Abstract
Midkine (MK) is a heparin-binding growth factor overexpressed in various human cancers. In the current study, a positive correlation was observed between MK expression and MICA/B serum levels of gastric cancer patients. In addition, MK transfection significantly increased MICA/B expression in gastric cancer cells. The soluble MICA/B expression was also elevated. Furthermore, MK transfection inhibited CD107a and Granzyme B expression, thereby suppressing the natural killer (NK) cell cytotoxicity in vitro. The phosphorylation of p38 MAPK and its promotion of CHOP expression were also observed after MK treatment and transfection. CHOP was indirectly bound to the MICA/B promoter region by interacting with AP-1, leading to MICA/B transcription. Overall, the current study shows that MK expression in tumor cells indirectly suppresses NK cytotoxicity by inducing MICA/B expression and suppressing NKG2D expression.
Keywords: MICA/B, Midkine, CHOP, P38, Tumor immune escape
Introduction
NKG2D is a stimulatory immunoreceptor expressed by natural killer (NK) cells and various T-cell subsets. It binds to several NKG2D ligands, including MICA and MICB, which are expressed at low levels on normal cells but frequently upregulated on tumor and virus-infected cells [1, 2]. MICA and MICB (MICA/B) activate NKG2D-expressing NK cells and T cells in vitro. Cleaved by matrix metalloproteases, MICA/B are released from the membrane as soluble molecules [3, 4], which directly block or downregulate NKG2D expression on lymphocytes [5–8]. Increased soluble MICA/B (sMICA/B) serum levels are always accompanied with impairment of T and/or NK cell functions and tumor development [6, 7, 9]. In addition, NKG2D-deficient mice show defective tumor cell clearance in spontaneous tumor models [10].
Midkine (MK) is a heparin-binding growth factor frequently expressed in many cancer cells. MK is involved in fibroblast transformation, cell growth, cell survival, cell migration, and angiogenesis [11, 12]. MK activates different signaling pathways in different cells using the receptor-like protein tyrosine phosphatase-ζ(PTPζ), low-density lipoprotein receptor-related protein (LRP) and α4β1-integrin and α6β1-integrin, mainly via the PI3 kinase and MAP kinase pathways [13]. In the present study, the effect of MK on the promotion of MIC/B expression is investigated using the human gastric cancer cell line BGC823. The microarray assay shows different gene expression profiles in gastric adenocarcinoma BGC823 cell after MK transfection. Significant differences in gene expression were found, including CHOP (C/EBP homologous protein 10), MICA/B, matrix metalloproteinase PLAUR, and MMPL1 genes [14]. The current study further confirms the positive correlation between MK and MICA/B in the sera of gastric cancer patients. In addition, MK upregulates CHOP expression via the P38-MAPK signal pathway and promotes the formation of more CHOP–AP-1 complexes to increase MICA/B expression. Increased sMICA/B inhibits the cytotoxicity of NK cells. Overall, the overexpression of MK plays an essential role in breaking up immune surveillance by upregulating the MICA/B expression and inhibiting NK cytotoxicity.
Materials and methods
Patients and serum sample
Serum from gastric cancer patients (n = 24) and healthy controls (n = 18) were collected from the Nanjing First Hospital (Nanjing, P. R. China). The mean age of the patients and the healthy controls was 56 and the male to female ratio was 1:1. None of the patients received any preoperative treatment. Informed consents were obtained from all patients. The current study was approved by the Research Ethical Committee of Nanjing First Hospital, China.
Cells culture and reagents
The BGC823 and K562 cells were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The human NK cell line NK-92 was a gift from Professor Haiming Wei (School of Life Sciences, University of Science and Technology of China). These cells were cultured in RPMI 1640 (Gibco BRL) supplemented with 10% fetal bovine serum (Gibco BRL), streptomycin (100 μg/mL), and penicillin (100 μg/mL) (Sigma). The NK-92 cells were cultured in minimum essential medium α-medium supplemented with 10% bovine calf serum, 10% equine serum, and 100 units/mL rhIL-2. The Anti-MICA/B-R-phycoerythrin (Clone # 6D4), anti-CD107a-fluorescein isothiocyanate (Clone # H4A3), anti-NKG2D-Allophycocyanin (Clone # 1D11), and anti-Granzyme B-FITC (Clone # GB11) were purchased from BD PharMingen. The isotype-matched negative control antibodies labeled with FITC and PE were purchased from BD PharMingen and used in all the experiments.
Knockdown of endogenous MK in BGC823 cells by small interfering RNA (siRNA)
The specific RNAi sequence for human MK (the same as that used in previous study) [15] was synthesized by Invitrogen (Life Technologies, USA). siRNA was transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In brief, approximately 3 × 104 × 104 cells/well were grown overnight in 24-well plate. When the cells reached 30–50% confluence, they were transfected with anti-MK-siRNA (100 nM) and negative control siRNA (100 nM) in serum-free medium using Lipofectamine 2000. After incubation for 4 h at 37°C, 400 μl RPMI 1640 with 10% FBS was added. The cells were collected for further testing after 48 h.
