Abstract
Objective
The objective of this study is to determine the validity and reliability of the red filter meibography by smartphone compared with infrared in assessing meibomian gland drop-out.
Methods and analysis
An analytical cross-sectional study was done with a total of 35 subjects (68 eyes) with suspected MGD based on symptoms and lid morphological abnormalities. Meibomian glands were photographed using two smartphones (Samsung S9 and iPhone XR) on a slit-lamp with added red filter. Images were assessed subjectively using meiboscore by the two raters and drop-out percentages were assessed by ImageJ.
Results
There was no agreement in meiboscore and a minimal level of agreement in drop-out percentages between red filter meibography and infrared. Inter-rater reliability showed no agreement between two raters. Intra-rater reliability demonstrated weak agreement in rater 1 and no agreement in rater 2.
Conclusion
Validity of the red filter meibography technique by smartphones is not yet satisfactory in evaluating drop-out. Further improvement on qualities of images must be done and research on subjective assessment was deemed necessary due to poor results of intrarater and inter-rater reliability.
Keywords: Imaging, Diagnostic tests/Investigation, Ocular surface
WHAT IS ALREADY KNOWN ON THIS TOPIC
Meibography is a technique to evaluate meibomian gland in vivo. Currently, it still uses infrared as the main modalities to visualise meibomian glands with excellent results. However, as MGD cases were still on the rises, we needed to develop inexpensive, simpler and readily available techniques using smartphone camera rapid improvement to perform meibography.
WHAT THIS STUDY ADDS
Although the validity and reliability in grading drop-outs are not comparable to infrared method, the meibomian gland can still be visualised using a red filter and captured by smartphones. This is promising and valuable, especially in rural and remote areas where infrared technology is not available.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Many can improve the affordable red filter technique to be comparable to the conventional infrared methods. In the future, artificial intelligence can be developed to analyse the red filter-meibography results.
Introduction
Meibomian gland dysfunction (MGD) is a chronic and diffuse disorder of the meibomian glands, characterised by obstruction of the terminal ducts and/or qualitative and quantitative changes in glandular secretion.1 2 MGD is often found in clinical practice and is a main cause of evaporative dry eye disease in the elderly.3–5 Morphological changes called meibomian gland drop-out (MG drop-out) can be seen in vivo with a technique called meibography.6 Meibography can help evaluate morphological changes, assess the degree of MG drop-out, guide treatment decisions and monitor therapeutic effects as well as patient education tools to improve adherence.
Most of the meibography modalities to date still use infrared techniques and the assessment of MG drop-out from meibography often uses subjective assessment by examiners with four scales called meiboscore although there is no agreement. However, infrared meibography is costly and not readily available in practices hence meibography is not routinely performed. Lee et al hypothesised that the visibility of the meibomian glands on non-contact infrared meibography is due to the loss of the shadow of the conjunctival blood vessels that are above the gland so that alternative methods that can remove the shadow of the conjunctival blood vessels can also increase the visibility of the meibomian glands without the infrared system.7 This study reports a moderate agreement between the meiboscore values of the images produced from the slit lamp with the addition of a red filter to infrared meibography.
The use of smartphone cameras to take pictures of the meibomian glands from a slit lamp with a red filter is expected to be a cheaper, non-invasive alternative to meibography and can be performed by most ophthalmologists. However, currently, there is no standardisation of image capture and reports on the validity of this technique. The purpose of this study was to determine the validity and reliability of the red filter meibography technique by smartphone compared with infrared meibography in assessing MG drop-out.
Materials and methods
This study is an analytical cross-sectional study to evaluate the validity and reliability of meibography examination using a red filter by a smartphone compared with infrared meibography. We investigated a total of 68 eyes of 35 patients aged more than 18 years and with suspected MGD based on history and eyelid morphology who came to Department of Ophthalmology, Cipto Mangunkusumo Hospital-Kirana, which is located in Jakarta in March 2021. Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research. We exclude patients with eyelid abnormalities that made it difficult to be everted, had a history of eyelid surgery due to trauma or tumour, had a history of eyelid trauma, showed severe conjunctival inflammation, a history of ocular surgery <4 weeks and patients with severe systemic disease.
All patients underwent slit-lamp examination of the anterior segment. Images of meibomian gland were then captured using the same slit-lamp biomiscroscopy, specifically the Haag-Streit BP 900-LED, set in a well-illuminated room with no windows. The slit-lamp was configured with background illumination light and positioned at a 30°–45° angle with 10 times magnification. A custom red filter was placed in front of the light source in the slit-lamp’s illumination system. Upper and lower eyelid images were acquired after everting the eyelid and photographs were taken by two types of smartphones (Samsung S9 and iPhone XR) consecutively. Patients also underwent infrared meibography using OCULUS Keratograph 5M. Photos from smartphones will be converted to black and white using filters settings embedded in camera application of the smartphone.
