinylated human MR1 monomers, which were loaded with 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU), were tetramerized using PE streptavidin (BD biosciences) and added to the cells along with a FITC-conjugated anti-CD3ε mAb (HIT3a, eBioscience). PE-conjugated, 6-formylpterin (6-FP)-loaded MR1 tetramers were used as a negative staining control. MR1 tetramer staining was performed at room temperature for 30 min.
To quantify the intracellular IFN-γ, IL-17A or granzyme B (GZM B) content of MAIT cells after their surface staining, cells were washed, resuspended in intracellular fixation and permeabilization buffer (eBioscience) and stored at room temperature, in dark, for 20 min. Cells were then washed and stained with eBioscience mouse anti-human mAbs against IFN-γ (clone 4S.B3), IL-17A (clone eBio64DEC17) and/or GZM B (clone GB11), or with appropriate isotype controls.
A BD FACSCanto II cytometer and FlowJo software (Tree Star, Ashland, OR) were used to acquire and analyze data, respectively.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6 software. Student’s t test, the Wilcoxon matched-pairs signed rank test and ANOVA were employed to compare grouped and paired data sets as appropriate. Differences with a p ≤ 0.05 were considered significant. *, **, *** and **** denote p ≤ 0.05, p ≤ 0.01, p ≤ 0.001 and p ≤ 0.0001, respectively.
Results
MAIT cells are readily detectable within the peripheral blood and liver of patients with CRLM
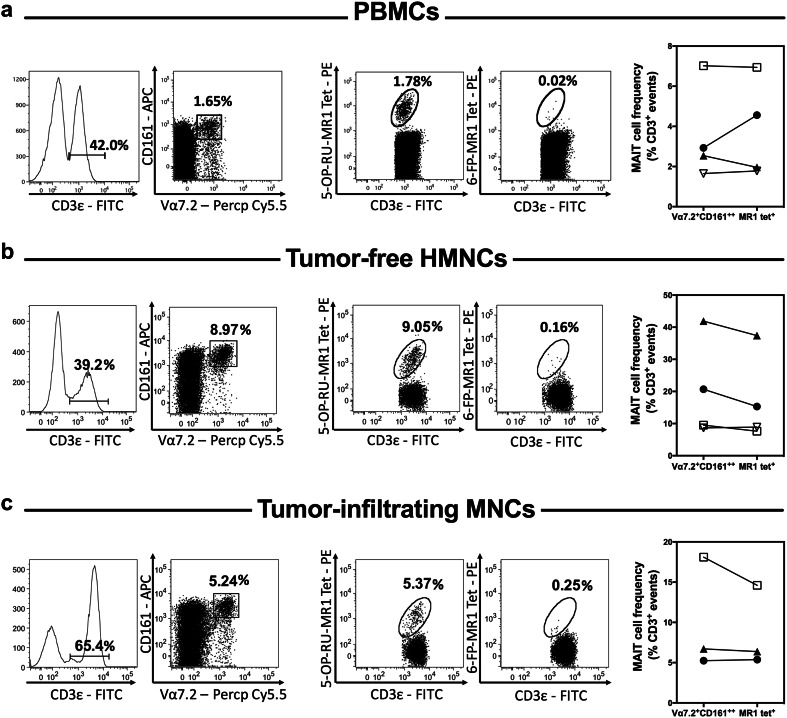
Normal human liver accommodates many MAIT cells [15, 16]. Whether these cells retain their predominant presence in the liver of CRLM patients and whether they penetrate the metastatic lesions are unknown. In our cohort, the mean frequency (±SEM) of total CD3+ cells among PBMCs (n = 21), tumor-free healthy tissue HMNCs (n = 21), tumor-margin MNCs (n = 18) and tumor-infiltrating MNCs (n = 21) were 44.0 (±4.2)%, 54.3 (±3.3)%, 56.9 (±4.3)% and 63.1 (±5.6)%, respectively. We found CD3+Vα7.2+CD161++ MAIT cells to be easily detectable among PBMCs and also within and outside hepatic tumor masses in the vast majority of our patients (Figs. 1a–c, 2).
Fig. 1.
