Abstract
Dendritic cell (DC)-based immunotherapy is explored worldwide in cancer patients. Several strategies have been employed to load DC with antigen, including peptide loading. To increase immunogenicity of peptides, major histocompatibility complex (MHC) class I binding affinity and stability of peptide–MHC complexes at the cell surface may be improved by modification of the amino acid sequence. In this study, we compared the capacity of DC loaded with wild-type versus modified gp100 peptides with higher binding affinities to induce an immune and clinical response in advanced melanoma patients. Metastatic HLA-A2.1+ melanoma patients were vaccinated intravenously (on average 25 × 106 DC) and intradermally (on average 11 × 106 DC) with mature DC loaded with keyhole limpet hemocyanin (KLH) together with tyrosinase peptide and either wild-type (15 patients) or modified (12 patients) gp100 peptides. All vaccinated patients showed a pronounced proliferative T cell or humoral response against KLH. Gp100-specific T cell responses were monitored in post-treatment delayed type hypersensitivity (DTH) skin biopsies by tetramer and functional analysis. Antigen-specific T cells were found in 2 of 15 patients vaccinated with wild-type gp100-loaded DC, versus 1 of 12 patients vaccinated with modified peptide-loaded DC. These three patients also had the best clinical response, with long-term (>8 years) complete responses in two patients, one in each group. We conclude that vaccination with peptide-loaded DC can result in long-term clinical responses in a minority of metastatic melanoma patients, and that the use of modified as compared to wild-type gp100 peptides for DC loading does not result in a relevant enhanced immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0942-x) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Immunotherapy, Altered-peptide ligands, Melanoma, Monitoring
Introduction
Dendritic cells (DC) are well known for their unique capacity to induce activation of naïve tumor-specific T cells [1]. They play a critical role in determining the quantity and quality of the immune response to the antigen. For this reason, a growing number of clinical trials were performed using tumor antigen-loaded DC as a therapeutic vaccine in cancer patients (reviewed in [2]). We and others gained ample experience with monocyte-derived DC in clinical immunization protocols [3–9]. Objective clinical responses and immune responses without significant toxicity have been observed after vaccination with tumor antigen-loaded DC [3, 4, 9]. Although the clinical outcome of vaccination studies with antigen-loaded DC are encouraging, DC vaccination is still in its infancy, with a lot of opportunity for optimization [10].
Several strategies were employed to load DC with antigen. The availability of class I-restricted peptides derived from tumor-associated antigens, such as gp100, tyrosinase, MAGE, and NY-ESO-1, led to the use of peptide-pulsed DC in anti-tumor vaccination trials [11]. However, natural epitopes are often poorly immunogenic. Major histocompatibility complex (MHC) class I binding affinity and stability of peptide–MHC complexes at the cell surface contributes to the immunogenicity of a cytotoxic T lymphocyte (CTL) epitope. The sequence at amino acid residues that are crucial for the interaction with HLA or with the specific T cell receptor (TCR) can be modified in order to increase the affinity for MHC class I and enhance the immunogenicity of peptides. In addition, modified peptides may increase the repertoire of CTLs reactive with the tumor antigens. For example, we described the altered HLA-A2.1-binding gp100:154 epitopes, which have an improved immunogenicity and elicit wild-type epitope-reactive CTL [12]. These modified peptides can subsequently be used for the preparation of more effective DC vaccines.