The resulting set of images was then masked by a research assistant and assessed by two raters (RLDN and SW) for a subjective assessment using the meiboscore. The assessment of randomised images was done in a dark room with a 22-inch LED monitor. Both raters will assess the same set of images 2 weeks apart after the first assessment for evaluation of intra-rater reliability. The highest meiboscore of the two assessments by rater 1 will be used in the statistical analysis. Then, for the evaluation of inter-rater reliability, a comparison of the meiboscore values between the two raters was analysed. Each set of images was digitally processed using ImageJ software by the researcher (GHA) to assess the drop-out percentage. The researcher and the two raters underwent training on subjective image assessment and computer application before conducting the study.
The meiboscore agreement analysis uses Cohen’s kappa (κ) while the agreement analysis of the drop-out percentage by ImageJ uses the intraclass correlation coefficient (ICC) in the form of a two-way mixed-effects absolute agreement. The guidelines used for interpretation of κ values were as follows: <0.20 indicated no agreement; 0.21–0.39, minimal; 0.40–0.59, weak; 0.60–0.79, moderate; 0.80–0.90, strong and >0.91, almost perfect as suggested previously.8 While guidelines used for interpretation of ICC values were as follows: <0.50 indicated poor value; 0.50–0.75, moderate; 0.75–0.90, good and >0.90, excellent as suggested previously.9
Results
A total of 68 eyes of 35 patients were included in this study. Demographic and general characteristics of all patients are summarised in table 1. Subjects ranged from 25 to 83 years with a mean of 60 years.
Table 1.
Demographic and clinical characteristics of subjects
| Characteristics | No. of patients (%) |
| Age in years (mean±SD) | 60.01±13.5 |
| 18–40 years | 6/68 (8.8) |
| 41–60 years | 24/68 (35.3) |
| >60 years | 38/68 (55.9) |
| Gender | |
| Male | 27/68 (39.7) |
| Female | 41/68 (60.3) |
| Predisposing factor | |
| Age >50 years | 50/68 (73.5) |
| Menopause (n=41) | 29/41 (70.7) |
| Antihistamine | 8/68 (11.8) |
| Sjogren syndrome | 8/68 (11.8) |
| Antidepressant | 4/68 (5.9) |
| Postmenopausal hormone (n=41) | 2/41 (4.9) |
| Contact lens use | 2/68 (2.9) |
| Isotretinoin | 2/68 (2.9) |
| Lid margin morphology | |
| Lid plugging | 68/68 (100) |
| Lid irregularities | 45/68 (66.2) |
| Lid telangiectasia | 36/68 (52.9) |
| Lid thickness | 9/68 (13.2) |
| Expressibility | |
| 0 | 3/68 (4.4) |
| 1 | 27/68 (39.7) |
| 2 | 34/68 (50) |
| 3 | 4/68 (5.9) |
| Stage MGD | |
| 1 | 1/68 (1.5) |
| 2 | 33/68 (48.5) |
| 3 | 32/68 (47.1) |
| 4 | 2/68 (2.9) |
| NIBUT (in seconds) | 5.64 (4.41–8.18)* |
| OSDI score | 38±22.2† |
| OSDI stage | |
| Normal | 16/68 (23.5) |
| Mild | 6/68 (8.8) |
| Moderate | 6/68 (8.8) |
| Severe | 40/68 (58.8) |
*Median with IQR.
†Mean±SD.
MGD, meibomian gland dysfunction; NIBUT, non-invasive break-up time; OSDI, Ocular Surface Disease Index.
The clinical characteristics of subjects are shown in table 1. Most of the subjects suffered from MGD in stages 2 and 3 based on clinical symptoms, clinical signs and ocular surface staining. The results of the Ocular Surface Disease Index questionnaire examination showed that most of the research subjects suffered from dry eyes with severe symptoms.
The kappa value appears to be less than 0.2, which means that there is no agreement for meiboscore between the two techniques (table 2). The same thing was found in the drop-out percentage analysis where there was a low agreement between the meibography with both types of smartphones to infrared meibography (table 3).
Table 2.