Peripheral blood and hepatic MAIT cells can be readily detected in CRC patients with liver metastasis. The frequency of MAIT cells was determined by flow cytometry among PBMCs (a), tumor-free, non-parenchymal hepatic mononuclear cells (b) and hepatic tumor-infiltrating mononuclear cells (c) isolated from patients with CRLM. MAIT cells were defined as CD3ε+Vα7.2+CD161++ or CD3ε+5-OP-RU-MR1 tetramer+ cells as indicated. 6-FP-MR1 tetramer was used as a staining control. Representative dot plots for each sample type are depicted (left panels). MAIT cell frequencies were calculated using both staining strategies for 3–4 patients (right panels). Values for matched samples (scatter plots with each patient being represented by a distinct symbol) are shown for comparison
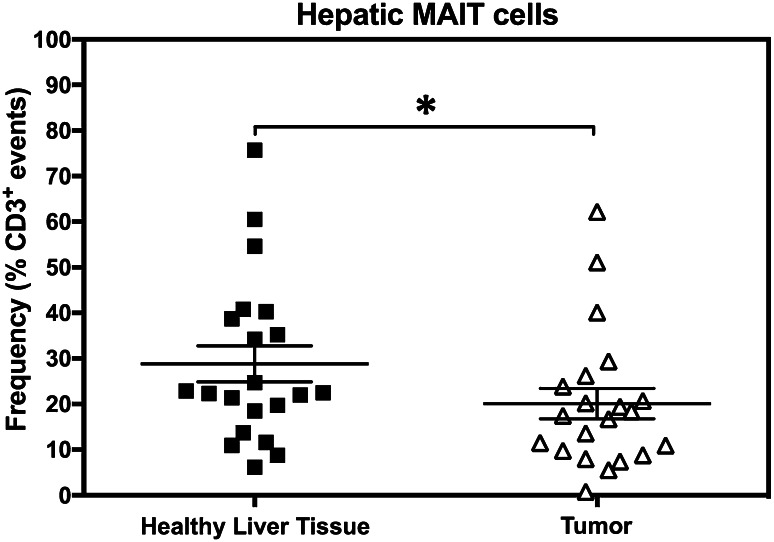
Fig. 2.
MAIT cell frequencies are diminished within liver metastases of CRC. Tumor-free and tumor-infiltrating non-parenchymal mononuclear cells were isolated from the liver of each CRLM patient. MAIT cells were then enumerated for each patient’s sample set, and the Wilcoxon matched-pairs signed rank test was used to analyze data statistically. Error bars represent standard error of the mean (SEM)
Although human MAIT cells are often defined by virtue of their CD3, Vα7.2 TCRα chain and CD161 co-expression, the relatively recent invention of MR1 tetramer reagents has provided powerful tools with which to validate MAIT cell identification [30, 31]. Having large enough samples from several patients (PBMCs and tumor-free HMNCs from 4 patients and tumor-infiltrating MNCs from 3 patients) enabled us to determine MAIT cell frequencies by MR1 tetramer staining as well. Using 5-OP-RU-loaded MR1 tetramers, MAIT cells could be detected in all samples with similar frequencies to those calculated after co-staining with a cocktail of anti-CD3ε, -Vα7.2 and -CD161 mAbs. As expected, there was no staining with 6-FP-loaded control MR1 tetramers. Figure 1a–c illustrate representative histograms and dot plots as well as the results of our head-to-head comparisons between the two staining protocols.
Together, these results indicate that hepatic metastasis of CRC does not compromise the presence of MAIT cells in the liver. Also importantly, MAIT cells can infiltrate the metastatic lesions in the liver of CRC patients.
MAIT cells are less abundant in CRLM tumor lesions than within healthy liver tissue
Since MAIT cells were found both within and outside the hepatic metastases of CRC, we determined their frequencies in paired sample sets harvested from each liver in an effort to avoid variations associated with inter-patient heterogeneity. To this end, we isolated tumor-free and tumor-infiltrating MNCs from each of the 21 liver samples and determined their MAIT cell content. Figure 2 demonstrates the outcome of our statistical comparison revealing a modest but still significant reduction in the MAIT cell compartment of the CRLM tumor microenvironment.