Here, we compare the capacity of DC loaded with wild-type gp100 peptides (gp100:154 KTWGQYWQV; gp100:280 YLEPGPVTA) or modified gp100 peptides (gp100:154 KTWGQYWAV; gp100:280 YLEPGPVTV), to induce an immune and clinical response in advanced melanoma patients. The modified gp100:154 peptide with alanine substitution at position 8 was selected from a panel of modified gp100:154 peptides based on enhanced MHC class I binding and superior immunogenicity compared to wild-type peptides in vitro and in vivo in HLA-A2.1 transgenic mice [12]. The modified gp100:280 peptide with valine replacement at position 9 was selected based on increased binding affinity to HLA-A2.1 and enhanced in vitro CTL induction in peripheral blood lymphocytes (PBLs) of HLA-A2.1+ melanoma patients [13]. CTLs raised against both modified gp100:154 and modified gp100:280 recognized wild-type gp100 [12, 13]. However, ultimate proof of increased immunogenicity can only be obtained in vivo. Therefore, in this study metastatic HLA-A2.1+ melanoma patients were vaccinated with mature DC loaded with keyhole limpet hemocyanin (KLH) together with tyrosinase peptide and either wild-type or modified gp100 peptides and immune and clinical responses were monitored.
Materials and methods
Patient criteria
Inclusion criteria were histologic evidence of metastatic melanoma, progressive disease, measurable disease parameters, focal or diffuse expression of gp100 (mandatory) and tyrosinase (optionally) in at least one metastasis as determined by immunohistochemistry, HLA-A2.1 phenotype, WHO performance status 0 or 1, and written informed consent. Patients were staged according to the 2001 AJCC staging system [14]. Patients with clinical signs of brain metastases, serious concomitant disease or a history of second malignancy were excluded. Prior treatment was allowed, provided a treatment-free period of at least 4 months was observed and all related toxicity had resolved. Approval from the local regulatory committee was obtained.
Clinical protocol and immunization schedule
In eligible patients a leukapheresis was performed from which DC were generated. Patients received treatment with either wild-type peptides-pulsed DC (wild-type group) or modified peptides-pulsed DC (modified group). The first 10 patients were included in the wild-type group, the next group of patients in the modified group, and the last five patients in the wild-type group. The first vaccination cycle consisted of two parts (Supplementary Fig. 1). In the first part, antigen-pulsed DC were administered three times at bi-weekly intervals, intravenously (i.v.) and intradermally (i.d.). In the second part, starting 4 weeks after the last vaccination of the induction phase, patients without tumor progression received 3 monthly i.d. vaccinations with peptides alone (100 μg) and KLH (2 μg), separately at different sites, as maintenance treatment for further T cell expansion. Prior to each vaccination 80 ml of blood was collected for immunological monitoring.
The first tumor response evaluation was performed after the induction phase. If no progression had occurred, patients continued treatment in the second part of the protocol. The second tumor evaluation took place after completion of the maintenance phase (i.e., 4 months after start of treatment). Disease control rate was defined as any partial or complete response or stable disease of at least 4 months, which appears clinically meaningful and is used more often in metastatic melanoma trials [15]. Stable disease and partial response were defined according to RECIST criteria [16]. Toxicity was assessed according to NCI common toxicity criteria. Progression free survival was calculated from the day of the first vaccination.
Patients who remained free of disease progression were eligible for two maintenance cycles, each at 6-month interval and each consisting of three bi-weekly intranodal vaccinations in a clinically tumor free, usually inguinal lymph node region under ultrasound guidance with mature DC, pulsed with gp100-, and tyrosinase peptides and KLH. Patients who received DC pulsed with wild-type gp100 peptides in the first cycle received modified peptide-pulsed DC in the first maintenance cycle and wild-type peptide-pulsed DC in the second maintenance cycle. Patients who received DC pulsed with modified gp100 peptides in the first cycle received wild-type peptide-pulsed DC in the first maintenance cycle and modified peptide-pulsed DC in the second maintenance cycle (Supplementary Fig. 1).