Enzyme-linked immunosorbent assay (ELISA)
The serum was immediately frozen and kept under −70°C for future measurements. The serum levels of soluble MK, MICA, and MICB from healthy donors and gastric cancer patients were detected via human MK, MICA, and MICB DuoSet ELISA development kits (R&D) following the manufacturer’s instructions.
Comparative gene expression profiling
Human genome arrays (CapitalBio Corp., P. R. China) were used for the comparative gene expression profiling study between the BGC823-3.1 and BGC823-MK cells. Total RNA was extracted from 1 × 107 cells using Trizol reagent (Invitrogen); the synthesis and labeling of target cRNA probes were performed using a cRNA amplification labeling kit (CapitalBio Corp., Beijing, China). The labeled cRNA target was fragmented and hybridized according to manufacturer’s standard protocols. The hybridized image was scanned using the LuxScan10KA dual channel laser scanner and quantified using the LuxScan 3.0 image analysis software (CapitalBio Corp., P. R. China). Analysis of differential gene expression was conducted and different genes with variation ratios of >2 or <0.5 were chosen for validation via real-time reverse transcriptase–polymerase chain reaction (RT-PCR).
RT-PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies) and reverse-transcribed using SuperScript III reverse transcriptase kit (Invitrogen, Life Technologies) according to standard protocols. Quantitative RT-PCR analysis was performed using a Brilliant SYBR Green QPCR Master kit (Stratagene) and run on Stratagene Mx3000P thermocycler. The primer sequences are as follows: MICA, 5′-CCTTGGCCATGAACGTCAGG-3′ and 5′-CCTCTGAGGCCTCRCTGCG-3′; MICB, 5′-ACCTTGGCTATGAACGTCACA-3′ and 5′-CCCTCTGAGACCTCGCTG CA-3′; MK, 5′-ATGCAGCACCGAGGCTTCCT-3′ and 5′-ATCCAGGCTTGGC GTCTAGT-3′; and CHOP, 5′-GCTCTAGAGGGCTGCAGAGATGGC-3′ and 5′-GGAATTCGGGACTGATGCTCCCA-3′. GAPDH, 5′-GGTGAAGGTCGGAGT CAACG-3′ and 5′-CAAAGTTGTCATGGATGACC-3′.
Flow cytometry analysis
The cells were collected by trypsination, washed twice with phosphate-buffered saline, and incubated under saturating anti-MICA/BmAbs-PE concentrations for 30 min at 4°C. The cells were then washed twice and analyzed on a FACS Calibur flow cytometer using Cell Quest software (Becton–Dickinson).
NK cytotoxicity assay
The cytotoxicity of the NK92 cells was measured as previously described [[2], [6]]. Briefly, the K562 cells were labeled using 5.0 μM carboxyfluorescein diacetate succinimidyl ester in 5% CO2 for 8 min at 37°C. The cells were then washed twice and resuspended in RPMI 1640. The target ratios were set to 5:1. The NK92 cells were treated with transfected cell supernate and MICA/B neutralizing antibodies at 37°C for 24 h. The target cells were mixed with conditioned medium to a final volume of 200 μL in a 96-well flat-bottom plate and incubated at 37°C in 5% CO2 for 4 h. The percentage of propidium iodide-stained cells was determined via flow cytometry.
Western blot analysis
The BGC823, BGC-3.1, BGC-MK, and BGC-MK (treated by SB203580) cells were lysed in buffer containing 50 mmol/L Tris–Cl, pH 8.0, 150 mmol/L NaCl, 0.02% NaN3, 0.1% SDS, 100 mg/L phenylmethylsulfonyl fluoride, 1 mg/L aprotinin, and 1% Triton. After centrifugation, the cell lysates (120 μg/lane) were subjected to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked for 1 h in PBST (10 mmol/L Tris–HCl, pH 7.4, 150 mmol/L NaCl, 0.05% Tween-20) containing 2% nonfat dry milk. Antibodies specific for CHOP (Santa Cruz Biotechnology), P38, phosphorylated P38 (Cell Signaling Technology), GAPDH (Santa Cruz Biotechnology), and horseradish peroxidase-conjugated goat anti-rabbit (and anti-mouse) secondary antibodies (Santa Cruz Biotechnology) were used. The proteins were detected using the enhanced chemiluminescence reaction (Kibbutz BeitHaemek).
