Abstract
Purpose
We assessed the prognostic significance of, and the relationship between, the pretreatment lymphocyte-to-monocyte ratio (LMR) and the TILs/tumor-associated macrophages (TAMs) ratio, in patients with esophageal squamous cell carcinoma (ESCC) of pathological stage T3N0M0 (pT3N0M0).
Methods
A total of 220 newly diagnosed ESCC patients of stage pT3N0M0 who had not undergone neoadjuvant therapy were included. Densities of CD8+ TILs, CD4+ TILs, CD45RO+ TILs, and CD68+ TAMs were assessed by immunohistochemical staining of tissue microarray cores from all 220 pT3N0M0 ESCC patients (who underwent radical resection). Hematological biomarkers including lymphocyte and monocyte counts were obtained from routine preoperative blood test data, and the LMR and TILs/TAMs ratios calculated. Cutoff finder for survival prediction was plotted to find out the optimal cutoff point for each parameter.
Results
The LMR and TILs/TAMs ratios were interrelated. On univariate analyses of data from the entire cohort, the LMR, CD45RO/CD68 ratio, and CD8/CD68 ratio were significantly associated with both OS and disease-free survival. Only the CD45RO/CD68 ratio was independently prognostic of survival on multivariate analysis.
Conclusions
The prognostic significance of the CD45RO/CD68 ratio was higher than that of the LMR. The CD45RO/CD68 ratio is a useful independent prognostic marker in patients with pT3N0M0 ESCC who have undergone complete resection without neoadjuvant therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1931-5) contains supplementary material, which is available to authorized users.
Keywords: Esophageal squamous cell cancer, Lymphocyte-to-monocyte ratio, TILs, Tumor-associated macrophages, Survival
Introduction
Esophageal squamous cell cancer (ESCC) is one of the deadliest cancers, associated with high rates of recurrence and distant metastasis [1]. Despite recent progress in diagnostic procedures and multimodal treatment approaches, prognosis differs even among patients of the same TNM stage, especially those of middle stage [2]. The new staging system (7th edition) of the American Joint Committee on Cancer (AJCC) incorporated non-anatomical esophageal cancer (EC) characteristics including the extent of tumor differentiation and tumor location [2]. For example, patients of pathological stage T3N0M0 (pT3N0M0) were divided into stages IB, IIA, and IIB, according to histologic grade and cancer location, implying that survival may differ greatly among such patients [2]. Thus, identification of promising prognostic factors contributing to risk classification and clinical management could improve long-term survival.
Apart from work on various molecular signals and genetic mutations associated with ESCC progression, the relationship between local and systemic immune responses and ESCC prognosis has attracted much recent attention. It has become clear over the past two decades that, in terms of disease progression, the tumor microenvironment (TME) is as important as are genetic and epigenetic changes in cancer cells [3]. The TME has many components including complexes of local stromal cells, distantly recruited cells, and immune cells [3]. Of these TME cells, TILs and tumor-associated macrophages (TAMs) may reflect tumor biology and predict patient outcome. The densities of CD8+ and CD4+ TILs have been shown to be of prognostic utility, and tumor TAMs enhanced tumor cell invasion and metastasis [3, 4]. The circulating lymphocyte-to-monocyte ratio (LMR), a marker of systemic inflammation, has been shown to be independently prognostic of progression of a variety of solid tumors [5, 6]. It has been hypothesized that the LMR may reflect the TILs/TAM ratio, as the circulating levels of lymphocytes and monocytes may indicate the formation or the presence of TILs and TAMs, respectively [5–7]. However, to the best of our knowledge, the reason why the LMR is prognostic and the relationship between the LMR and the TILs/TAMs ratio remain poorly studied. Few reports have combined biomarkers to refine outcome predictions for ESCC patients. Our objective was to explore a possible correlation between the LMR and the TILs/TAMs ratio and to compare the prognostic utilities of these ratios in patients with ESCC.
Materials and methods
Patients
The study was approved by the Ethics Committee of our hospital. The inclusion criteria were: (1) ESCC that was histopathologically confirmed and pathologically staged as T3N0M0 after curative esophagectomy; (2) availability of blood test data obtained within 3 days prior to surgery; and (3) performance of a complete preoperative evaluation including endoscopic esophageal ultrasonography, computed tomography, and liver function testing. The exclusion criteria were prescription of any neoadjuvant therapy, immunosuppressive therapy (e.g., recent steroids), or immunotherapy, acute infection, any hematological disorders, and any prior history of a malignancy or an autoimmune disease. We finally included 220 newly diagnosed ESCC patients who were pathologically staged as T3N0M0 between June 2004 and December 2012. All patients provided written informed consent and agreed to their tumor tissue and clinical data being used for research purposes.
Clinical and laboratory parameters
The 7th edition of the AJCC TNM staging system was used to classify the tumor stage [2]. Tumor length (to the nearest 1 mm) was defined as the longest dimension measured on general postoperative pathological specimens. Tumor locations were categorized into the upper, middle, and lower esophagus. Tumor differentiation was graded as poor/not differentiated, moderately differentiated, or well differentiated.
All blood samples (collected from the forearm veins within 3 days prior to surgery) were placed in tubes containing ethylenediaminetetraacetic acid (EDTA) and immediately sent for analysis. Peripheral lymphocytes and monocytes were counted by an automated hematology analyzer (XE-5000, Sysmex, Kobe, Japan). Each LMR was calculated by dividing the absolute lymphocyte count (ALC) by the absolute monocyte count (AMC). The optimal prognostic cutoffs for lymphocyte counts, monocyte counts, and the LMR were determined using the method established by Budczies et al. (http://molpath.charite.de/cutoff/) [8].
