Abstract
Checkpoint blockade therapy utilizing monoclonal antibodies to reactivate T cells and recover their antitumor activity makes an epoch in cancer immunotherapy. The role of B7-H4, a novel negative immune checkpoint, in oral squamous cell carcinoma (OSCC) has still not been elucidated. In this study, tissue samples from human OSCC, which contains 165 primary OSCC, 48 oral epithelial dysplasia and 43 normal oral mucosa specimens, and Tgfbr1/Pten 2cKO mice OSCC model were stained with B7-H4 antibody to analyze the correlations between B7-H4 expression and clinicopathological characteristics. Kaplan–Meier analysis was used to compare the survival of patients with high B7-H4 expression and patients with low B7-H4 expression. We found B7-H4 is highly expressed in human OSCC tissue, and the B7-H4 expression level was associated with the clinicopathological parameters containing pathological grade and lymph node status. Moreover, we confirmed that B7-H4 was overexpressed in Tgfbr1/Pten 2cKO mice OSCC model. Our data also indicated that patients with high B7-H4 expression had poor overall survival compared with those with low B7-H4 expression. Furthermore, this study demonstrated that B7-H4 was positively associated with PD-L1, CD11b, CD33, PI3Kα p110, and p-S6 (S235/236). Taken together, these findings suggest B7-H4 is a potential target in the treatment of OSCC.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1867-9) contains supplementary material, which is available to authorized users.
Keywords: B7-H4, Immune checkpoint, Immunotherapy, Oral squamous cell carcinoma, Transgenic mice, Tissue microarray
Introduction
Oral squamous cell carcinoma (OSCC), with an incidence of approximately 48,000 Chinese and mortality rate of approximately 50 % in 2015, is one of the ten most common cancers globally [1, 2]. There are many important risk factors so far identified for OSCC, among which are smoking, alcohol use, and immune status, with smoking and alcohol having synergistic effects [3, 4]. In spite of advances in conventional treatment, the mortality of OSCC has not been markedly improved for the past several decades [5]. It is now evident that poor prognosis of OSCC patients is attributed to recurrence, cervical lymph node metastasis, and radiotherapy and chemotherapy resistance [6], which speaks to the limitations in curative effect of the currently available standard of care treatment approaches employing combinations of surgery, radiotherapy, and chemotherapy. Therefore, the need for new therapeutic modalities and approaches in OSCC should be exploited.
In recent years, immune therapy as an effective treatment for several cancers, such as advanced melanoma and non-small-cell lung cancer, has emerged [7, 8]. As is the case with many cancers, OSCC is an immunosuppressive disease, with recruitment of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in tumor microenvironment, which provides the premise for cancer immunotherapy [9, 10]. Among the most promising approaches to immune therapy is the blockade of immune checkpoints [11]. Recent clinical successes in cancer immunotherapy of blocking cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1) have leaded us to extend the potential of cancer immunotherapy by inhibiting more recently identified checkpoint ligands and receptors [12].
It is now evident that members of the B7 family of coregulatory ligands play a vital role in the control and fine tuning of antigen-specific T cell-mediated immune responses [13]. B7-H4 (also known as B7S1, B7x, and VTCN1), a member of the Ig superfamily, is a negative checkpoint ligand that shares homology with B7 family and is a potent T cell suppressor [14]. B7-H4 has been implied in the delivery of an inhibitory signal to T cells, thereby abrogating T cell proliferation, cytokine production, and cell cycle progression [15, 16]. B7-H4 is predominantly expressed in tumor cells in human cancer tissues, such as ovarian, breast, melanoma, gastric and renal cell carcinoma, non-small-cell lung cancer, esophageal and endometrial cancer, and its protein levels are found to be correlated with various clinicopathological features and postoperative prognosis [17–19]. To date, however, studies pertaining to expression of B7-H4 in OSCC tumor are not clearly elucidated.
In this study, we aimed to characterize the expression of B7-H4 in human OSCC tissue microarray as well as in mice model and the association of B7-H4 expression with clinicopathological parameters was analyzed. In addition, the correlation among B7-H4 and Programmed death-ligand 1 (PD-L1), CD11b, CD33, PI3Kα p110, and p-S6 (S235/236) is evaluated. These findings, in summary, indicate B7-H4 is a new prognostic marker and a potential immunotherapeutic target of OSCC.
Materials and methods
Ethical statement
This study was approved by the School and Hospital of Stomatology of Wuhan University Medical Ethics Committee.
Patients, tumor samples and human OSCC tissue array
All patients were diagnosed with OSCC with pathological confirmation at our institution from January 2008 to August 2015, and informed consent was obtained from the patients before they underwent surgery. The clinical stages of their OSCC were classified in accordance with the guidelines of the International Union Against Cancer (UICC 2002), and we adopted the scheme of the World Health Organisation to determine the histological grading. Paraffin-embedded tissue arrays from OSCC were constructed with 1.5-mm core from each patient. These tissue microarray slides (T12-412, T12-412-TMA2, T15-411) included 165 confirmed cases of primary OSCC (exclude recurrent, pre-operation chemotherapy, or pre-operation radiotherapy), 43 normal oral mucosa, 48 oral epithelial dysplasia, 41 metastasis lymph node, 17 OSCC with pre-operation inductive TPF (cisplatin, docetaxel, and fluorouracil) chemotherapy, 10 cases with pre-operation radiotherapy treatment, and 12 recurrence tumor cases of OSCC.
