Abstract
Myeloid-derived suppressor cells (MDSCs) are known as key immune regulators in various human malignancies, and it is reported that CD14+HLA-DR−/low MDSCs are increased in hepatocellular carcinoma (HCC) patients. However, the host factors that regulate the frequency and the effect on the prognosis of HCC patients are still unclear. We investigated these issues and clarified the relationships between a feature of MDSCs and host factors in HCC patients. We examined the frequency of MDSCs in 123 HCC patients, 30 chronic liver disease patients without HCC, and 13 healthy controls by flow cytometric analysis. The relationships between the clinical features and the frequency of MDSCs were analyzed. In 33 patients who received curative radiofrequency ablation (RFA) therapy, we examined the impact of MDSCs on HCC recurrence. The frequency of MDSCs in HCC patients was significantly increased. It was correlated with tumor progression, but not with the degree of liver fibrosis and inflammation. In terms of serum cytokines, the concentrations of IL-10, IL-13, and vascular endothelial growth factor were significantly correlated with the frequency of MDSCs. In HCC patients who received curative RFA therapy, the frequency of MDSCs after treatment showed various changes and was inversely correlated with recurrence-free survival time. The frequency of MDSCs is correlated with tumor progression, and this frequency after RFA is inversely correlated with the prognosis of HCC patients. Patients with a high frequency of MDSCs after RFA should be closely followed and the inhibition of MDSCs may improve the prognosis of patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1447-1) contains supplementary material, which is available to authorized users.
Keywords: Myeloid-derived suppressor cells, Hepatocellular carcinoma, Radiofrequency ablation, Recurrence, Cancer
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer mortality globally [1, 2]. Current treatment options including surgical resection, radiofrequency ablation (RFA), liver transplantation, chemotherapy, transcatheter arterial chemoembolization (TACE), and sorafenib are reported to improve survival in HCC patients [3–7]. However, despite curative treatments for HCC, tumor recurrence rates remain high and the survival of those who have advanced HCC remains unsatisfactory [3–7]. Therefore, the development of new anti-tumor treatments for HCC remains an urgent and important field of research.
To overcome the limitations of these treatments, several immunotherapies have been developed as attractive strategies for HCC. In several studies of HCC immunotherapy, effective induction of immune-mediated cells by tumor antigen-derived peptides or antigen-presenting cells showed anti-tumor effects, but the population of patients who exhibited such effects was very small [8–12].
In previous studies, it was reported that many kinds of tumor generate a number of immune-suppressive mechanisms [13–15]. Recently, myeloid-derived suppressor cells (MDSCs) have been characterized as key immune regulators in various human cancers [15–24]. They show the capacity to inhibit T cell function and promote tumor development [15, 25]. Human MDSCs are a heterogeneous population that shows CD11b+, CD33+, HLA-DR−/low and can be divided into granulocytic CD14− and monocytic CD14+ subtypes [26–28]. In most recent studies, it has been reported that CD14+HLA-DR−/low MDSCs were increased in HCC patients and the cells inhibited the function of T cells through the induction of regulatory T cells (Tregs) [24]. Tregs represent 5–10 % of CD4+ T cells and can suppress the activation and proliferation of CD4+ and CD8+ T cells [14, 29]. It was reported that an increased frequency of circulating Tregs was associated with poor survival of HCC patients [30]. Understanding the inhibitory mechanism of MDSCs and controlling their function are very important to develop more effective immunotherapy for HCC.
In this study, we investigate the host factors that are associated with the frequency of MDSCs in HCC patients and the effect of MDSCs on the prognosis of patients and clarify the relationships between a feature of MDSCs and host factors in HCC patients.
Materials and methods
Patients and healthy controls
Blood samples were obtained from a total of 123 HCC patients, 26 chronic liver disease (CLD) patients without HCC, and 13 healthy controls. The diagnosis of HCC was histologically confirmed in 68 patients. For the remaining 55 patients, diagnosis was made by dynamic CT or MRI. Patient characteristics and disease classification are shown in Suppl. table 1. All CLD patients without HCC underwent percutaneous liver biopsy to evaluate the disease severity according to the Metavir scoring system. In 33 patients treated with curative percutaneous RFA, blood samples were obtained on the day of treatment and 2–4 weeks after treatment, and we observed recurrence of these patients with periodic imaging studies. All subjects provided written informed consent to participate in this study in accordance with the Declaration of Helsinki. This study was approved by the regional ethics committee (Medical Ethics Committee of Kanazawa University).