Activation of intracellular signaling pathways by exogenous MK
A total of 3 × 106 BGC823 cells in 10 mL 10% FBS medium per dish were plated in 100 mm dishes. After 16 h, the medium was replaced with serum-free DMEM, and culture was allowed to stand for another 16 h. Recombinant human MK (0, 25, 50, 100, and 200 ng/mL) was added to the cells, and the culture was allowed to stand for 4 h. The cells were then harvested for Western blot and flow cytometric analyses.
Chromatin immunoprecipitation (ChIP) and immunoprecipitation (IP)
The BGC823, BGC-3.1, and BGC-MK cells were processed for ChIP (the CHIP kit, Beyotime Institute of Biotechnology, China) and IP assays following the manufacturer’s instructions. IPs used mixed rabbit and mouse IgG and anti-CHOP monoclonal antibodies (Santa Cruz Biotechnology). The immunocomplexes were collected using protein A/G agarose beads and sequentially washed, as detailed in the manufacturer’s protocol. The chromatin was eluted with 1% SDS/0.1 mM NaHCO3 and digested with proteinase K; cross-linking was reversed for 5 h in 200 mM NaCl at 65°C. The DNA was purified using Qiaquick Gel Extraction kit (Qiagen) and dissolved in 100 μL Tris–EDTA. The fractions of precleaved chromatin were processed similar to the immunoprecipitated chromatin for the input DNA controls. The forward and reverse primers for PCR amplification were 5′-CTTCTAAATCTCCCCAGGTCTCCAG-3′ and 5′-TGCCAGCCAGAAGCAGG AAGACC-3′ for MICA, and 5′-CGTGGCCCCGCCCTCTCCACTC-3′ and 5′-AGGCGACGGCCAGAAACAGCAG-3′ for MICB.
Statistical analysis
The SPSS statistical program was used to test the correlation between MK and MICA/B. Statistical significance was assessed via the one-way ANOVA and ANOVA post hoc Bonferroni test using the SPSS 12.0 software (Statistical Product and Service Solutions Inc). Differences with P < 0.05 were considered statistically significant.
Results
MK induced elevated MICA/B expression in gastric patients
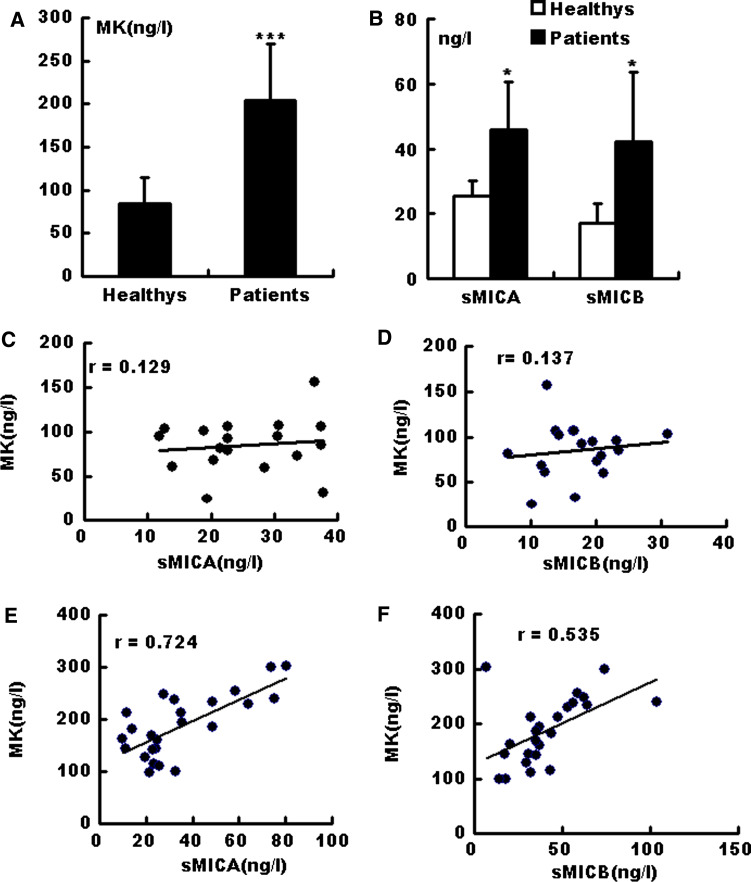
First, the MK, MICA, and MICB concentrations were measured in healthy individuals (18 cases) and gastric cancer patients (24 cases). The MK levels in the gastric cancer patients were significantly higher than those in healthy donors (Fig. 1a). The MICA/B levels in gastric cancer patients were also higher compared with those in healthy (Fig. 1b). A correlation test was performed on the sera levels of MICA/B and MK. No correlation was observed between MK and MICA/B expression in the healthy donors (Fig. 1c, d). However, the expression of MICA (Fig. 1e) and MICB (Fig. 1f) was positively correlated with the MK levels in cancer patients.
Fig. 1.