Knock out mice OSCC model
Tissue specific and time inducible Tgfbr1/Pten 2cKO mice (K14-Cre ERtam+/−; Tgfbr1 flox/flox; Pten flox/flox) were gifted by Dr. Ashok B. Kulkarni in National Institute of Dental and Craniofacial Research. All experiments were conducted according to guidelines of Institutional Animal Care and Use Committee of Wuhan University and the NIH guidelines for the Care and Use of Laboratory Animals. The mice were maintained and genotyped in accordance to published protocols [20, 21]. All the mice were FVBN/CD1/129/C57 mixed background.
Immunohistochemical staining
All paraffin-embedded specimens were cut into 4-µm sections. The slides were dried at 60 °C for 2 h, deparaffinized and dehydrated orderly. The sections were then boiled in 0.01 M citric acid buffer solution (pH 6.0) or 1 mM EDTA buffer solution (pH 8.0) for 1.5 min at high pressure. Then 3 % hydrogen superoxide was incubated for 20 min to quench endogenous peroxidase activity and 10 % normal goat serum was used to block non-specific binding. The sections were incubated with polyclonal anti-human B7-H4 (Cell Signaling Technology, 1:800), PD-L1 (Cell Signaling Technology, 1:100), PI3Kα p110 (Cell Signaling Technology, 1:200), p-S6 (S235/236) (Cell Signaling Technology, 1:200), CD11b (Abcam, 1:400), CD33 (Zymed, 1:200), or isotype-matched IgG controls overnight at 4 °C. Then, a secondary biotinylated immunoglobulin G antibody solution and an avidin–biotin–peroxidase reagent were incubated into the slides. After being washed with phosphate buffer saline, 3,3′-diaminobenzidine tetrachloride was incubated into the sections and then lightly counterstained with Mayer’s hematoxylin.
Scoring system, hierarchical clustering, and data visualization
Scoring system, hierarchical clustering, and data visualization were performed according to our previous studies [21, 22]. All slices were scanned using an Aperio ScanScope CS scanner (Vista, CA, USA) and quantified for pixel quantification by Aperio Quantification software (Version 9.1) [23]. An area of concern was sorted, scanning and quantifying, then the formula (3+) × 3+(2+) × 2+ (1+) × 1 was utilized to count histoscore of pixel quantification [21]. The scaled values of expression scores were converted in Microsoft excel. The results were presented by the Cluster 3.0 [24] and Java TreeView 1.0.5 [25]. Finally, the clustered data on behalf of markers were displayed on the vertical axis and representing tissue samples were arranged on the horizontal axis. Closely related biomarkers were placed tightly together [24].
Statistical analysis
The data analysis was conducted with one-way analysis of variance for multiple group comparison and unpaired t test for two-group comparison. Two-tailed Pearson’s statistics was utilized for correlated expression of PD-L1, CD11b, CD33, PI3Kα p110, and p-S6 (S235/236) based on confirmation of the sample with Gaussian distribution. Survival curves were exhibited by the method of Kaplan–Meier, and log-rank test was used to access the significance of observed differences. Data analyses were operated using Graph Pad Prism version 5.0 (GraphPad Software Inc, La Jolla, CA). The quantified results are presented as mean ± SEM, and P < 0.05 was considered statistically significant.
