Abstract
In order to grow within an immunocompetent host, tumour cells have evolved various strategies to cope with the host’s immune system. These strategies include the downregulation of surface molecules and the secretion of immunosuppressive factors like IL-10 and PGE2 that impair the maturation of immune effector cells, among other mechanisms. Recently, tumour exosomes (TEX) have also been implicated in tumour-induced immune suppression as it has been shown that TEX can induce apoptosis in T lymphocytes. In this study, we extend our knowledge about immunosuppressive features of these microvesicles in that we show that TEX efficiently bind and sequester tumour-reactive antibodies and dramatically reduce their binding to tumour cells. Moreover, we demonstrate that this antibody sequestration reduces the antibody-dependent cytotoxicity by immune effector cells, which is among the most important anti-tumour reactions of the immune system and a significant activity of therapeutic antibodies. Taken together, these data point to the fact that tumour-derived exosomes interfere with the tumour-specific function of immune cells and constitute an additional mechanism how tumours escape from immune surveillance.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-0979-5) contains supplementary material, which is available to authorized users.
Keywords: Exosomes, ADCC, Trastuzumab, Immune escape
Introduction
In order to grow and progress within a functional immune system, tumours have evolved several strategies to evade and counteract immune surveillance. For example, cancer cells downregulate MHC molecules and co-stimulatory surface molecules and release immunosuppressive factors like IL-10, TGF-β and prostaglandin E2 that impair anti-tumour function of immune cells ([1] for review). Also, tumour-infiltrating cells display a reduced immune function [2] or even tolerance [3]. In consequence, cancer patients can only very rarely mount effective anti-tumour responses, despite the presence of immune effector cells in significant numbers and tumour-reactive antibodies within the tumour vicinity. More general, the tumour educates immune cells in its vicinity thereby creating a microenvironment with immunosuppressive properties.
Recently, tumour-derived exosomes (TEX) became a focus of particular interest to tumour immunologists when it has been shown that tumours release TEX constitutively and that TEX skew the function of the cellular immune system. For instance, they impair lymphocyte responses to IL-2 [4], inhibit monocyte differentiation into dendritic cells [5], express the Fas ligand, induce apoptosis in activated T lymphocytes [6, 7] and downmodulate the cytolytic activity of NK-cells [8, 9]. Also, it has been shown that tumour exosomes contain angiogenesis-promoting factors and functional genetic information [10].
The precise immunological function of TEX, however, is still not fully understood. Intriguingly, TEX carry different tumour antigens known to provoke cellular and humoral immune responses in cancer-bearing patients such as EpCAM [11, 12], Melan-A/MART-1 [13, 14] and the epidermal growth factor receptor 2 (HER2) [15, 16], and the sera from cancer patients often exhibit significantly higher levels of immune responses to TEX-derived proteins [17, 18]. HER2 is a tumour-associated membrane antigen with protein-tyrosine kinase activity that is over-expressed in several types of adenocarcinoma and plays a key role in tumour growth and progression [19, 20]. The ectopic over-expression on cancer cells regularly induces humoral immune responses in patients so that HER2− and EpCAM−specific antibodies are detectable in the sera of patients with HER2+ and EpCAM+ cancer [21–25]. Both antigens are targets for a number of therapeutic monoclonal antibodies and their derivatives.
Therefore, the release of immunogenic vesicles by tumour cells seems paradoxical, since tumours tend to evade the host’s immune system. Here, we provide a possible explanation for this obvious contradiction in that we show that TEX bind and sequester tumour-reactive antibodies resulting in a reduced binding of these antibodies to the tumour cells. Furthermore, sequestration of tumour-reactive antibodies by exosomes impaired antibody-dependent cellular cytotoxicity (ADCC), which is a major immune effector function towards tumour cells. In summary, we confirm here that TEX are released by tumour cells in order to manipulate the immune system.