IHC
Postoperative specimens of all patients were subjected to immunohistochemical analysis performed using standard automated protocols. Sections (4 μm thick) were deparaffinized in xylene and rehydrated through baths with a graded alcohol series. The antigens were retrieved by microwaving under high pressure for 2 min. Sections were stained with the following primary antibodies: anti-CD68 (clone OTI4G1, Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China); anti-CD8 (clone SP16, Beijing Zhongshan Golden Bridge Biotechnology Company); anti-CD4 antibody (clone OTI6E10, Beijing Zhongshan Golden Bridge Biotechnology Company); and anti-CD45RO (clone OTI2E7, Beijing Zhongshan Golden Bridge Biotechnology Company) in a humidified chamber at 37 °C for 60 min and subsequently incubated with secondary goat antirabbit and goat antimouse antibodies (Beijing Zhongshan Golden Bridge Biotechnology Company, Beijing, China) at 37 °C for 15 min. Reaction products were visualized by color reaction with 3,3′-diaminobenzidine and counterstained with hematoxylin.
Two independent pathologists blinded to clinical data reviewed all slides. The densities of CD68+ TAMs, CD8+ TILs, CD4+ TILs, or CD45RO+ TILs were evaluated in a medium-power field (200×) in a manner similar to recent recommendations in the literature [9]. In brief, TILs were scored as a percentage of stained lymphocytes in the stromal compartment on a semiquantitative scale (results in 5% steps beginning with 0%). The optimal prognostic cutoffs for TILs and TAMs were determined using the method established by Budczies et al. (http://molpath.charite.de/cutoff/) [8].
Statistical analysis
A Chi-square test was used to compare the differences in baseline and clinicopathological characteristics between the groups. Disease-free survival (DFS) was defined as time elapsed from date of surgery to that of local recurrence/distant metastasis, or to the date of last follow-up. OS was the time from the date of surgery to death from ESCC or the last follow-up. The Kaplan–Meier method was used to estimate the survival curves, and differences between subgroups were compared with the aid of the log-rank test. Cox’s proportional hazards models were used to perform univariate and multivariate analysis defining hazard ratios (HRs) for variables relevant to DFS and OS. Two-sided p values and HRs with 95% confidence intervals (CIs) are reported. The optimal cutoff values for certain variables were determined using the method of Budczies et al. (http://molpath.charite.de/cutoff/) [8]. All statistical analyses were conducted with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). A two-sided p value <0.05 was considered to reflect statistical significance.
Results
Immune markers and clinical variables
Immunohistochemically, CD45RO+, CD4+, and CD8+ T cells, as well as CD68+ TAMs infiltrating ESCC tissue, were evident in the stroma (Fig. 1). Supplementary Figure S1 illustrates representative examples of CD8, CD4, CD45RO, and CD68 expression in ESCC samples. The immunohistochemical and hematological variables are shown in Supplementary Table S1. The optimal cutoffs for survival prediction were 36.80, 16.90, 5.00, and 15.10% for the CD68, CD8, CD4, and CD45RO counts, respectively. Similarly, an ALC of 1.431 × 109 /L and an AMC of 0.630 × 109 /L were the respective optimal cutoffs for prediction of survival. Ratios were defined as the proportions of CD45RO+ , CD4+ , and CD8+ T cells divided by the proportion of CD68+ TAMs. The LMR was calculated by dividing the lymphocyte count by the monocyte count. The optimal cutoffs were 3.364, 1.960, 1.671, and 1.980 for the LMR, and the CD4/CD68, CD8/CD68, and CD45RO/CD68 ratios, respectively. Representative examples of high and low CD8/CD68, CD45RO/CD68, and CD4/CD68 ratios are shown in Fig. 1.
Fig. 1.
Representative examples of high and low CD8/CD68 (a, b), CD4/CD68 (c, d), and CD45RO/CD68 ratios (e, f) expression (×200 magnification) in esophageal squamous cell carcinoma (ESCC) samples
The complete baseline characteristics of all patients (data on low- and high-level immune marker groups are shown separately) are presented in Table 1. High CD68 + TAM infiltrates correlated with longer tumor length and vascular invasion (Table 1). No significant correlation was evident between CD68 status and gender, tumor location, or any other parameters, whereas a low AMC (<0.630 × 109 /L) and a high LMR (>3.364) were more frequent in patients with shorter tumors and tumors that were well or moderately differentiated (Table 1). Also, high CD45RO/CD68 and CD4/CD68 ratios were associated with both tumor differentiation (p = 0.006 and 0.036, respectively) and recurrence (p < 0.001 and p = 0.006, respectively) (Table 2). In contrast, we found no significant difference in age, tumor location, TNM stage, or any other variable between patients with high or low CD8, CD4, or CD45RO counts, and ALCs or CD8/CD68 ratios.
Table 2.