Correlation analysis of serum MK and sMICA/B in healthy donors and gastric cancer patients. Serum MK (a), sMICA and sMICB (b) levels were measured via ELISA. Data are shown as mean ± SEM from 18 healthy individuals or 24 gastric cancer patients. Correlation between MK and MICA (c, d)/MICB (e, f) levels in healthy individuals (c, e) and gastric cancer patients (d, f) were further analyzed. The Pearson correlation coefficient R is shown for each pair. ***P < 0.01; *P < 0.05
Microarray analysis of the BGC823 cells before and after MK overexpression
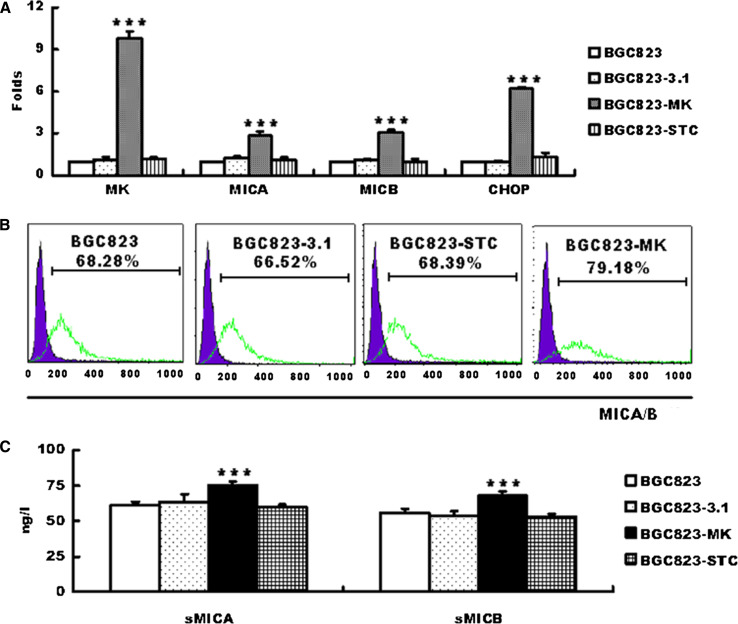
BGC823 cells (gastric cancer cell line) were transfected with the pcDNA3.1-MK or pcDNA3.1-vector plasmids, and microarray analysis was conducted to verify further whether MK induces MICA/B expression. There are 550 differentially expressed genes, 407 upregulated genes, 143 downregulated genes, including CHOP, MICA, MICB, and two matrix metalloproteinases (MMPL1, PLAUR). 18 genes were confirmed via quantitative RT-PCR in transfected cells [16] (Fig. 2a).
Fig. 2.
Midkine induces MICA/B expression in BGC823 cells. BGC823 cells were transfected with pcDNA3.1/vector, pcDNA3.1/MK, and pcDNA3.1/STC. Total RNA was then extracted and used for quantitative RT-PCR. MK, MICA/B, and CHOP mRNA expression were normalized to the expression of the housekeeping gene GAPDH and displayed as fold expression relative to the control (a). The graph shows the percentages of apoptotic cells, which represent the mean ± SEM of three independent experiments. Expression of the surface MICA/B on BGC823, BGC823-3.1, BGC823-STC, BGC823-SCT, and the sMICA and sMICB levels were detected in the cultured supernates via ELISA. The data are shown as means ± SEM of three independent experiments. ***P < 0.01 versus control
The soluble and the membrane-bound MICA/B protein expression induced by MK transfection were then tested. MICA/B expression dramatically increased on the MK-transfected cell surface (Fig. 2b). In addition, the culture supernate from the MK-transfected cells showed significantly higher MICA/B levels (Fig. 2c). Exposure to high MK levels did not increase the expression of other NKG2D ligands (ULBP1-4) (Data not shown).