Results
Breast cancer-derived exosomes carry the tumour antigens HER2 and EpCAM
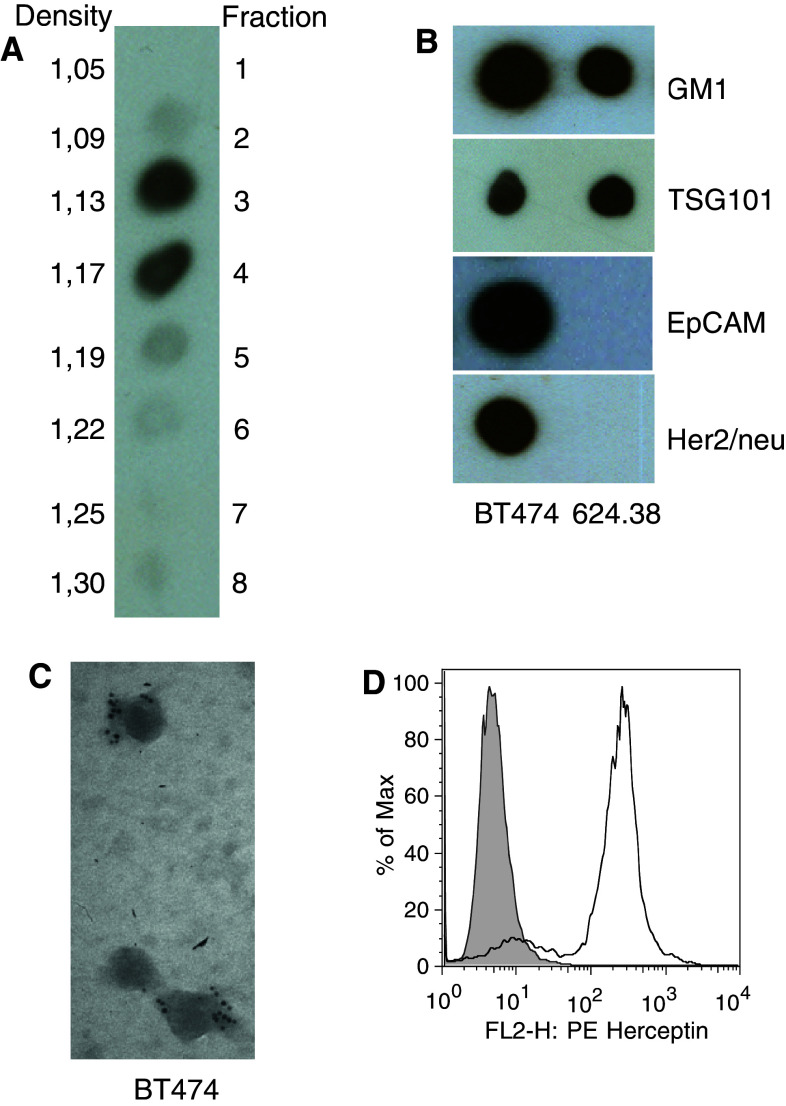
We used the tumour-associated antigens HER2 and EpCAM as model antigens in order to address the question whether tumour-derived exosomes (TEX) interact with, and sequestrate, tumour-reactive antibodies thereby hampering their interaction with tumour cells. Such a sequestration may have profound consequences for the efficacy of both natural autoantibodies and therapeutic antibodies applied for adjuvant cancer treatment. To investigate the interaction of TEX with specific antibodies, we first isolated exosomes from the conditioned cell culture supernatants of BT474 breast cancer cells and Mel624.38 melanoma cells. We pelleted the exosomes from the supernatants by differential centrifugation and further purified them by floating into a linear sucrose gradient. After fractionation of the gradient, we performed immunoblots with cholera toxin that specifically binds to the ganglioside M1 (GM1), a typical marker for exosomes [26]. GM1-positive material was detected almost exclusively in fractions 3 and 4 of the gradient, corresponding to densities of 1.13 and 1.17 g/ml, respectively, (Fig. 1a), and thus to densities typical for exosomes [27]. Next, we tested these fractions for the presence of TSG101, another typical marker for exosomes as well as for EpCAM and HER2. As expected, exosomes from both cell lines contained TSG101 but only BT474 exosomes contained also detectable amounts of EpCAM and HER2 (Fig. 1b). As a final proof that the isolated material mainly consisted of exosomes, we performed immune electron microscopy, which clearly confirmed that the particles were of the typical size of exosomes and that HER2 was present on BT474 exosomes (Fig. 1c). Also, HER2 present in exosomal preparations efficiently bound the therapeutic antibody trastuzumab (Fig. 1d) and was of full-length (185kD) and thus most probably integrated in the exosomal membrane (Fig. S1).
Fig. 1.
Tumour exosomes isolated from conditioned cell culture supernatants carry HER2 and EpCAM. a Exosomes can be fractionated by sucrose gradient centrifugation and identified with cholera toxin that specifically binds to GM1. b Material isolated from fractions 3 and 4 carry the exosome markers GM1 and TSG101 as proven by dot blots. Exosomes from the breast cancer cell line BT474 also carry the tumour antigens EpCAM and HER2 whereas Mel624.38 exosomes do not. c Immune electron microscopy revealed that HER2 is associated with particles isolated from fractions 3 and 4. d FACS analysis of BT474 exosomes coupled to latex beads showed that trastuzumab binds to TEX-associated HER2 efficiently
Tumour exosomes sequester tumour-reactive antibodies
After having shown that HER2 and EpCAM are present on BT474 exosomes, we next questioned whether TEX can sequester antibodies specific to these two antigens and thereby inhibit binding of the antibodies to tumour cells. Given the fact that tumours release TEX constitutively and TEX can readily be detected systemically, i.e. in the blood and body fluids of cancer patients [28, 29], their local concentration within the tumour might be significant. Although the intratumoural TEX concentration is still largely unknown, the sequestration of tumour-reactive antibodies by TEX may constitute an important new mechanism of tumour immune escape serving as an antibody decoy. This is an important point given that HER2 and EpCAM are targets for both autoantibodies as well as for therapeutic antibodies and their sequestration may contribute to tumour growth and therapy resistance, respectively.
