Abstract
The immune system has evolved regulatory mechanisms to control immune responses to self-antigens. Regulatory T (Treg) cells play a pivotal role in maintaining immune tolerance, but tumour growth is associated with local immunosuppression, which can subvert effector immune responses. Indeed, the induction and recruitment of Treg cells by tumours is a major barrier in the development of effective immunotherapeutics and vaccines against cancer. Retinoic acid (RA) has been shown to promote conversion of naïve T cells into Treg cells. This study addresses the hypothesis that blocking RA receptor alpha (RARα) may enhance the efficacy of a tumour vaccine by inhibiting the induction of Treg cells. We found that RA significantly enhanced TGF-β-induced expression of Foxp3 on naïve and committed T cells in vitro and that this was blocked by an antagonist of RARα (RARi). In addition, RARi significantly suppressed TGF-β and IL-10 and enhanced IL-12 production by dendritic cells (DC) in response to killed tumour cells or TLR agonists. Furthermore, RARi augmented the efficacy of an antigen-pulsed and TLR-activated DC vaccine, significantly attenuating growth of B16 tumours in vivo and enhancing survival of mice. This protective effect was associated with significant reduction in tumour-infiltrating FoxP3+ and IL-10+ Treg cells and a corresponding increase in tumour-infiltrating CD4+ and CD8+ T cells that secreted IFN-γ. Our findings demonstrate that RARα is an important target for the development of effective anti-tumour immunotherapeutics and for improving the efficacy of cancer vaccines.
Keywords: Tumour immunity, Retinoic acid, Regulatory T cells, TGF-β, Dendritic cell vaccine
Introduction
Vitamin A deficiency compromises many aspects of innate and adaptive immune responses, with defects in immune control of bacterial, viral and protozoan infections [1]. The synthesis of dietary vitamin A into retinoic acid (RA) is mediated at immune-privileged sites, by bona fide RA-producing cells, such as gut-associated lymphoid tissue dendritic cells (DCs) and macrophages. CD103+ DCs in gut-associated lymphoid organs express retinal dehydrogenases an aldehyde dehydrogenase (ALDH), which produces the active metabolite RA independent of TLR activation [2]. While multiple isoforms of RA exist, all-trans retinoic acid (ATRA) is the predominant isoform in most tissues [3, 4]. ATRA binds to a family of nuclear hormone receptors, termed RA receptors (RAR), and retinoid X receptors. Ligand-activated heterodimers of these receptors act as transcriptional regulators by binding to retinoic acid response elements (RARE) in gene promoters [5]. There are three types of RAR: RARα, RARβ and RARγ, but RARα is the dominant type in immune cells. RARα signalling can modulate the function of T cells and DCs [6].
It has recently been discovered that ATRA is a key regulator of TGF-β-dependent immune homoestasis, contributing to the reciprocal regulation of Treg and IL-17-producing CD4+ T helper cell (Th17) development. It has been shown that RA synthesized by DCs and macrophages can synergize with TGF-β to enhance the conversion of naïve T cells into functionally competent FoxP3+-induced Treg (iTreg) cells [7–10]. Furthermore, RA inhibits the differentiation of naïve T cells into Th17 cells [9, 11]. RA production by intestinal CD103+ DCs has been shown to play a role in the maintenance of intestinal tolerance. However, RA can also promote effector T cell responses during infection or autoimmune inflammation [6, 12]. Furthermore, mice reared on a vitamin A-deficient diet and RARα-knockout mice have defective Th1 and Th17 responses [6, 13].
One of the fundamental challenges in the development of effective immunotherapeutics and vaccines against cancer is the identification of strategies to overcome the suppressive environment created by the tumour. Suppressive mechanisms elicited by the tumour include the secretion of immunosuppressive molecules, such as TGF-β, and the substantial recruitment and activation of Treg cells locally within the tumour microenvironment, but also systemically, with increased Treg cells commonly detected in peripheral blood and unrelated organs in cancer patients [14, 15]. Removal or depletion of Treg cells in murine tumour models has been shown to dramatically enhance tumour immune surveillance, greatly improving the efficacy of therapeutic cancer vaccines and in some cases allowing spontaneous tumour rejection [14–17]. However, since Treg cells are an essential component of a healthy immune system, approaches that persistently suppress Treg cell function may not be generally applicable for the treatment of human tumours. While blocking CTLA4, which is constitutively expressed on Treg cells, has shown efficacy against a range of human tumours, this treatment has been associated with the induction of colitis and grade 3 and 4 autoimmune manifestations [18, 19].
An alternative strategy and less harmful approach than the systemic inhibition of Treg cells may be to block or limit the activation and expansion of Treg cells at the site of the tumour, while simultaneously boosting effector T cell responses via tumour-antigen-loaded DC vaccines that promote the induction of antigen-specific effector T cells. This combined approach should facilitate attempts to enhance the efficacy of cancer vaccines, while preventing the induction of autoimmune or inflammatory disorders. Much of the published work on active immunotherapy, including studies from our laboratory, has highlighted the promising potential of using an autologous DC pulsed in vitro, away from the immunosuppressive environment of the growing tumour, with tumour-antigens and immunomodulatory molecules, such as TLR agonists, prior to being adoptively transferred in vivo [20, 21]. Since RA, which is metabolised by DCs, has a central role in promoting the generation of TGF-β-dependent iTreg cells, targeting RA metabolism or RAR signalling in the DC could be an attractive therapeutic target to integrate into DC immunotherapies. Therefore, the aim of the present study is to investigate whether antagonising the RA-RARα axis could inhibit the induction of FoxP3+ Treg cells and improve the efficacy of a DC vaccine for the treatment of tumours by reducing the ratio of Treg cells to effector T cells.
Materials and methods
Mice
Six- to eight-week-old female C57BL/6 mice were purchased from Harlan Laboratories (Oxen, UK). Animal experiments and maintenance were approved and regulated by the university ethics committee and the Irish Department of Health.
Tumour models
The B16F10 murine melanoma cell line was purchased from the American Type Culture Collection (Manassas, VA) and used for tumour induction in C57BL/6 mice, by subcutaneous (s.c.) administration into the flank. Tumour growth was recorded every 2–3 days, and animals were killed when tumours measured 15 mm in diameter. Tumour volume (V) was calculated as V = (π/6) (d12 × d2) where d1 is the shortest diameter measurement.
