Abstract
T cell Ig and ITIM domain (TIGIT) is a newly identified inhibitory receptor expressed on T and natural killer (NK) cells. Cytokine-induced killer (CIK) cells express CD3 and CD56 molecules, and share functional properties with both NK and T cells. However, it remains unknown whether TIGIT is expressed in CIK cells. Here, we show that TIGIT is expressed by CIK cells and interacts with CD155. By blocking TIGIT using an anti-TIGIT functional antibody, we demonstrate that CIK cells display increased proliferation; higher cytotoxic targeting of tumor cells expressing CD155; and higher expression of interferon-γ (IFN-γ), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Furthermore, increases in IFN-γ and cytotoxicity by blockade of TIGIT were reduced by blocking DNAX accessory molecule-1 (DNAM-1) signaling, implying that TIGIT exerts immunosuppressive effects by competing with DNAM-1 for the same ligand, CD155. Our results provide evidence that blockade of TIGIT may be a novel strategy to improve the cytotoxic activity of CIK cells.
Keywords: TIGIT, CD155, DNAM-1, CIK cells, Immunotherapy
Introduction
Cytokine-induced killer cells were first characterized by Schmidt-Wolf et al. [1–3]. These authors expanded the cells in culture from peripheral blood mononuclear cells (PBMCs) that were primed with IFN-γ and activated with interleukin-2 (IL-2) and monoclonal antibody to OKT3. CIK cells share phenotypic and functional properties with both natural killer (NK) and T cells by co-expressing CD3 and CD56 [4, 5]. CD3+CD56+ double-positive cell subsets are the most relevant effector cells in the bulk CIK cell population and show major histocompatibility complex (MHC)-unrestricted cytotoxicity toward neoplastic cells but not normal cells [2, 6, 7].
The ease of CIK cell production in vitro and their antitumor potential have made them suitable candidates for adoptive cell immunotherapy programs. Indeed, both autologous and allogeneic CIK cells have been evaluated in phase I and II clinical trials for the treatment for various hematopoietic and solid tumors [8–17]. However, clinical efficacy has been modest to date and limited by suboptimal tumor cell killing [18, 19]. Therefore, novel approaches to improve the antitumor activity of CIK cells are needed to achieve better clinical efficiency.
T cell Ig and ITIM domain is a newly identified inhibitory receptor that is mainly expressed on NK and T cells, particularly memory T cells, follicular T helper cells, and Treg cells [20–25]. It is a CD28 family protein consisting of an extracellular IgV domain, transmembrane domain, and cytoplasmic tail containing two ITIMs (immunoreceptor tyrosine-based inhibition motifs) [25, 26]. Poliovirus receptor (PVR or CD155) was identified as the ligand of TIGIT [22, 24]. Kuchroo et al. [18, 27–29] showed that TIGIT has intrinsic effects on T cells and directly inhibits T cell proliferation. Moreover, TIGIT inhibits NK cell cytolysis through engagement of CD155 [24, 30]. DNAM-1 expression on activated NK cells has a role in enhancing cytotoxic functions by competing with TIGIT for CD155 [31].
Here, we examined in detail the function of TIGIT on CIK cells. We show that TIGIT and CD155 are expressed by CIK cells. Using an anti-TIGIT antibody, we demonstrated a direct inhibitory effect on CIK cell proliferation by TIGIT. In addition, we found that antibody blockade of TIGIT or CD155 enhanced the cytotoxicity of CIK cells upon encountering CD155-expressing tumor cells. We also demonstrate TIGIT-mediated inhibition of cytokine production with decreases in IFN-γ, IL-6, and TNF-ɑ. Furthermore, we found that the increases in IFN-γ and cytotoxicity by TIGIT blockade were suppressed by blocking DNAM-1 signaling.
Materials and methods
Cell lines and antibodies
The human melanoma cell line A375 was grown in DMEM (GIBCO BRL, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (FBS, GIBCO BRL) as described [32]. The human renal carcinoma cell line 786-O and human chronic myelogenous leukemia cell line K562 were obtained from the Shanghai Cell Collection (Shanghai, China). 786-O and K562 cells were cultured at 37 °C with 5 % CO2 in RPMI-1640 medium (GIBCO BRL) containing 10 % FBS, 2 mM l-glutamine, and 100 U/ml penicillin/streptomycin. Antibodies used were as follows: anti-TIGIT and antihuman TIGIT functional-grade purified (eBioscience, San Diego, USA); anti-CD155, anti-DNAM-1, and purified antihuman CD155 (BioLegend, CA, USA); and anti-CD3, anti-CD4, anti-CD8, and anti-CD56 (BD PharMingen, San Diego, CA, USA).