Hepatic tumor-infiltrating MAIT cells are functionally impaired in CRLM patients
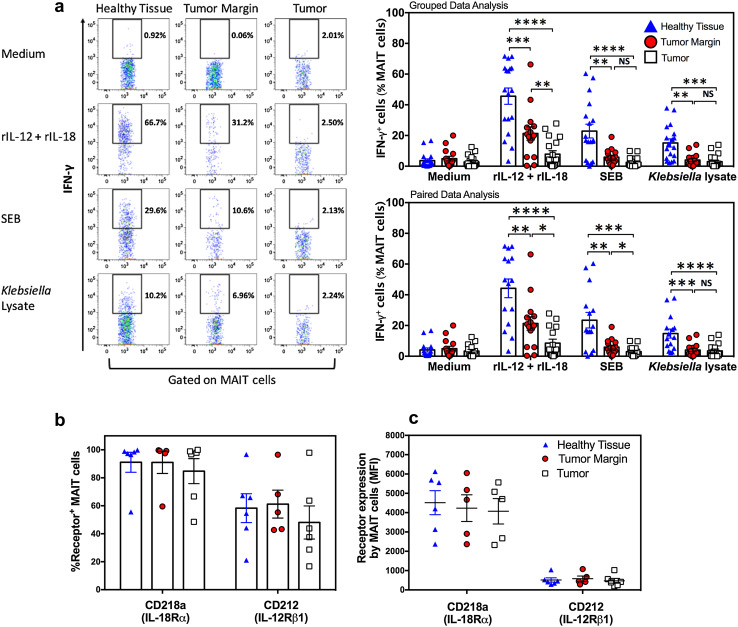
While slightly depressed, the frequencies of MAIT cells in CRLM ranged between 0.2 and 15.3% in peripheral blood and between 6.2 and 75.7% in healthy liver tissue. The calculated MAIT cell averages in these compartments (Figs. 1, 2) are not markedly different from the expected values, and the noted variation is not unusual either [15, 16]. However, given that MAIT cells constituted a substantial fraction of TILs in CRLM, the more important question was whether they retain their effector functions and/or contribute to the inflammatory cytokine milieu within the local tumor microenvironment. To address this question, we obtained PBMCs, TILs, HMNCs in the tumor margin (within 1-cm distance from tumors) and HMNCs residing in the healthy liver tissue distal to the metastatic lesions (>10 cm away from tumors). Isolated cells were then exposed to a panel of non-specific and cognate MAIT cell stimuli followed by surface staining for several markers and intracellular staining for IFN-γ, a prototypic pro-inflammatory cytokine whose levels are associated with a better prognosis in CRC [32]. Using a limited number of samples, we were also able to assess the expression of IL-17A and GZM B, a potent cytotoxic effector molecule that mediates target cell destruction by activated MAIT cells [33].
The stimuli we employed to either directly or indirectly activate MAIT cells included rIL-12 plus rIL-18 (TCR-independent) [23], SEB (TCR/cytokine-dependent but MR1-independent) [34] and Klebsiella pneumoniae lystate (TCR- and MR1-dependent) [35, 36]. It is noteworthy that we also attempted to activate MAIT cells using an anti-CD3 mAb (OKT3) as a source of signal 1 in conjunction with rIL-12 that can replace CD28-mediated costimulation, thus supplying signal 2 for T cell activation [37]. However, this stimulation mode proved to be too strong and made MAIT cells in all 12 PBMC samples, all 12 healthy HMNC samples, all 10 tumor-margin HMNC samples, and in 11 out of 12 tumor-infiltrating HMNC samples undetectable (data not shown) due, perhaps, to TCR downregulation or cell death. Interestingly and in partial contrast, conventional CD3+Vα7.2− T cells showed various degrees of detectability, from none to complete, in the same cohort. Supplementary Figure 1 depicts dot plots from 3 representative patients displaying no detectable MAIT cells along with absent, partially present or fully detectable CD3+Vα7.2− cells. Therefore, OKT3 plus rIL-12 could not be used as an optimal stimulus in our functional studies on MAIT cells.
We examined whether and to what extent tumor-infiltrating MAIT cells and those residing in the surrounding liver tissue, near to or far from the tumor(s), react to TCR/MR1-dependent and -independent stimuli. We found that in stark contrast with their healthy hepatic tissue counterparts, tumor-infiltrating MAIT cells had a complete or near-complete failure to produce IFN-γ in response to rIL-12/rIL-18, SEB or Klebsiella lysate (Fig. 3a). This was evident not only by grouped data set assessments but also through paired t tests comparing samples obtained from each individual patient. Of note, the IFN-γ response magnitude of tumor-margin MAIT cells was at an intermediate level, lower than that of their healthy tissue counterparts but higher than that of tumor-infiltrating MAIT cells. This was especially evident after stimulation with rIL-12 plus rIL-18 or with SEB (Fig. 3a). Next, we sought to ascertain whether intratumoral MAIT cell hyporesponsiveness to rIL-12 and rIL-18 could be due to weak cytokine receptor expression. We found the frequencies of MAIT cells expressing CD212 (IL-12Rβ1) or CD218a (IL-18Rα) to be comparable across all the three hepatic compartments (Fig. 3b). In addition, CD212 and CD218a expression on a per cell basis, as judged by their MFI, were equivalent (Fig. 3c). Therefore, the drastic functional impairment of tumor-infiltrating MAIT cells is not due to a loss of IL-12 and/or IL-18 receptors.