RA and RAR inhibitor
ATRA was purchased from Enzo Life Science (Exeter, UK) and unless otherwise stated was used at a concentration of 100 nM. The RAR inhibitor (RARi) Ro 41-5253, a synthetic retinoid and selective small molecule antagonist of RARα, was purchased from Enzo Life Science. R0 41-5253 competes with ATRA for binding to RARα. Unless otherwise stated, it was used at a concentration of 5 μM.
DC tumour vaccine
We have previously shown that DCs that have been matured in vitro with TLR agonists, such as CpG, and pulsed with heat-shocked irradiated tumour cells as a source of antigen are effective cell-based vaccines against murine tumours [14, 20]. Murine bone marrow-derived immature DCs were generated as described [14]. For blocking RARα, DCs were pretreated with RARi (Ro41-5253) for 15 min. DCs were then loaded at a 1:1 ratio with heat-shocked (43 °C for 1 h), γ-irradiated (200 Gy) (hs/irr) tumour cells followed by stimulation with CpG (5 μg/ml) for 18 h. DCs were washed and injected (5 × 105) s.c. in a volume of 100 μl into the tumour site 3, 10 and 17 days after tumour inoculation. IL-10, IL-12p70, TGF-β, and IL-6 (R&D Systems) concentrations were quantified in supernatant by ELISA.
Aldehyde dehydrogenase (ALDH) activity
ALDH, the enzyme involved in RA production, can be quantified by cleavage of a synthetic substrate (ALDEFLUOR). ALDH activity in DCs was quantified using the ALDEFLUOR® staining kit (STEMCELL Technologies, Vancouver, British Columbia, Canada) according to the manufacturer’s protocol. Briefly, DCs were suspended at 1 × 106 cells/ml in ALDEFLUOR assay buffer containing 5 μl of activated ALDEFLUOR substrate (150 nM) with or without the ALDH inhibitor diethylaminobenzaldehyde (DEAB) (5 μl of 1.5 mM). Samples were incubated for 30–60 min at 37 °C. Cells were then centrifuged at 1200 rpm for 5 min and the samples stained with anti-CD11c-APC in cold ALDEFLUOR assay buffer. Dead cells were excluded using propidium iodide. Samples were analysed by flow cytometry, gating on the FL1 (530/40)-positive population of ALDEFLUOR cells.
Real-time PCR
RNA was extracted from homogenized tissue or purified cells using RNeasy kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription was performed using high-capacity cDNA reverse transcription kit (Applied Biosystems) followed by real-time PCR using an ABI PRISM7500 Sequence Detection System (Applied Biosystems). Analysis of Rarα mRNA levels was performed using commercially available primer/probe sets (Applied Biosystems). Relative levels of expression were determined by normalization to 18S rRNA.
Treg cell conversion assay
CD4+CD25− cells were purified from the spleens of naïve mice using MACS cell sorting. Cells were cultured with plate-bound anti-CD3 (1 μg/ml; BD), soluble anti-CD28 (5 μg/ml BD) and recombinant (r)IL-2 (10 ng/ml; BD) and, were indicated, rTGF-β (5 ng/ml; R&D Systems) and ATRA. Alternatively, DO11.10 ovalbumin (OVA) TCR transgenic (Tg) mice were immunized with OVA protein (100 μg; Hyglos) and CpG (50 μg) into the footpad and popliteal lymph nodes removed 7 days post-immunization. Lymph node cells were cultured with OVA peptide 323–339 (1 μg/ml; New England Peptide) and rIL-2 (10 ng/ml) and, were indicated, recombinant rTGF-β (5 ng/ml) and ATRA.
Flow cytometric analysis
For conversion assay, T cells were stained with antibodies specific for CD4, CD25 and FoxP3 after 3-day incubation and analysed by flow cytometry. To assess DC maturation, bone marrow-derived DCs were cultured for 18 h with CpG (5 μg/ml), hs/irr B16 (1 × 106/ml) or both, or with medium only as a control. Cells were labelled with anti-CD11c, CD40 and CD86 and analysed by flow cytometry. For in vivo experiments, tumours were removed on appropriate days and single-cell suspensions were prepared. Cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 2 h and then brefeldin A (5 μg/ml) was added for a further 4 h at 37 °C. Cells were stained with antibodies to CD4 or CD8 (eBioscience). Cells were fixed and permeabilized, then incubated with anti-IL-10, anti-IFN-γ, anti-FoxP3 or anti-T-bet (eBioscience). Data were acquired using a CyAn ADP flow cytometer (Beckman Coulter) and analysed with FlowJo software (TreeStar Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad Instat. ANOVA or Student’s t test was used to compare statistical differences of means between groups.