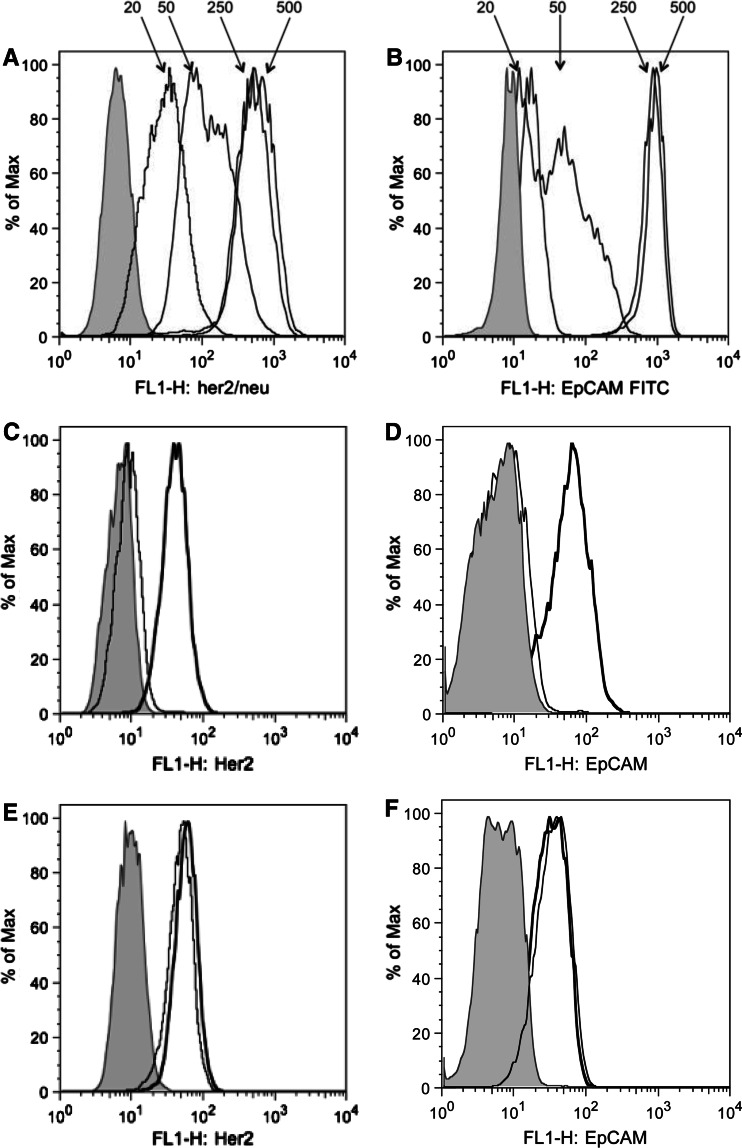
To address this question, we performed FACS-based binding assays with the HER2-specific therapeutic antibody trastuzumab (Herceptin®) and the EpCAM-specific antibody C215. In a first series of experiments, we determined the antibody concentrations that gave approximately half-maximum staining of tumour cells as measured by FACS in order to avoid that sequestration effects are masked by excess amounts of antibody and found 50 ng/ml antibody to be appropriate concentrations (Fig. 2). Next, we incubated trastuzumab and C215 at concentrations of 50 ng/ml with BT474 and MEL624.38 exosomes. We then added BT474 cells and performed staining for 30 min. As shown in Fig. 2, binding of trastuzumab and C215 to BT474 cells was clearly reduced when the antibodies had been pre-incubated with BT474 exosomes but not with MEL624.38 exosomes. In contrast, BT474 exosomes did not inhibit the binding of a CD40-specific antibody to a human B cell line (Fig. S2). Similar results were obtained with TEX from another HER2+EpCAM+ breast cancer cell line, SkBr3, and the HER2−EpCAM− melanoma cell line A375 (Fig. S3). These results demonstrate that TEX efficiently and specifically sequester tumour-reactive antibodies and reduce their binding to tumour cells.
Fig. 2.
Pre-incubation with HER2+EpCAM+ TEX prevents binding of trastuzumab and C215 to HER2+EpCAM+ cancer cells. a trastuzumab and b C215 were titrated on BT474 cells to identify the antibody concentration that gives half-maximal staining. Trastuzumab c and e or C215 d and f at concentrations of 50 ng/ml were pre-incubated with 100 μg/ml HER2+EpCAM+ BT474 exosomes (c and d) or with the same amount of HER2−EpCAM− Mel624.38 exosomes (e and f). As a positive control, cells were stained with antibodies that were not pre-incubated with TEX. It became clear that pre-incubation with BT474 exosomes but not with Mel624.38 exosomes prevented trastuzumab and C215 from binding to BT474 cells. Grey histogram unstained cells. Bold line Maximal staining of trastuzumab and C215 on BT474 cells. Thin line staining of BT474 cells with pre-incubated trastuzumab. One out of the seven independent experiments is shown
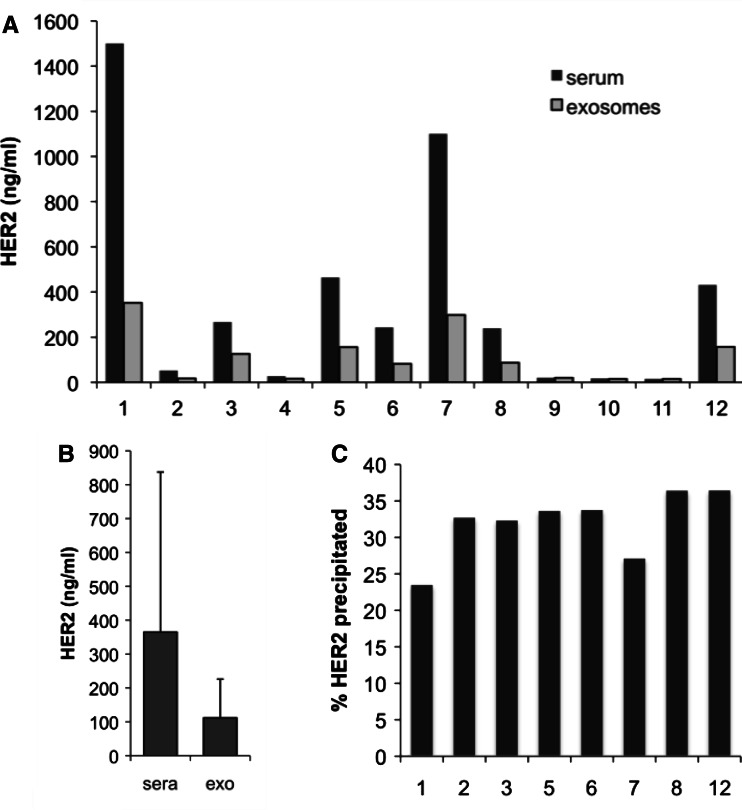
Next, we wondered whether HER2+ exosomes are also present in the sera of breast cancer patients. For this, we isolated exosomes by ultracentrifugation as described from 200 μl of sera of twelve patients. We then resuspended the exosomes in the same volume of lysis buffer and determined the HER2 concentration in an ELISA assay. As shown in Fig. 3a, HER2-containing material could be precipitated from sera applying the protocol for the isolation of exosomes. The amount of HER2 that was precipitated varied from patient to patient, ranging between 20 and 60% of the total HER2 concentration (Fig. 3b). Thus, HER2-carrying exosomes most likely contribute to the total HER2 levels in the blood of patients with HER2+ cancer.