CIK cell culture
Human PBMCs were isolated from four healthy volunteers using Ficoll separation solution (Biochrom, Berlin, Germany). This study was approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical College, Xuzhou, China, according to the guidelines of the Declaration of Helsinki. To generate CIK cells, the PBMCs were cultured in complete medium consisting of RPMI-1640, 10 % FBS, and 1000 U/ml recombinant human IFN-γ, (PeproTech, Rocky Hill, USA). After 24 h, the cells were treated with 30 ng/ml anti-CD3 monoclonal antibody (OKT-3, eBioscience) and 300 IU/ml recombinant human IL-2 (PeproTech). Fresh complete medium containing 300 IU/ml IL-2 was added every 2–3 days. Cells were harvested at days 0, 4, 7, 10, 14, 18, 21, 28, and 35 and characterized for standard CIK cell markers by flow cytometry. On day 10, CIK cells were treated with 2 μg/ml anti-TIGIT functional-grade purified antibody, or 2 μg/ml mouse IgGl isotype control.
Flow cytometric analysis
Flow cytometry was performed on a FACS Canto (Becton–Dickinson, San Jose, USA). Briefly, 1 × 106 cells were harvested, washed, and resuspended in 100 μl PBS, and then, 5 μl FITC- and/or PE-conjugated antibodies were added to each sample. After incubation at 4 °C for 30 min, the cells were washed with PBS, resuspended in 300 μl buffer, and analyzed by flow cytometry.
Cell proliferation assay
Cell proliferation was assessed by staining with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Carlsbad, USA). CIK cells were stained with CFSE prior to treatment with the anti-TIGIT antibody at day 10. The cells were analyzed by flow cytometry after 8 days in culture. Briefly, on day 10, CIK cells were harvested, washed, and resuspended in 1 ml PBS with 1 % BSA at a final concentration of 5 × 106 cells/ml, and then labeled for 10 min at 37 °C with 2 mM CFSE. The staining was stopped by incubation on ice for 5 min in five volumes of ice-cold complete medium. The cells were then washed three times with ice-cold PBS containing 1 % BSA and returned to culture under appropriate conditions. CFSE staining was analyzed using a FACScan.
Apoptotic cell detection
Apoptotic cells were detected by an Annexin-V Apoptosis Detection Kit FITC (eBioscience, Santiago, USA) according to the manufacturer’s instructions. The cell pellet was resuspended in 1 × annexin-binding buffer (ABB). An aliquot of 5 µl Annexin-V fluorescent dye was added to the sample, followed by incubation for 15 min at room temperature. The sample was washed and resuspended in 200 µl ABB. Then, 2 µl of 10 mg/ml propidium iodide dye was added to the sample, followed by incubation for 15 min on ice. After incubation, the sample was subjected to flow cytometric analysis.
Cytotoxicity assay
To determine specific cytotoxicity, we used the CytoTox 96 Nonradioactive Cytotoxicity assay (Promega, Madison, USA) based on calorimetric detection of the released enzyme lactate dehydrogenase (LDH). Target cells were harvested, washed, counted, and diluted to 2 × 105 cells/ml, and then 50 µl/well was added to a 96-well plate. Effector CIK cells were added at effector-to-target cell ratios of 5:1, 10:1, 20:1 and co-cultured for 4 h. All conditions were assayed in quadruplicate. After 4 h of incubation at 37 °C, 50 µl of supernatants was assayed for LDH activity following the manufacturer’s protocol.
Determination of cytokine levels
Secreted levels of IFN-γ, IL-6, and TNF-α were determined by ELISA using commercially available ELISA kits (R&D, Minneapolis, MN USA) as described [33].
Statistical analysis
Values are expressed as the mean ± standard deviation. Statistical analysis was performed using the Student’s t test, one-way analysis of variance followed by Duncan’s new multiple range method, or the Newman–Keuls test. P values of less than 0.05 were considered significant.