Correlation between LMR and TILs/TAM ratio and clinicopathological characteristics
| LMR | CD45RO/CD68 ratio | CD8/CD68 ratio | CD4/CD68 ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | Low | High | p | Low | High | p | |
| Age (years) | 0.787 | 0.250 | 0.683 | 0.455 | ||||||||
| ≤60 | 72 | 52 | 87 | 37 | 98 | 26 | 97 | 27 | ||||
| >60 | 54 | 42 | 74 | 22 | 78 | 18 | 79 | 17 | ||||
| Gender | 0.998 | 0.303 | 0.893 | 0.636 | ||||||||
| Male | 67 | 50 | 89 | 28 | 94 | 23 | 95 | 22 | ||||
| Female | 59 | 44 | 72 | 31 | 82 | 21 | 81 | 22 | ||||
| KPS | 0.216 | 0.253 | 0.651 | 0.407 | ||||||||
| <80 | 39 | 22 | 48 | 13 | 50 | 11 | 51 | 10 | ||||
| ≥80 | 87 | 72 | 113 | 46 | 126 | 33 | 125 | 34 | ||||
| Tumor location | 0.896 | 0.382 | 0.054 | 0.464 | ||||||||
| Upper | 16 | 10 | 21 | 5 | 21 | 5 | 23 | 3 | ||||
| Middle | 50 | 38 | 67 | 21 | 77 | 11 | 71 | 17 | ||||
| Lower | 60 | 46 | 73 | 33 | 78 | 28 | 82 | 24 | ||||
| Tumor differentiation | 0.016 | 0.006 | 0.248 | 0.036 | ||||||||
| Well | 44 | 44 | 54 | 34 | 67 | 21 | 64 | 24 | ||||
| Moderate | 46 | 38 | 67 | 17 | 72 | 12 | 74 | 10 | ||||
| Poor | 36 | 12 | 40 | 8 | 37 | 11 | 38 | 10 | ||||
| Tumor length | 0.023 | 0.300 | 0.629 | 0.408 | ||||||||
| ≤4 cm | 68 | 65 | 94 | 39 | 105 | 28 | 104 | 29 | ||||
| >4 cm | 58 | 29 | 67 | 20 | 71 | 16 | 72 | 15 | ||||
| Perineural invasion | 0.702 | 0.195 | 0.602 | 1.000 | ||||||||
| No | 123 | 91 | 155 | 59 | 170 | 44 | 171 | 43 | ||||
| Yes | 3 | 3 | 6 | 0 | 6 | 0 | 5 | 1 | ||||
| Vascular invasion | 0.701 | 0.194 | 0.349 | 0.349 | ||||||||
| No | 121 | 92 | 154 | 59 | 169 | 44 | 169 | 44 | ||||
| Yes | 5 | 2 | 7 | 0 | 7 | 0 | 7 | 0 | ||||
| Treatment | 0.820 | 0.178 | 0.161 | |||||||||
| Surgery only | 61 | 41 | 77 | 25 | 85 | 17 | 84 | 18 | 0.330 | |||
| Adjuvant chemotherapy | 25 | 21 | 30 | 16 | 32 | 14 | 37 | 9 | ||||
| Adjuvant radiation | 23 | 16 | 26 | 13 | 30 | 9 | 27 | 12 | ||||
| Adjuvant chemoradiation | 17 | 16 | 28 | 5 | 29 | 4 | 28 | 5 | ||||
| Recurrence | 0.319 | <0.001 | 0.130 | 0.016 | ||||||||
| No | 19 | 19 | 19 | 19 | 27 | 11 | 25 | 13 | ||||
| Yes | 107 | 75 | 142 | 40 | 149 | 33 | 151 | 31 | ||||
Values in bold signify p < 0.05
LMR lymphocyte-to-monocyte ratio, TILs tumor-infiltrating lymphocytes, TAM tumor-associated macrophages
Table 1.
Correlation between AMC and CD68+ TAM and clinicopathological characteristics
| No. of patients | % | AMC | CD68+ TAM | |||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | |||
| Age (years) | 0.472 | 0.170 | ||||||
| ≤60 | 124 | 56.36 | 65 | 59 | 109 | 15 | ||
| >60 | 96 | 43.64 | 55 | 41 | 78 | 18 | ||
| Gender | 0.554 | 0.558 | ||||||
| Male | 117 | 53.18 | 66 | 51 | 101 | 16 | ||
| Female | 103 | 46.82 | 54 | 49 | 86 | 17 | ||
| KPS | 0.322 | 0.833 | ||||||
| <80 | 61 | 27.73 | 30 | 31 | 51 | 10 | ||
| ≥80 | 159 | 72.27 | 90 | 69 | 136 | 23 | ||
| Tumor location | 0.745 | 0.061 | ||||||
| Upper | 26 | 11.82 | 15 | 11 | 22 | 4 | ||
| Middle | 88 | 40.00 | 50 | 38 | 69 | 19 | ||
| Lower | 106 | 48.18 | 55 | 51 | 96 | 10 | ||
| Tumor differentiation | 0.001 | 0.886 | ||||||
| Well | 88 | 40.00 | 52 | 36 | 76 | 12 | ||
| Moderate | 84 | 38.18 | 53 | 31 | 71 | 13 | ||
| Poor | 48 | 21.82 | 15 | 33 | 40 | 8 | ||
| Tumor length | 0.019 | 0.022 | ||||||
| ≤4 cm | 133 | 60.45 | 81 | 52 | 119 | 14 | ||
| >4 cm | 87 | 39.54 | 39 | 48 | 68 | 19 | ||
| Perineural invasion | 1.000 | 0.222 | ||||||
| No | 214 | 97.27 | 117 | 97 | 183 | 31 | ||
| Yes | 6 | 2.73 | 3 | 3 | 4 | 2 | ||
| Vascular invasion | 0.250 | 0.011 | ||||||
| No | 213 | 96.82 | 118 | 95 | 184 | 29 | ||
| Yes | 7 | 3.18 | 2 | 5 | 3 | 4 | ||
| Treatment | 0.550 | 0.304 | ||||||
| Surgery only | 102 | 46.36 | 51 | 51 | 91 | 11 | ||
| Adjuvant chemotherapy | 46 | 20.91 | 25 | 21 | 39 | 7 | ||
| Adjuvant radiation | 39 | 17.73 | 24 | 15 | 31 | 8 | ||