DC: preparation and characterization
DC were generated from peripheral blood mononuclear cells (PBMC) as described previously [17, 18]. After leukapheresis, PBMC were used for the generation of monocyte-conditioned medium (MCM) and plastic-adherent monocytes were cultured in X-VIVO 15 medium (BioWhittaker, Walkersville, MD) supplemented with 2% pooled human serum (Bloodbank Rivierenland, Nijmegen, The Netherlands), IL-4 (500 U/ml) and granulocyte macrophage colony stimulating factor (GM-CSF, 800 U/ml) (both from Schering-Plough, International, Kenilworth, NJ). After the addition of KLH (10 μg/ml; Calbiochem, San Diego, CA), which is free of LPS and does not induce maturation of DC, on day 3–4, autologous MCM with prostaglandin E2 (PGE2, 10 μg/ml, Pharmacia & Upjohn, Puurs, Belgium) and 10 ng/ml recombinant TNF-α (kindly provided by Dr. Adolf Bender, Vienna, Austria) were added on day 7 (30%, v/v). This procedure resulted in mature DC on day 9 as demonstrated by high expression levels of MHC class I and II, CD80, CD83 and CD86 and absence of CD14 [17].
Cryopreservation of PBMC and DC
PBMC and DC were frozen using a cryo 1°C freezing container (Nalgene, Rochester, NY, USA) which was put in −80°C for 24 h, in freezing medium consisting of 50% XVIVO-15 (5% HS), 40% human serum albumin, and 10% DMSO (final concentration, Sigma). Cells were frozen in 1 ml per vial containing a maximum of 40 × 106 cells [17].
Cells were thawed in a 37°C water bath after which the cells were washed once in cold medium and once in medium of room temperature before further use. The recovery of viable mature DC was 65 ± 10% [17].
Peptide pulsing
DC were pulsed with HLA-A2.1-restricted clinical grade wild-type peptides, gp100:154–167, gp100:280–288 or modified peptides (gp100:154–167 Q → A, gp100:280–288 A → V) and wild-type tyrosinase 369–376. Peptides were generated in our research facility according to GMP standards. Peptides were approved by the local pharmacist after analysis of purity (>90%), absence of pyrogen, sterility, toxicity, and HLA-binding capacity. Pulsing was done directly after harvesting or after thawing [19–21]. On the day of vaccination, we added peptides (50 μg/ml) for 90 min and kept DC at 37°C/5% CO2. Thereafter, fresh peptides (25 μg/ml) were added and DC were kept at room temperature for 60 min. After peptide loading, DC were washed twice in 0.9% sodium chloride and resuspended in 0.2 ml (on average 25 × 106 DC) and 1 ml (on average 11 × 106 DC) for i.d and i.v. injections, respectively [4].
Antibodies and immunostaining
To characterize and compare the phenotype of the DC populations, flow cytometry was performed using either FITC-conjugated or PE-conjugated mAbs. The following FITC-conjugated mAbs were used: anti-HLA class I (W6/32) and anti-HLA-DR/DP (Q5/13); and PE-conjugated mAbs: anti-CD80 (Becton–Dickinson, Mountain View, CA), anti-CD14, anti-CD83 (both Beckman Coulter, Mijdrecht, The Netherlands), and anti-CD86 (Pharmingen, San Diego, CA). Flow cytometric analysis was performed with a FACS Scan (Becton–Dickinson). FACS data were analyzed with CellQuest Pro (Becton–Dickinson).
Delayed type hypersensitivity
One to two weeks after each of the three cycles of three bi-weekly injections with peptide-loaded DC a delayed type hypersensitivity (DTH) skin test was performed [6]. Briefly, unpulsed DC, DC pulsed with peptides (tyrosinase and/or wild-type gp100:154 plus wild-type gp100:280), DC pulsed with KLH, and DC pulsed with peptides plus KLH (2 × 105 DC each) were injected i.d. in the skin of the back of the patients at four different sites. The diameter (mm) of induration was measured after 48 h, each time by the same investigator (M.J.P.G.), and an induration of more than 2 mm was considered positive.
From positive DTH sites, punch biopsies (6 mm) were obtained under local anesthesia. Biopsies were cut small pieces and cultured in RPMI/7%HS supplemented with IL-2 (100 U/ml). Every 7 days, half of the medium was replaced by fresh IL-2-containing RPMI/7%HS. After 2–4 weeks of culturing, DTH-infiltrating lymphocytes were tested for antigen recognition in a cytotoxicity assay or tested for tetramer binding.