Results
Characterization of CIK cells
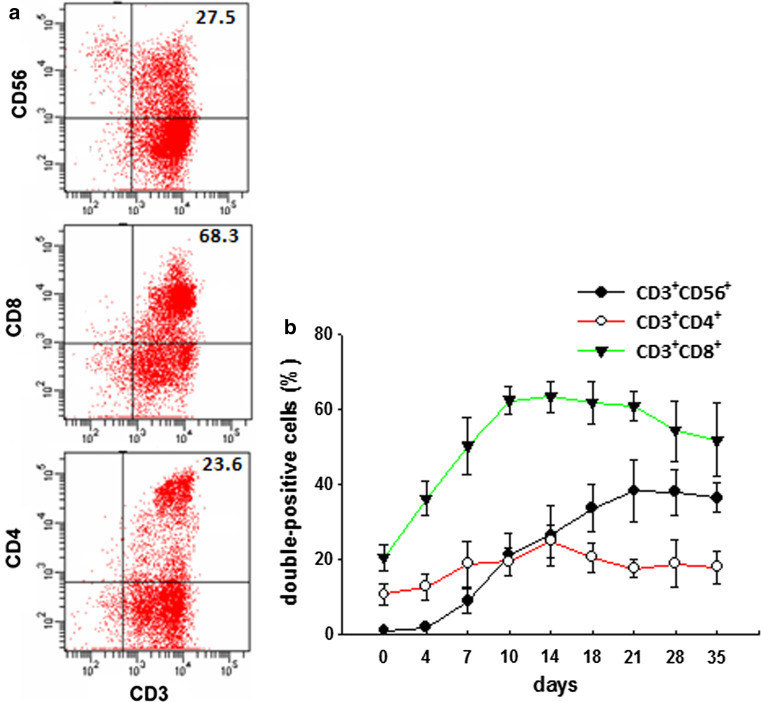
The phenotype of CIK cell was analyzed by flow cytometry at days 0, 4, 7, 10, 14, 18, 21, 28, and 35 of culture. The percentages of CD3+CD8+, CD3+CD4+, and CD3+CD56+ double-positive cells are shown in Fig. 1. The proportion of CD3+ CD56+ cells steadily increased during culture, peaked at 21 days, and was maintained thereafter (Fig. 1b).
Fig. 1.
Phenotypic analysis of CIK cells during culture. PBMCs were isolated from four healthy human volunteers and stimulated with IFN-γ, OKT-3, and IL-2, and cultured for 35 days. FACS analysis was performed at days 0, 4, 7, 10, 14, 18, 21, 28, and 35 of culture. a The percentage of CD3+CD8+, CD3+CD4+, CD3+CD56+ double-positive cells is shown at day 14 of culture. Data shown are representative of at least four independent experiments. b The line graphs display time course of expression of CD3+CD8+, CD3+CD4+, and CD3+CD56+ double-positive cells in the CIK cell population. Data are representative of four different donors. Mean values are shown ± SD
TIGIT, DNAM-1, and CD155 are expressed on CIK cells
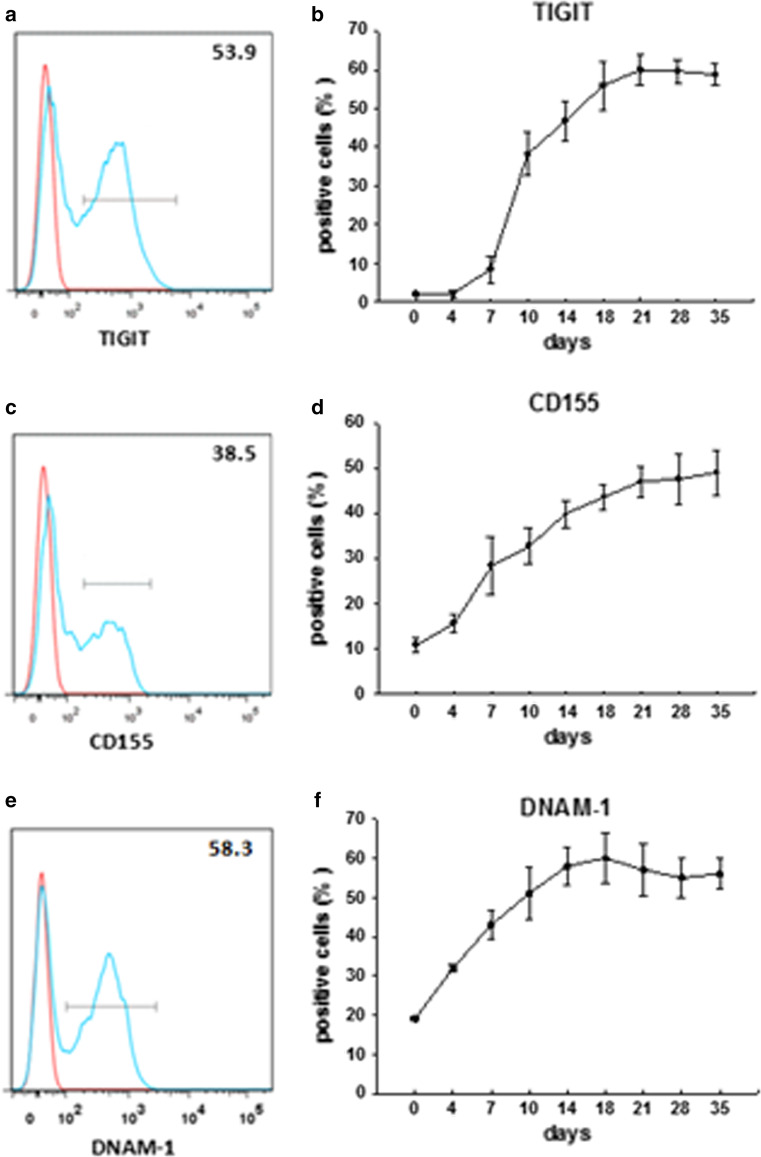
We examined TIGIT expression in CIK cells as depicted in Fig. 2a, b. TIGIT expression levels steadily increased starting at day 7 post-CIK cell activation, peaked at 21 days, and was maintained thereafter. CD155, the ligand of TIGIT, was induced after activation of CIK cells (Fig. 2c, d). Because CD155 is also a ligand of DNAM-1, we determined the expression of DNAM-1 on CIK cells (Fig. 2e, f).