Cell isolation and flow cytometric analysis
Peripheral blood mononuclear cells (PBMCs) were separated as described below; heparinized venous blood was diluted in phosphate-buffered saline (PBS) and loaded on Ficoll-Histopaque (Sigma, St. Louis, Mo.) in 50 ml tubes. After centrifugation at 2,000 rpm for 20 min at room temperature, PBMCs were harvested from the interphase, resuspended in PBS, centrifuged at 1,400 rpm for 10 min, and finally resuspended in complete culture medium consisting of RPMI (GibcoBRL, Grand Island, NY), 10 % heat inactivated FCS (Gibco BRL), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL). PBMCs were resuspended in RPMI 1,640 medium containing 80 % FCS and 10 % dimethyl sulfoxide and cryopreserved until use. The viability of cryopreserved PBMCs was 60–70 %. In some patients, fresh and cryopreserved PBMCs were obtained from the same sample. To determine the frequency and phenotype of MDSCs and Tregs, multicolor fluorescence-activated cell sorting analysis was carried out using the Becton–Dickinson FACSAria II system. The following anti-human monoclonal antibodies were used: anti-CD4 (Becton–Dickinson), anti-CD11b (Becton–Dickinson), anti-CD14 (Becton–Dickinson), anti-CD15 (Becton–Dickinson), anti-CD25 (Becton–Dickinson), anti-CD33 (Becton–Dickinson), anti-CD127 (Becton–Dickinson), and anti-HLA-DR (Becton–Dickinson).
Suppression assay
CD14+HLA-DR−/low MDSCs and CD14+HLA-DR+ cells were sorted using the Becton–Dickinson FACSAria II system. 2 × 104 PBMCs were cultured and stimulated with 1 μg/ml plate-bound anti-CD3 (eBioscience) and 1 μg/mL soluble anti-CD28 (eBioscience) in 96-well round-bottomed plates. 24 h later, to determine the suppressive ability of MDSCs, increasing concentrations of MDSCs were added to the stimulated PBMCs. Proliferation was measured by 3Hincorporation after 72 h. [3H] thymidine was added, and cell proliferation was measured by incorporation of radiolabeled thymidine for 24 h.
Cytokine and chemokine profiling
Blood samples were collected from patients at the same time of PBMC isolation. After centrifugation at 3,000 rpm for 10 min at 4 °C, serum fractions were obtained and stored at −20 °C until use. Serum levels of various cytokines and chemokines were measured using the Bio-Plex Protein Array System. Briefly, frozen serum samples were thawed at room temperature and diluted 1:4 in sample diluents; 50 μl aliquots of the diluted sample was added in duplicate to the wells of 96-well microtiter plates containing the coated beads for a validated panel of human cytokines and chemokines according to the manufacturer’s instructions. The following 27 cytokines and chemokines were targeted: IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, basic fibroblast growth factor (FGF), eotaxin (chemokine C–C motif ligand (CCL) 11), G-CSF, GM-CSF, IFN-γ, interferon gamma-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, platelet-derived growth factor (PDGF)-BB, regulated upon activation, normal T cell expressed and secreted (RANTES), TNF-α, and vascular endothelial growth factor (VEGF). Nine standards (ranging from 0.5 to 32,000 pg/ml) were used to generate calibration curves for each cytokine. Data acquisition and analysis were performed using Bio-Plex Manager software version 4.1.1.
Statistical analysis
Data are expressed as the mean ± SD. Chi-squared test with Yates’ correction, unpaired t test, Mann–Whitney U test, and Kruskal–Wallis were used for univariate analysis of two groups that were classified according to the frequency of MDSCs. The probability of tumor recurrence-free survival was estimated using the Kaplan–Meier method. The Mantel-Cox log-rank test was used to compare curves between groups. The prognostic factors for tumor recurrence-free survival were analyzed for statistical significance by the Kaplan–Meier method (univariate) and the Cox proportional hazard model (multivariate). Variables with p < 0.1 were entered into multivariate logistic regression analysis. A level of p < 0.05 was considered significant.