Results
RA enhances TGF-β-induced Foxp3 expression on naïve and committed CD4+ T cells
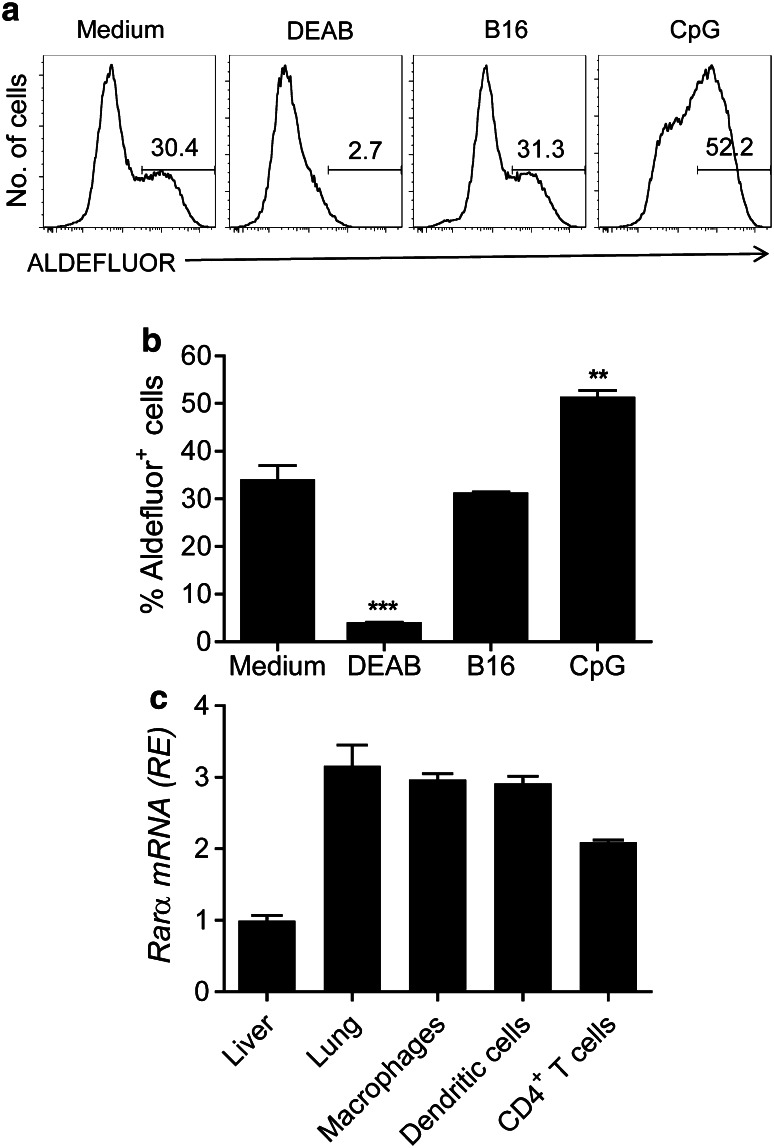
We investigated the possibility that murine bone marrow-derived DCs could produce RA. RA is synthesized via a multi-step pathway where retinol (Vitamin A) is converted by alcohol dehydrogenase into retinal and then converted into RA by a family of ALDH enzymes [22]. Active ALDH expression in DCs was quantified by flow cytometry following conversion of substrate ALDEFLUOR (ALD) into a fluorescent product. The results indicate that DCs endogenously express functional ALDH enzymes, and this expression was significantly enhanced by stimulation with the TLR9 ligand CpG, but not by killed B16 tumour cells and significantly reduced by the ALDH inhibitor DEAB (Fig. 1a, b). We next examined expression of RARα on DC, CD4+ T cells and other tissues. We found that RARα was expressed by bone marrow-derived DCs, macrophages and CD4+ T cells from spleen of naïve mice and was also expressed in lung tissue (Fig. 1c).
Fig. 1.
Dendritic cells express ALDH and RARα a DCs were incubated with medium, hs/irr B16 tumour cells or CpG (5 μg/ml). After 24 h, cells were incubated with ALDEFLOUR in the presence or absence of the ALDH inhibitor DEAB and analysed by flow cytometry gating on CD11c+ cells. Data are representative FACS plots (a) or mean ± SE values from three independent experiments (b). c Relative mRNA expression of Rarα in liver, lungs or purified macrophages, DCs or CD4+ T cells. Mean ± SE values for triplicate samples. **p < 0.01, ***p < 0.001 versus medium control by ANOVA
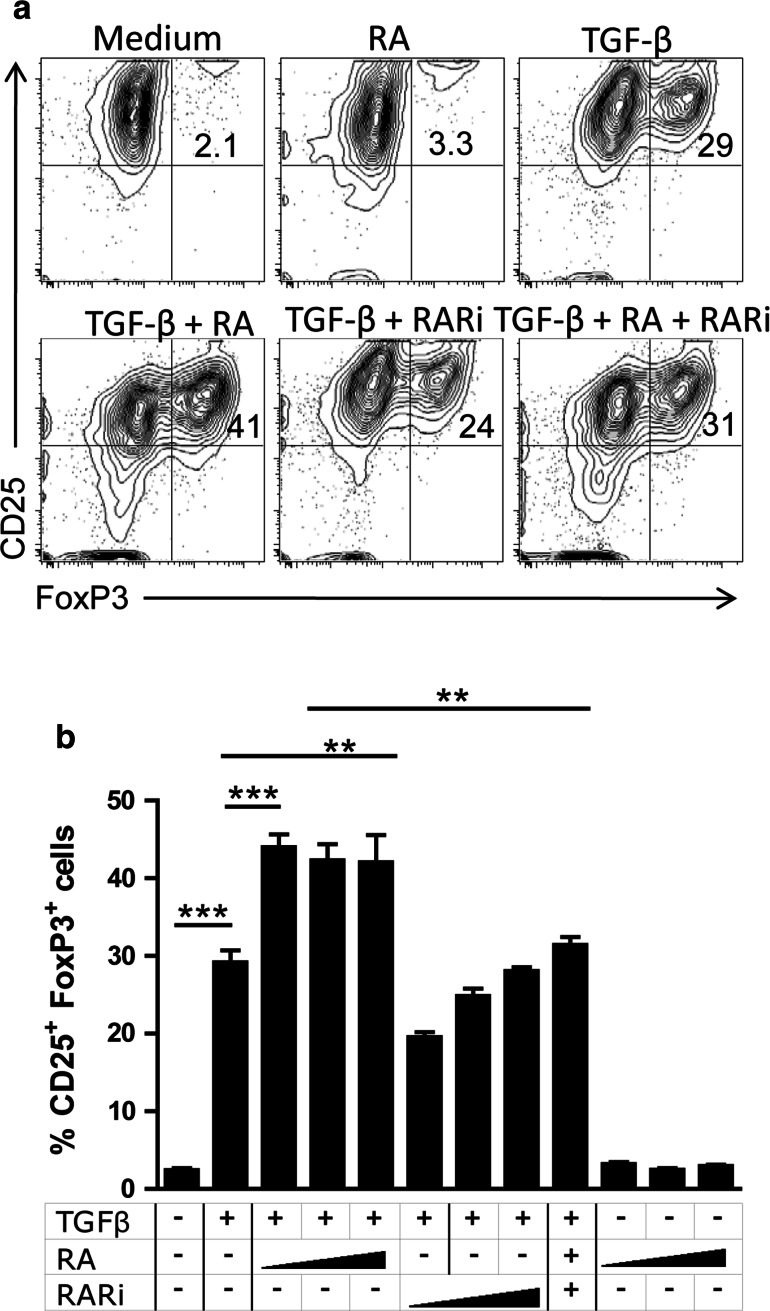
It has previously been demonstrated that RA can promote peripheral conversion of naïve T cells into FoxP3+ Treg cells [7–10]. Here, we examined the effect of RA in combination with TGF-β on Foxp3 expression on CD4+CD25− T cells and whether blocking RARα with the antagonist Ro41-5253 (RARi) could inhibit FoxP3 expression. Purified CD4+CD25− T cells were stimulated with anti-CD3, anti-CD28 and rIL-2, in the presence or absence of rTGF-β and/or ATRA for 3 days and analysed for CD25 and FoxP3 expression. Addition of ATRA alone had little effect on FoxP3 expression (Fig. 2a). In contrast, rTGF-β significantly enhanced the frequency of T cells expressing CD25 and FoxP3, and this was further enhanced by the addition of RA over a range of concentrations (Fig. 2a, b). Furthermore, the cooperative effect of TGF-β and RA on Treg cell conversion was significantly inhibited by a range of concentrations of RARi (Fig. 2b).