Results
B7-H4 over-expression in primary human OSCC specimens and is significantly correlated with patients’ survival
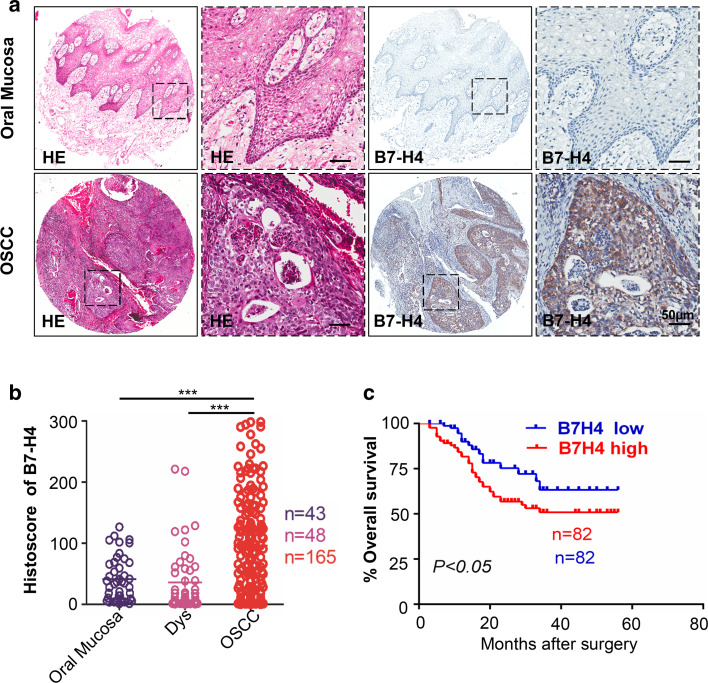
For the purpose to confirm whether B7-H4 expression was associated with OSCC in humans, we inquired the Oncomine database, a publicly available dataset of cancer [26]. In a meta-analysis of gene expression profiling, VTCN1 (gene encoding B7-H4) DNA copy number along with the level of mRNA expression of this gene was significantly increased in OSCC compared with the normal counterpart (P < 0.05, Supplementary Fig. 1a–c). Subsequently, we performed immunohistochemistry in human OSCC tissue microarray to appraise the protein expression of B7-H4 in human OSCC tissues (Fig. 1a). We observed that cytoplasmic and membranous patterns of B7-H4 staining were found in the tumor cells (Fig. 1a). Meanwhile, B7-H4 expression analysis showed immunoreactivity of B7-H4 in human primary OSCC (n = 165) was significantly higher than in dysplasia (n = 48, P < 0.001) and in normal oral mucosa (n = 43, P < 0.001, Fig. 1a with quantification in Fig. 1b). Furthermore, we used Kaplan–Meier method to explore the prognosis value of B7-H4 in OSCC and the median of B7-H4 expression (the histoscore = 88.12) is used as cutoff. As shown in Fig. 1c, log-Rank analysis indicated that overall rate of the patients with higher B7-H4 expression had a significantly poorer survival rate than those with lower B7-H4 expression (P < 0.05, Hazard ratio 1.784, 95 % CI 1.018–3.017).
Fig. 1.
High expression of B7-H4 in oral squamous cell carcinoma. a Representative hematoxylin-eosin (HE, left) and immunohistochemistry staining (right) of B7-H4 in oral mucosa and in primary OSCC tissue. Scale bar 50 μm. b Quantification of immunohistochemical histoscore of B7-H4 among oral mucosa (n = 43), dysplasia (Dys, n = 48) and primary oral squamous cell carcinoma (OSCC, n = 165) (all data are presented as mean ± SEM, one-way ANOVA with post Tukey test. ***P < 0.001). c Kaplan–Meier curve showing head neck squamous cell carcinoma patient with low B7-H4 expression (n = 82) survival longer than B7-H4 high expression patient, which log-Rank analysis reveal the difference was significant (n = 82, P < 0.05)
The expression of B7-H4 is correlated with pathological grade and lymph node status
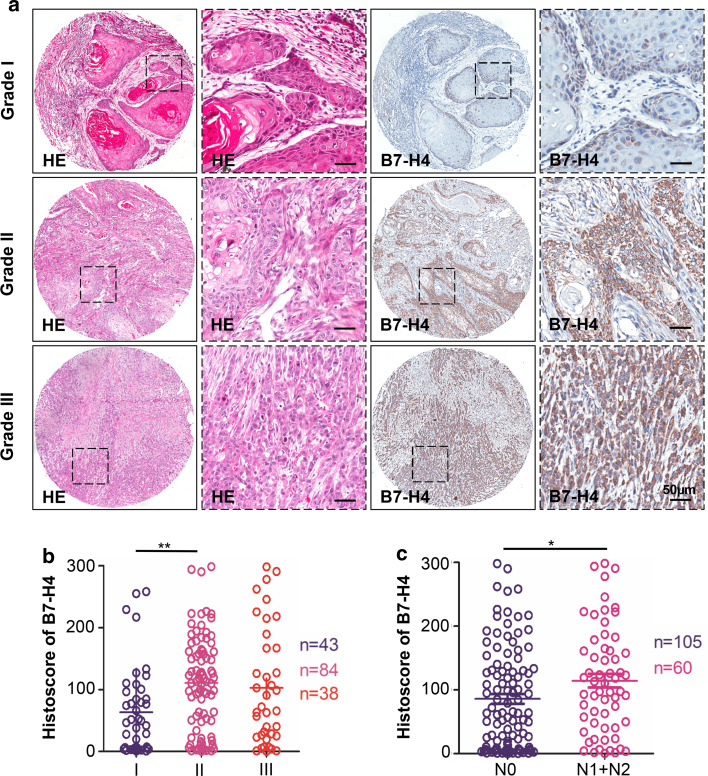
On further assessment of the relationships among B7-H4 and pathological features, we found that B7-H4 was significantly associated with pathological grade. High-grade (Grade II, mean ± SEM = 110.8 ± 8.349, n = 84) OSCC exhibited intense B7-H4 immunoreactivity compared with low-grade (Grade I, mean ± SEM = 63.40 ± 10.76, n = 43) OSCC (P < 0.01). The comparison of Grade III (mean ± SEM = 103.0 ± 15.29, n = 38) with Grade I did not highlight significant differences in B7-H4 expression, which may be due to sample size issue, although a trend (P = 0.0683) toward an upregulation of B7-H4 expression in Grade III is observable (Fig. 2a with quantification in Fig. 2b). It has also been noticed a noteworthy increase in B7-H4 immunoreactivity in the primary tumor patient with the pathological lymph node-positive (N1 + N2, n = 60, P < 0.05, Fig. 2c) in comparison with the pathological lymph node-negative patients (N0, n = 105). The expression of B7-H4 was not significantly increased in T1, T2, T3, and T4 category (Supplementary Fig. 2a). All the above results indicated that B7-H4 protein expression was correlated with advanced OSCC.