Humoral response to KLH
Antibodies against KLH were measured in the serum of vaccinated patients by ELISA as previously described [4]. Briefly, 96-well plates were coated overnight at 4°C with the protein KLH (25 μg/ml) in phosphate buffered saline (0.1 ml/well). After washing the plates, different concentrations of patient serum (range 1 in 100 to 1 in 500,000) were added for 1 h at room temperature. After extensive washing, specific Ab (total IgG, IgG1, IgG2, and IgG4) labeled with horseradish peroxidase were allowed to bind for 1 h at room temperature. Peroxidase activity was revealed using 3,3′,5,5-tetramethyl-benzidine as substrate and measured in a microtiter plate reader at 450 nm. A positive signal at a ≥1 in 400 dilution of the patients’ serum was considered positive. An OD of 2× the value before vaccination at a ≥1 in 400 dilution was considered positive.
Proliferative response and cytokine production to KLH
Cellular responses against KLH were measured in a proliferation assay. Briefly, 1 × 105 PBMC, isolated from blood samples taken before each vaccination, were plated per well of a 96-well tissue culture microplate either in the presence of KLH or without. After 16 h of culture, supernatants (50 μl) were taken and IL-2, IL-4, IL-5, IL-10, TNF-α, and interferon (IFN)-γ were measured by a cytometric bead array (Th1/Th2 Cytokine CBA 1; BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions. After 4 days of culture, 1 μCi/well of tritiated thymidine, incorporation of tritiated thymidine was measured in a beta-counter. A proliferation index >2 was considered positive.
MHC tetramer staining
Tetrameric–MHC complexes were kindly provided by Dr. R. Luiten and Dr. H. Spits from the Netherlands Cancer Institute (Amsterdam, The Netherlands) [22]. Each tetramer was validated by staining against a CTL line specific for HLA-A2 in association with the peptide of interest. Tetramer staining was performed as described previously [4]. PBMC (1 × 105 cells in 10 μl) were incubated with PE-labeled tetrameric–MHC complexes for 1 h at RT. After washing the samples were analyzed by flow cytometry.
Antigen recognition
Antigen recognition was determined by the production of cytokines or cytotoxic activity of DTH-infiltrating lymphocytes in response to T2 cells pulsed with the indicated peptides or BLM (a melanoma cell line expressing HLA-A2.1 but no endogenous expression of gp100 and tyrosinase), transfected with control antigen G250, with gp100 or tyrosinase, or an allogeneic HLA-A2.1-positive, gp100-positive, and tyrosinase-positive tumor cell line (MEL624). Cytotoxic activity of DTH-infiltrating lymphocytes of patient wt-9 was measured using the chromium release assay [23]. Cytokine production was measured in supernatants after 16 h of coculture by the cytometric bead array (Th1/Th2 Cytokine CBA; BD Pharmingen).
Analysis of T cell frequencies in peripheral blood
The presence of tumor antigen-specific CD8+ T cells was analyzed using mixed lymphocyte-peptide cultures as described previously [24]. Briefly, PBMC, isolated from peripheral blood before vaccination and after one cycle of three DC vaccinations, were thawed, divided over three groups, and incubated for 1 h at room temperature in Iscove’s medium (Life Technologies, Carlsbad, CA, USA) with 1% HS and 10 μM of tyrosinase:369 peptide (YMDGTMSQV), wild-type gp100:154 peptide (KTWGQYWQV), or wild-type gp100:280 peptide (YLEPGPVVTA). Next, these pulsed cells were washed, pooled, and distributed at 2 × 105 cells/0.2 ml in round-bottom microwells in Iscove’s with 10% HS, l-arginine (116 mg/l), l-asparagine (36 mg/l), l-glutamine (216 mg/l), IL-2 (20 U/ml), IL-4 (1.5 ng/ml), and IL-7 (10 ng/ml). On day 7, 50% of the medium was replaced by fresh medium containing IL-2 and wild-type gp100:154 peptide. Wild-type gp100:280 peptide was added to the cultures at day 8 and tyrosinase:369 peptide at day 9. Tetramer labeling was performed on day 14 as described previously [24].