Meiboscore agreement between red filter meibography and infrared meibography
| Meiboscore | Kappa value (95% CI) | P value | |
| Smartphone 1 | Lower lid | −0.036 (−0.089 to 0.027) | 0.338 |
| (Samsung S9) | Upper lid | 0.094 (0.008 to 0.186) | 0.038 |
| Lower and upper lid | −0.022 (−0.060 to 0.023) | 0.468 | |
| Smartphone 2 | Lower lid | 0.146 (−0.012 to 0.322) | 0.054 |
| (iPhone XR) | Upper lid | 0.090 (0.003 to 0.196) | 0.027 |
| Lower and upper lid | 0.041 (−0.069 to 0.153) | 0.422 | |
Table 3.
Drop-out percentage using ImageJ agreement between red filter meibography and infrared meibography
| Drop-out percentage | ICC (95% CI) | P value | |
| Smartphone 1 | Lower lid | 0.231 (−0.088 to 0.511) | 0.001 |
| (Samsung S9) | Upper lid | 0.057 (−0.055 to 0.203) | 0.049 |
| Mean lower and upper lid | 0.120 (−0.069 to 0.369) | 0.001 | |
| Smartphone 2 | Lower lid | 0.215 (−0.074 to 0.473) | 0.001 |
| (iPhone XR) | Upper lid | 0.046 (−0.054 to 0.176) | 0.116 |
| Mean lower and upper lid | 0.096 (−0.069 to 0.301) | 0.006 | |
ICC, intraclass correlation coefficient.
There is a positive correlation between meiboscore and drop-out percentage using ImageJ (table 4), which means the increase of meiboscore is directly proportional to the increase in the drop-out percentage even though the correlation value is low (Spearman correlation value is less than 0.5). However, meiboscore correlation value with drop-out percentage using ImageJ of smartphone 2 (iPhone XR) images appears slightly higher than images produced by smartphone 1 (Samsung S9).
Table 4.
Correlation between meiboscore and drop-out percentage using ImageJ in red filter meibography
| Spearman correlation coefficient (ρ) | P value | ||
| Smartphone 1 | Lower lid | 0.230 | 0.06 |
| (Samsung S9) | Upper lid | 0.269 | 0.027 |
| Lower and upper lid | 0.331 | 0.006 | |
| Smartphone 2 | Lower lid | 0.492 | 0.001 |
| (iPhone XR) | Upper lid | 0.354 | 0.003 |
| Lower and upper lid | 0.491 | 0.001 |
The results of the inter-rater reliability analysis showed minimal to weak agreement between the two raters in assessing images produced by standard infrared meibography techniques (online supplemental table 1). There is also no agreement between the two raters in assessing the images produced by smartphone 1 (Samsung S9) while there is only minimal agreement between two raters in assessing the images produced by smartphone 2 (iPhone XR).
bmjophth-2023-001266supp001.pdf (60KB, pdf)
Intra-rater reliability analysis of the two raters showed a weak to moderate agreement value in assessing the images produced by the infrared meibography. Rater 1 shows a weak agreement in assessing the image produced by smartphone 1 (Samsung S9) and rater 2 shows no agreement between the two assessments. Then, in assessing the image produced by smartphone 2 (iPhone XR), rater 1 showed weak agreement in assessing the upper eyelid but obtained minimal agreement in assessing the lower lid. Rater 2 also showed a minimal agreement in assessing the upper and lower lids of the photos produced by smartphone 2 (iPhone XR).
Discussion
This study showed that there is no agreement in meiboscore and drop-out percentage between red filter meibography technique by the Samsung S9 and iPhone XR smartphones compared with infrared. However, there is a positive correlation between the meiboscore and the drop-out percentage using ImageJ on the red filter meibography technique by both types of smartphones, although the correlation value is weak. Furthermore, inter-rater and intrarater reliability for the meiboscore assessment of the red filter meibography technique by both types of smartphones showed no agreement to weak agreement between the two raters.
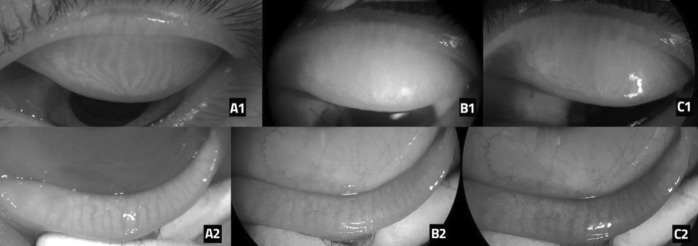
None to low agreement found in this study indicates that the red filter meibography technique is not comparable to the infrared method in displaying the Meibomian glands (figure 1). The images produced by red filter meibography using smartphones have shortcomings, including the presence of very bright light reflections that make the intact Meibomian glands look like areas with drop-outs (figure 1A1–C1). In addition, the visualisation of the meibomian glands in the upper lid is also more difficult than the lower lid. Meibomian gland contrast in the image produced by the red filter meibography also appears so low that it is indistinguishable from the surrounding area (figure 1A2–C2). Such difficulties were also found in study by Lee et al although they found moderate agreement in meiboscore between red filter and infrared meibography.7
Figure 1.