Fig. 2.
Human OSCC tissue array analysis revealed that B7-H4 correlated with high-grade OSCC. a Representative hematoxylin-eosin staining (HE, left) and immunohistochemical staining (right) of B7-H4 in human oral cancer tissues with different grades (I–III) (Scale bars 50 μm). b Quantitative analysis of histoscore of B7-H4 expression in grade I, grade II and grade III. B7-H4 levels in high-grade OSCC were significantly higher when compared with low-grade OSCC (one-way ANOVA with post Tukey test, P < 0.01). c The expression of B7-H4 was correlated with lymph node status of human OSCC (t test, P < 0.05). *P < 0.05; **P < 0.01. Scale bars 50 μm
The correlation between the expression of B7-H4 and clinical parameters
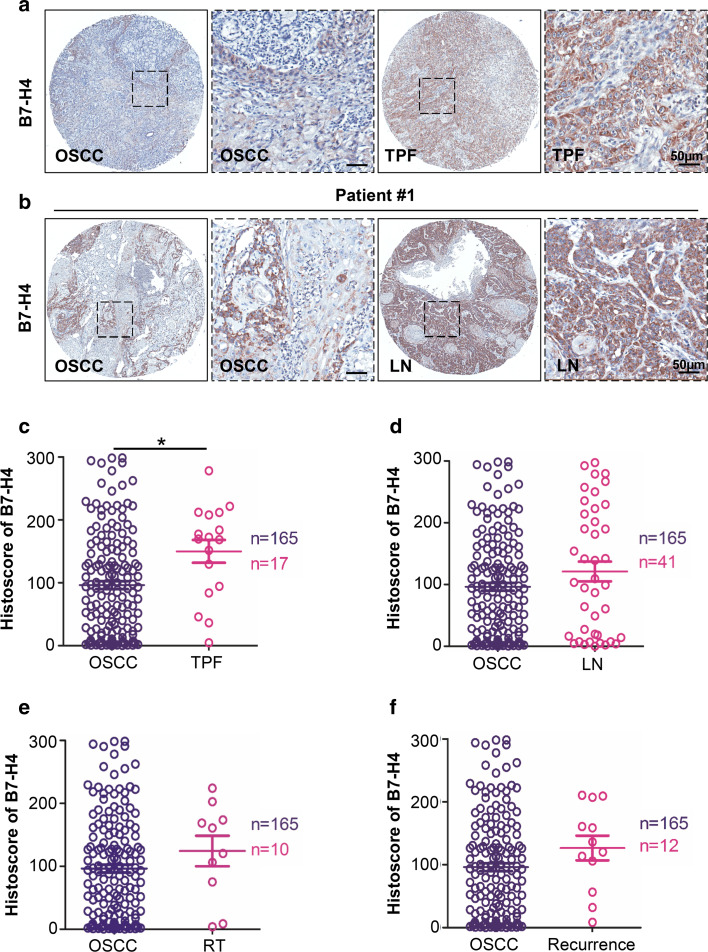
To further investigate the protein expression of B7-H4 in metastasis lymph node, chemotherapeutic response, radiotherapy treatment and recurrence of OSCC, 17 OSCC with pre-operation inductive TPF (cisplatin, docetaxel, and fluorouracil) chemotherapy, 41 metastasis lymph node cases, 10 cases with pre-operation radiotherapy treatment and 12 recurrent tumor cases of OSCC were selected for immunohistochemistry analysis. Representative immunohistochemistry images are shown in Fig. 3a, b. Immunohistochemistry showed a significant increase of B7-H4 expression in OSCC with pre-operation inductive TPF chemotherapy (P < 0.05, Fig. 3a with quantification in Fig. 3c). There was no significant difference between the primary tumor and metastasis lymph node (P > 0.05, Fig. 3b and quantification in Fig. 3d). Meanwhile, there was no significant influence on the level of B7-H4 expression in patients with pre-operation radiotherapy treatment (Fig. 3e) and recurrence (Fig. 3f). No significant association was found between B7-H4 in primary OSCC and cigarette smoking or alcohol consumption (Supplementary Fig. 2b–c).
Fig. 3.