| Adjuvant chemoradiation | 33 | 15.00 | 20 | 13 | 26 | 7 | ||
| Recurrence | 1.000 | 0.466 | ||||||
| No | 38 | 17.27 | 21 | 17 | 34 | 4 | ||
| Yes | 182 | 82.73 | 99 | 83 | 153 | 29 | ||
Values in bold signify p < 0.05
AMC absolute monocyte count, TAM tumor-associated macrophages
Correlation between tumor-infiltrating and hematological immune markers
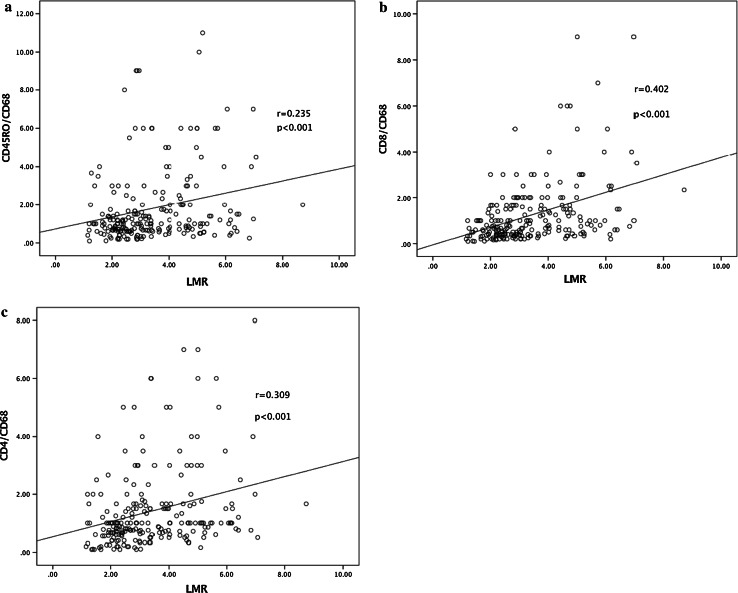
A significant correlation was evident between the CD68 score and the AMC (r = 0.315, p <0.001) (Table 3). Also, the ALC exhibited a weak positive association with CD4 and a negative correlation with CD68 counts (r = 0.115 and −0.149; p = 0.021 and 0.027, respectively) (Table 3). The LMR was significantly correlated with the CD8/CD68, CD4/CD68, and CD45RO/CD68 ratios (r = 0.402, 0.309, and 0.235, all p values <0.001) (Table 4; Fig. 2a–c).
Table 3.
Correlation of hematological and immunohistochemical variables with each other
| CD8 | CD45RO | CD68 | ALC | AMC | |
|---|---|---|---|---|---|
| CD4 | |||||
| r | 0.050 | 0.151 | 0.048 | 0.155 | −0.101 |
| p | 0.462 | 0.025 | 0.482 | 0.021 | 0.136 |
| CD8 | |||||
| r | 0.128 | −0.078 | 0.129 | −0.293 | |
| p | 0.058 | 0.250 | 0.056 | <0.001 | |
| CD45RO | |||||
| r | 0.024 | 0.011 | −0.022 | ||
| p | 0.729 | 0.870 | 0.748 | ||
| CD68 | |||||
| r | −0.149 | 0.315 | |||
| p | 0.027 | <0.001 | |||
| ALC | |||||
| r | −0.118 | ||||
| p | 0.082 | ||||
Spearman’s r coefficient test; values in bold signify p < 0.05
ALC absolute lymphocyte count, AMC absolute monocyte count
Table 4.
Correlation of LMR and TILs/TAM ratio
| LMR | CD45RO/CD68 | CD8/CD68 | CD4/CD68 |
|---|---|---|---|
| r | 0.235 | 0.402 | 0.309 |
| p | <0.001 | <0.001 | <0.001 |
Spearman’s r coefficient test; values in bold signify p < 0.05
LMR lymphocyte-to-monocyte ratio, TILs tumor-infiltrating lymphocytes, TAM tumor-associated macrophages
Fig. 2.
Correlations between the CD45RO/CD68 ratio (a), the CD8/CD68 ratio (b), the CD4/CD68 ratio (c), and the lymphocyte-to-monocyte ratio (LMR)
Survival analysis
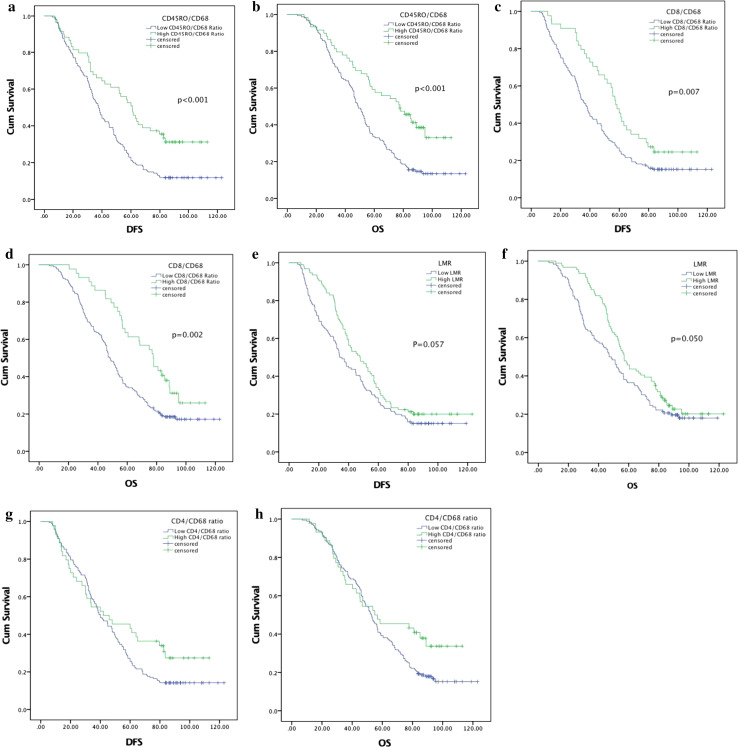
Median follow-up time was 53.25 months. In total, 175 (79.09%) patients died from ESCC before the end of the follow-up. The median DFS and OS for the entire population were 40.0 months (95% CI 34.2–45.8 months) and 53.0 months (95% CI 48.0–58.0 months), respectively. Univariate analysis showed that conventional tumor histopathological features, including tumor length, tumor location, extent of differentiation, and vascular invasion, were prognostically significant (Table 5). No type of lymphocyte infiltrating the stroma and no hematological markers alone were of any prognostic significance. In contrast, except for the CD4/CD68 ratio (Table 5; Fig. 3g, h), high LMR and high CD8/CD68 and CD45RO/CD68 ratios were significantly associated with a favorable DFS (p = 0.059, 0.008, <0.001, respectively) and OS (p = 0.050, 0.002, <0.001, respectively) (Table 5; Fig. 3a–f). All of these factors (p < 0.1) were entered into multivariate analysis using the Cox’s proportional hazards model. The CD45RO/CD68 ratio was strongly prognostic of DFS, with HRs of 0.913 (95% CI 0.841–0.990, p = 0.028) when used as a continuous variable (per point of increase) and 0.600 (95% CI 0.418–0.861, p = 0.006) when employed as a categorical variable (Table 5). In addition, the ratio was a good indicator of improved OS, with HRs of 0.899 (95% CI 0.825–0.979, p = 0.014) when used as a continuous variable (per point of increase) and 0.494 (95% CI 0.342–0.715, p < 0.001) when employed as a categorical variable (Table 5). Vascular invasion, extent of tumor differentiation, tumor location, and preoperative Karnofsky performance score (KPS) were independently associated with both DFS (p = 0.027, 0.003, 0.018 and 0.036, respectively) and OS (p = 0.020, <0.001, 0.009, and 0.011, respectively) (Supplementary Figures S2–S5).