Fig. 2.
RA enhances and RARi inhibits TGF-β-induced expression of Foxp3 on naïve T cells. Naïve murine CD4+CD25− cells were cultured at 1 × 106 cells/ml in anti-CD3-coated wells (1 μg/ml), in medium containing rIL-2 (10 ng/ml) and soluble anti-CD28 (5 μg/ml) with or without rTGF-β (5 ng/ml), ATRA (RA; 50, 100 or 200 nM) or both in the presence or absence RARi (2.5, 5, or 10 μM). After 3 days, cells were analysed for expression of FoxP3 and CD25 after gating on CD4+ T Cells. Data are representative FACS plots (a) and or mean ± SE values from 3 independent experiments (b) **p < 0.01, ***p < 0.001 by ANOVA
We also assessed the effect of ATRA on committed T cells using lymph node cells from OVA TCR Tg mice that had been immunized with OVA protein with CpG as an adjuvant. Culture of OVA-stimulated lymph node cells with TGF-β enhanced FoxP3 expression on CD4+ T cells (Fig. 3a). Addition of ATRA alone did not enhance FoxP3 expression, but did significantly augment TGF-β-induced Foxp3 in antigen-activated CD4+ T cells (Fig. 3b). Antigen-activated CD4+ T cells expressed high levels of the transcription factor T-box-containing protein expressed in T cells (T-bet), the master regulator of Th1 cells, but did not express FoxP3 (Fig. 3c). FoxP3 expression was enhanced by TGF-β and further augmented by addition of RA, with significant proportion of cells expressing both Foxp3 and T-bet. Interestingly, the frequency of Foxp3+T-bet− cells was low following stimulation with TGF-β, but was substantially increased following addition of RA (Fig. 3c). These findings demonstrate that in combination with TGF-β, ATRA enhances FoxP3 expression on committed as well as naïve T cells.
Fig. 3.
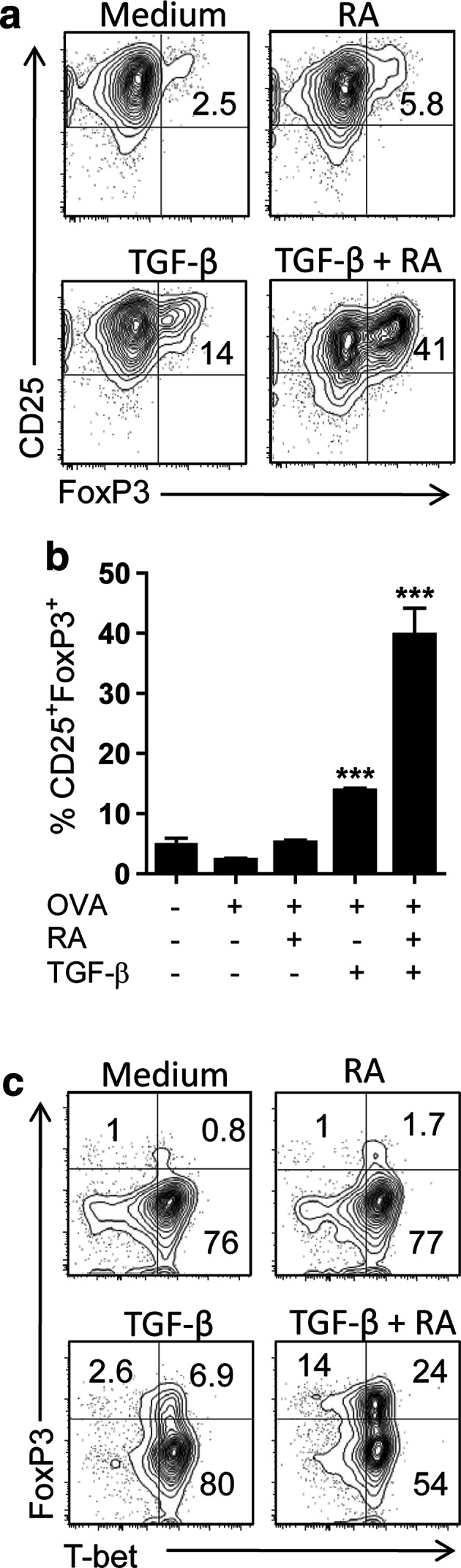
RA enhances TGF-β-induced expression of Foxp3 on committed antigen-stimulated T cells. Lymph node cells from OVA TCR transgenic mice immunized with OVA protein were cultured at 1 × 106 cells/ml with OVA peptide and medium only, TGF-β (5 ng/ml), ATRA (RA; 100 nM) or TGF-β and ATRA. After 3 days, cells were analysed for expression of FoxP3, CD25 and T-bet after gating on CD4+ T Cells. Data are representative FACS plots (a) and or mean ± SE values from three independent experiments for CD25 versus Foxp3 (b) or FACS plots for Foxp3 versus T-bet (c) ***p < 0.01 versus OVA only by ANOVA
Inhibition of RARα suppresses anti-inflammatory cytokine production by DC and enhances their ability to prime Th1 cells
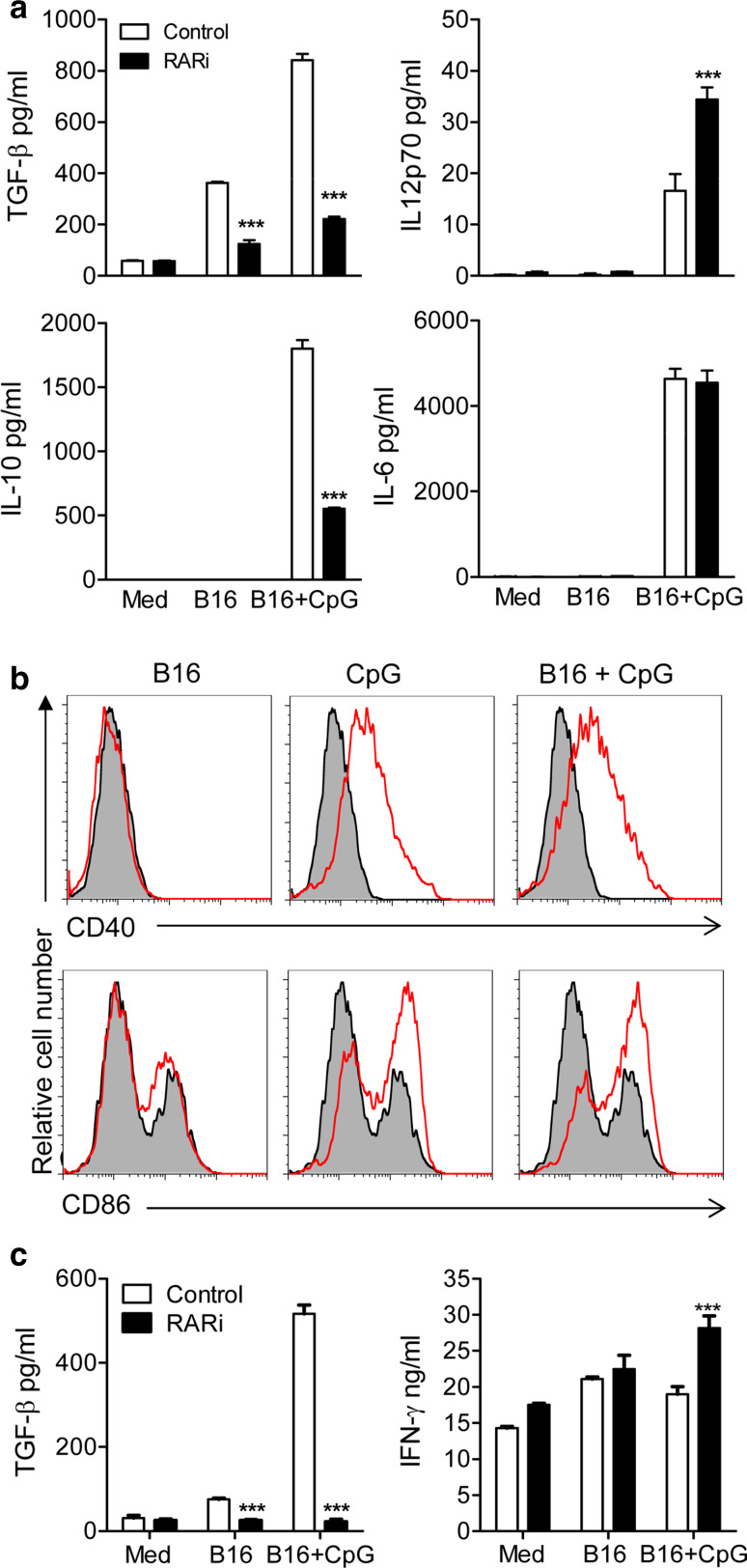