Statistical analysis
Data were analyzed statistically by means of analysis of variance and Student–Newman–Keuls test, or by means of Mann–Whitney U nonparametric statistics. Statistical significance was defined as P < 0.05.
Results
Patient characteristics
Twenty-seven patients were included in the study. In the first DC vaccination cycle, 15 patients received wild-type peptide-pulsed DC (partly previously reported in [4]), and 12 received modified peptide-pulsed DC (Supplementary Fig. 1). The clinical characteristics are presented in Table 1. Median age in the wild-type group was 54 years (22–73) and in the modified group 48 years (30–70). All patients had performance status 0, according to WHO criteria. Serum lactate dehydrogenase (LDH) was elevated in four patients in the wild-type group and in four patients in the modified group. According to AJCC criteria, nine patients had stage M1c, 4 M1b, and 2 M1a in the wild-type group and 7 M1c and 4 M1b in the modified group, respectively. In the modified group 1, patient with stage IIIC disease was incorrectly included.
Table 1.
Patient characteristics
| Patient | Age/sex | AJCC stage | Localization metastases | LDH | Response | PFS (m) |
|---|---|---|---|---|---|---|
| Wild-type group | ||||||
| wt-1 | 73/m | IV-M1b | Lung, skin | − | PD | – |
| wt-2 | 56/m | IV-M1b | Lung | − | PD | – |
| wt-3 | 50/f | IV-M1c | sc, gut | − | SD | 4.5 |
| wt-4 | 66/m | IV-M1a | Bulky LN | − | SD | 13b |
| wt-5 | 35/f | IV-M1c | Kidney, spleen, LN | + | SD | 4.5 |
| wt-6 | 54/m | IV-M1c | sc, gut | − | PD | – |
| wt-7 | 22/m | IV-M1c | sc, testicle | − | SD | 4 |
| wt-8 | 59/m | IV-M1c | Bulky LN, sc | + | PD | – |
| wt-9 | 33/f | IV-M1a | LN | − | SDa | >124b |
| wt-10 | 63/m | IV-M1b | LN, lung | − | PD | – |
| wt-11 | 72/m | IV-M1c | Liver | + | SD | 8 |
| wt-12 | 52/m | IV-M1a | sc | − | PD | – |
| wt-13 | 34/m | IV-M1b | Lung, LN | − | PD | – |
| wt-14 | 56/m | IV-M1c | Liver | − | SD | 6 |
| wt-15 | 51/m | IV-M1c | Lung, sc | + | PD | – |
| Modified group | ||||||
| mod-1 | 48/f | IIIC | LN, in-transits | − | PD | – |
| mod-2 | 55/f | IV-M1c | sc, LN, soft tissue | + | PD | – |
| mod-3 | 43/m | IV-M1c | sc, LN, soft tissue | + | PD | – |
| mod-4 | 31/f | IV-M1c | Liver | − | CR | >100c |
| mod-5 | 38/f | IV-M1c | Lung, liver, sc, LN | + | PD | – |
| mod-6 | 64/m | IV-M1b | Lung | − | PD | – |
| mod-7 | 48/f | IV-M1c | Skin, liver, LN | + | PD | – |
| mod-8 | 47/f | IV-M1b | Skin, lung | − | PD | – |
| mod-9 | 30/m | IV-M1c | PD | – | ||
| mod-10 | 70/m | IV-M1c | LN, skin, lung, adrenal | − | PD | – |
| mod-11 | 32/f | IV-M1b | Lung, sc | − | PD | – |
| mod-12 | 56/m | IV-M1b | Lung | − | SD | 4 |
m male, f female, sc subcutaneous, LN lymph nodes, LDH lactate dehydrogenase, PD progressive disease, SD stable disease, CR complete remission, PFS progression free survival