Table 5.
Univariate analysis of clinicopathological and immunohistochemical parameters associated with disease-free survival and overall survival
| Variable | Disease-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age (≤60 vs. >60) | 0.973 (0.726–1.304) | 0.855 | 1.033 (0.765–1.393) | 0.834 | ||||
| Gender (male vs. female) | 0.866 (0.646–1.161) | 0.336 | 0.881 (0.653–1.188) | 0.405 | ||||
| Pre-op KPS (<80 vs. ≥80) | 0.737 (0.534–1.017) | 0.063 | 0.695 (0.495–0.976) | 0.036 | 0.700 (0.505–0.971) | 0.032 | 0.638 (0.451–0.902) | 0.011 |
| Tumor location | ||||||||
| Upper | Ref. | 0.044 | Ref. | 0.018 | Ref. | 0.036 | Ref. | 0.009 |
| Middle | 0.979 (0.614–1.561) | 0.928 | 0.952 (0.587–1.546) | 0.843 | 0.943 (0.589–1.507) | 0.805 | 0.879 (0.539–1.434) | 0.607 |
| Lower | 0.678 (0.427–1.076) | 0.099 | 0.623 (0.387–1.002) | 0.051 | 0.646 (0.406–1.029) | 0.066 | 0.560 (0.346–0.906) | 0.018 |
| Tumor length (≤4 cm vs.> 4 cm) | 1.366 (1.017–1.834) | 0.038 | 1.191 (0.870–1.630) | 0.275 | 1.331 (0.984–1.799) | 0.063 | 1.145 (0.828–1.582) | 0.413 |
| Differential grade | ||||||||
| Well | Ref. | 0.016 | Ref. | 0.003 | Ref. | 0.004 | <0.001 | |
| Moderate | 1.272 (0.914–1.770) | 0.153 | 1.360 (0.962–1.925) | 0.082 | 1.296 (0.922–1.182) | 0.135 | 1.389 (0.972–1.986) | 0.071 |
| Poor | 1.763 (1.196–2.597) | 0.004 | 1.997 (1.333–2.990) | 0.001 | 1.964 (1.325–2.912) | 0.001 | 2.294 (1.516–3.470) | <0.001 |
| Perineural invasion (no vs. yes) | 1.545 (0.683–3.492) | 0.296 | 1.734 (0.766–3.925) | 0.187 | ||||
| Vascular invasion (no vs. yes) | 3.175 (1.474–6.839) | 0.003 | 2.451 (1.200–5.802) | 0.027 | 3.457 (1.603–7.453) | 0.002 | 2.577 (1.160–5.728) | 0.020 |
| Treatment regimens | ||||||||
| Surgery alone | Ref. | 0.857 | Ref. | 0.915 | ||||
| Adjuvant chemotherapy | 1.087 (0.742–1.592) | 0.669 | 1.042 (0.705–1.539) | 0.836 | ||||
| Adjuvant radiation | 1.071 (0.721–1.591) | 0.734 | 0.943 (0.623–1.427) | 0.782 | ||||
| Adjuvant chemoradiation | 0.887 (0.571–1.380) | 0.595 | 0.878 (0.560–1.377) | 0.572 | ||||
| Hematological markers | ||||||||
| ALC (low vs. high) | 0.891 (0.613–1.294) | 0.545 | 0.917 (0.624–1.347) | 0.659 | ||||
| AMC (low vs. high) | 1.173 (0.876–1.570) | 0.285 | 1.262 (0.937–1.700) | 0.126 | ||||
| LMR (low vs. high) | 0.753 (0.560–1.011) | 0.057 | 0.945 (0.686–1.303) | 0.732 | 0.739 (0.546–1.000) | 0.050 | 0.995 (0.716–1.384) | 0.979 |
| Immunohistochemical markers | ||||||||
| CD8+ TILs (low vs. high) | 0.921 (0.687–1.235) | 0.584 | 0.865 (0.641–1.169) | 0.345 | ||||
| CD4+ TILs (low vs. high) | 0.877 (0.583–1.321) | 0.531 | 0.851 (0.564–1.282) | 0.440 | ||||
| CD45RO+ TILs (low vs. high) | 1.079 (0.787–1.479) | 0.639 | 1.066 (0.774–1.469) | 0.696 | ||||
| TAM (CD68) (low vs. high) | 1.318 (0.886–1.962) | 0.173 | 1.352 (0.896–2.039) | 0.151 | ||||
| CD8/CD68 (low vs. high) | 0.601 (0.411–0.877) | 0.007 | 0.766 (0.473–1.240) | 0.279 | 0.540 (0.364–0.802) | 0.002 | 0.688 (0.421–1.126) | 0.137 |
| CD4/CD68 (low vs. high) | 0.717 (0.486–1.057) | 0.093 | 1.149 (0.714–1.851) | 0.567 | 0.683 (0.455–1.025) | 0.066 | 1.138 (0.694–1.866) | 0.609 |
| CD45RO/CD68 (low vs. high) | 0.529 (0.371–0.754) | <0.001 | 0.600 (0.418–0.861) | 0.006 | 0.494 (0.342–0.715) | <0.001 | 0.567 (0.389–0.827) | 0.003 |
| CD45RO/CD68 (as a continuous variable) | 0.891 (0.820–0.968) | 0.006 | 0.913 (0.841–0.990) | 0.028 | 0.876 (0.803–0.956) | 0.003 | 0.899 (0.825–0.979) | 0.014 |
Values in bold signify p < 0.05
TILs tumor-infiltrating lymphocytes, TAM tumor-associated macrophages, ALC absolute lymphocyte count, AMC absolute monocyte count, LMR lymphocyte-to-monocyte ratio, HR hazard ratio, CI confidence interval, Ref. reference
Fig. 3.