Since both IL-10 and TGF-β production by innate immune cells is known to promote the induction of Treg cells and these cytokines are induced in DCs by tumour cells and TLR agonists, we tested the effects of blocking RARα on cytokine production by DCs treated with killed tumour cells or CpG. DCs were pretreated with medium or the RARα antagonist for 15 min prior to incubation with hs/irr B16 tumour cells for 2 h before the addition of CpG. Pretreatment with RARi significantly suppressed secretion of TGF-β and IL-10 by DCs stimulated with hs/irr B16 tumour cells and CpG (Fig. 4a; p < 0.001). In contrast, RARi significantly enhanced IL-12p70 production from CpG-activated DCs pulsed with hs/irr B16 cells (Fig. 4a; p < 0.001), while having no effect on IL-6 production. The modulatory effect of RARi on cytokine production by DC was observed when the antagonist was added 15 or 60 min before, 15 min after or at the same time as the hs/irr B16 + CpG, but the optimum effect was observed when the cells were preincubated for 15 min with RARi (data now shown). Stimulation of DC with CpG enhanced CD40 and CD86 expression (Fig. 4b). In contrast, culture with hs/irr B16 did not modulate endogenous or CpG-induced co-stimulatory molecule expression on DC (Fig. 4b).
Fig. 4.
Inhibition of RA suppresses TGF-β and IL-10 production by DCs and induction of TGF-β-secreting T cells. a DCs were stimulated with the indicated combinations of hs/irr B16, CpG (5 μg/ml) and RARi. After 24 h in culture, TGF-β, IL-10, IL-12p70 and IL-6 concentrations were quantified in supernatants by ELISA. Results are means (±SD) of triplicate assays. Data are representative of six independent experiments. b DCs were stimulated with hs/irr B16, CpG (5 μg/ml) or both. After 24 h in culture, CD40 and CD86 expression was assessed by flow cytometry analysis. Results are presented as stimulated (red line) versus medium control (grey). c DCs were stimulated with the indicated combinations of hs/irr B16, CpG and RARi as in (a). After 24 h, DCs were washed and added to purified CD4+ T cells activated with αCD3 at a T cell: DC ratio of 5:1. After 3 days, supernatants were recovered and concentrations of TGF-β and IFN-γ were determined in triplicate assays by ELISA. Results are mean ± SE values for triplate assays and are representative of three independent experiments. ***p < 0.001 medium control versus RARi by Student’s t test
We next examined the ability of these modulated DCs to direct CD4+ T cell responses in vitro. DCs were incubated with a combination of RARi, hs/irr B16 tumour cells and CpG. After 24 h, DCs were washed and added to activated CD4+ T cells. Pretreatment of DCs with RARi resulted in significant inhibition of TGF-β from CD4+ T cells following co-culture with CpG-activated DCs pulsed with hs/irr B16 cells (Fig. 4c; p < 0.001). In contrast, blocking RARα signalling in CpG-activated DC significantly enhanced the ability of the DCs to induce IFN-γ production from anti-CD3-activated CD4+ T cells (Fig. 4c; p < 0.001). Collectively, these experiments suggest that the inhibition of RARα in tumour Ag-pulsed and CpG-activated DCs directed CD4+ T cells away from immunosuppressive responses and towards a pro-inflammatory Th1 response.