Fig. 3.
The physical location of MAIT cells within CRLM-afflicted livers dictates their functional capacity. Non-parenchymal mononuclear cells harvested from healthy liver tissue, tumor margin and metastatic tumors of CRLM patients were left untreated or exposed to rIL-12+rIL-18, SEB or Klebsiella lysate. Twenty-four hours later, the frequencies of IFN-γ+ MAIT cells were determined by flow cytometry. Representative dot plots are illustrated, and statistical analyses of grouped and paired data sets were performed using two-way ANOVA and paired t tests, respectively (a). Baseline frequencies of CD212+ and CD218a+ hepatic MAIT cells (b) and the MFI of CD212 and CD218a staining (c) are also shown
Other than IFN-γ production that was used as the main readout in our functional assays, we also determined the frequencies of GZM B+ cells among healthy liver, tumor-margin and tumor-infiltrating MAIT cells. We found that on average, GZM B+ cells constituted a relatively high proportion of hepatic MAIT cells in their steady state (Supplementary Figure 2). Healthy tissue MAIT cells responded rigorously to rIL-12/r-IL-18, SEB or Klebsiella lysate. By contrast, there were fewer GZM B+ MAIT cells within the CRLM lesions although the noted difference did not reach statistical significance due, likely, to the small number of samples examined.
Finally, we evaluated samples obtained from 3 patients for IL-17A expression and found only a negligible percentage of both healthy liver and tumor-infiltrating MAIT cells to be positive for this cytokine (Supplementary Table 1).
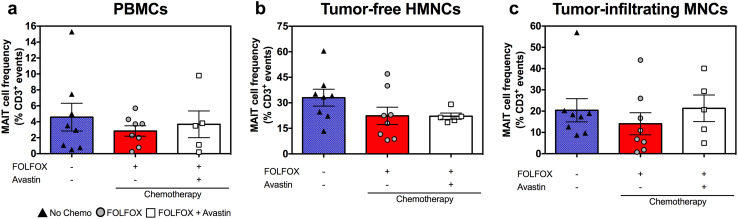
MAIT cell functional abnormalities of CRLM patients are not caused by preoperative chemotherapy
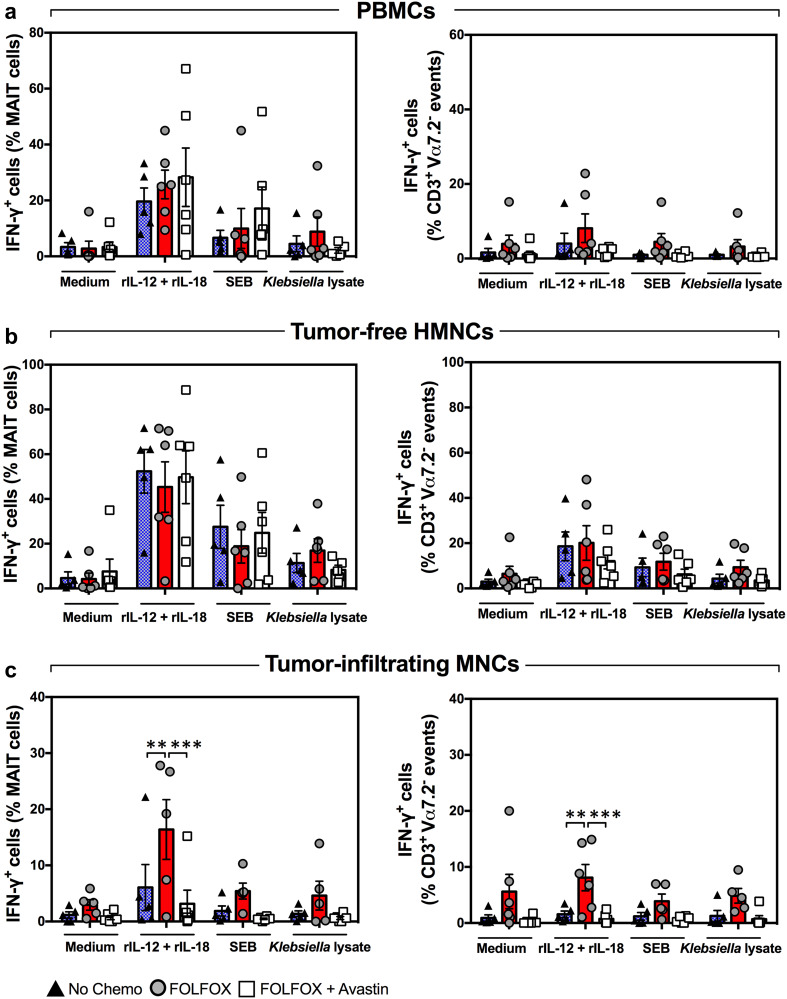
Many chemotherapeutic agents interfere with normal biological functions of various cell types. Since 13 out of the 21 patients enrolled in this study received FOLFOX, either alone or in combination with Avastin, before liver resection (Table 1), it was important to investigate the effect(s) of these agents, if any, on MAIT cells. We found similar circulating MAIT cell frequencies in patients with and without preoperative chemotherapy (Supplementary Figure 3). Therefore, the chemotherapeutic regimens used in approximately 62% of our patients appear not to have a negative impact on their peripheral blood MAIT cell proportion. In subsequent analyses, we determined both the frequency (Fig. 4) and the IFN-γ production capacity (Fig. 5) of MAIT cells among PBMCs, tumor-free, healthy tissue HMNCs and tumor-infiltrating MNCs isolated from three patient subgroups, namely those receiving no chemotherapy, those treated with FOLFOX and those given FOLFOX plus Avastin. These analyses revealed no differences between the three subgroups with the sole exception of moderately heightened, rather than depressed, IFN-γ production in response to rIL-12/rIL-18 by tumor-infiltrating MAIT cells in the ‘FOLFOX only’ subgroup (Fig. 5c). Collectively, these