Fig. 3.
Sera from breast cancer patients contain particle-associated HER2. a HER2 levels present in the exosome preparations were quantified with an commercial ELISA assay in comparison with whole sera. It became clear that a part of total HER2 could be isolated by ultracentrifugation applying the protocol for the isolation of exosomes. b Average HER2 levels from the 12 patients shown in A as measured in complete serum and precipitated material. c Fractions ranging from 23 to 37% of total HER2 were precipitated from the high-HER2 sera, implicating that TEX-associated HER2 contributes by roughly one-third to the total HER2 serum levels in patients. Fractions were not calculated for low-HER2 sera that were out of the linear range of the assay
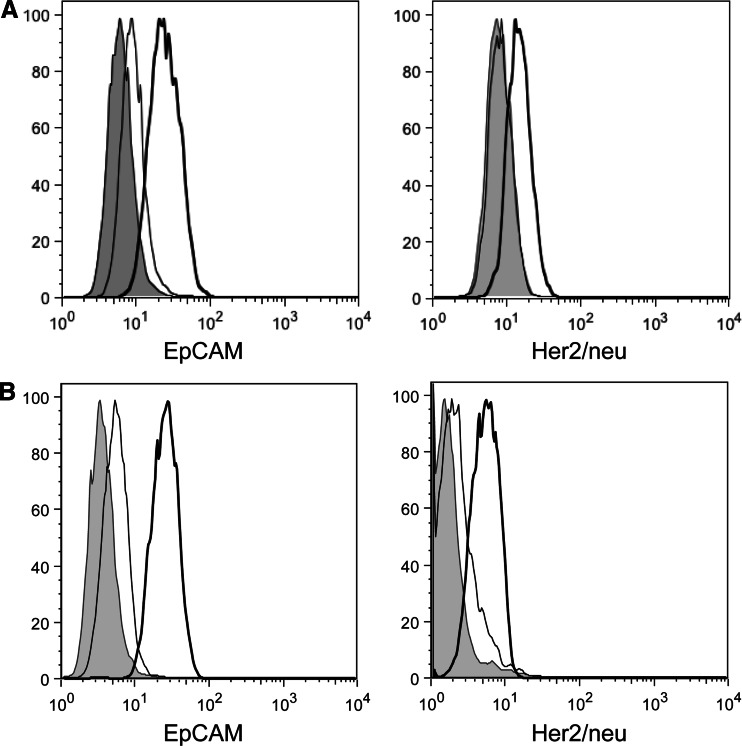
We also isolated exosomes from the malignant ascites of two patients with ovarian cancer. As with BT474 exosomes, ascites exosomes were HER2+ and EpCAM+ (Fig. S4). As shown in Fig. 4, exosomes isolated from malignant ascites sequestered the tumour-reactive antibodies trastuzumab and C215 as efficient as exosomes from BT474 cells. Importantly and in contrast to patients’ sera, malignant ascites yielded sufficient amounts of TEX to perform functional assays.
Fig. 4.
Primary tumour-derived exosomes prevent binding of tumour-reactive antibodies. a and b Exosomes were isolated from the malignant ascites of two patients with ovarian cancer. C215 (left histograms) or of trastuzumab (right histograms) at 50 ng/ml each were pre-incubated with ascites exosomes (200 μg each) and used to stain BT474 cancer cells. It became clear that exosomes from malignant ascites efficiently prevented binding of the two antibodies. Grey histogram unstained BT474 cells. Bold line Maximal staining of trastuzumab on BT474 cells. Thin line staining of BT474 cells with pre-incubated trastuzumab
Tumour exosomes inhibit ADCC
The mechanism of HER2 antibody therapy is not fully understood, but FcR+ immune effector cells are essential for the therapeutic effect [30]. Engagement of FcR+ cells and induction of ADCC are a substantial anti-tumour component of therapeutic antibodies in vivo. However, ADCC is impaired in cancer patients [31–33] and, even more interesting, sera from patients with malignant diseases have been shown to actively impair ADCC [32].