Soluble MICA/B decreases NKG2D expression and NK cytotoxicity
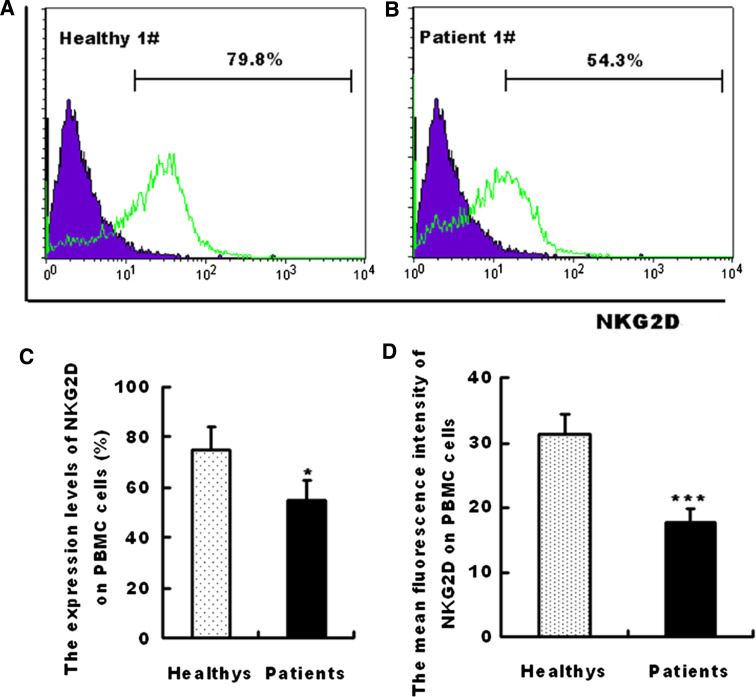
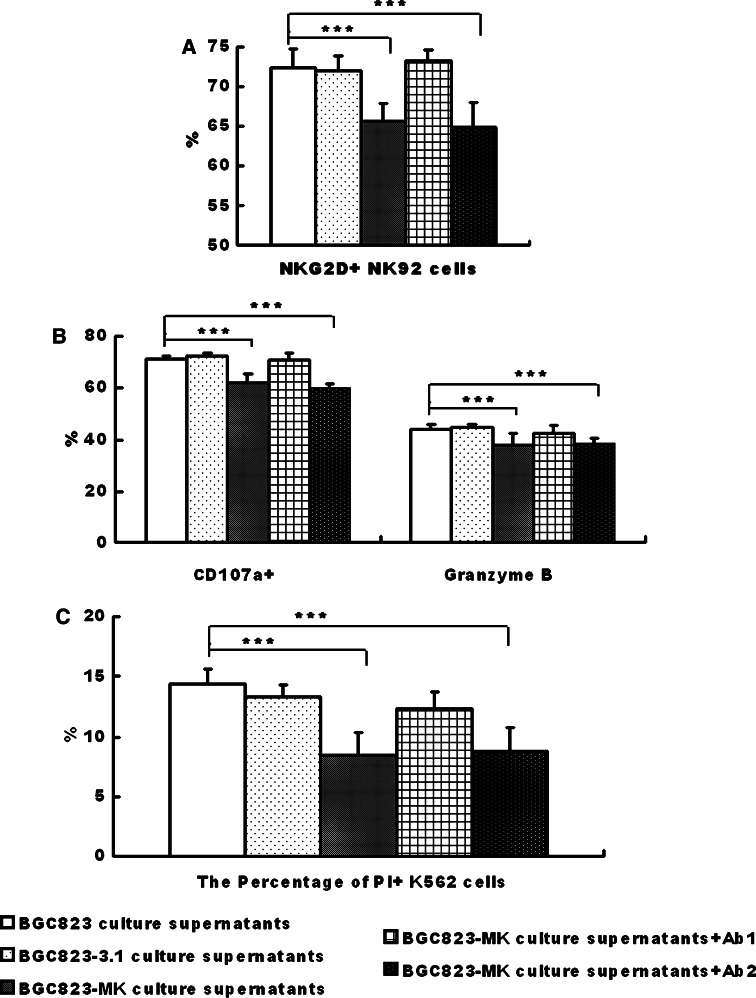
The NKG2D expression levels on the peripheral blood mononuclear cells (PBMCs) in the serum were compared between healthy subjects (Fig. 3a) and patients (Fig. 3b). Interestingly, the NKG2D expression on the PBMCs of the gastric cancer patients was significantly lower than that from healthy subjects (Fig. 3c, d). The soluble MICA/B induces endocytosis and degradation of NKG2D on NK cells [8]. The NK92 cells were incubated with the cultured supernates of BGC823-3.1 and BGC823-MK cells to test whether the decreased NKG2D was due to MK overexpression on the cancer cells. The NKG2D expression on NK92 cells was significantly lower in the MK-transfected groups compared with the control group (Fig. 4a). The decreased NKG2D expression caused by the soluble MICA/B expression was validated, wherein the neutralizing MICA/B antibodies blocked this decline (Fig. 4a).
Fig. 3.
Decreased NKG2D expression levels in gastric cancer patients. NKG2D expression on the peripheral blood mononuclear cells of a healthy individual (a) and a patient (b) were quantified via flow cytometry. The percentage of positive expression and mean fluorescence intensity were also represented as the mean ± SEM of 18 healthy individuals and 24 gastric patients. ***P < 0.01; *P < 0.05
Fig. 4.