results demonstrate that preoperative chemotherapy is not responsible for MAIT cell dysfunction in CRLM patients. The above experiments enabled us to also assess IFN-γ production by CD3+Vα7.2− conventional T cells in response to the same stimuli (Fig. 5, right panels). Although unfractionated conventional T cells were less potent in this capacity, their ability to produce IFN-γ followed a similar pattern in the three patient subgroups.
Fig. 4.
Preoperative chemotherapy with FOLFOX or with FOLFOX plus Avastin does not lower blood and hepatic MAIT cell frequencies. PBMCs (a), tumor-free, non-parenchymal hepatic mononuclear cells (b) and hepatic tumor-infiltrating mononuclear cells (c) were isolated from CRLM patients who had received no chemotherapy (filled triangles), FOLFOX (filled circles) or FOLFOX plus Avastin (open squares) before they underwent liver resection surgery. The percentage of MAIT cells in each cell population was determined by flow cytometry. Error bars represent SEM
Fig. 5.
Baseline and inducible IFN-γ production by blood and hepatic MAIT cells is refractory to treatment with FOLFOX ± Avastin. PBMCs (a), tumor-free hepatic mononuclear cells (b) and tumor-infiltrating mononuclear cells (c) were isolated from CRLM patients who had received no chemotherapy (filled triangles), preoperative FOLFOX (filled circles) or FOLFOX plus Avastin (open squares). The ability of MAIT cells (left panels) and CD3+Vα7.2− conventional T cells (right panels) to produce IFN-γ in response to rIL-12+rIL-18, SEB or Klebsiella lysate was evaluated by flow cytometry. Error bars represent SEM
Discussion
In this work, we have investigated for the first time, to our knowledge, the frequencies and several important functional parameters of MAIT cells in the liver of patients with CRLM. Two relatively recent studies have demonstrated the presence of MAIT cells within primary colon adenocarcinomas [38, 39]. However, the role of MAIT cells in metastatic disease, especially in locations where these cells are highly enriched (e.g., human liver) has not been explored before.
We found that MAIT cells heavily infiltrate the hepatic metastases of CRC. However, MAIT cells extracted from the tumors, and to a lesser extent those adjacent to the lesions, were hampered in their ability to make IFN-γ and also showed a tendency for failure to upregulate GZM B. MAIT cell stimuli we used were carefully chosen to either mimic therapeutic scenarios or to shed mechanistic light on potential MAIT cell defects. A combination of IL-12 and IL-18 was used because: (i) these cytokines are potent inducers of IFN-γ and TH1-type inflammatory responses [40]; (ii) they are implicated in immune surveillance against CRC and considered attractive candidates in cytokine-based or combination immunotherapy of CRC [41]; and (iii) they are known to activate MAIT cells [23]. SEB and Klebsiella lysate were utilized to enable examination of TCR- and/or MR1-dependent MAIT cell activation.