Increased expression of B7-H4 in OSCC with pre-operation cisplatin based chemotherapy. a Representative HE staining (left) and immunohistochemical staining (right) of OSCC with pre-operation inductive TPF (cisplatin, docetaxel, and fluorouracil) chemotherapy. b Representative HE staining (left) and immunohistochemical (right) staining of primary OSCC and metastasis lymph node (LN). c Quantitative of immunohistochemistry staining using digital scanner and histoscore of B7-H4 expression with pre-operation inductive TPF was significantly higher than primary OSCC (t test, P < 0.05). d There was no significant difference between the expression level of B7-H4 in primary OSCC and metastasis lymph node (t test, P > 0.05). e There was no significant difference between the expression level of B7-H4 in primary OSCC and OSCC with pre-operation radiotherapy treatment (RT) (t test, P > 0.05). f There was no significant difference between the expression level of B7-H4 in primary OSCC and recurrence (t test, P > 0.05). Scale bars 50 μm
Protein expression of B7-H4 was remarkably correlated with PD-L1, CD11b, CD33, PI3Kα p110, and p-S6 (S235/236) in human OSCC tissue
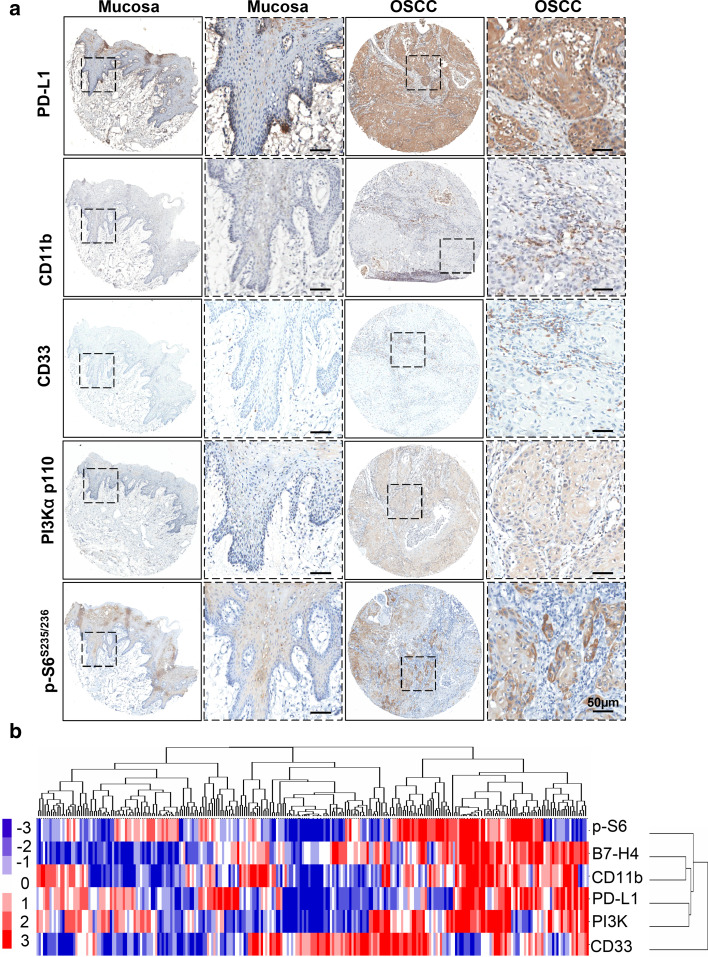
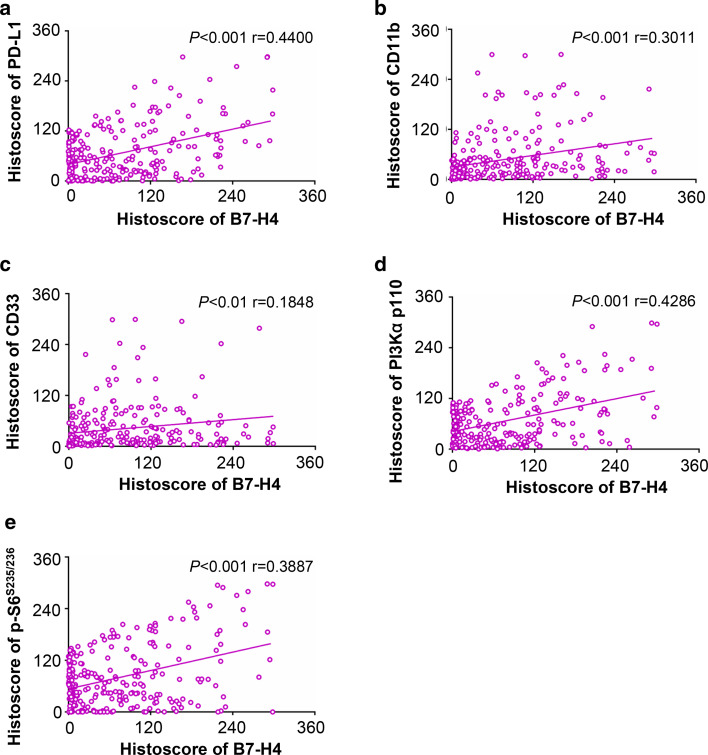
As detected in silico prediction of B7-H4 related genes and STRING, B7-H4 may correlate with PD-L1 and many other immune molecules (Supplementary Table 1, Supplementary Fig. 3). We focused on the use of immunohistochemistry with specific antibody to stain the human OSCC tissue microarrays. As shown in Fig. 4a, an increase in PD-L1, PI3Kα p110, and p-S6 (S235/236) (a downstream molecule of mTOR) in the tumor cells of OSCC compared with normal mucosa was observed. The specific antibodies for CD11b and CD33 were used to stain MDSCs [27], and expression of CD11b and CD33 was mainly in the immune cells of OSCC, which is higher than in normal mucosa. Furthermore, using hierarchical clustering analysis, we demonstrated that the expression of CD11b was close to the expression of B7-H4 (Fig. 4b). In addition, Spearman rank correlation coefficient test and linear tendency test were conducted. We found that the protein expression of B7-H4 in OSCC was significantly correlated with PD-L1 (P < 0.001, r = 0.4400), CD11b (P < 0.001, r = 0.3011), CD33 (P < 0.01, r = 0.1848), PI3Kα p110 (P < 0.001, r = 0.4286), and p-S6 (S235/236) (P < 0.001, r = 0.3887) (Fig. 5). The observation above suggested that PI3K–AKT–mTOR pathway may influence the expression of B7-H4 in OSCC and B7-H4 may bind to a receptor on MDSCs to promote tumor growth.