The soluble MICA/B in the supernate reduced the NKG2D receptor expression in the NK92 cells and inhibited the cytotoxicity of NK92 cells against K562 cells. NK92 cells were incubated with the cultured supernates of BGC823, BGC823-3.1, and BGC823-MK cells. The cytotoxicity (a) and the expression of CD107a (b) and Granzyme B (c) were measured via flow cytometry. The bar graph shows means ± SD from three independent experiments. ***P < 0.01; *P < 0.05
The cytotoxicity of the NK92 cell cultured with the supernates was further checked. The cytolytic activity of the NK92 cells was significantly decreased when the K562 cells were cultured with the BGC823-MK supernate (Fig. 4b). Simultaneously, the expression of CD107a and Granzyme B on NK92 cells was also inhibited in the culture with the BGC823-MK supernate (Fig. 4c). In addition, the MICA/B neutralizing antibodies reversed these inhibitory effects (Fig. 4c).
MK-p38-CHOP/AP1-MICA/B signal pathway analysis
Microarray analysis revealed that several transcription factors are altered by MK overexpression. A notable transcription factor was CHOP (Ratio = 8.6).
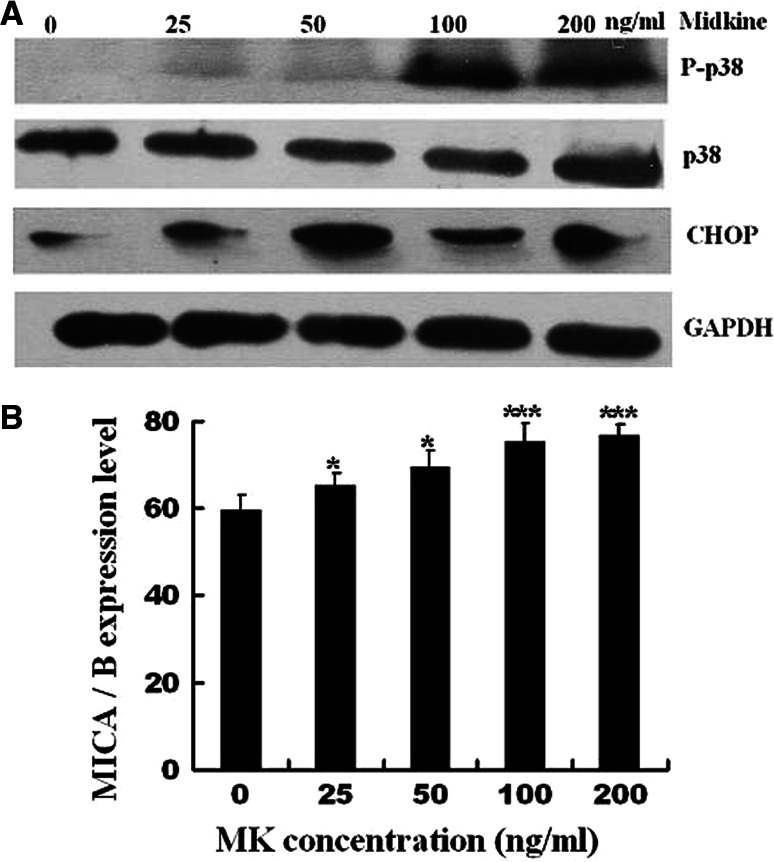
Figure 5a, b show that exogenous recombinant MK caused p38 phosphorylation of the BGC823 cells starting 25 ng/mL and gradually increasing to a peak at 100 ng/mL. CHOP and MICA/B gradually increased in a dose-dependent manner.
Fig. 5.
Activation of intracellular signaling pathways by exogenous MK. CHOP, P38, and phosphorylated P38 expression in BGC823 cells treated with 0, 25, 50, 100, and 200 ng/mL exogenous MK were analyzed via Western blot analysis (a). MICA/B expression in BGC823 cells treated with 0, 25, 50, 100, and 200 ng/mL exogenous MK were analyzed via flow cytometry. The bar graph shows the means ± SD from three independent experiments. ***P < 0.01; *P < 0.05
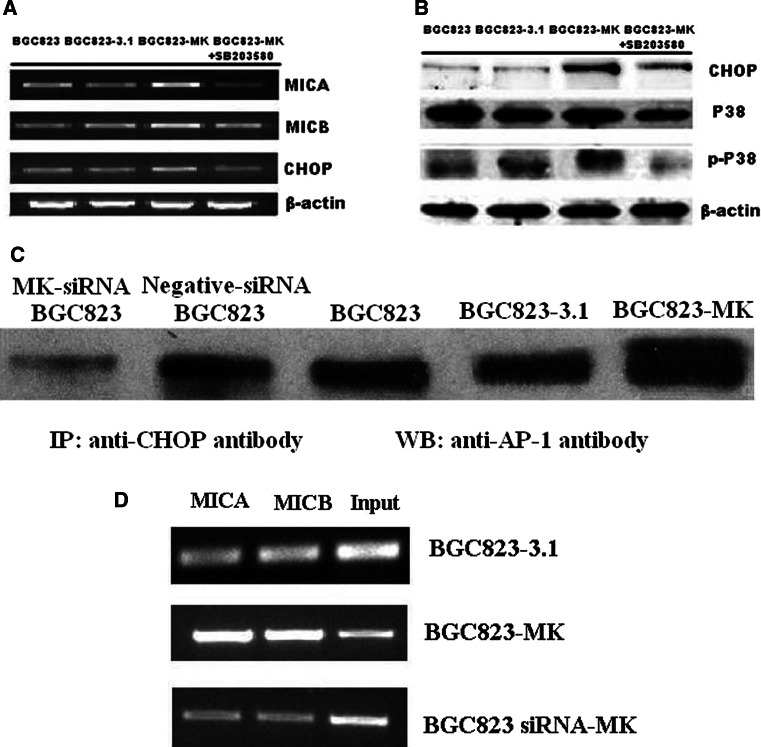
The upregulation of CHOP on the BGC823-MK cells at the mRNA level was tested via RT-PCR and the protein levels by Western blot analysis (Fig. 6a, b). However, the levels of MICA/B mRNA and protein did not decline in MK-siRNA BGC823 cells (Data not shown). The binding of the AP-1/CHOP complex to the promoter region of MICA and MICB was investigated via IP and ChIP assays using BGC823, BGC823-3.1, BGC823-MK, MK-siRNA, and negative-siRNA BGC823 cells. As shown in Fig. 6c, CHOP binds to the transcription factor AP-1 in BGC823, BGC823-3.1, and BGC823-MK cells, and the CHOP/AP-1 polymers in BGC823-MK cells were prominent. The DNA fragments were purified using CHOP Abs. A PCR was done to check whether the CHOP/AP-1 polymers cross-linked with the MICA/B promoter regions. In the ChIP assay, both the MICA/B promoter fragments and fragment copies were positively correlated with MK expression (Fig. 6d). These results indicate that the CHOP/AP-1 protein complex bind to the MICA/B promoter region and regulates MICA/B expression in BGC823 cells.
Fig. 6.