We found that unlike healthy liver tissue MAIT cells, tumor-infiltrating MAIT cells were incapable of producing IFN-γ in response to rIL-12/rIL-18 despite amply expressing the receptors for these cytokines. In addition, SEB that activates MAIT cells in a TCR- and IL-12/IL-18-dependent but MR1-independent manner [34] failed to elicit an appreciable IFN-γ response by tumor-infiltrating MAIT cells neither did Klebsiella crude lysate as a source of MR1-restricted, TCR-binding MAIT cell antigens [35, 36]. Therefore, MAIT cell defects within the metastatic lesions are wide-ranging and involve TCR and cytokine receptor signaling. Consequently, targeting each pathway alone may not be adequate to overcome MAIT cell dysfunctions.
MAIT cells residing in certain tissues express IL-17A [15, 42], a cytokine that has been linked to tumorigenesis and suppressor cell accumulation in tumor microenvironments [43–46]. Therefore, it is important to determine whether tumor-infiltrating MAIT cells’ failure to produce IFN-γ represented a global defect or simply results from a shift from an IFN-γ- to an IL-17A-producing phenotype. We favor the former scenario because we found only a negligible proportion of MAIT cells to be IL-17A+ within the hepatic metastases of 3 CRLM patients. This finding needs to be validated in a larger cohort.
At least three lines of evidence suggest that MAIT cell insufficiencies of CRLM patients are not caused by preoperative chemotherapy. First and foremost, we found no marked reduction in peripheral blood and hepatic MAIT cell frequencies and IFN-γ synthesis in patients who had been treated with FOLFOX (±Avastin) (Figs. 4, 5). Second, even in patients receiving systemic chemotherapy, healthy liver tissue MAIT cells were still able to produce copious amounts of IFN-γ and GZM B (Fig. 5b, Supplementary Figure 2). Third, hepatic MAIT cells residing in close proximity to CRLM masses were only partially effective in producing IFN-γ and GZM B, suggesting that it is indeed the tumor microenvironment, and not chemotherapy, that incapacitates MAIT cells in CRLM patients.
MAIT cells’ chemoresistance is not surprising since they express high levels of multi-drug resistance protein 1 (MDR1) [aka. ATP-binding cassette subfamily B member 1 (ABCB1)], which confers upon the cells the ability to pump out certain toxins and drugs including chemotherapeutics [15, 47]. Therefore, it is possible that the chemotherapeutic agent(s) administered before liver resection for CRLM (i.e., FOLFOX in this study) may be actively expelled by MAIT cells. From a therapeutic standpoint, our findings suggest that MAIT cell stimuli, including select cytokines and MR1-restricted TCR agonists, can be potentially combined with chemotherapy to form novel and more efficacious treatments for CRLM and similar malignancies.
In their study, Sundström et al. found tumor-derived MAIT cells to be only slightly impaired such that ~50% of the cells were still able to produce IFN-γ in response to a combination of PMA and ionomycin [38], which activates the cells in a TCR-independent fashion only. In our study involving multiple TCR-dependent and -independent stimuli, tumor-infiltrating MAIT cells were almost completely dysfunctional. This discrepancy cannot be attributed to cellular stimulation protocol differences because we also used PMA plus ionomycin in pilot experiments to restore IFN-γ production by MAIT cells, albeit to no avail (data not shown). Instead, we propose that distinct properties of the tumor microenvironments in the two studies (i.e., primary tumors in the gut versus metastatic tumors in the liver) and/or intrinsically unique features of gut- and liver-resident MAIT cells may account for the above-noted difference.
An intriguing side observation in the course of our study was substantial GZM B expression by hepatic MAIT cells (Supplementary Figure 2), which is unlike resting, blood-derived human MAIT cells that reportedly express no GZM B [33]. This is consistent with a partially activated phenotype in hepatic MAIT cells’ steady state due, perhaps, to their constant exposure to gut microbes through the portal venous system [48].
Once/if restored, IFN-γ production by MAIT cells should activate NK and T cells as well as macrophages among other cell types, thus indirectly contributing to antitumor immunity. Furthermore, since MAIT cells are armed with a cytolytic arsenal of their own [33] and also express NKG2D [15] that is known to facilitate anticancer immune surveillance [25], it will be crucial to explore if MAIT cells can directly sense tumor cells or antigens.