Fig. 2.
Expression of TIGIT, CD155, and DNAM-1 on CIK cells. CIK cells were taken at the indicated time points, and surface expression of TIGIT and CD155 was assessed by flow cytometry. The line graphs display the cell surface expression of TIGIT (a), CD155 (c), and DNAM-1 (e) at day 14 of culture, and the time course of expression of TIGIT (b), CD155 (d), and DNAM-1 (f) on CIK cells. Data are representative of four different donors. Mean values are shown ± SD
TIGIT inhibits CIK cell proliferation
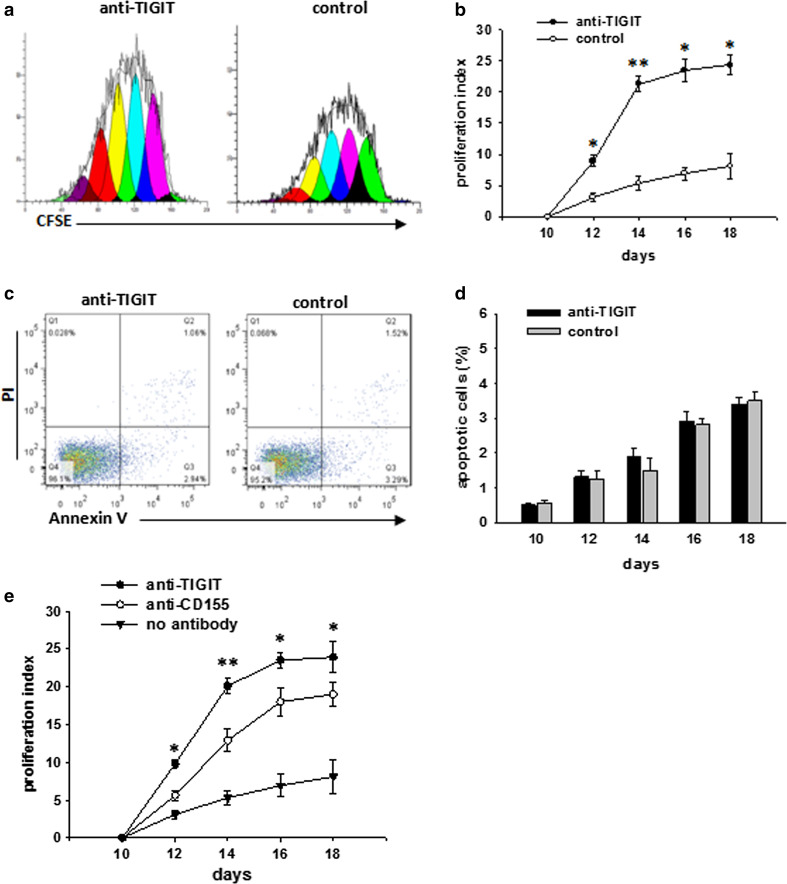
We investigated whether TIGIT directly inhibited CIK cell proliferation. On day 10 of CIK culture, we used the anti-TIGIT antibody to block TIGIT in CIK cells for 8 days, and then, CIK cell proliferation was assessed. As shown in Fig. 3a, b, antibody blockade of TIGIT enhanced the proliferation of CIK cells. Furthermore, TIGIT blockade did not cause a significant increase in the frequency of early or late apoptotic CIK cells (Fig. 3c, d).
Fig. 3.