Kaplan–Meier analysis of disease-free survival and overall survival in terms of the CD45RO/CD68 ratio (a, b), the CD8/CD68 ratio (c, d), the LMR (e, f), and the CD4/CD68 ratio (g, h)
Discussion
In the present study, we performed integrated analyses of local and systemic immune factors, including the LMR (based on the pretreatment peripheral blood cell counts), and tumor-infiltrating immune markers (evaluated using a tissue microarray method), including CD4+ , CD8+ , CD45RO+ TILs, and CD68+ TAMs, in 220 pT3N0M0 ESCC patients. We sought to evaluate the correlation between, and compare the prognostic abilities of, the LMR and the TILs/TAMs ratio. We found that LMR and TILs/TAM ratio are interrelated. Patients with low stromal CD45RO/CD68 ratios tended to exhibit reduced DFS and OS. Furthermore, a high CD45RO/CD68 ratio afforded better discrimination than did the LMR of subgroups with significantly poorer DFS and OS. Therefore, the stromal CD45RO/CD68 ratio may serve as a novel biomarker predicting the outcomes of patients with pT3N0M0 ESCC.
Tumor immune microenvironments can be analyzed effectively by performing IHC methods on resected tumors. Previous studies found that high or low levels of TILs and chemokines were of significant prognostic utility in ESCC patients [4, 10]. Moreover, two prior studies on EC patients evaluated the relationships among local and systemic inflammation, standard clinicopathological factors, and survival; most patients had esophageal adenocarcinoma (EAC); only a small proportion had ESCC [11, 12]. Although tumor inflammatory infiltration was irrelevant in terms of tumor necrosis, such infiltration played an important role in suppressing of tumor progression and metastasis in EAC, but not ESCC, patients [11, 12]. However, as the number of ESCC patients was small, and as the carcinogenesis of EAC and ESCC differs, any role played by inflammatory tumor infiltrates requires re-evaluation [11, 13]. In our present study, we found that the densities of single types of TILs or TAMs did not correlate with prognosis, but the combination of TAMs and TIL densities did show a correlation. This suggests that interactions among various immune cells in the TME may significantly affect ESCC progression.
CD45RO has been generally accepted as the optimal single marker for the entire memory T cell population, with the exception of T memory stem cells [14]. Memory T cells are acknowledged to respond faster upon re-stimulation with antigen compared with naive T cells [15]. Immunochemically, high-level expression of CD45RO+ T memory cells has been associated with better disease-related outcomes in various human cancers, including EAC and ESCC [16–18]. Chronic inflammation has long been regarded as a key risk factor for many human cancers [19]. Recently, a close association between chronic inflammation-associated genomic instability and esophageal carcinogenesis was reported in ESCC patients [20]. TAMs constitute prominent inflammatory cell populations in many types of tumor, playing pivotal roles in cancer-related inflammation [3, 19]. Although the roles played by TAMs in cancer development and progression are disputed, there is increasing evidence that a TAM-rich microenvironment increases the probability of metastasis and is associated with poor survival in patients with various cancers, including ESCC [11, 21–24]. VEGFA and EGF produced by TAMs foster angiogenic tissue programming and tumor growth, respectively, compatible with the positive relationship evident between CD68 expression, and both vascular invasion and tumor length [25–27]. Moreover, TAMs compromise effector cell functions in TMEs; TAMs express inhibitory receptors and secrete several cytokines, chemokines, and enzymes [25, 28]. The advantage of the CD45RO/CD68 index is that it combines the information on the status of both antitumor immunity and inflammation, thus comprehensively indicating the extent of the host immune response.
The preoperative, absolute peripheral monocyte count was suggested to be a useful prognostic marker in a large cohort of patients with resected ESCCs [29]. Moreover, recent studies have shown that a reduced LMR was associated with poor survival of cancer patients, including those with ESCC [30–32]. Our findings in a large cohort of ESCC patients are consistent with those of previous studies. The OS of patients with low LMRs was much poorer than that of those with high LMRs (median OS, 47.0 vs. 57 months). Also, the LMR was associated with both tumor differentiation and tumor length in our cohort. The LMR is a potentially valuable clinical biomarker, being easily calculated, reproducible, and low cost. Moreover, the LMR correlated with the TILs/TAMs ratio, suggesting that a systemic inflammatory response may reflect concurrent focal inflammation in the tumor, in agreement with data from previous studies on hepatocellular carcinoma [33]. However, LMR was not an independent prognostic indicator in our study. This warrants further investigation in a large prospective study of patients with ESCC.