Inhibition of RARα suppresses induction of Treg cells and enhances the efficacy of a DC vaccine against B16 melanoma in mice
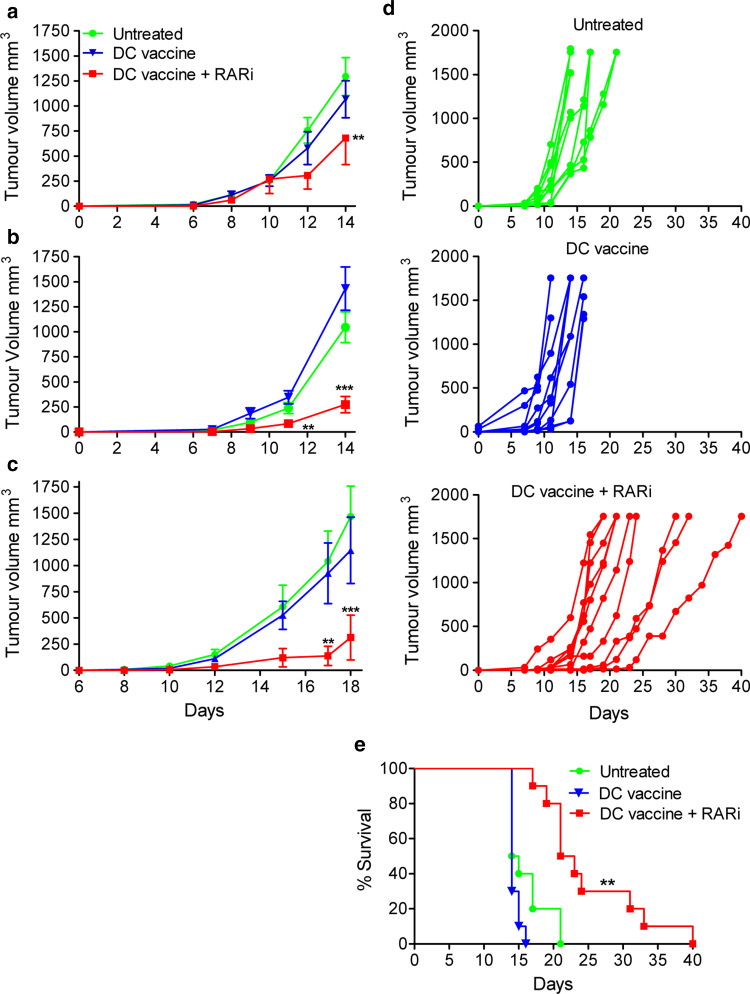
Having shown that RA promotes induction of Treg cells in vitro, we examined the hypothesis that whether inhibition of RARα could improve the efficacy of DC-based immunotherapy against the poorly immunogenic B16 melanoma in mice by suppressing the induction of Treg cells in vivo. Following s.c. challenge with B16 cells, mice were injected into the region of the tumour site with either one (day 10), two (day 3 and 10) or three (days 3, 10 and 17) dosage of a DC vaccine, comprising of CpG-activated DCs pulsed with hs/irr B16 tumour cells, in the presence or absence of RARi. Analysis of the DCs prior to injection revealed that CpG stimulation induced maturation (CD80, CD86 and CD40 expression) and IL-12 and IL-10 production (Fig. 4b and data not shown). Treatment of tumour-bearing mice with the DC vaccine alone had no significant effect on tumour growth (Fig. 5a). However, when RARα was blocked in the DCs, one treatment of this vaccine 10 days after tumour challenge significantly attenuated tumour growth (Fig. 5a; p < 0.01). Furthermore, two dosage of the RARi-treated DC vaccine enhanced the potency of this vaccine (Fig. 5b; p < 0.01–0.001), while treatment with three dosage of the RARi-treated DC vaccine demonstrated a further attenuation of tumour growth, with a significant reduction in tumour growth when compared with untreated mice or mice treated with the DC vaccine alone (Fig. 5c; p < 0.01–0.001). When the experiment was repeated where tumours were allowed to progress to a maximum size of 15 mm in diameter when individual mice had to be killed, the results demonstrated that addition of RARi to the DC vaccine reduced the rate of tumour growth and significantly enhancing survival of the mice (Fig. 5d, e). These findings demonstrate that blocking RARα enhances the efficacy of a DC-based vaccine for cancer.
Fig. 5.