Results
CD14+HLA-DR−/low MDSCs are increased in the peripheral blood of HCC patients
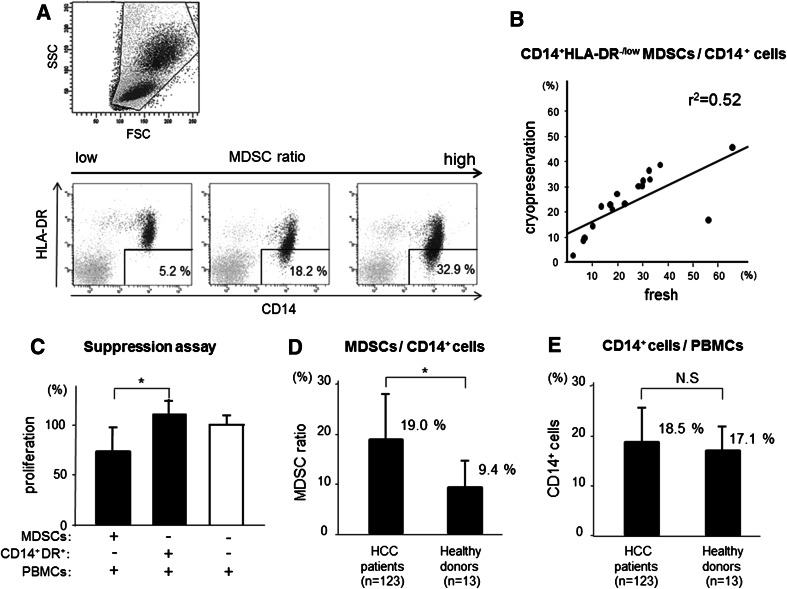
We analyzed the peripheral blood of 123 patients with HCC, 26 CLD patients without HCC, and 13 healthy donors for the prevalence of CD14+HLA-DR−/low MDSCs. Because the PBMCs are tested after Ficoll, some cells may be lost. Therefore, we examined the population of MDSCs as a percentage of total CD14+ cells by flow cytometry after cell surface labeling for the expression of HLA-DR (Fig. 1a). CD14+HLA-DR−/low population in PBMCs of HCC patients represented 3.2–56.8 % of the CD14+ cells. The frequency of CD14+HLA-DR−/low MDSCs/CD14+ cells in cryopreserved PBMCs correlated with that in fresh PBMCs (Fig. 1b). Therefore, we analyzed the frequency of CD14+HLA-DR−/low MDSCs/CD14+ cells using cryopreserved PBMCs.
Fig. 1.
a Flow cytometry shows CD14+HLA-DR−/low+ MDSCs. PBMCs from patients and healthy donors were labeled with anti-CD14 and HLA-DR. Three staining examples of HCC patients are shown in the order from a small number (left) to a large number (right). b The increase in CD14+HLA-DR−/low MDSCs/CD14+ cells in cryopreserved PBMC correlated with that in fresh PBMC (r 2 = 0.52). c Proliferation of PBMCs stimulated by anti-CD3/28 in the presence or absence of MDSCs was measured by 3Hincorporation assay. CD14+HLA-DR−/low MDSCs significantly decreased autologous PBMC proliferation (n = 4; *, p < 0.05). d The frequency of MDSCs was significantly higher in HCC patients than healthy donors (*, p < 0.01). e Overall frequencies of CD14+ cells did not differ significantly
To confirm the function of these cells, sorted CD14+HLA-DR−/low MDSCs and CD14+HLA-DR+ (control) cells were added at different ratios to autologous anti-CD3/CD28-stimulated PBMCs, and the proliferation was measured by 3Hincorporation. CD14+HLA-DR−/low MDSCs of HCC patients significantly decreased autologous PBMC proliferation (Fig. 1c). On the other hand, CD14+HLA-DR+ (control) cells could not suppress the autologous PBMC proliferation.
As shown in Fig. 1d, the frequency of MDSCs was significantly higher in HCC patients (19.0 %) than in healthy donors (9.4 %) (p < 0.01). Overall frequencies of CD14+ cells did not differ significantly between the groups (Fig. 1e). Individual frequencies of MDSCs of all the patients and healthy donors are represented as scatter plots (Fig. 2a). The frequency of MDSCs was correlated with the stage of HCC (stage III and IV: 22.3 % (n = 46) vs. stage I and II: 17.0 % (n = 77), p < 0.01) and was significantly higher in HCC patients than CLD patients without HCC and healthy donors. Interestingly, there was no difference between CLD patients without HCC and healthy donors. Moreover, these numbers did not change depending on the degree of fibrosis or inflammatory activity of the liver (Fig. 2b, c).