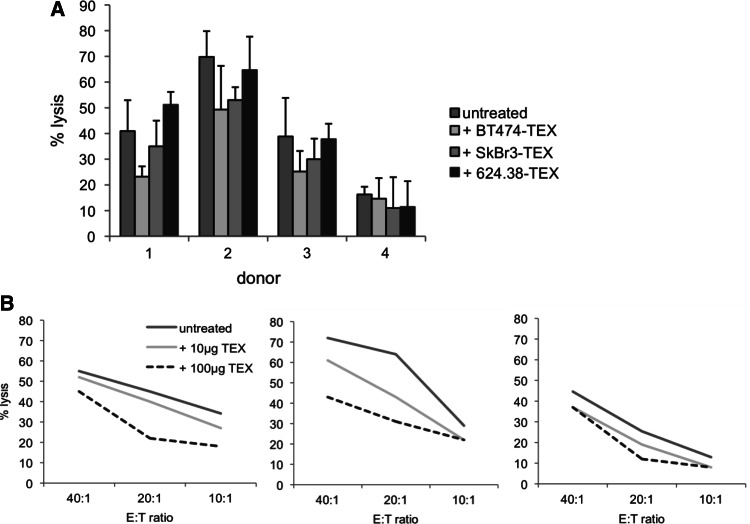
To evaluate whether antibody sequestration by TEX affects immune functions, we quantified ADCC of PBMCs against tumour cells. ADCC is a typical effector mechanism of antibodies and is considered one of the most important mechanisms of action of therapeutic antibodies. In this assay, we used calcein-AM-labelled ADCC-sensitive BT474 cells as targets and PBMCs from healthy donors as effector cells. We used trastuzumab, which is known to efficiently promote ADCC [34–36], as initiating antibody and quantified target cell lysis by measuring the amount of calcein-AM in the supernatants. Apparently as a result, we found that the pre-incubation of trastuzumab with HER2+ BT474 and SkBr3 exosomes resulted in a statistically significant lower ADCC activity of PBMCS from three out of the four different donors against BT474 tumour cells (Fig. 5). In contrast, we did not observe ADCC reduction in PBMCs upon pre-incubation with HER2– MEL624.38 exosomes. In the same set of experiment, we were not able to reproducibly induce ADCC by human PBMCs with the EpCAM-specific murine IgG2a antibody C215 (data not shown).
Fig. 5.
Tumour-derived exosomes prevent ADCC initiated by trastuzumab. a Trastuzumab-promoted ADCC with PBMCs from three out of the four different donors was significantly impaired after pre-incubation of the antibody with HER+ exosomes from BT474 and SkBr3 cells when compared to pre-incubation with HER2− exosomes from MEL624.38 cells or to untreated antibody. b Titration of effector cells and BT474 exosomes demonstrated that the inhibition of trastuzumab-initiated ADCC is dose dependent. Data with PBMCs from three different donors are shown
Discussion
The immune system follows different routes to block tumour progression. It generates tumour-specific cytolytic T cells and attacks cancer cells by immune reactions like ADCC, which is initiated by the binding of tumour-reactive antibodies to the surface of cancer cells. However, these anti-tumour immune responses are often blunted and hence can only rarely be raised successfully. Cancer patients usually suffer from impaired immune functions and it is clear that the tumour, by different means, is responsible for this dysfunction, which is best illustrated by the anergy and immaturity of tumour-infiltrating T lymphocytes and myeloid cells, respectively. Many immunosuppressive activities of tumours are attributable to substances like IL-10, TGF-β or adenosine, and other mechanisms are probably still to be elucidated. Among these are exosomes that are released by cancer cells at levels detectable in peripheral blood [37, 38]. The intratumoural concentration of exosomes is unknown and one can only speculate that it is probably higher than that measured in the peripheral circulation. The reason for this phenomenon is unclear but like for normal cells, exosomes probably constitute a route of secretion of unnecessary proteins also to malignant cells. On the other hand, tumour-derived exosomes are a source of tumour antigens that regularly evoke immune responses like tumour-reactive autoantibodies in cancer-bearing hosts. Over-expression of proteins in cancer cells is thought to be the major reason for the generation of tumour-reactive autoantibodies. Whether tumour-derived exosomes even contribute to the induction of these autoantibodies is not known.
Tumour-reactive antibodies directed against tumour-associated membrane antigens are present at detectable levels in the sera of many cancer patients [17, 39]. For example, HER2-specific antibodies are significantly higher in sera from patients with HER2-expressing mammary carcinoma compared to patients with HER2-negative cancer and healthy controls [21, 23, 40], and EpCAM-specific autoantibodies have been found in patients with colorectal and ovarian carcinoma [25, 41, 42]. The role of these autoantibodies is not fully understood. On the one hand, HER2-specific antibodies have been shown to block HER2 signalling and to inhibit tumour growth [22], and p53 autoantibodies have been described as prognostic biomarkers for improved outcome in patients with ovarian cancer [43]. On the other hand, it has been demonstrated that B cells and humoral immunity promote chronic inflammation and thus foster cancer development [44], As we show above, HER2 is also present on TEX derived from HER2-expressing cancer cells.