Inhibition of RA enhances the efficacy of a TLR-activated DC vaccine against B16 tumours. C57BL/6 mice were injected s.c. into the flank with 2 × 105 B16 tumour cells. Mice were injected s.c. in the region of the tumour on days 10 (a) 3 and 10 (b) or 3, 10 and 17 days (c) post-tumour induction with 1–5 × 105 DC that were preincubated with medium only (control) or RARi (5 μM) for 15 min and then pulsed for 24 h with hs/irr B16 tumour cells (ratio 1:1) and CpG (5 μg/ml). Tumour growth was quantified; experiment was terminated when tumours in the control group reached a diameter of 15 mm. (a n = 6, b n = 15, c n = 8; **p < 0.01, ***p < 0.001 by ANOVA). d, e Mice were immunized on days 3, 10 and 17, and the experiment was allowed to progress for 40 days, with individual mice killed when the tumour reached a diameter of 15 mm. Results are shown as tumour growth curves for individual mice (d) and survival (e). **p < 0.01, by log-rank test (n = 10). Results for each panel are representative of 3–6 independent experiments
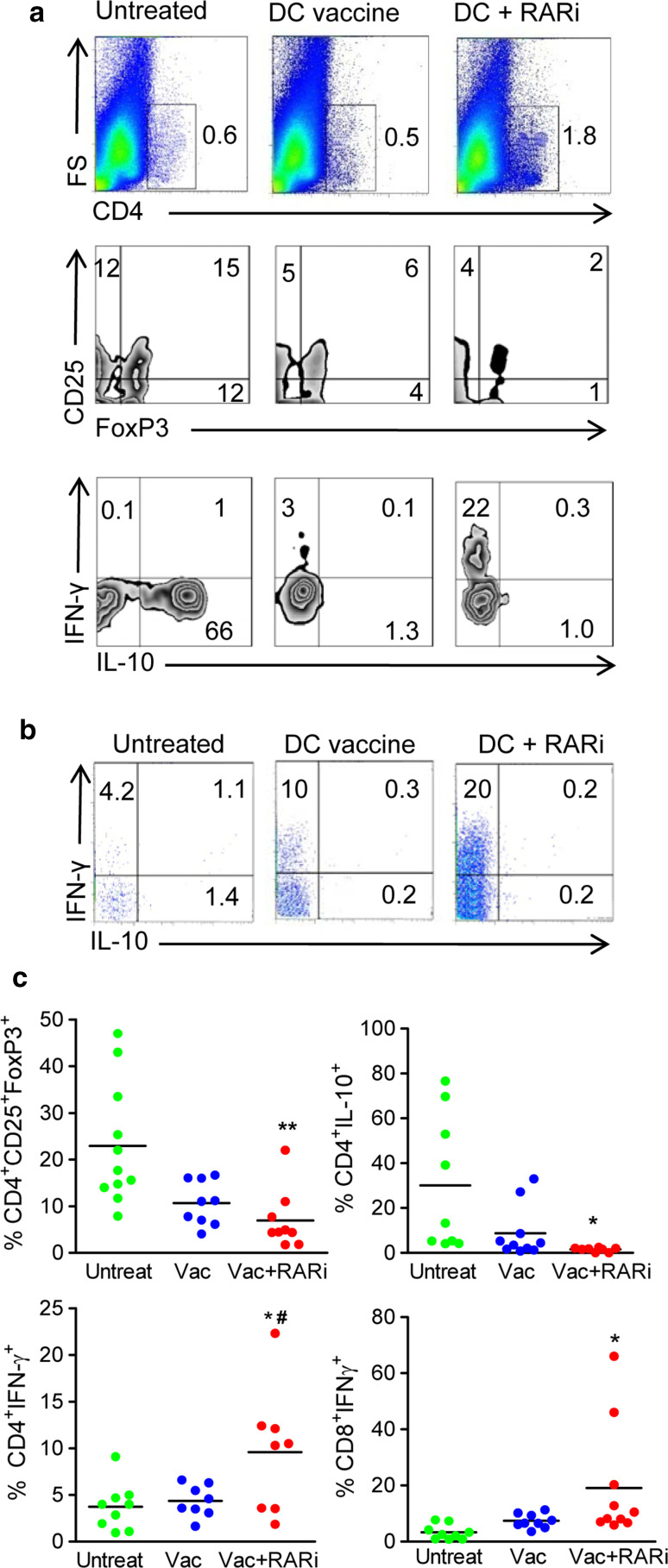
In order to determine whether blocking RARα in the DC vaccine attenuated tumour growth by inhibiting Treg cells and boosting effector immune responses in vivo, tumours were removed and tumour-infiltrating lymphocytes (TIL) were assessed for cytokine production. Attenuation of tumour growth by treatment with the RARi-treated DC vaccine was associated with a substantial increase in the percentage of tumour-infiltrating CD4+ T cells when compared with untreated mice or mice treated with DC vaccine only (Fig. 6a). Furthermore, intracellular cytometric analysis on TIL demonstrated that the increase in infiltrating CD4+ T cells in mice treated with the RARi-treated DC vaccine was associated with a significant reduction in the frequency of CD4+CD25+FoxP3+ T cells and CD4+IL-10+ Tr1 type Treg cells infiltrating the tumours when compared with control groups (Fig. 6; p < 0.01). The protective effect of treatment with the RARi-treated DC vaccine was also associated with a significant enhancement in the frequency of tumour-infiltrating IFN-γ-expressing CD4+ T cells and CD8+ T cells (Fig. 6). Collectively, these data suggest that blocking RARα enhances the efficacy of a DC vaccine by significantly inhibiting the induction of Treg cells, allowing the infiltration and activation of protective IFN-γ-secreting CD4+ and CD8+ T cells.
Fig. 6.
The protective effect of inhibiting RA is associated with the induction of tumour-infiltrating Treg cells. C57BL/6 mice were injected s.c. into the flank with 2 × 105 B16 tumour cells. Mice were injected s.c. into the region of the tumour on days 3, 10 and 17 post-tumour induction with DC vaccine with and without RARi as described in Fig. 5c. The experiment was terminated when tumours reached a diameter of 15 mm. a Representative dot plot of tumour-infiltrating CD4+ T cells, CD25+FoxP3+ T cells (gated on CD4), IL-10+ CD4 T cells or IFN-γ+ CD4 T cells from each treatment group. b Representative dot plot of tumour-infiltrating IFN-γ+ and IL-10+ CD8+ T cells from each treatment group. c Mean frequency of CD4+CD25+FoxP3+ T cells, CD4+IL-10+ T cells, CD4+IFN-γ+ T cells amd CD8+IFN-γ+ T cells from each treatment group (n = 8 from 2 experiments, *p < 0.05, **p < 0.01 versus untreated; #p < 0.05 versus DC vaccine, by one way ANOVA)
Discussion
In this study, we have demonstrated that blocking RARα in a TLR-activated DC vaccine significantly augments the efficacy of this cell-based therapy against murine tumours. The reduction in tumour growth following administration of the vaccine was associated with a significant reduction in tumour-infiltrating CD4+CD25+FoxP3+ T cells and CD4+IL10+ T cells and a corresponding significant increase in the frequency of tumour-infiltrating IFN-γ-secreting CD4+ and CD8+ T cells.