Fig. 2.
a Scatter plots of MDSC ratio (CD14+HLA-DR−/low MDSCs/CD14+ cells) in patients and healthy donors. The frequency of MDSCs was significantly increased in HCC patients compared with that in non-HCC controls. Moreover, the frequency of MDSCs was correlated with tumor progression (stage III and IV: 22.3 % (n = 46) vs. stage I and II: 17.0 % (n = 77); *, p < 0.05). In non-HCC controls, there was no significant difference in the frequency of MDSCs. b, c In non-HCC patients, the frequency of MDSCs did not change depending on the degree of fibrosis or inflammatory activity of the liver according to the Metavir scoring system
In previous reports, granulocytic MDSCs were defined in combination with several surface markers including CD14, CD15, CD11b, CD33, CD66b, and HLA-DR in several cancers. Therefore, we examined the frequency of CD15+CD14−CD11b+CD33+ cells in 37 HCC patients and 11 healthy donors (Suppl. figure 1A). Although there was no statistical significant difference, the frequency of CD15+CD14−CD11b+CD33+ cells in HCC patients was higher than that in healthy donors (2.84 vs. 2.06 %, p = 0.073) (Suppl. figure 1B). The frequency was correlated with the stage of HCC (stage III and IV: 3.69 % (n = 13) vs. stage I and II: 2.39 % (n = 24), p = 0.022) (Suppl. figure 1C).
Relationship between the frequency of Tregs and MDSCs
It is well known that the frequency of circulating Tregs is increased and correlated with disease progression in HCC patients. The frequency of CD4+ CD25+ CD127−/low Tregs was significantly increased in HCC patients (Suppl. figure 2A) and associated with tumor progression (Suppl. figure 2B). However, there was not a strong correlation between the frequency of MDSCs and Tregs in our study (Suppl. figure 2C).
Identification of host factors related to the frequency of MDSCs in HCC patients
We divided the HCC patients into two groups using the threshold of an MDSC ratio of 22 %. This threshold is the average +2SD of the MDSC ratio in non-HCC patients. In the group with high frequency, the tumor factors including size, multiplicity, and stage were significantly worse (tumor size, 28.3 vs. 24.4 mm; tumor multiplicity (multiple/solitary), 27/12 vs. 42/42; TNM stage (I and II vs. III and IV), 17/22 vs. 60/24, p < 0.05) (Table 1). Moreover, hepatic reserve was also worse in the group with high frequency (Child-Pugh classification (A/B/C), 20/17/2 vs. 64/16/4, p < 0.05). In addition, overall survival was significantly shortened in the group with high frequency (hazard ratio 2.67, p = 0.008) (Suppl. figure 3A), and recurrence-free survival was also significantly shortened (hazard ratio 1.94, p = 0.010) (Suppl. figure 3B).
Table 1.
Clinical findings and MDSCs
| Clinical characteristics | MDSC ratio ≥22 | MDSC ratio <22 | p value |
|---|---|---|---|
| (n = 39) | (n = 84) | ||
| Age (year) | 68.5 | 70.1 | 0.646 |
| Sex (M/F) | 29/10 | 54/30 | 0.267 |
| AST (IU/l) | 62.0 | 61.5 | 0.543 |
| ALT (IU/l) | 47.1 | 53.9 | 0.759 |
| LDH (IU/l) | 225 | 218 | 0.832 |
| γGTP (IU/l) | 78.0 | 76.0 | 0.252 |
| Platelet (104/μl) | 10.9 | 10.6 | 0.884 |
| Prothrombin time (%) | 75.2 | 82.3 | 0.045 |
| Serum albumin (g/dl) | 3.53 | 3.68 | 0.120 |
| Total bilirubin (mg/dl) | 1.21 | 0.94 | 0.286 |
| WBC (/μl) | 3910 | 3610 | 0.235 |
| Neutrophil (%) | 63.2 | 59.4 | 0.093 |
| Lymphocyte (%) | 26.1 | 29.5 | 0.047 |
| Total cholesterol (mg/dl) | 151 | 149 | 0.926 |
| HbA1c (%) | 5.27 | 5.43 | 0.197 |
| Type IV collagen 7S (ng/ml) | 8.2 | 7.3 | 0.086 |
| DCP (mAU/ml) | 5157 | 432 | 0.561 |
| AFP (ng/ml) | 1301 | 934 | 0.240 |
| Tumor size (mm) | 28.3 | 24.4 | 0.014 |
| Tumor multiplicity (multiple/solitary) | 27/12 | 42/42 | 0.046 |