Trastuzumab is a humanized antibody that is used for the adjuvant treatment of breast cancer. Trastuzumab binds to the HER2 receptor, amplified in 20% of breast cancers. The most relevant anti-tumour activity of trastuzumab is thought to be the induction of ADCC. Hence, the findings we describe here may be of clinical relevance in that the sequestration of the antibody by tumour exosomes may contribute to phenomenon that many patients do not respond to trastuzumab [45, 46]. Resistance to trastuzumab treatment is frequent but predictive biomarkers are still lacking. Patients with HER2+ cancer also have elevated HER2 serum levels (>15 ng/ml) that are thought to be attributable to the shed extracellular domain of HER2, although ELISA assays, which are normally used to quantify serum HER2, do not discriminate between soluble and particulate protein [47, 48]. We show here that at least a part of the total HER2 is present in the form of TEX and that TEX-associated HER2 is capable of binding and thus sequestrating trastuzumab. As a consequence, it is tempting to speculate that exosomal sequestration contributes to trastuzumab resistance, particularly because it is believed that the antibody has a higher binding affinity to full-sized HER2 than to soluble HER2 extracellular domains generated by proteolytic cleavage [49]. In essence, sequestration by TEX of tumour-reactive antibodies is a realistic concept given the high systemic and presumably even much higher intratumoural concentration of TEX and hence constitutes a more general problem with resistance of tumours to the activities of the immune system and also to therapeutic molecular antibodies.
Taken together, we show here that tumour-derived exosomes efficiently bind and sequester tumour-reactive antibodies, including the therapeutic antibody trastuzumab, resulting in reduced binding to tumour cells and ADCC. This sequestration is a new tumour escape mechanism and may equally contribute to the resistance of tumours to targeted immunological therapies.
Materials and methods
Cell culture
BT474 [50] and SkBr3 (ATCC HTB-30) are human breast cancer cell line, and Mel 624.38 [51] and A375 (ATCC CRL-1619) are derived from human melanomas. RZ-LCL is a human EBV-immortalized B cell line established in our own laboratory. All cell lines were maintained in D-MEM (Gibco®, Invitrogen, Karlsruhe, Germany) supplemented with 10% foetal bovine serum (PAA-Laboratories, Pasching, Austria) at 37°C in a humidified CO2 atmosphere. Tumour lines were negative for mycoplasma as routinely tested by PCR (Venor® GeM Mycoplasma Detection Kit, Minerva Biolabs, Berlin, Germany).
Antibodies and ELISA assays
Trastuzumab (Herceptin®, Roche Inc.) is a humanized antibody that binds the extracellular domain of HER2. The following antibodies were used for FACS analysis: HER2 2502 (Trion Research, Munich, Germany), EpCAM (C215, Trion Research), TSG101 (NB200-112, Acris) and CD40 (Immunotools, Friesoyte, Germany). GM1 in exosomes was detected with cholera toxin conjugated with horseradish peroxidase (Sigma–Aldrich, Germany). Antibody L87 (NeoMarkers, Fremont, CA) was used for HER2 immunoblots. For the detection of HER2+ exosomes in sera from patients with invasive breast cancer, 200 μl serum each from 12 patients were diluted 1:3 with PBS, and exosomes were isolated by ultracentrifugation as described and resuspended in 200 μl PBS/1% Triton X-100. A HER2 ELISA assay (Sino Biological, Beijing, China) was performed according to the manufacturer’s instructions. Patients’ sera were obtained from the Dep. of Clinical Chemistry of the University Hospital Munich.
Flow cytometry
For flow cytometry analysis cells were stained with appropriate antibodies diluted in PBS/2% foetal bovine serum for 15 min. After three washes in PBS/2%, foetal bovine serum cells were incubated for 15 min with secondary antibody and washed again. For decoy experiments, 50 ng/ml trastuzumab or C215 were pre-incubated with 200 μg/ml exosomes for 1 h. Flow cytometry was done with a FACSCalibur flow cytometer (Becton–Dickinson, Franklin Lakes, NJ) and analysed with FlowJo (Tree Star Inc., Ashland, OR). For direct FACS analysis, TEX were coupled to latex–aldehyde beads (IDC, Eugene, OR). For this, 5 μl particles were mixed with 20 μg TEX in 100 μl PBS for 30 min at room temperature and then for 2 h at 4°C. Then, 100 mM glycine was added, and the beads were incubated for another 10 min and washed. Trastuzumab on TEX-coated latex beads was detected with a FITC-labelled anti-human IgG antibody (Dianova, Hamburg, Germany).
Isolation of exosomes
Exosomes were isolated from cell culture supernatants as described previously [52]. Briefly, cells were grown to approximately 60% confluency, rinsed in PBS and incubated in D-MEM without FCS for 48 h. Then, supernatants were collected and depleted of cells and cellular debris by centrifugation at 1,200 rpm for 10 min and subsequently passed through a 0.2 μm PES filter. The filtrate was concentrated by ultrafiltration with a Centricon Plus-70 centrifugal filter device (Millipore, Billerica, MA). The concentrate was then pelleted in a TL-100 rotor at 100,000 g for 2 h at 4°C and resuspended in 100 μl PBS. The protein concentration was measured with a BCA assay (Pierce, Rockford, IL). Where necessary, exosomes were further purified as previously described [26]. Briefly, pellets after ultracentrifugation were resuspended in 2 M sucrose in 20 mM NaOH, pH 7.4. A discontinuous linear sucrose gradient (1.75–0.25 M Sucrose, 20 mM HEPES/NaOH, pH 7.4) was layered on top of the exosome suspension and centrifuged for 22 h at 39.000 rpm in a Beckman SW60Ti rotor (Beckman Coulter, Fullerton, CA). Finally, 500 μl fractions were collected from the top of the tube and analysed by dot blot. Malignant ascites was obtained from patients with ovarian cancer who signed an informed consent, approved by the Ethical Committee of the University of Essen. Exosomes from ascites were isolated as described above and tested by dot blots for the presence of HER2 and EpCAM (not shown).