MAIT cells may represent attractive therapeutic targets in CRC because: (i) they home to mucosal layers (including the primary site of CRC); (ii) they circulate with high frequencies in blood and are highly enriched in the liver (a common site of CRC metastasis); (iii) MAIT cells are among very few T cell types that launch rapid responses and can, as such, be viewed as ‘emergency responders’ not only to infection but also to other stimuli (natural or synthetic); (iv) MAIT cell agonists and antagonists have been recently described [19, 49] and the possibility of ex vivo MAIT cell expansion for therapeutic purposes is not far-fetched [50]; (v) MAIT cells are restricted by the monomorphic antigen-presenting molecule MR1 [51]. As such, MR1-restricted agonists should work beyond the HLA restriction barrier to target MAIT cells in genetically diverse human populations. It remains to be elucidated whether systemic administration or local, tumor-targeted delivery of MR1-restricted antigens alone or in combination with other immuno- or chemotherapeutic agents will overcome MAIT cell unresponsiveness in CRC. Future investigations will address the clinical outcome(s) of novel MAIT cell-based interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was funded by a Canadian Institutes of Health Research (CIHR) operating Grant (MOP-130465) to S.M. Mansour Haeryfar and by a Dean’s Research Initiative Award from Schulich School of Medicine and Dentistry, Western University, to Roberto Hernandez-Alejandro and S.M. Mansour Haeryfar. Khashayarsha Khazaie is supported by grant R01CA160436 from NIH, and Christopher R. Shaler is a CIHR postdoctoral fellowship recipient. We thank members of the Haeryfar laboratory for helpful discussions, Delfina Mazzuca for production and purification of staphylococcal enterotoxin B, and Katie Bain for technical assistance with preparation of Klebsiella lysate.
Abbreviations
- ABCB1
ATP-binding cassette subfamily B member 1
- ASA
American Society of Anesthesiologists
- CRC
Colorectal carcinoma
- CRLM
Colorectal liver metastasis
- ECOG
Eastern cooperative oncology group
- FOLFOX
Leucovorin calcium (folinic acid)/5-fluorouracil/oxaliplatin
- 6-FP
6-formylpterin
- GZM B
Granzyme B
- HMNCs
Hepatic [non-parenchymal] mononuclear cells
- MAIT
Mucosa-associated invariant T [cell]
- MDR1
Multi-drug resistance protein 1
- MNCs
[non-parenchymal] mononuclear cells
- MR1
MHC-related protein 1
- NKG2D
Natural-killer group 2, member D
- NKT
Natural killer T [cell]
- 5-OP-RU
5-(2-oxopropylideneamino)-6-D-ribitylaminouracil
- rIL
Recombinant [human] interleukin
- SEB
Staphylococcal enterotoxin B
- TH1
T helper 1
- TH17
T helper 17
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Christopher R. Shaler, Mauro E. Tun-Abraham contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour AL, Stein KD, Sharpe KB, Brooks DD, Cowens-Alvarado RL. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65(6):428–455. doi: 10.3322/caac.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17(36):4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Castanon M, Er TK, Bujanda L, Herreros-Villanueva M. Immunotherapy in colorectal cancer: what have we learned so far? Clin Chim Acta. 2016;460:78–87. doi: 10.1016/j.cca.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79(12):2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ. Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol. 2005;42(4):541–546. doi: 10.1016/j.molimm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11(20):7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 9.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13(5):1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 10.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 13.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howson LJ, Salio M, Cerundolo V. MR1-restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front Immunol. 2015;6:303. doi: 10.3389/fimmu.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 16.Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, Lee KH, Gehring AJ, De Libero G, Bertoletti A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 20.Meermeier EW, Laugel BF, Sewell AK, Corbett AJ, Rossjohn J, McCluskey J, Harriff MJ, Franks T, Gold MC, Lewinsohn DM. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat Commun. 2016;7:12506. doi: 10.1038/ncomms12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C, Lantz O. Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol. 2013;191(12):6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci USA. 2009;106(20):8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, Willberg CB. CD161++CD8+T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, Szekeres-Bartho J, Szereday L, Illes Z. Invariant Vα7.2-Jα33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008;20(12):1517–1525. doi: 10.1093/intimm/dxn111. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Soto A, Huergo-Zapico L, Acebes-Huerta A, Villa-Alvarez M, Gonzalez S. NKG2D signaling in cancer immunosurveillance. Int J Cancer. 2015;136(8):1741–1750. doi: 10.1002/ijc.28775. [DOI] [PubMed] [Google Scholar]