| TNM stage (I plus II/III plus IV) | 17/22 | 60/24 | 0.003 |
| Child-Pugh (A/B/C) | 20/17/2 | 64/16/4 | 0.015 |
| Etiology (HCV/HBV/others) | 21/11/7 | 61/11/12 | 0.081 |
| CD4+ CD25+ CD127−/low Tregs/CD4+ cells (%) | 7.04 | 6.70 | 0.281 |
AST aspirate aminotransferase, ALT alanine aminotransferase, LDH lactic dehydrogenase, γGTP gamma glutamyltransferase, WBC white blood cell, Hb hemoglobin, DCP des-gamma-prothrombin, AFP alpha-fetoprotein, HCV hepatitis C virus, HBV hepatitis B virus, Tregs regulatory T cells
Chi-squared test with Yates’ correction, unpaired t test, Mann–Whitney U test, and Kruskal–Wallis test were used for univariate analysis of two groups that were classified according to the frequency of MDSCs
Relationship between serum cytokine levels and the frequency of MDSCs
In previous studies, the balance of circulating cytokines was thought to promote accumulation and activation of MDSCs [18, 31–34]. Therefore, we examined the relationship between serum cytokine levels and the frequency of MDSCs in HCC patients. In 54 HCC patients, serum levels of cytokines and chemokines were measured using the Bio-Plex Protein Array system. Serum concentrations of IL-10, IL-13, and VEGF were significantly increased in the group with a high frequency of MDSCs (Table 2). In addition, there was a positive correlation between these cytokine levels in serum and the frequency of MDSCs. We also examined the relationship between serum cytokine levels and the frequency of Tregs. We divided the HCC patients into two groups using the threshold of 7 %, which is the average +2SD of the % of Tregs among CD4+ cells in non-HCC patients. Serum concentration of IL-10 was significantly increased in the group with a high frequency of Tregs (Suppl. table 2).
Table 2.
Serum cytokines and MDSCs
| Cytokine | Healthy donor (mean) | MDSC ratio ≥ 22 (mean) | Range | MDSC ratio < 22 (mean) | Range | p value |
|---|---|---|---|---|---|---|
| (n = 13) | (n = 21) | (n = 31) | ||||
| IL-1ra | 34.2 | 97.0 | (21.5–600) | 40.3 | (3.4–151) | 0.057 |
| IL-2 | 10.5 | 38.1 | (4.3–54.3) | 11.3 | (0.9–49.7) | 0.055 |
| IL-4 | 2.6 | 5.75 | (1.47–11.9) | 5.03 | (0.71–10.9) | 0.159 |
| IL-6 | 9.9 | 21.5 | (1.2–130) | 10.1 | (0.2–97.2) | 0.065 |
| IL-8 | 24.5 | 64.7 | (10.9–291) | 35.1 | (6.2–142) | 0.156 |
| IL-10 | 2.76 | 6.01 | (0.8–11.5) | 2.81 | (0.1–12.0) | 0.003 |
| IL-12(p70) | 14.6 | 33.3 | (0.6–140) | 17.6 | (1.4–57) | 0.058 |
| IL-13 | 7.6 | 13.1 | (1.2–33.6) | 8.2 | (2.7–22.9) | 0.015 |
| IL-17 | 15.7 | 23.5 | (4.6–70) | 20.8 | (2.1–119) | 0.115 |
| Eotaxin | 104 | 141 | (51.9–493) | 124 | (26.3–331) | 0.675 |
| G-CSF | 7.9 | 13.2 | (2.7–41.3) | 8.7 | (0.5–17.9) | 0.050 |
| IFN-γ | 52.6 | 95.4 | (23.1–417) | 69.9 | (2.5–238) | 0.136 |
| MCP-1 | 20.2 | 26.8 | (8.4–114) | 23.8 | (3.5–77) | 0.744 |
| MIP-1b | 97.6 | 120 | (58.3–490) | 108 | (39.7–263) | 0.508 |
| PDGF | 4012 | 4375 | (1,312–10,136) | 4013 | (831–13,557) | 0.484 |
| RANTES | 2978 | 2890 | (1,040–4,826) | 3184 | (599–6,165) | 0.186 |
| TNF-α | 10.5 | 34.9 | (0.1–175) | 27.6 | (2.9–105) | 0.756 |
| VEGF | 34.6 | 101.7 | (22.5–371) | 59.5 | (9.3–183) | 0.045 |
IL interleukin, G-CSF granulocytic colony stimulating factor, IFN interferon, MCP monocyte chemoattractant protein, MIP macrophage inflammatory protein, PDGF platelet-derived growth factor, RANTES regulated upon activation, normal T cell expressed and secreted, TNF tumor necrosis factor, VEGF vascular endothelial growth factor
Mann–Whitney U test was used for univariate analysis of two groups that were classified according to the frequency of MDSCs
Kinetics of MDSCs before and after curative RFA therapy