The FDA approval of Sipuleucel-T, a patient-specific autologous cell-based vaccine for the treatment of prostate cancer, has highlighted the promise of active immunotherapy for the treatment of cancer [23]. However, despite encouraging results, DC-based immunotherapeutic approaches have had limited success in the clinic to date. This has been attributed in part to tumour-derived immunosuppressive molecules, including TGF-β, and tumour-induced Treg cells. While it is clear that inhibiting or limiting Treg cells in cancer patients has the potential to confer protective anti-tumour immunity, current monotherapies that limit Treg cell functions can also compromise host immune tolerance against self-antigens, which can lead to the development of autoimmune disease. Our approach aimed to improve vaccine efficacy by manipulating the balance of regulatory and effector T cell responses at the site of the tumour, attenuating the activation, expansion and/or conversion of Treg cells at the site of the tumour while boosting Th1 responses.
A number of studies, including DC vaccine trials in humans, have demonstrated that antigen-pulsed immature DC are very poorly immunogenic with a tendency to induce Treg cells [24]; however, with optimal maturation conditions, they can induce antigen-specific Th1 responses [25]. TLR agonists are potent inducer of DC maturation and promote adaptive immune responses [26], and the TLR7 agonist imiquimod is an effective therapy for basal cell carcinoma [27]. However, other TLR agonists have not been very effective monotherapies against human tumours [28]. We and others have reported that active immunotherapy using TLR ligands can induce Treg cells as well as protective Th1 cells [20, 29]. Here, we show that blocking RARα in the DC vaccine reduces Treg cell induction, while allowing the CpG-activated DCs to drive effector T cell responses. We found that blocking RARα in CpG-activated DCs pulsed with hs/irr B16 cells significantly improved the efficacy of the CpG-activated DC therapy, significantly attenuating tumour growth and enhancing survival in vivo. Attenuation of tumour growth was associated with a substantial increase in the percentage of tumour-infiltrating CD4+ T cells, an effect that correlates with a good prognosis in the clinic [30]. In addition, therapeutic treatment with the DC vaccine in combination with RARi results in a significant increase in tumour-infiltrating IFN-γ-secreting CD4+ and CD8+ T cells. It is well established that CD8+ CTLs and CD4+ Th1 cells have a protective role in anti-tumour immunity, with the capacity to mediate direct killing of tumour cells and to activate other immune cells via the secretion of IFN-γ [31]. Furthermore, inhibition of RARα in the DC vaccine resulted in a significant reduction in the frequency of tumour-infiltrating CD4+CD25+FoxP3+ T cells and CD4+IL-10+ T cells. Indeed, Treg cells play a major role in the failure of many immunotherapeutic approaches against cancer, by inhibiting the protective anti-tumour immune responses involving CD4+ T cells and CD8+ T cells [32].
The results of the present study confirm previous reports that RA works with TGF-β to enhance conversion of naïve T cells into Treg cells [7–10], but also provides evidence that RA can enhance TGF-β-mediated induction of Foxp3 expression in T-bet+-committed T cells. Furthermore, suppression of RARα inhibited anti-inflammatory cytokine production by DCs and attenuated the induction of Treg cells, while enhancing Th1-type responses. Our findings suggest that inhibiting RARα in a DC vaccine is a promising immunotherapeutic approach to overcome local immune tolerance and promote effector T cell responses that confer significant protection against tumour growth in vivo. Blocking RARα in an Ag-pulsed and CpG-activated DC vaccine has the capacity to drive potent anti-tumour immune responses by limiting the activation and expansion of tumour infiltration Treg cells and is less likely to result in systemic side effects associated with global suppression of Treg cells.
Acknowledgments
This work was supported by Science Foundation Ireland (SFI) PI grants (06/IN.1/B87 and 11/PI/1-36) to Kingston Mills.
Conflict of interest
Kingston Mills is a co-founder and shareholder in Opsona Therapeutics Ltd and TriMod Therapeutics Ltd, university spin-out companies involved in the development of immunotherapeutics. All other authors do not have any conflict of interest.
References
- 1.Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]
- 2.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186:1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 3.Mic FA, Molotkov A, Fan X, Cuenca AE, Duester G. RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech Dev. 2000;97:227–230. doi: 10.1016/S0925-4773(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 4.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre B, Brand C, Lefebvre P, Ozato K. Chromosomal integration of retinoic acid response elements prevents cooperative transcriptional activation by retinoic acid receptor and retinoid X receptor. Mol Cell Biol. 2002;22:1446–1459. doi: 10.1128/MCB.22.5.1446-1459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JA, Cannons JL, Grainger JR, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103 + DCs induces Foxp3 + regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 9.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 12.DePaolo RW, Abadie V, Tang F, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- 14.Jarnicki AG, Lysaght J, Todryk S, Mills KHG. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cell. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 15.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58:948–961. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 17.Coe D, Addey C, White M, Simpson E, Dyson J, Chai JG. The roles of antigen-specificity, responsiveness to transforming growth factor-beta and antigen-presenting cell subsets in tumour-induced expansion of regulatory T cells. Immunology. 2010;131:556–569. doi: 10.1111/j.1365-2567.2010.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 20.Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+ IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72:581–591. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 21.Conroy H, Galvin KC, Higgins SC, Mills KH. Gene silencing of TGF-beta1 enhances antitumor immunity induced with a dendritic cell vaccine by reducing tumor-associated regulatory T cells. Cancer Immunol Immunother CII. 2012;61:425–431. doi: 10.1007/s00262-011-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol. 1999;63:139–188. doi: 10.1016/S0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- 23.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 24.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuler-Thurner B, Schultz ES, Berger TG, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 27.Lacarrubba F, Potenza MC, Gurgone S, Micali G. Successful treatment and management of large superficial basal cell carcinomas with topical imiquimod 5% cream: a case series and review. J Dermatolog Treat. 2011;22:353–358. doi: 10.3109/09546634.2010.548503. [DOI] [PubMed] [Google Scholar]
- 28.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 29.Jarnicki AG, Conroy H, Brereton C, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 30.Oble DA, Loewe R, Yu P, Mihm MC., Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 31.Berard F, Blanco P, Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]