Results of meibography photos taken on research subjects. The top row shows the upper lid and the bottom row shows the bottom lid. (A1 and A2) Infrared meibography, (B1 and B2): Red filter meibography by smartphone 1 (Samsung S9), (C1 and C2) Red filter meibography by smartphone 2 (iPhone XR).
We encountered several limitations during implementation that may have caused suboptimal images. This includes the lack of characterisation of red light passing through the red filter due to the unavailability of spectroscopic equipment owned by the institution or nearby optical laboratories. We conclude that the red-filtered light source does not adequately reach the spectrum for visualising the meibomian glands. Research by Peral et al found that there are three optimal wavelengths for observation of Meibomian glands, namely at 600 nm (visible red light wavelength), 725 nm and 950 nm (infrared wavelength) and concluded that meibomian gland contrast decreases rapidly when the wavelength is less than 600 nm.10 The red filter used in this study may not fully absorb light other than red and infrared wavelengths, thus unabsorbed light with wavelengths lower than the red light spectrum disturbs the visualisation and contrast of meibomian glands.
Second, the smartphone camera sensor is also not optimal in capturing invisible infrared light because it is embedded with an anti-infrared optical filter. Smartphone cameras have a complementary metal-oxide-semiconductor detector that is sensitive to visible light and near-infrared (NIR) light, but the camera is equipped with a filter that blocks NIR wavelengths for ordinary photography because it will affect image quality.11
Lastly, settings contrast, brightness, colour composition on smartphone cameras are done automatically by software so that the visualisation of the meibomian glands is not optimal. Infrared meibography is also difficult to assess if the resulting image is low in contrast, the illumination is not uniform and the gland area is out of focus.12
However, there are several things that can be done to overcome the lack of quality of the photos mentioned above for further research. First, using a tool capable of performing infrared imaging or often referred to as NIR optical imaging. Several studies on smartphone-based medical imaging use NIR imaging techniques. Meibography research using alternative sources of infrared light, among others, was carried out by Osae et al with a simple infrared video camera usually used for closed circuit television and by Wang et al who also added an infrared camera module to Android-based smartphones.13 14 Research by Osae et al stated that the use of a homemade meibographer was able to depict meibomian glands and could be used in developing countries with limited access to complex and expensive imaging systems.13 Research by Wang et al uses a 2-megapixel infrared camera module which has a lamp array with a light wavelength of 850 nm and is connected to an Android-based smartphone.14 The smaller size is said to be able to visualise the meibomian glands quickly and more easily.14 However, these two studies did not compare the results of the images with standard meibography.
We found that inter-reliability scores showed low agreement in assessing images produced by infrared meibography. This may be due to the difficulty of using meiboscore in MG drop-out scoring. When compared with several meibography with different percentages of atrophy, the images near the grading transition limits (0%, 33% and 66%) are very similar and difficult to classify.15 Meiboscore method was interesting because of its simplicity but it is the biggest drawback because it fails to take into account changes in the meibomian glands before drop-out occurs.16 The use of meiboscore for subjective assessment of MG drop-out is difficult for untrained raters and discussion of raters is needed to reach a good agreement.
The new meibography method with smartphones still has hope for improvement and room for innovation. The use of the red filter in this study was able to display the meibomian glands in a relatively easy and inexpensive way, although the visualisation was not optimal for grading drop-outs both subjectively and with the help of computer applications. Future research can use more optimal methods in visualising the meibomian glands, for example, using additional cameras and infrared sensors on smartphones. The image quality is expected to improve with this method so that later the image can be segmented automatically by a computer, as in the research by Celik et al and Koh et al.17 18 In the near future, processing and analysis of meibography images can also be carried out by artificial intelligence systems which offer various advantages such as reduced time for analysis, increased diagnostic efficiency and help to overcome intrarater and inter-rater variability of subjective assessment by clinicians.12 15 19 Then, the use of smartphones as a diagnostic tool for point-of-care test is also very promising and important to be studied further because it is compact, portable and relatively inexpensive.20 Despite the many weaknesses in this study, it is hoped to provide the knowledge that there is an alternative to meibomian gland visualisation if infrared meibography is not available and forms the basis for further studies of MGD.
Acknowledgments
The authors would like to thank the staffs of Ciptomangunkusumo Hospital-Kirana for their suppport in data acquisition.