TIGIT cell-intrinsic signaling inhibits CIK cell proliferation. a Proliferation of CFSE-labeled CIK cells at day 10 of culture, and then stimulated with anti-TIGIT or isotype-matched control antibody in the presence of IL-2. CFSE dilution was analyzed by flow cytometry at day 14 in the presence of anti-TIGIT antibody. Data are representative of at least four independent experiments. b CIK cell proliferation was assessed at days 10, 12, 14, 16, and 18 of culture in the same conditions as in (a), and proliferation indices were determined using FlowJo software (n = 5). c Flow cytometry analysis based on Annexin-V/PI staining was performed to evaluate the percentage of apoptotic cells at day 18 of CIK culture in the presence of anti-TIGIT antibody. Data are representative of at least four independent experiments. d The percentage of apoptotic CIK cells was assessed at days 10, 12, 14, 16, and 18 of culture in the same conditions as in (c) (n = 5). e Blocking of TIGIT or CD155 activity enhances the proliferation of CIK cells. CIK cell proliferation was assessed in the presence of anti-TIGIT, anti-CD155 or no antibody at days 10, 12, 14, 16, and 18 in the same conditions as in (a), and proliferation indices were determined using the FlowJo software (n = 5). Mean values are shown ± SD. *p < 0.05, **p < 0.001 compared to control group
TIGIT and CD155 were highly expressed on activated CIK cells (Fig. 2). Therefore, we further tested whether the TIGIT–CD155 interaction led to the inhibition of CIK cell proliferation. To this end, we blocked TIGIT and CD155 in CIK cells using anti-TIGIT and anti-CD155 antibodies, respectively. As shown in Fig. 3e, antibody blockade of CD155 or TIGIT directly enhanced CIK cell proliferation. These results suggest that TIGIT also has intrinsic effects on CIK cells and inhibits CIK cell proliferation by binding to its ligand, CD155.
TIGIT inhibits CIK cell cytotoxicity
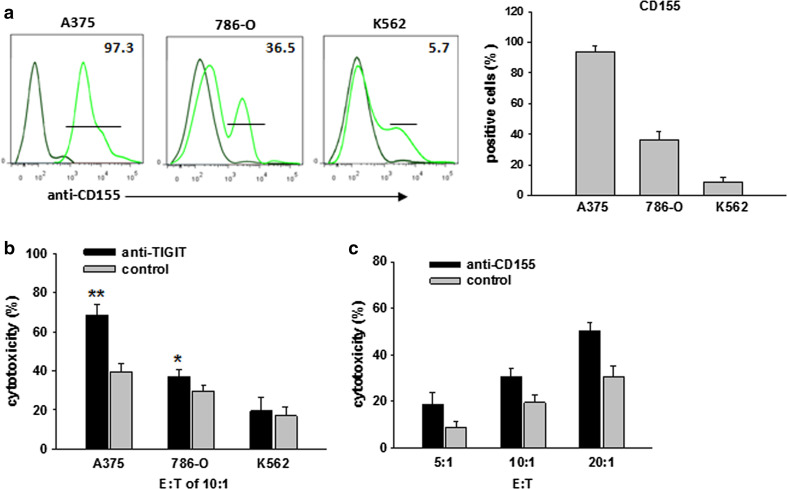
To test whether TIGIT directly inhibited CIK cell cytotoxicity, we evaluated CIK cell killing of tumor cells expressing various levels of CD155. We used three tumor cell lines, A375, 786-O, and K562, with diverse expression levels of CD155 (Fig. 4a). Blocking the TIGIT–CD155 interaction resulted in significantly increased killing of A375 cells (high CD155-expressing cells), only slightly increased killing of 786-O cells (low CD155-expressing cells), and had no effect on K562 cells (lowest CD155-expressing cells) (Fig. 4b). We further investigated whether the activity of TIGIT depends on binding to CD155. As demonstrated in Fig. 4c, blocking of CD155 on A375 cells significantly increased killing by CIK cells. These results suggest that the TIGIT interaction with CD155 directly inhibits CIK cell cytotoxicity.
Fig. 4.
TIGIT inhibits CIK cell cytotoxicity upon interaction with CD155. a Flow cytometric analysis of A375, 786-O, and K562 cell expression of CD155 stained with anti-CD155 antibody. b Blocking of TIGIT activity enhances the killing of CIK cells. Starting on day 10, CIK cells were stimulated with anti-TIGIT or isotype-matched control antibody in the presence of IL-2 for 4 days. The CIK cells were then incubated with A375, 786-O, and K562 cells at an E:T ratio of 10:1, and specific lysis was determined 4 h later. Data are shown as mean ± SD of four replicates. c Blocking of TIGIT–CD155 interactions. A375 cells were pre-incubated with anti-CD155 or isotype-matched control antibody overnight, and then incubated with CIK cells expressing TIGIT at the indicated E:T ratios, and specific lysis was determined 4 h later. Data are shown as mean ± SD of four replicates. *p < 0.05, **p < 0.001 compared to control group
TIGIT inhibits cytokine productions in CIK cells
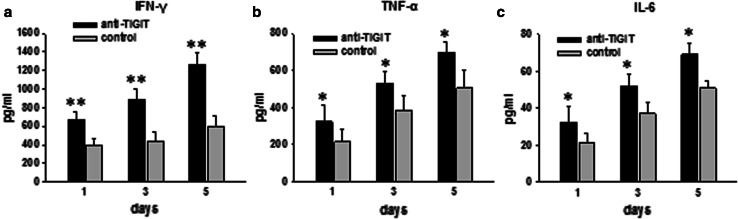
To further examine the direct mechanism of the inhibitory effect of TIGIT on CIK cell function, we investigated the role of TIGIT signaling in cytokine production by CIK cells. CIK cells were incubated with plate-bound anti-TIGIT or isotype control antibodies and stimulated for 5 days. TIGIT blockade significantly increased the production of IFN-γ by CIK cells (Fig. 5a). To a lesser extent, production of TNF-α and IL-6 was also increased in CIK cells exposed to the anti-TIGIT antibody (Fig. 5b, c). These data demonstrate that TIGIT suppresses CIK cell responses.