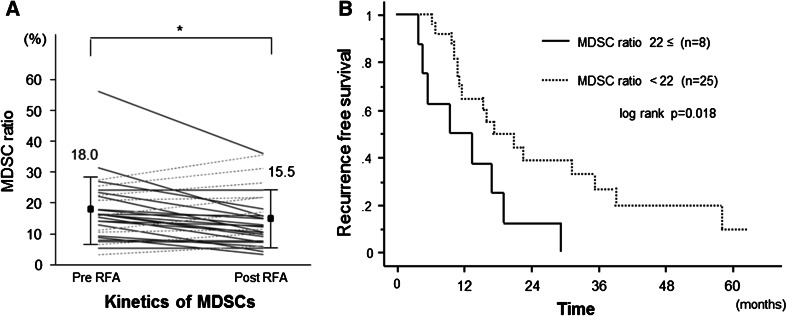
We examined the frequency of MDSCs before and after curative RFA therapy in 33 patients. For this analysis, blood samples were obtained on the day of treatment (before) and 2–4 weeks after treatment (after). The frequency of MDSCs was significantly decreased after RFA therapy (18.0 to 15.5 %, p < 0.05) (Fig. 3a). However, in several patients, the frequency of MDSCs remained at a high level compared with that in non-HCC patients. The clinical parameters before RFA were not statistically different between the patients with and without a high frequency of MDSCs after RFA (Suppl. table 3).
Fig. 3.
a In 33 HCC patients who received curative RFA therapy, the frequency of MDSCs was significantly decreased after treatment. However, in several patients, the frequencies were increased after treatment (dotted lines) (*, p < 0.05). b Kaplan–Meier curve for recurrence-free survival after RFA therapy. The patients with high frequency of MDSCs (solid line) relapsed
Next, we followed up these patients for recurrence and analyzed the risk factors. If a high frequency of MDSC was observed after curative RFA therapy, the recurrence-free survival was significantly shortened (Fig. 3b). In contrast, the frequency of MDSCs before treatment did not affect the recurrence. In univariate analysis for recurrence, post-treatment MDSC ratio ≥22 % (p = 0.023) and tumor multiplicity (p = 0.010) were significantly associated with HCC recurrence (Table 3). In multivariable analysis for recurrence, considering the variables in the univariate analysis with p < 0.1, only post-treatment MDSC ratio ≥22 % (HR 3.906, p = 0.014) was extracted as a significant risk factor for recurrence.
Table 3.
Cox proportional hazards regression for recurrence
| Variant | Univariate HR (95 % CI) | p value | Multivariable HR (95 % CI) | p value |
|---|---|---|---|---|
| Sex: female | 0.763 (0.446–1.308) | 0.326 | ||
| Age: ≥70 years | 1.111 (0.671–1.840) | 0.683 | ||
| Pre-MDSC ratio: ≥22 % | 1.210 (0.698–2.096) | 0.497 | ||
| Pre-neutrophil | 0.990 (0.969–1.012) | 0.385 | ||
| Pre-lymphocyte | 1.014 (0.987–1.043) | 0.311 | ||
| Pre-neutrophil/lymphocyte | 0.978 (0.787–1.216) | 0.844 | ||
| Pre-ALT | 1.001 (0993–1.008) | 0.882 | ||
| Pre-serum albumin: <3.5 mg/dl | 1.143 (0.665–1.982) | 0.647 | ||
| Pre-prothrombin time: <70 % | 1.662 (0.961–2.903) | 0.073 | 1.881 (0.522–6.777) | 0.101 |
| Post-MDSC ratio: ≥22 % | 2.795 (1.150–6.792) | 0.023 | 3.906 (1.313–11.616) | 0.014 |
| Post-neutrophil | 1.005 (0.975–1.035) | 0.762 | ||
| Post-lymphocyte | 0.993 (0.960–1.027) | 0.678 | ||
| Post-neutrophil/lymphocyte | 1.003 (0.810–1.242) | 0.980 | ||
| Post-ALT | 0.995 (0.981–1.010) | 0.501 | ||
| Type IV collagen 7S | 1.122 (0.992–1.268) | 0.067 | 1.192 (0.907–1.566) | 0.207 |
| AFP: ≥100 ng/ml | 1.357 (0.743–2.480) | 0.321 | ||
| Tumor size: ≥20 mm | 1.29 (0.78–2.12) | 0.328 | ||
| Tumor multiplicity: multiple | 2.00 (1.18–3.40) | 0.010 | 1.851 (0.721–4.753) | 0.201 |
HR hazard ratio, CI confidence interval, ALT alanine aminotransferase, AFP alpha-fetoprotein
Discussion
MDSCs are expanded in pathological conditions such as malignancy, infection, or trauma and consist of a heterogeneous population of immature myeloid cells [15, 25]. In pathological conditions, immature myeloid cells are blocked to differentiate into mature macrophages, dendritic cells, or granulocytes; as a result, MDSCs are accumulated [15, 25]. MDSCs strongly inhibit anti-tumor immune response through a number of mechanisms [15, 25]. As monocytic subsets of MDSCs, CD14+HLA-DR−/low MDSCs have been reported in various malignancies, including melanoma, multiple myeloma, prostate cancer, and bladder cancer [18, 20, 22, 35]. In the most recent study, Hoechst et al. [24] reported that CD14+HLA-DR−/low MDSCs were significantly increased in HCC patients and they suppressed T cell functions through the induction of CD4+CD25+Foxp3+ Treg.