Footnotes
Contributors: All the authors participated in the planning, research and writing of the manuscript. GHA: guarantor, planning, conduct, acquisition of data, analysis and interpretation of data, and accepts full responsibility for the work. RLDN: planning, analysis of data for the work, revising the work for important intellectual content, final approval of the version to be published. SW: planning, analysis of data for the work, revising the work for important intellectual content. RS: planning, revising the work for important intellectual content, final approval of the version to be published. PY: planning, revising the work for important intellectual content. AK: planning, revising the work for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Faculty of Medicine, University of Indonesia—Cipto Mangunkusumo Hospital KET-1377/UN2.F1/ETIK/PPM.00.02/2020. Participants gave informed consent to participate in the study before taking part.
References
- 1. Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005. 10.1167/iovs.10-6997e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols KK. The international workshop on meibomian gland dysfunction: introduction. Invest Ophthalmol Vis Sci 2011;52:1917–21. 10.1167/iovs.10-6997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf 2003;1:107–26. 10.1016/s1542-0124(12)70139-8 [DOI] [PubMed] [Google Scholar]
- 4. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31:472–8. 10.1097/ICO.0b013e318225415a [DOI] [PubMed] [Google Scholar]
- 5. Tong L, Chaurasia SS, Mehta JS, et al. Screening for meibomian gland disease: its relation to dry eye subtypes and symptoms in a tertiary referral clinic in Singapore. Invest Ophthalmol Vis Sci 2010;51:3449–54. 10.1167/iovs.09-4445 [DOI] [PubMed] [Google Scholar]
- 6. Pult H, Nichols JJ. A review of meibography. Optom Vis Sci 2012;89:E760–9. 10.1097/OPX.0b013e3182512ac1 [DOI] [PubMed] [Google Scholar]
- 7. Lee S-M, Park I, Goo YH, et al. Validation of alternative methods for detecting meibomian gland dropout without an infrared light system: red filter for simple and effective meibography. Cornea 2019;38:574–80. 10.1097/ICO.0000000000001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 9. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peral A, Alonso J, Gomez-Pedrero JA. Effect of illuminating wavelength on the contrast of meibography images. OSA Continuum 2018;1:1041. 10.1364/OSAC.1.001041 [DOI] [Google Scholar]
- 11. Ghassemi P, Wang B, Wang J, et al. Evaluation of mobile phone performance for near-infrared fluorescence imaging. IEEE Trans Biomed Eng 2017;64:1650–3. 10.1109/TBME.2016.2601014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Setu MAK, Horstmann J, Schmidt S, et al. Deep learning-based automatic meibomian gland segmentation and morphology assessment in infrared meibography. Sci Rep 2021;11:7649. 10.1038/s41598-021-87314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osae EA, Ablorddepey RK, Horstmann J, et al. Assessment of meibomian glands using a custom-made meibographer in dry eye patients in Ghana. BMC Ophthalmol 2018;18:201. 10.1186/s12886-018-0869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang DN, Patel Y, Luong M. Portable meibography technology using a smartphone device. Can J Ophthalmol 2020;55:e211–3. 10.1016/j.jcjo.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Yeh TN, Chakraborty R, et al. A deep learning approach for meibomian gland atrophy evaluation in meibography images. Transl Vis Sci Technol 2019;8:37. 10.1167/tvst.8.6.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wise RJ, Sobel RK, Allen RC. Meibography: a review of techniques and technologies. Saudi J Ophthalmol 2012;26:349–56. 10.1016/j.sjopt.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Celik T, Lee HK, Petznick A, et al. Bioimage informatics approach to automated meibomian gland analysis in infrared images of meibography. J Optom 2013;6:194–204. 10.1016/j.optom.2013.09.001 [DOI] [Google Scholar]
- 18. Koh YW, Celik T, Lee HK, et al. Detection of meibomian glands and classification of meibography images. J Biomed Opt 2012;17:086008. 10.1117/1.JBO.17.8.086008 [DOI] [PubMed] [Google Scholar]
- 19. Dai Q, Liu X, Lin X, et al. A novel meibomian gland morphology analytic system based on a convolutional neural network. IEEE Access 2021;9:23083–94. 10.1109/ACCESS.2021.3056234 [DOI] [Google Scholar]
- 20. Huang W, Luo S, Yang D, et al. Applications of smartphone-based near-infrared (NIR) imaging, measurement, and spectroscopy technologies to point-of-care (POC) diagnostics. J Zhejiang Univ Sci B 2021;22:171–89. 10.1631/jzus.B2000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2023-001266supp001.pdf (60KB, pdf)
Data Availability Statement
No data are available.