Fig. 5.
TIGIT inhibits CIK cell cytokine production. Starting on day 10, CIK cells were stimulated with anti-TIGIT or isotype-matched control antibody in the presence of IL-2 for 5 days. Production of IFN-γ (a), TNF-ɑ (b), and IL-6 (c) was determined by ELISA at days 1, 3, and 5. Data are shown as mean ± SD of four replicates. *p < 0.05, **p < 0.001 compared to control group
Enhancement of CIK cell responses after TIGIT blockade is mediated by CD155 engagement of DNAM-1
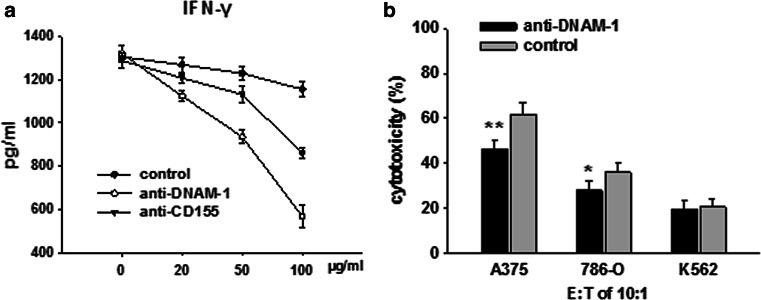
In addition to the inhibitory cell-intrinsic role of TIGIT in CIK cells, we examined whether TIGIT had a second mechanism of negative immunoregulation by competing with DNAM-1 for CD155. To examine the mechanism of the increased production of IFN-γ by blockade of TIGIT, we cultured CIK cells with antibodies against either CD155 or DNAM-1. As shown in Fig. 6a, adding either the anti-CD155 or anti-DNAM-1 antibody blocked the increased production of IFN-γ induced by TIGIT blockade. Next, we performed a killing assay to assess the direct effect of DNAM-1 receptors on CIK cells interacting with CD155-expressing tumor cells. The activating receptor DNAM-1 was blocked on CIK cells blockaded for TIGIT. As shown in Fig. 6b, blocking DNAM-1 inhibited CIK-mediated lysis of A375 cells, but had no effect on lysis of K562 cells. These data indicate that in the absence of TIGIT, the DNAM-1–CD155 interaction induces IFN-γ production and increases cytotoxicity of CIK cells.
Fig. 6.
Blocking of DNAM-1 signaling with anti-DNAM-1 or anti-CD155 decreased the effects of TIGIT blockade. (a) Starting on day 10, CIK cells were stimulated with anti-TIGIT or isotype-matched control antibody in the presence of IL-2 for 3 days, and then cultured with at the indicated concentration of anti-DNAM-1 or anti-CD155 antibodies for 2 days. Production of IFN-γ was determined by ELISA. (b) Starting on day 10, CIK cells were stimulated with anti-TIGIT or isotype-matched control antibody in the presence of IL-2 for 3 days, and then cultured with 50 μg/ml anti-DNAM-1 antibodies for 2 days. CIK cells were then incubated with A375, 786-O or K562 cells at an E:T ratio of 10:1, and specific lysis was determined 4 h later. Data are shown as mean ± SD of four replicates. *p < 0.05, **p < 0.001 compared to control group
Discussion
Despite the various reports on CIK cells, including clinical trials of adoptively transferred bulk-expanded CIK cells, the molecular mechanism by which CIK cells kill tumor cells has not been clearly defined. This study aimed to evaluate the effect of the inhibitory receptor TIGIT on PBMC-derived CIK cells.