The CHOP-AP-1 complex in the MICA promoter region. MICA, MICB, and CHOP mRNA expression in BGC823, BGC823-3.1, BGC823-MK, and BGC823-MK cells treated with the P38 signal pathway inhibitor SB203580 were analyzed via RT-PCR (a). CHOP, P38, and phosphorylated P38 expression in BGC823, BGC823-3.1, BGC823-MK, and BGC823-MK cells treated with SB203580 were analyzed via Western blot analysis (b). Immunoprecipitates obtained using antibodies against CHOP were further blotted with antibodies against AP-1 (c). The ChIP assay shows that increased amounts of the CHOP–AP-1 complex were present near the promoter of MICA/B in the BGC823-MK cells compared with the control cells (d)
Discussion
The activating receptor NKG2D and its ligands play an important role in NK, γδ+, and CD8+ T-cell-mediated immune response to tumors [14, 17]. The engagement of NKG2D ligands onto tumor cells triggers the elimination of tumor cells by effector cells. However, with NKG2D ligand-expressing cells, tumor still develops in immunocompetent animals. Therefore, a mechanism to help MICA/B expression on tumor cells escape immune surveillance must be investigated.
Firstly, a positive correlation between MK and soluble MICA/B levels in cancer patients, which were higher than healthy people [18, 19], was confirmed and a hypothesis regarding the upregulation of MICA/B production was made. The BGC823 cells were transfected with the MK gene to investigate the role of MK in MICA/B expression. The results of the microarray, RT-PCR, ELISA, and FCM analysis indicated that MK enhances membrane and secreted MICA/B expression on transfected cells.
The MK and MICA/B expression levels in the MKN-28, MKN-45, AGS, and BGC823 cells were analyzed via Q-PCR. The endogenous MK was lowest in MKN-28, which had lowest MICA/B expression. However, no such clear correspondence was note in other three cells, possibly because MICA/B expression was also regulated by other factors (data not shown).
Although the microarray analysis confirmed that MK upregulates matrix metalloproteinase expression (MMPL1 and PLAU), the expression of these genes was not confirmed by Western blot analysis and quantitative PCR studies.
The current study also confirmed that NKG2D expression on PBMC cells in gastric cancer patients is significantly lower than that in the healthy controls. However, the NKG2D expression on the PBMC cells showed no difference between advanced and early gastric cancer. The decreased NKG2D expression on the immune cells may be one of the key mechanisms responsible for immune cell dysfunction in gastric cancer.
Membrane MICA/B triggers the cytotoxicity of NK and γδT cells via the engagement of NKG2D. Soluble MICA/B induces the endocytosis and degradation of NKG2D to promote the immunologic escape [4, 8]. Both membrane and soluble MICA/B were increased by MK. The increased membrane MICA/B did not enhance NK92 cell cytotoxicity to BGC823-MK cells (Data not shown). The NKG2D expression on NK92 cells was significantly decreased when the NK92 cells were preincubated with BGC823-MK cell culture medium, in which sMICA/B was increased. The cytotoxicity of the preincubated NK92 cells against the K562 target cells was also significantly decreased. Moreover, the anti-MICA/B neutralizing antibodies blocked this decline. These results support the hypothesis that soluble MICA/B inhibits the cytotoxicity of NK cells.