The reason why lower level of LMR is associated with poor cancer outcomes remains unknown. Lymphopenia might weaken the efficacy of the immune system; cell-mediated cytotoxicity may be attenuated if the level of effector T cells is insufficient [6, 34]. Circulating monocytes may contribute to both tumor growth and reduced immunosurveillance. Serum monocytes are recruited locally and differentiate into macrophages only after infiltrating a tumor; the cells respond to the wide spectrum of chemokines and growth or differentiation factors, such as CCL-2 and CSF-1, produced by tumor and stromal cells in the TME. This may explain the moderate correlation evident between the CD68 and AMC scores, and the correlation between the LMR and the TILs/TAMs ratio [35, 36].
Previous studies evaluated the extent of T cell infiltration by using automated imaging software. However, such software distinguishes cells by color depths only, and cell shape, features, or position are not considered. Moreover, CD68 has recently been shown to not be a specific macrophage marker, but a lysosomal protein enriched not only in macrophages but also in non-myeloid cells including carcinomas [37]. In the present study, two independent pathologists blinded to clinical and pathological information evaluated the densities of TILs and TAMs. The data are superior to those obtained using automated imaging software; the pathologists considered cell morphology and position during the assessment. Moreover, our patients were more homogeneous than those of other cohorts; i.e., they were all of the same TNM stage (pT3N0M0). As the CD45RO/CD68 ratio was an independently favorable prognostic factor, it may be that this ratio will be a useful non-anatomical marker complementing TNM staging. However, studies on patients at other disease stages, and those who receive different initial treatments (including neoadjuvant therapy and definitive therapies), are required.
There were certain limitations to our study. First, a previous study on other tumor types has specifically identified infiltrating inflammatory cells as “invasive margin” or “cancer cell nests” by location [38]. We could not analyze ESCC specimens in this manner because the primary infiltration pattern of ESCC is perivascular stromal infiltration; the diffuse pattern is very rare. Therefore, most lymphocytes are located in the stroma, and few infiltrate into cancer cell nests (which are thus difficult to evaluate). Moreover, we found that patients with high CD4/CD68 status had a propensity toward reduced recurrence and longer survival. The associations did not reach statistical significance (Table 5; Fig. 3g, h). This is probably because different subpopulations of CD4+ TILs exert distinct functions [15]. More detailed classification is required in the future. In addition, in our study, we did not classify circulating lymphocytes in detail; this may cause us to underestimate (to some extent) the correlation between the levels of circulating lymphocytes and TILs, and the impact of circulating lymphocyte levels on survival. However, the levels of circulating lymphocyte subpopulations, such as CD4+ , CD8+ ,and CD45RO+ lymphocytes, supposedly correlate with survival in patients with several types of solid tumors embracing nasopharyngeal carcinoma, cervical cancer, and hepatocellular carcinoma (most of which are virus-associated tumors) [39–41]. Virus-specific immunity may increase the numbers of T cell subpopulations, thus improving survival [42]. However, the etiology and pathogenesis of ESCC remain unclear. Recently, the InterSCOPE study, the largest sero-epidemiological study on HPV in ESCC, revealed the absence of viral biological activity for 51 mucosotropic HPV types in ESCC from high-incidence regions [43]. However, it remains true that the impact of circulating lymphocytes on survival of ESCC patients requires further study.
In summary, we show that the tumor-infiltrating CD45RO/CD68 ratio may more comprehensively indicate host immune response status than do the LMR and other single markers. The CD45RO/CD68 ratio may be a useful prognostic biomarker in patients with pT3N0M0 ESCC and is a promising candidate marker of the immunoscore. Further research on the local and systemic immune responses of ESCC patients treated in different centers is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by a grant from Natural Science Foundation of Shandong Province, China (Grant No. ZR2015HZ004).
Abbreviations
- AJCC
American Joint Committee on Cancer
- ALC
Absolute lymphocyte count
- AMC
Absolute monocyte count
- CI
Confidence interval
- DFS
Disease-free survival
- EAC
Esophageal adenocarcinoma
- EC
Esophageal cancer
- ESCC
Esophageal squamous cell cancer
- HR
Hazard ratio
- KPS
Karnofsky performance score
- LMR
Lymphocyte-to-monocyte ratio
- TAMs
Tumor-associated macrophages
- TME
Tumor microenvironment
Compliance with ethical standards
Conflict of interest
No author has any conflict of interest.
Contributor Information
Minghuan Li, Phone: +86-531-67626112, Email: lminghuan@sina.com.
Jinming Yu, Phone: +86-531-87984729, Email: sdyujinming@sina.cn.