Fig. 4.
Correlation of B7H4 with PD-L1, CD11b, CD33, PI3Kα p110, and p-S6 (S235/236) in human OSCC tissue microarray. a Representative immunohistochemical staining of PD-L1, CD11b, CD33, PI3Kα p110 and p-S6 (S235/236) in human oral cancer tissue (right) compared with normal oral mucosa (left) (Scale bars 50 μm). b Hierarchical clustering presents the protein expression correlation of PD-L1, CD11b, CD33, PI3K, and p-S6 in human OSCC tissue microarray
Fig. 5.
Quantification and correlation of B7-H4 with PD-L1, CD11b, CD33, PI3Kα p110 and p-S6 (S235/236) in human OSCC tissue. Correlation of B7-H4 with PD-L1 (a), CD11b (b), CD33 (c), PI3Kα p110 (d), and p-S6(S235/236) (e) in human OSCC tissue array
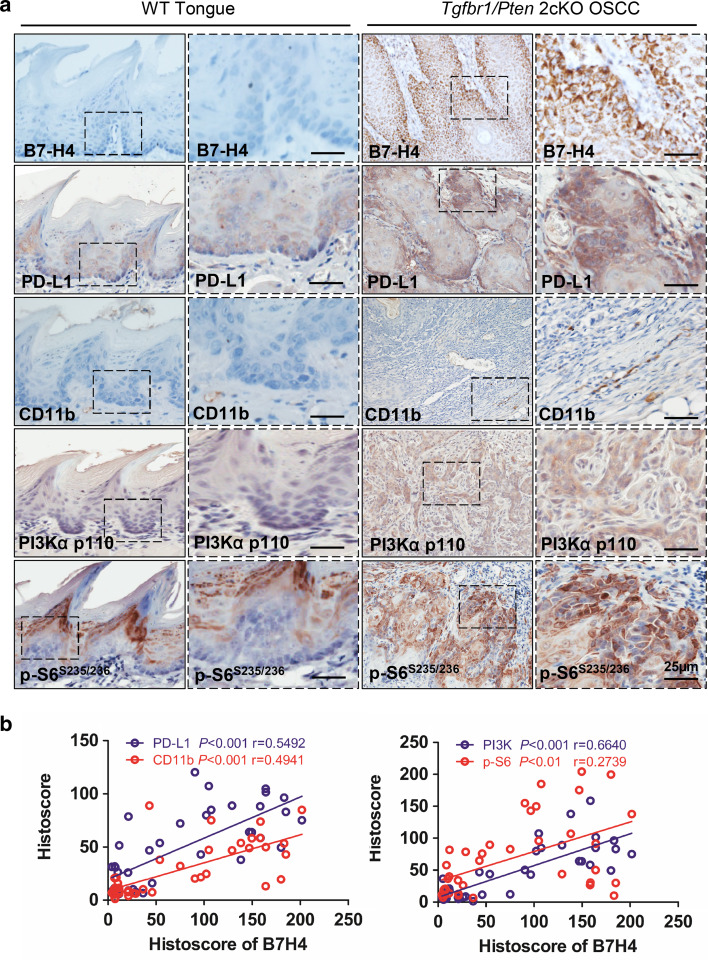
B7-H4 was over-expressed in Tgfbr1/Pten 2cKO mice OSCC model and was remarkably correlated with PD-L1, CD11b, PI3Kα p110, and p-S6 (S235/236) in mice OSCC tissue
Our previous work reported mice with tissue specific deletion of tumor suppressor gene Pten and Tgfbr1 in epithelia spontaneously develop OSCC in a mouse model [28]. To determine whether over-expression of B7-H4 was associated with high-grade squamous cell carcinoma in mice, we performed B7-H4 immunohistochemistry and found that B7-H4 was located mostly in the membrane and cytoplasm of the cancer cell of Tgfbr1/Pten 2cKO mice (n = 20, respectively). However, B7-H4 staining was negative in wild-type (WT) mice tongue (Fig. 6a). Meanwhile, we use specific antibody to stain the mice OSCC tissues. As shown in Fig. 6a, an increase in PD-L1, CD11b, PI3Kα p110, and p-S6 (S235/236) in the Tgfbr1/Pten 2cKO mice OSCC compared with WT tongue was observed. Furthermore, we found that the protein expression of B7-H4 in mice OSCC was significantly correlated with PD-L1 (P < 0.001, r = 0.5492), CD11b (P < 0.001, r = 0.4941), PI3Kα p110 (P < 0.001, r = 0.6640), and p-S6 (S235/236) (P < 0.01, r = 0.2739) (Fig. 6b).