Immune electron microscopy
Droplets of exosomes were placed on formvar-coated grids, left to adsorb for 1 h and fixed with 2.2% formaldehyde and 0.1% glutaraldehyde for 1 h. After washing with PBS/1%, BSA-C (BIOTREND, Koeln, Germany) grids were blocked with Aurion Block for 30 min. Immunolabelling was performed at room temperature for 2 h with a mouse monoclonal anti-HER2 antibody. Unbound antibody was removed by five washes, and antibody binding was detected by anti-mouse IgG-gold particles. After an additional fixation with 2% glutaraldehyde for 10 min and further washing, grids were stained with 2% phosphotungstic acid for 2 min.
Dot blot
For each dot, 2 μl of exosome suspension were spotted on a nitrocellulose membrane (Protran®, Whatman, Dassel, Germany). After spots had dried, the membrane was blocked and incubated with primary antibodies and peroxidase-conjugated secondary antibody (anti-mouse from Promega, Madison, WI). Dots were detected with chemiluminescence method using Roti®-Lumin (Carl Roth GmbH, Karlsruhe, Germany) and Amersham Hyperfilm ECL (GE Healthcare, Buckinghamshire, UK).
Sequestration of antibodies by exosomes
Exosomes were isolated as described. Antibodies specific for HER2 (trastuzumab) and EpCAM (C215) at concentrations that gave half-maximal staining in flow cytometry were incubated with different amounts of exosomes for 30 min at room temperature in a total volume of 100 μl PBS with 2% FCS. As a control, antibodies were incubated without exosomes. Then, 1 × 105 cells were added and incubation was continued for another 30 min on ice. Cells were washed, incubated with a FITC-labelled secondary antibody for another 30 min, and binding of trastuzumab and C215 was measured by FACS.
Antibody-dependent cellular cytotoxicity (ADCC)
Antibody-dependent cellular cytotoxicity was determined using a calcein-acetyloxymethyl (calcein-AM) cytotoxicity assay as described [53]. BT474 target cells were labelled with calcein-AM (10 mg/ml; Invitrogen, Karlsruhe, Germany) for 30 min at 37°C, washed three times in PBS and delivered to a 96-well plate at a concentration of 5 × 103 cells/well. Effector PBMCs from normal human donors were obtained by density gradient centrifugation over Biocoll Separating Solution (Biochrom, Berlin, Germany). PBMCs were washed three times in PBS and then added to the target cells at an E:T ratio of 10:1–40:1. Trastuzumab, pre-incubated with 100 μg/ml exosomes for 1 h at room temperature, was added to a final concentration of 50 ng/ml. All components were diluted in standard cell culture medium with 10% FCS to a final volume of 225 μl/well. After 6 h at 37°C, 150 μl of the supernatants were transferred into a new 96-well plate and fluorescence was determined using a Wallac 1,420 Victor2 multi-label counter (Perkin Elmer, Waltham, MA). Cytotoxicity was calculated by the formula % lysis = 100 × (A – B)/(C – B), where A represents fluorescence of test supernatant, B represents fluorescence of target alone and C represents maximum calcein release from target cells lysed with 0.5% Triton X-100. Results represent the average of triplicate samples.
Statistics
Gaussian distribution of values was tested using D’Agostino and Pearson omnibus normality test. Statistics evaluation was performed using the paired t test.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by institutional grants.
Conflict of interest
The authors declare no conflict of interest.