In the present study, in addition to an increase in the number of MDSCs in HCC patients, we observed that the frequency was correlated with the progression of HCC. Consistent with our results, it has also been reported that the frequency of CD14+HLA-DR−/low MDSCs was correlated with tumor progression in patients with other cancers, such as melanoma, prostate cancer, and bladder cancer [22, 35, 36]. However, the mechanisms behind the increase in MDSCs in advanced cancer patients are still unclear. As is well known, there is a close relationship between hepatocarcinogenesis and histological status of underlying liver [37, 38]. Therefore, the advance of hepatic fibrosis and the increase in inflammatory cell infiltration into liver might result in an increase in MDSCs following the progression of HCC. However, there was no relationship between the frequency of CD14+HLA-DR−/low MDSCs and underlying liver status in our study. From our observations, increase in MDSCs was only correlated with tumor progression, but not with hepatic fibrosis or disease activity of CLD. This finding suggests that the expansion of CD14+HLA-DR−/low MDSCs was mostly derived from the tumor environment itself, but not from inflammation or fibrosis of liver tissue around the tumor. The finding that a significant decrease in the frequency of circulating CD14+HLA-DR−/low MDSCs is observed in most patients with curative treatment in this study supports this hypothesis. On the other hand, Tregs were also increased in HCC patients and associated with the progression of HCC. Though it was reported that MDSCs suppressed T cell function through the induction of Tregs, there was not a strong correlation between the frequencies of these two immunosuppressive cells.
Regarding the mechanism of MDSC expansion, we also analyzed the relationship between the serum cytokine levels and the frequency of MDSCs. We observed that the serum concentrations of IL-10, IL-13, and VEGF were significantly increased in the group with high frequency of MDSCs and there was a positive correlation between these cytokine levels and the frequency of MDSCs. Moreover, although there was no significant difference, the serum concentrations of IL-1ra, IL-2, IL-6, IL-12(p70), and G-CSF tended to be increased in the group with high frequency of MDSCs. In accordance with our results, various cytokines, including IL-6, IL-10, IL-13, G-CSF, and VEGF, that trigger Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathways have been reported to be associated with the frequency of MDSCs [39]. In particular, the cytokines involved in the JAK2-STAT3 signaling pathway are considered to be the main regulators of the expansion of MDSCs, which leads to stimulation of myelopoiesis and inhibition of myeloid-cell differentiation [40–42].
Another important finding of our study is that the frequency of MDSCs showed various changes after curative RFA and this frequency is an independent risk factor of HCC recurrence. In most of the patients, the frequency of MDSCs decreased after RFA. A similar phenomenon has also been reported in other cancer treatments [19, 21, 36]. Liu et al. [21] reported that MDSCs were decreased in non-small cell lung cancer patients who had clinical benefit from chemotherapy or who received curative surgery. These results suggest that a decrease in the frequency of MDSCs is due to tumor eradication.