Fig. 6.
High expression of B7-H4 in Tgfbr1/Pten 2cKO mice OSCC and Correlation of PD-L1, CD11b, PI3Kα p110 and p-S6 (S235/236) in mice OSCC tissue. a Immunohistochemistry of B7-H4, PD-L1, CD11b, PI3Kα p110, and p-S6(S235/236) in tongue of the wild-type (WT) mice and the tumor of Pten/Tgfbr1 conditional knock out mice (Scale bar 25 µm). b Correlation of B7-H4 with PD-L1, CD11b, PI3Kα p110 and p-S6 (S235/236) in mice OSCC tissue
Discussion
The expression and functional relevance of B7-H4 in patients with malignancies, such as renal cell carcinoma (RCC), human melanoma, esophageal squamous cell carcinoma (ESCC), have been recently reported [18, 29, 30]. In this study, our results demonstrated that B7-H4 was highly and aberrantly expressed in human primary OSCC tissue and the B7-H4 expression level was associated with the clinicopathological parameters containing pathological grade, lymph node status, and poor patient outcome.
It has been event that B7-H4 is commonly detectable in the many human tumors and predicts patients’ survival [31]. Quandt et al. [29] reported that B7-H4 expression was found with a high frequency in the primary tumors and in the metastases of melanoma and a survival benefit for patients with B7-H4 low expressing melanoma was detected. Consistently, immunohistochemistry showed B7-H4 staining in RCC and B7-H4 expression was related with adverse clinical and pathologic parameters, among which are constitutional symptoms, tumor necrosis, tumor size, advanced cancer stage, grade, and patient’s survival [30]. However, up to now, the expression of B7-H4 and its clinical significance in OSCC are not known. Our study timely reveals that B7-H4 is over-expressed in primary OSCC, and its expression level is significantly associated with pathological grade and advanced lymph node status. Survival analysis indicated that patients with high B7-H4 expression had poor overall survival compared with those with low B7-H4 expression. Our findings support that B7-H4 may be a prognostic indicator in OSCC.
PI3K–AKT–mTOR pathway plays an important role in the pathogenesis of OSCC [32]. It has also been demonstrated that PI3K–AKT–mTOR pathway may enhance the expression of specific immune-inhibitory molecules on tumors, such as PD-L1 [33] and B7-H3 [34]. In this study, we found that the expression of B7-H4 in human OSCC was positively correlated with PD-L1, PI3Kα p110, and p-S6 (S235/236). Loss of Tgfbr1/Pten signaling in head and neck epithelia activates the PI3K/Akt signaling [28, 35]. We also observed B7-H4 was over-expressed in Tgfbr1/Pten 2cKO mice and was positively correlated with PI3Kα p110 and p-S6 (S235/236), which is consistent with human OSCC tissue. Therefore, we hypothesized that this pathway may also influence the expression of B7-H4 in OSCC. Jeon et al. demonstrated that B7-H4 promotes immune escape and develop tumor growth by binding to receptor on MDSCs. Consistent with prior study, we exhibited B7-H4 is positively related with CD11b and CD33, which implied that B7-H4 functioning its negative regulator may be associated with MDSCs. Loss of Tgfbr1 and Pten signaling in head and neck epithelia enhances chemokine production and recruitment of tumor promoting MDSCs [28]. We found the expression of B7-H4 on mice OSCC was positively correlated with CD11b, which suggested that the interaction between B7-H4 and MDSCs may weaken T cell antitumor immunity. Collectively, findings from all above display B7-H4 may correlate with PI3K–AKT–mTOR pathway and immune suppressive status, and provide some preliminary work for making B7-H4 become a novel target for immune therapy of OSCC. Additional work is needed to further clarify the immunological mechanisms of B7-H4.
Recent studies have reported promising activity of checkpoints in OSCC, such as PD-1 and PD-L1 [36–38]. Krambeck et al. [30] have demonstrated that the combined expression of B7-H4 and PD-L1 was associated with an increased risk of death from RCC beyond the risk of either molecule alone. In our study, we have found that B7-H4 is positively associated with PD-L1, which may indicate that B7-H4 and PD-L1 as negative costimulatory molecules collaborate to undermine host antitumoral immunity. Our previous studies have demonstrated that PD-1/PD-L1 was over-expressed in Tgfbr1/Pten 2cKO mice OSCC model [39]. In this study, we also observed that B7-H4 was over-expressed in Tgfbr1/Pten 2cKO mice OSCC model, which is consistent with prior findings. However, we did not perform mouse experiments due to the lack of reagent of B7-H4 monoclonal antibody. In the future study, we will utilize our mice OSCC model to explore the function of B7-H4 and the relationship between B7-H4 and PD-L1 before clinical trial with available anti-B7-H4 monoclonal antibody.