Abbreviation
- TEX
Tumour-derived exosomes
References
- 1.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonia SJ, Extermann M, Flavell RA. Immunologic nonresponsiveness to tumors. Crit Rev Oncog. 1998;9:35–41. doi: 10.1615/critrevoncog.v9.i1.30. [DOI] [PubMed] [Google Scholar]
- 3.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 4.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 5.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 6.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 7.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–5119. [PubMed] [Google Scholar]
- 8.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 9.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 10.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WTJ, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Trojan A, Tun-Kyi A, Odermatt B, Nestle FO, Stahel RA. Functional detection of epithelial cell adhesion molecule specific cytotoxic T lymphocytes in patients with lung cancer, colorectal cancer and in healthy donors. Lung Cancer. 2002;36:151–158. doi: 10.1016/S0169-5002(01)00478-0. [DOI] [PubMed] [Google Scholar]
- 13.Bioley G, Jandus C, Tuyaerts S, Rimoldi D, Kwok WW, Speiser DE, Tiercy JM, Thielemans K, Cerottini JC, Romero P. Melan-A/MART-1-specific CD4 T cells in melanoma patients: identification of new epitopes and ex vivo visualization of specific T cells by MHC class II tetramers. J Immunol. 2006;177:6769–6779. doi: 10.4049/jimmunol.177.10.6769. [DOI] [PubMed] [Google Scholar]
- 14.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 15.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 16.Kono K, Rongcun Y, Charo J, Ichihara F, Celis E, Sette A, Appella E, Sekikawa T, Matsumoto Y, Kiessling R. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer. 1998;78:202–208. doi: 10.1002/(SICI)1097-0215(19981005)78:2<202::AID-IJC14>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor DD, Gercel-Taylor C, Parker LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Oncol: Gynecol; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 20.Tagliabue E, Balsari A, Campiglio M, Pupa SM. HER2 as a target for breast cancer therapy. Expert Opin Biol Ther. 2010;10:711–724. doi: 10.1517/14712591003689972. [DOI] [PubMed] [Google Scholar]
- 21.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 23.Ward RL, Hawkins NJ, Coomber D, Disis ML. Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum Immunol. 1999;60:510–515. doi: 10.1016/S0198-8859(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 24.Heubner M, Errico D, Kasimir-Bauer S, Herlyn D, Kimmig R, Wimberger P. EpCAM-autoantibody levels in the course of disease of ovarian cancer patients. Oncol: Med; 2010. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Herlyn D, Wong KK, Park DC, Schorge JO, Lu KH, Skates SJ, Cramer DW, Berkowitz RS, Mok SC. Identification of epithelial cell adhesion molecule autoantibody in patients with ovarian cancer. Clin Cancer Res. 2003;9:4782–4791. [PubMed] [Google Scholar]
- 26.de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 27.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 29.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 30.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 31.Park TK, Kim SN. Cell-mediated immunity in patients with invasive carcinoma of the cervix. Yonsei Med J. 1989;30:164–172. doi: 10.3349/ymj.1989.30.2.164. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki H, Kagimoto T, Oda T, Kawano F, Takatsuki K. Natural killer activity and antibody-dependent cell-mediated cytotoxicity in multiple myeloma. Jpn J Clin Oncol. 1985;15:611–617. [PubMed] [Google Scholar]
- 33.Dallegri F, Ballestrero A, Ottonello L, Patrone F. Defective antibody-dependent tumour cell lysis by neutrophils from cancer patients. Clin Exp Immunol. 1989;77:58–61. [PMC free article] [PubMed] [Google Scholar]
- 34.Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28:4–11. doi: 10.1016/S0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- 35.Katsumi Y, Kuwahara Y, Tamura S, Kikuchi K, Otabe O, Tsuchiya K, Iehara T, Kuroda H, Hosoi H, Sugimoto T. Trastuzumab activates allogeneic or autologous antibody-dependent cellular cytotoxicity against malignant rhabdoid tumor cells and interleukin-2 augments the cytotoxicity. Clin Cancer Res. 2008;14:1192–1199. doi: 10.1158/1078-0432.CCR-07-1661. [DOI] [PubMed] [Google Scholar]
- 36.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Skog J, Hsu CH, Lessard RT, Balaj L, Wurdinger T, Carter BS, Breakefield XO, Toner M, Irimia D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10:505–511. doi: 10.1039/b916199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller S, Konig AK, Marme F, Runz S, Wolterink S, Koensgen D, Mustea A, Sehouli J, Altevogt P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–1394. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 40.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164:1825–1829. doi: 10.1016/S0022-5347(05)67114-5. [DOI] [PubMed] [Google Scholar]
- 41.Mosolits S, Harmenberg U, Ruden U, Ohman L, Nilsson B, Wahren B, Fagerberg J, Mellstedt H. Autoantibodies against the tumour-associated antigen GA733–2 in patients with colorectal carcinoma. Cancer Immunol Immunother. 1999;47:315–320. doi: 10.1007/s002620050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosolits S, Steinitz M, Harmenberg U, Ruden U, Eriksson E, Mellstedt H, Fagerberg J. Immunogenic regions of the GA733–2 tumour-associated antigen recognised by autoantibodies of patients with colorectal carcinoma. Cancer Immunol Immunother. 2002;51:209–218. doi: 10.1007/s00262-002-0272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, Cramer D. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859–868. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calabrich A, Fernandes Gdos S, Katz A. Trastuzumab: mechanisms of resistance and therapeutic opportunities. Oncology (Williston Park) 2008;22:1250–1258. [PubMed] [Google Scholar]
- 46.Mehta K, Osipo C. Trastuzumab resistance: role for Notch signaling. Sci World J. 2009;9:1438–1448. doi: 10.1100/tsw.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx D, Fattahi-Meibodi A, Kudelka R, Uebel T, Kuhn W, Meden H. Detection of p105 (c-erbB-2, HER2/neu) serum levels by a new ELISA in patients with ovarian carcinoma. Anticancer Res. 1998;18:2891–2894. [PubMed] [Google Scholar]
- 48.Streckfus C, Bigler L, Dellinger T, Dai X, Kingman A, Thigpen JT. The presence of soluble c-erbB-2 in saliva and serum among women with breast carcinoma: a preliminary study. Clin Cancer Res. 2000;6:2363–2370. [PubMed] [Google Scholar]
- 49.Maple L, Lathrop R, Bozich S, Harman W, Tacey R, Kelley M, Danilkovitch-Miagkova A. Development and validation of ELISA for herceptin detection in human serum. J Immunol Methods. 2004;295:169–182. doi: 10.1016/j.jim.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Lasfargues EY, Coutinho WG, Redfield ES. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst. 1978;61:967–978. [PubMed] [Google Scholar]
- 51.Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, Parmiani G. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065X.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 52.Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol, Chap. 3, Unit 3.22 [DOI] [PubMed]
- 53.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.