A recent research has shown that MICA expression can be modulated by the transcription factor STAT-3 [20]. In the present study, CHOP, another transcription factor, was elevated in the BGC823-MK cells. However, no CHOP binding site was found in the MICA/B promoter regions. An AP-1-TATA-like motif was then found upstream of their transcriptional initiation. Previous studies have shown that CHOP interacts with AP-1 transcription factor to activate promoter elements [21, 22]. The p38 signal pathway in BGC823 was dramatically activated by exogenously recombinant human MK in a dose-dependent manner. In addition, a higher number of CHOP forms a complex with AP-1, binding to the MICA/B promoter regions to activate the expression of MICA/B. The current study demonstrates that MK regulates the MICA/B expression through the P38-CHOP/AP pathway and inhibits NK cell cytotoxicity.
In summary, the molecular mechanism that triggers the regulation of MICA and MICB expression by MK has been proven. MK induces CHOP expression by activating the P38 signaling pathway, and the increasing CHOP forms a complex with AP-1 to increase MICA/B transcription. These data elucidated a new role for MK in tumor development and it provides new insights into the regulation of MICA/B during immune surveillance.
Acknowledgments
This work was supported by Medical Science and technology development Foundation, Nanjing Department of Health (Grant #: YKK09054), Science and Technology Project of Jiangsu Provincial Department of Health (Grant #: H201037, KF200928). We thank Dr. Hong Zhou for his helpful discussions and comments on the manuscript.
Footnotes
Shuli Zhao and Huiuan Wang contributed equally to this work.
Contributor Information
Xingguo Chen, Phone: +86-25-52887029, FAX: +86-25-52887029, Email: chenxingguo16@yahoo.com.cn.
Yayi Hou, Email: yayihou@nju.edu.cn.
References
- 1.Obeidy P, Sharland AF. NKG2D and its ligands. Int J Biochem Cell Biol. 2009;41:2364–2367. doi: 10.1016/j.biocel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 3.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 4.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 5.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006;66:2520–2526. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 6.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 7.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother. 2006;55:1584–1589. doi: 10.1007/s00262-006-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebmann V, Schutt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR, Grosse-Wilde H. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin Immunol. 2007;123:114–120. doi: 10.1016/j.clim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Rao GS, Groh V, Spies T, Gattuso P, Kaufman HL, Plate J, Prinz RA. Major histocompatibility complex class I-related chain A/B (MICA/B) expression in tumor tissue and serum of pancreatic cancer: role of uric acid accumulation in gemcitabine-induced MICA/B expression. BMC Cancer. 2011;11:194–205. doi: 10.1186/1471-2407-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 12.Dai LC. Midkine translocated to nucleoli and involved in carcinogenesis. World J Gastroenterol. 2009;15:412–416. doi: 10.3748/wjg.15.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muramatsu T (2010) Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. In: Proceedings of the Japan Academy, Series B, 86, 2010 [DOI] [PMC free article] [PubMed]
- 14.Andresen Lars. Karen Aagaard Hansen, Helle Jensen, Stine Falsig Pedersen, Peter Stougaard, Helle Rusz Hansen, Jesper Jurlander and Søren Skov. Propionic Acid Secreted from Propionibacteria Induces NKG2D Ligand Expression on Human-Activated T Lymphocytes and Cancer Cells. J Immunol. 2009;183:897–906. doi: 10.4049/jimmunol.0803014. [DOI] [PubMed] [Google Scholar]
- 15.Wang Qingling, Huang Yahong, Ni Yanhong, Wang Hui, Hou Yayi. siRNA targeting midkine inhibits gastric cancer cells growth and induces apoptosis involved caspase-3,8,9 activation and mitochondrial depolarization. J Biomed Sci. 2007;14:783–795. doi: 10.1007/s11373-007-9192-0. [DOI] [PubMed] [Google Scholar]
- 16.Yayi H. Molecular basis of the effect of midkine on tumor growth in human gastric cancer cell BGC823. Biomed Pharmacother. 2008;62:422. [Google Scholar]
- 17.Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother. 2009;58:641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 20.Bedel R, Thiery-Vuillemin A, Grandclement C, Balland J, Remy-Martin JP, Kantelip B, Pallandre JR, Pivot X, Ferrand C, Tiberghien P, Borg C (2011) Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res [DOI] [PubMed]
- 21.Ubeda M, Vallejo M, Habener JF. CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol Cell Biol. 1999;19:7589–7599. doi: 10.1128/mcb.19.11.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]