In conclusion, B7-H4 expression in OSCC may represent a new predictor of prognosis. Meanwhile, the positive correlation among B7-H4 and PD-L1, CD11b, CD33, PI3Kα p110, and p-S6 (S235/236) is analyzed to preliminarily outline the B7-H4 associated molecules which may play a potential immune modulating role in tumor microenvironment. Our data also support the evidence that B7-H4 may be a novel target for molecular-targeted therapy against OSCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by National Natural Science Foundation of China 81272963, 81472528 (Zhi-Jun Sun), 81272964, 81472529 (Wen-Feng Zhang). Zhi-Jun Sun was supported by program for new century excellent talents in university (NCET-13-0439), Ministry of Education of China.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- ESCC
Esophageal squamous cell carcinoma
- MDSCs
Myeloid-derived suppressor cells
- OSCC
Oral squamous cell carcinoma
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- RCC
Renal cell carcinoma
- TPF
Cisplatin, docetaxel, and fluorouracil
- Tregs
Regulatory T cells
- WT
Wild type
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Lei Wu and Wei-Wei Deng have contributed equally to this work.
References
- 1.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ. He J (2016) Cancer statistics in China. CA Cancer J Clin. 2015;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65(5):401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Kong W, Peng Y, Miao Q, Mackillop WJ. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int J Cancer. 2009;125(9):2159–2165. doi: 10.1002/ijc.24533. [DOI] [PubMed] [Google Scholar]
- 6.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Costa AM, Schuyler CA, Walker DD, Young MR. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2012;61(6):927–939. doi: 10.1007/s00262-011-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreno BM, Collins M. BTLA: a new inhibitory receptor with a B7-like ligand. Trends Immunol. 2003;24(10):524–527. doi: 10.1016/j.it.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Fauci JM, Straughn JM, Jr, Ferrone S, Buchsbaum DJ. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127(2):420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 16.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Shibata K, Koya Y, Kajiyama H, Senga T, Yamashita M, Kikkawa F. B7-H4 overexpression correlates with a poor prognosis for cervical cancer patients. Mol Clin Oncol. 2014;2(2):219–225. doi: 10.3892/mco.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59(11):1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Sun ZJ, Bian Y, Kulkarni AB. MicroRNA-135b acts as a tumor promoter by targeting the hypoxia-inducible factor pathway in genetically defined mouse model of head and neck squamous cell carcinoma. Cancer Lett. 2013;331(2):230–238. doi: 10.1016/j.canlet.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 2012;18(19):5304–5313. doi: 10.1158/1078-0432.CCR-12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget. 2015;6(11):8807–8821. doi: 10.18632/oncotarget.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CF, Zhang L, Ma SR, Zhao ZL, Wang WM, He KF, Zhao YF, Zhang WF, Liu B, Sun ZJ. Clinical significance of Keap1 and Nrf2 in oral squamous cell carcinoma. PLoS One. 2013;8(12):e83479. doi: 10.1371/journal.pone.0083479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16(1):53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, Waes CV, Kulkarni AB. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2012;31(28):3322–3332. doi: 10.1038/onc.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17(10):3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 30.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 32.D’Amato V, Rosa R, D’Amato C, Formisano L, Marciano R, Nappi L, Raimondo L, Di Mauro C, Servetto A, Fusciello C, Veneziani BM, De Placido S, Bianco R. The dual PI3K/mTOR inhibitor PKI-587 enhances sensitivity to cetuximab in EGFR-resistant human head and neck cancer models. Br J Cancer. 2014;110(12):2887–2895. doi: 10.1038/bjc.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Yu S, Li H, Liu C, Li J, Lin W, Gao A, Wang L, Gao W, Sun Y. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett. 2015;589(17):2248–2256. doi: 10.1016/j.febslet.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Bian Y, Terse A, Du J, Hall B, Molinolo A, Zhang P, Chen W, Flanders KC, Gutkind JS, Wakefield LM, Kulkarni AB. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69(14):5918–5926. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, Chen L, Drake CG, Topalian SL, Pardoll DM, Pai SI. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, Gey A, Rotem-Yehudar R, Pere H, Tran T, Guerin CL, Chauvat A, Dransart E, Alanio C, Albert S, Barry B, Sandoval F, Quintin-Colonna F, Bruneval P, Fridman WH, Lemoine FM, Oudard S, Johannes L, Olive D, Brasnu D, Tartour E. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 38.Malaspina TS, Gasparoto TH, Costa MR, de Melo EF, Jr, Ikoma MR, Damante JH, Cavassani KA, Garlet GP, da Silva JS, Campanelli AP. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):965–974. doi: 10.1007/s00262-011-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ, Gutkind JS, Kulkarni AB, Sun ZJ. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPalpha axis in HPV negative head and neck squamous cell carcinoma. Oncotarget. 2015;6(39):42067–42080. doi: 10.18632/oncotarget.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.