It is well known that tumor factors including multiplicity, tumor diameter, serum levels of tumor marker, and hepatic reserve are risk factors of HCC recurrence after RFA [43, 44], but it has not been reported that the frequency of circulating MDSCs is also a risk factor. From our findings, there was a clear inverse correlation between the frequency of MDSCs after RFA and recurrence-free survival. Consistent with our results, in the patients with pancreatic, esophageal, and gastric cancer, Gabitass et al. [23] reported that an increase in MDSCs was associated with an increased risk of death and that the frequency of MDSCs was an independent prognostic factor for patient survival. Taken together with these findings, our results suggest that the frequency of MDSCs might be one of the prognostic factors of patients after cancer treatments.
As we showed, the frequency of MDSCs is primarily correlated with tumor progression. However, between the patients with high and low frequency of MDSCs after RFA, there was no significant difference in hepatic reserve and tumor factors before treatment. Although an incomplete HCC eradication at a microscopic level may allow a high frequency of MDSCs after RFA, there may be other mechanisms such as subsequently tumor-specific immune responses after RFA. In addition, there is a limitation of the present study because we used cryopreserved PBMCs for phenotypic analysis of MDSCs. Further studies using fresh PBMCs are needed for precise phenotypic analysis of MDSCs and elucidation of the mechanism to regulate the frequency of MDSCs after HCC treatment.
In conclusion, the frequency of MDSCs in HCC patients is correlated with tumor progression, and the frequency after RFA is inversely correlated with the prognosis of HCC patients. HCC patients who show a high frequency of MDSCs after RFA should be closely followed, and the inhibition or elimination of MDSCs after HCC treatments may improve the prognosis of HCC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- MDSCs
Myeloid-derived suppressor cells
- HCC
Hepatocellular carcinoma
- CLD
Chronic liver disease
- RFA
Radiofrequency ablation
- TACE
Transcatheter arterial chemoembolization
- PBMC
Peripheral blood mononuclear cell
- Tregs
Regulatory T cells
- HLA
Human leukocyte antigen
- FGF
Fibroblast growth factor
- CCL
Chemokine C–C motif ligand
- G-CSF
Granulocyte colony stimulating factor
- GM-CSF
Granulocyte macrophage colony stimulating factor
- IP
Interferon gamma-induced protein
- MCP
Monocyte chemoattractant protein
- MIP
Macrophage inflammatory protein
- PDGF
Platelet-derived growth factor
- RANTES
Regulated upon activation, normal T cell expressed and secreted
- TNF
Tumor necrosis factor
- VEGF
Vascular endothelial growth factor
- JAK
Janus kinase
- STAT
Signal transducer and activator of transcription
References
- 1.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42–52. doi: 10.1634/theoncologist.2010-S4-42. [DOI] [PubMed] [Google Scholar]
- 4.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishizaki Y, Kawasaki S. The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future) J Gastroenterol. 2008;43:18–26. doi: 10.1007/s00535-007-2141-x. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita T, Arai K, Sunagozaka H, Ueda T, Terashima T, Mizukoshi E, Sakai A. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:281–290. doi: 10.1159/000334439. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232–S241. doi: 10.1053/j.gastro.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Wang L, Zhang Y. Dendritic cell as therapeutic vaccines against tumors and its role in therapy for hepatocellular carcinoma. Cell Mol Immunol. 2006;3:197–203. [PubMed] [Google Scholar]
- 10.Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–1146. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 11.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 12.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 13.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4 + CD25 + T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 17.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Clin Cancer Res. 2006;12:1515–1524. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 18.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased level of both CD4 + FOXP3 + regulatory T cells and CD14 + HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b +/CD14−/CD15 +/CD33 + myeloid-derived suppressor cells and CD8 + T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14 + HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2011;61(2):255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 30.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 33.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan XK, Zhao XK, Xia YC, Zhu X, Xiao P. Increased circulating immunosuppressive CD14(+)HLA-DR(-/low) cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J Int Med Res. 2011;39:1381–1391. doi: 10.1177/147323001103900424. [DOI] [PubMed] [Google Scholar]
- 36.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 37.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 38.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 39.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 42.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izumi N, Asahina Y, Noguchi O, Uchihara M, Kanazawa N, Itakura J, Himeno Y. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer. 2001;91:949–956. doi: 10.1002/1097-0142(20010301)91:5<949::AID-CNCR1084>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 44.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.