- 26.McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res. 2009;15(22):6993–7002. doi: 10.1158/1078-0432.CCR-09-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 29.Chau TA, McCully ML, Brintnell W, An G, Kasper KJ, Vines ED, Kubes P, Haeryfar SM, McCormick JK, Cairns E, Heinrichs DE, Madrenas J. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat Med. 2009;15(6):641–648. doi: 10.1038/nm.1965. [DOI] [PubMed] [Google Scholar]
- 30.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, Kostenko L, Meehan B, Kedzierska K, Liu L, Fairlie DP, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J, Kjer-Nielsen L. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van Sinderen D, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 32.Vayrynen JP, Kantola T, Vayrynen SA, Klintrup K, Bloigu R, Karhu T, Makela J, Herzig KH, Karttunen TJ, Tuomisto A, Makinen MJ. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer. 2016;139(1):112–121. doi: 10.1002/ijc.30040. [DOI] [PubMed] [Google Scholar]
- 33.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8(2):429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaler CR, Choi J, Rudak PT, Memarnejadian A, Szabo PA, Tun-Abraham ME, Rossjohn J, Corbett AJ, McCluskey J, McCormick JK, Lantz O, Hernandez-Alejandro R, Haeryfar SMM. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol. 2017;15(6):e2001930. doi: 10.1371/journal.pbio.2001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 36.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makrigiannis AP, Musgrave BL, Haeryfar SM, Hoskin DW. Interleukin-12 can replace CD28-dependent T-cell costimulation during nonspecific cytotoxic T lymphocyte induction by anti-CD3 antibody. J Leukoc Biol. 2001;69(1):113–122. [PubMed] [Google Scholar]
- 38.Sundstrom P, Ahlmanner F, Akeus P, Sundquist M, Alsen S, Yrlid U, Borjesson L, Sjoling A, Gustavsson B, Wong SB, Quiding-Jarbrink M. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-γ. J Immunol. 2015;195(7):3472–3481. doi: 10.4049/jimmunol.1500258. [DOI] [PubMed] [Google Scholar]
- 39.Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, Li M, Ni J, Li C, Wang L, Jiang Y. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. 2016;6:20358. doi: 10.1038/srep20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25(6):439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Tasaki K, Yoshida Y, Maeda T, Miyauchi M, Kawamura K, Takenaga K, Yamamoto H, Kouzu T, Asano T, Ochiai T, Sakiyama S, Tagawa M. Protective immunity is induced in murine colon carcinoma cells by the expression of interleukin-12 or interleukin-18, which activate type 1 helper T cells. Cancer Gene Ther. 2000;7(2):247–254. doi: 10.1038/sj.cgt.7700094. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, Broliden K, Sandberg JK, Tjernlund A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10(1):35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Simone V, Pallone F, Monteleone G, Stolfi C. Role of TH17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2(12):e26617. doi: 10.4161/onci.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Z, Qu Y, Leng Y, Sun W, Ma S, Wei J, Hu J, Zhang X. Human colon carcinogenesis is associated with increased interleukin-17-driven inflammatory responses. Drug Des Dev Ther. 2015;9:1679–1689. doi: 10.2147/DDDT.S79431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, Iyadorai T, Malik AA, Roslani AC, Vadivelu JS, Van Meerbeke S, Huso DL, Pardoll DM, Sears CL. Redundant innate and adaptive sources of IL17 production drive colon tumorigenesis. Cancer Res. 2016;76(8):2115–2124. doi: 10.1158/0008-5472.CAN-15-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu X, Shao X, Wu D, Ye J, Zhang T, Wang X, Qiu F, Yan J, Huang J. Tumor-infiltrating CD39+γδ Tregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. 2017;6(2):e1277305. doi: 10.1080/2162402X.2016.1277305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novak J, Dobrovolny J, Brozova J, Novakova L, Kozak T. Recovery of mucosal-associated invariant T cells after myeloablative chemotherapy and autologous peripheral blood stem cell transplantation. Clin Exp Med. 2015;16(4):529–537. doi: 10.1007/s10238-015-0384-z. [DOI] [PubMed] [Google Scholar]
- 48.Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunol. 2016;5(8):e98. doi: 10.1038/cti.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, Blanot D, Herve M, Schmidt F, Mengin-Lecreulx D, Lantz O. In vitro and in vivo analysis of the gram-negative bacteria-derived riboflavin precursor derivatives activating mouse MAIT cells. J Immunol. 2015;194(10):4641–4649. doi: 10.4049/jimmunol.1403224. [DOI] [PubMed] [Google Scholar]
- 50.Guo T, Chamoto K, Hirano N. Adoptive T cell therapy targeting CD1 and MR1. Front Immunol. 2015;6:247. doi: 10.3389/fimmu.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.