TIGIT is an inhibitor of T cell priming and NK cell killing, but its importance in CIK cells had not been tested. We analyzed the kinetics of TIGIT expression in CIK cells by flow cytometry. TIGIT expression was induced upon activation of CIK cells and peaked at day 21 of CIK cell activation. The kinetics of surface expression of TIGIT were similar to the increase in CD3+CD56+ double-positive cells during CIK cell culture. These results suggest that TIGIT expression is closely associated with CIK cell activation. Antibody blockade of TIGIT strongly enhanced CIK cell proliferation in a time-dependent manner. Similarly, culturing CIK cells with anti-TIGIT antibody resulted in higher production levels of IFN-γ. These data showed that TIGIT acts as a critical and specific regulator of the effector functions of stimulated CIK cells in addition to its roles in other immune cell lineages.
T cell Ig and ITIM domain inhibits T cell responses and NK cell cytotoxicity by binding its ligand CD155 [27, 34]. We tested whether the inhibition of CIK cell responses by TIGIT was mediated through binding to CD155. CD155 was highly expressed on activated CIK cells. Blocking the TIGIT–CD155 interaction by antibody blockade of CD155 or TIGIT enhanced the proliferation of CIK cells. We further observed that TIGIT interacts with CD155, and this interaction leads to inhibition of CIK cell cytotoxicity. These results demonstrated that TIGIT acts as a negative regulator by binding to its ligand, CD155, to inhibit CIK cell responses.
TIGIT may also directly compete with costimulatory receptor DNAM-1 for binding to the same ligand, CD155. However, the molecular relationship between TIGIT and DNAM-1 has not been fully elucidated [28]. The anti-DNAM-1 antibody was sufficient to abolish the effects of anti-TIGIT, suggesting that TIGIT signaling directly inhibits CIK cells by competing with DNAM-1 for binding to CD155. The TIGIT/DNAM-1 pathway is similar to the CD28/CTLA4 pathway that involves binding to CD80 and CD86 ligands.
The TIGIT-intrinsic mechanism of negative regulation of T and NK cell functions may also have implications in tumor immunity. Ipilimumab, an antibody that blocks a negative regulatory pathway, augments antitumor immunity [35–38]. Thus, the TIGIT pathway is an attractive target for therapeutic manipulation.
Our data demonstrate that TIGIT is an inhibitory receptor expressed by CIK cells and directly inhibits CIK cell activation by binding CD155. This mechanism may enable tumors overexpressing CD155 to escape immune attack by CIK cells. Several recent reports have demonstrated enhanced expression of CD155 in various tumors such as colorectal carcinomas, gastric cancer, and neuroblastomas [34, 39–43]. Although further studies are needed to test the therapeutic benefits of targeting TIGIT, modulation of the TIGIT–CD155 interaction may allow more targeted manipulation of CIK cells for antitumor immunotherapy.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81202015), Jiangsu Provincial Special Program of Medical Science (BL2012020), and the Natural Science Foundation of the Jiangsu Province Higher Education Institutions (No. 12KJB320014).
Abbreviations
- ABB
1 × annexin-binding buffer
- BSA
Bovine serum albumin
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- CIK
Cytokine-induced killer
- DNAM-1
DNAX accessory molecule-1
- ELISA
Enzyme-linked immunosorbent assay
- IFN-γ
Interferon-γ
- IL-2
Interleukin-2
- IL-6
Interleukin-6
- ITIM
Immunoreceptor tyrosine-based inhibition motif
- MHC
Major histocompatibility complex
- NK
Natural killer
- OKT-3
Activating monoclonal antibody against CD3
- PBMCs
Peripheral blood mononuclear cells
- PVR
Poliovirus receptor
- TIGIT
T cell Ig and ITIM domain
- TNF-α
Tumor necrosis factor-α
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Chao Gao, Phone: +86-516-8558-2513, Email: chg200811@126.com.
Junnian Zheng, Phone: +86-516-8558-2513, Email: jnzheng@xzmc.edu.cn.