References
- 1.Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198(2):205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer Staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63(7):1555–1559. [PubMed] [Google Scholar]
- 5.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Gu L, Li H, Chen L, Ma X, Li X, Gao Y, Zhang Y, Xie Y, Zhang X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7(22):31926–31942. doi: 10.18632/oncotarget.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TWG The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JY, Li F, Wang LP, Chen XF, Wang D, Cao L, Ping Y, Zhao S, Li B, Thorne SH, Zhang B, Kalinski P, Zhang Y. CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2015;113(5):747–755. doi: 10.1038/bjc.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta S, Going JJ, Crumley AB, Mohammed Z, Orange C, Edwards J, Fullarton GM, Horgan PG, McMillan DC. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106(4):702–710. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crumley AB, Going JJ, Hilmy M, Dutta S, Tannahill C, McKernan M, Edwards J, Stuart RC, McMillan DC. Interrelationships between tumor proliferative activity, leucocyte and macrophage infiltration, systemic inflammatory response, and survival in patients selected for potentially curative resection for gastroesophageal cancer. Ann Surg Oncol. 2011;18(9):2604–2612. doi: 10.1245/s10434-011-1658-7. [DOI] [PubMed] [Google Scholar]
- 13.Tao CJ, Chen YY, Jiang F, Feng XL, Jin QF, Jin T, Piao YF, Chen XZ. A prognostic model combining CD4/CD8 ratio and N stage predicts the risk of distant metastasis for patients with nasopharyngeal carcinoma treated by intensity modulated radiotherapy. Oncotarget. 2016 doi: 10.18632/oncotarget.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto K, Sho M, Wakatsuki K, Takayama T, Matsumoto S, Nakamura S, Akahori T, Tanaka T, Migita K, Ito M, Nakajima Y. Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol. 2012;168(2):186–191. doi: 10.1111/j.1365-2249.2012.04565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauser S, Langer R, Tschernitz S, Gais P, Jutting U, Feith M, Hofler H, Walch A. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I–IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10:608. doi: 10.1186/1471-2407-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsen EE, Kilvaer T, Khanehkenari MR, Maurseth RJ, Al-Saad S, Hald SM, Al-Shibli K, Andersen S, Richardsen E, Busund LT, Bremnes R, Donnem T. CD45RO(+) memory T lymphocytes—a candidate marker for TNM-immunoscore in squamous non-small cell lung cancer. Neoplasia. 2015;17(11):839–848. doi: 10.1016/j.neo.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin R, Zhang C, Zheng J, Tian D, Lei Z, Chen D, Xu Z, Su M. Chronic inflammation-associated genomic instability paves the way for human esophageal carcinogenesis. Oncotarget. 2016;7(17):24564–24571. doi: 10.18632/oncotarget.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutterer GC, Pichler M, Chromecki TF, Strini KA, Klatte T, Pummer K, Remzi M, Mannweiler S, Zigeuner R. Tumour-associated macrophages might represent a favourable prognostic indicator in patients with papillary renal cell carcinoma. Histopathology. 2013;63(3):309–315. doi: 10.1111/his.12163. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, He MY, Zhu LF, Yang CC, Zhou ML, Wang Q, Zhang W, Zheng YY, Wang DM, Xu ZQ, Wu YN, Liu LK. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:12. doi: 10.1186/s13046-015-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111(6):752–759. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 24.Guo SJ, Lin DM, Li J, Liu RZ, Zhou CX, Wang DM, Ma WB, Zhang YH, Zhang SR. Tumor-associated macrophages and CD3-zeta expression of tumor-infiltrating lymphocytes in human esophageal squamous-cell carcinoma. Dis Esophagus. 2007;20(2):107–116. doi: 10.1111/j.1442-2050.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 25.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8 + T cells. J Clin Investig. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han L, Jia Y, Song Q, Wang N, Wang J, Bai B, Chen X, Wang C, Cheng Y. Prognostic significance of preoperative absolute peripheral monocyte count in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(7):740–746. doi: 10.1111/dote.12401. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8:137–145. doi: 10.2147/OTT.S73794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji H, Xuan Q, Yan C, Liu T, Nanding A, Zhang Q. The prognostic and predictive value of the lymphocyte to monocyte ratio in luminal-type breast cancer patients treated with CEF chemotherapy. Oncotarget. 2016;7(23):34881–34889. doi: 10.18632/oncotarget.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Friesenbichler J, Trajanoski S, Stojakovic T, Eberhard K, Leithner A, Pichler M. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer. 2014;135(2):362–370. doi: 10.1002/ijc.28677. [DOI] [PubMed] [Google Scholar]
- 33.Liao R, Jiang N, Tang ZW, Li DW, Huang P, Luo SQ, Gong JP, Du CY. Systemic and intratumoral balances between monocytes/macrophages and lymphocytes predict prognosis in hepatocellular carcinoma patients after surgery. Oncotarget. 2016;7(21):30951–30961. doi: 10.18632/oncotarget.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao WW, Zhang LN, You KY, Huang R, Yu X, Ding PR, Gao YH. A low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Cancer. 2015;6(7):616–622. doi: 10.7150/jca.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 36.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67(5):453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 38.Ling A, Edin S, Wikberg ML, Oberg A, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110(10):2551–2559. doi: 10.1038/bjc.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS ONE. 2015;10(2):e0117458. doi: 10.1371/journal.pone.0117458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu T, Huang Z, Su B, Wang S, Wang D, Wang C, Wei W, Jiang J, Zhang G, Yang H, Hu W. Prognostic significance of circulating CD19+ B lymphocytes in EBV-associated nasopharyngeal carcinoma. Med oncol. 2014 doi: 10.1007/s12032-014-0198-y. [DOI] [PubMed] [Google Scholar]
- 41.Ordonez R, Henriquez-Hernandez LA, Federico M, Valenciano A, Pinar B, Lloret M, Bordon E, Rodriguez-Gallego C, Lara PC. Radio-induced apoptosis of peripheral blood CD8 T lymphocytes is a novel prognostic factor for survival in cervical carcinoma patients. Strahlenther Onkol. 2014;190(2):210–216. doi: 10.1007/s00066-013-0488-x. [DOI] [PubMed] [Google Scholar]
- 42.Turksma AW, Bontkes HJ, van den Heuvel H, de Gruijl TD, von Blomberg BME, Braakhuis BJM, Leemans CR, Bloemena E, Meijer C, Hooijberg E. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013;19(6):577–584. doi: 10.1111/odi.12037. [DOI] [PubMed] [Google Scholar]
- 43.Halec G, Schmitt M, Egger S, Abnet CC, Babb C, Dawsey SM, Flechtenmacher C, Gheit T, Hale M, Holzinger D, Malekzadeh R, Taylor PR, Tommasino M, Urban MI, Waterboer T, Pawlita M, Sitas F. Mucosal alpha-papillomaviruses are not associated with esophageal squamous cell carcinomas: lack of mechanistic evidence from South Africa, China and Iran and from a world-wide meta-analysis. Int J Cancer. 2016;139(1):85–98. doi: 10.1002/ijc.29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.