References
- 1.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174(1):139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3 + CD56 + killer cells. Blood. 1995;86(9):3493–3499. [PubMed] [Google Scholar]
- 3.Jakel CE, Schmidt-Wolf IG. An update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cells. Expert Opin Biol Ther. 2014;14(7):905–916. doi: 10.1517/14712598.2014.900537. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21(13):1673–1679. [PubMed] [Google Scholar]
- 5.Gutgemann S, Frank S, Strehl J, Schmidt-Wolf IG. Cytokine-induced killer cells are type II natural killer T cells. Ger Med Sci. 2007;5:Doc07. [PMC free article] [PubMed] [Google Scholar]
- 6.Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology. 2009;126(3):423–435. doi: 10.1111/j.1365-2567.2008.02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiolo D, Martinuzzi E, Todorovic M, Vitaggio K, Vallario A, Jordaney N, Carnevale-Schianca F, Capaldi A, Geuna M, Casorzo L, Nash RA, Aglietta M, Cignetti A. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol. 2008;20(7):841–848. doi: 10.1093/intimm/dxn042. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M, Dorbic T, Neubauer A, Wittig B, Huhn D. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81(6):1009–1016. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF, Feng YY, Fan ZP, Xu DP, Wang FS. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10(8):1146–1151. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11(3):181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, Dander E, Gaipa G, D’Amico G, Biagi E, Parma M, Pogliani EM, Spinelli O, Baronciani D, Grassi A, Golay J, Barbui T, Biondi A, Rambaldi A. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]
- 12.Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41(1):36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(10):1497–1502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, Hao X, Ren X. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012;18(6):1751–1759. doi: 10.1158/1078-0432.CCR-11-2442. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61(11):2125–2133. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L, Zhou Q, Wu J, Ji M, Li G, Jiang J, Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–2259. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung MJ, Park JY, Bang S, Park SW, Song SY. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63(9):939–946. doi: 10.1007/s00262-014-1566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olioso P, Giancola R, Di Riti M, Contento A, Accorsi P, Iacone A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27(3):130–139. doi: 10.1002/hon.886. [DOI] [PubMed] [Google Scholar]
- 19.Linn YC, Yong HX, Niam M, Lim TJ, Chu S, Choong A, Chuah C, Goh YT, Hwang W, Loh Y, Ng HJ, Suck G, Chan M, Koh M. A phase I/II clinical trial of autologous cytokine-induced killer cells as adjuvant immunotherapy for acute and chronic myeloid leukemia in clinical remission. Cytotherapy. 2012;14(7):851–859. doi: 10.3109/14653249.2012.694419. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 21.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289(25):17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Wang J, Zhou X, Liang R, Bai Q, Yang L, Gu H, Gao G, Dong B, Zhu H, Chen X. Increased expression of TIGIT on CD4 + T cells ameliorates immune-mediated bone marrow failure of aplastic anemia. J Cell Biochem. 2014;115(11):1918–1927. doi: 10.1002/jcb.24862. [DOI] [PubMed] [Google Scholar]
- 24.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41(4):902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, Comps-Agrar L, Wiesmann C, Bazan JF, Eaton DL, Grogan JL. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci USA. 2012;109(14):5399–5404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186(3):1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188(8):3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foks AC, Ran IA, Frodermann V, Bot I, van Santbrink PJ, Kuiper J, van Puijvelde GH. Agonistic anti-TIGIT treatment inhibits T cell responses in LDLr deficient mice without affecting atherosclerotic lesion development. PLoS One. 2013;8(12):e83134. doi: 10.1371/journal.pone.0083134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43(8):2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Andrade LF, Smyth MJ, Martinet L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol Cell Biol. 2014;92(3):237–244. doi: 10.1038/icb.2013.95. [DOI] [PubMed] [Google Scholar]
- 32.Jiang G, Zhang K, Jiang AJ, Xu D, Xin Y, Wei ZP, Zheng JN, Liu YQ. A conditionally replicating adenovirus carrying interleukin-24 sensitizes melanoma cells to radiotherapy via apoptosis. Mol Oncol. 2012;6(4):383–391. doi: 10.1016/j.molonc.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang BF, Liu JJ, Pei DS, Yang ZX, Di JH, Chen FF, Li HZ, Xu W, Wu YP, Zheng JN. Potent antitumor effect elicited by RGD-mda-7, an mda-7/IL-24 mutant, via targeting the integrin receptor of tumor cells. Cancer Biother Radiopharm. 2011;26(5):647–655. doi: 10.1089/cbr.2011.0984. [DOI] [PubMed] [Google Scholar]
- 34.Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 2005;65(23):10930–10937. doi: 10.1158/0008-5472.CAN-05-1890. [DOI] [PubMed] [Google Scholar]
- 35.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18(2):206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, Ng S, Maio M, Franke FA, Sundar S, Agarwal N, Bergman AM, Ciuleanu TE, Korbenfeld E, Sengelov L, Hansen S, Logothetis C, Beer TM, McHenry MB, Gagnier P, Liu D, Gerritsen WR. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarhini A. Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica. 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49(2):236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castriconi R, Dondero A, Corrias MV, Lanino E, Pende D, Moretta L, Bottino C, Moretta A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64(24):9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 42.Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, Smyth MJ. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184(2):902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 43.Atsumi S, Matsumine A, Toyoda H, Niimi R, Iino T, Sudo A. Prognostic significance of CD155 mRNA expression in soft tissue sarcomas. Oncol Lett. 2013;5(6):1771–1776. doi: 10.3892/ol.2013.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]