aPartial remission of a distant LN metastasis, CR after surgery for >10 years
bPatients received maintenance treatment with modified peptide-pulsed DC in cycle 2 and wild-type peptide-pulsed DC in cycle 3
cPatient received maintenance treatment with wild-type peptide-pulsed DC in cycle 2 and modified peptide-pulsed DC in cycle 3
Characteristics and number of injected DC
Patients vaccinated with wild-type peptide-pulsed DC received an average of 27 × 106 DC i.v. and 11 × 106 DC i.d. per vaccination during the first cycle. Patients vaccinated with modified peptide-pulsed DC received an average of 22 × 106 DC i.v. and 12 × 106 DC i.d. (Table 2). The amount of injected DC was dependent on the yield after DC culture. We did not observe a correlation between the amount of injected cells and the induction of immune responses. In a previous study, we observed robust T cell responses upon DC vaccination with as little as two million cells [8]. After maturation, DC of all patients showed a mature phenotype with high HLA-ABC, HLA-DR/DP, HLA-DQ, CD80, CD83, and CD86 expression (Online Resource 1). There was no difference in the amount of injected DC (P = 0.3 for i.v. injected DC, P = 0.7 for i.d. injected DC) or maturation status (P = 0.2 to P = 0.4), except for CD80 (P = 0.02) and CD83 (P = 0.01) that were expressed at lower levels in DC pulsed with wild-type peptide.
Table 2.
Vaccine-induced antigen-specific immune responses
| Patient no. | Injected DC (×106) (i.v./i.d.) | Proliferative response against KLHa | Humoral response (IgG) against KLHb | Tetramer positive CD8+ T cells in DTHc | Tetramer positive CD8+ T cells in blood | ||
|---|---|---|---|---|---|---|---|
| Vacc 1 | Vacc 2 | Vacc 3 | |||||
| Wild-type group | |||||||
| wt-1 | 12/10 | 12/12 | 10/10 | +++ | + | – | nt |
| wt-2 | 70/25 | 50/17 | 60/30 | +++ | + | – | nt |
| wt-3 | 30/14 | 18/8 | 20/10 | +++ | + | nt | nt |
| wt-4 | 36/12 | 15/8 | 24/12 | +++ | + | +c1 | + |
| wt-5 | 50/15 | 17/5 | 30/15 | +++ | – | – | − |
| wt-6 | 15/7 | 26/9 | 13/13 | + | + | – | nt |
| wt-7 | 22/4 | 30/8 | 55/7 | ++ | + | nt | – |
| wt-8 | 45/15 | 34/6 | 50/13 | – | + | nt | nt |
| wt-9 | 30/7 | 30/8 | 30/10 | +++ | + | +c2 | – |
| wt-10 | 33/15 | 19/12 | 27/15 | +++ | + | – | nt |
| wt-11 | 13/6 | 22/11 | 16/8 | + | – | – | – |
| wt-12 | 22/13 | – | – | nt | nt | nt | nt |
| wt-13 | 22/11 | 15/8 | 13/7 | +++ | – | – | – |
| wt-14 | 26/13 | 24/12 | 23/11 | +++ | – | – | – |
| wt-15 | 30/15 | 20/10 | 20/10 | + | – | nt | – |
| Modified group | |||||||
| mod-1 | 20/10 | 16/8 | 24/12 | +++ | + | – | nt |
| mod-2 | 26/12 | 23/12 | 22/11 | +++ | – | no | nt |
| mod-3 | 27/14 | 38/19 | 31/16 | + | – | – | nt |
| mod-4 | 16/8 | 14/7 | 14/7 | +++ | + | +c3 | nt |
| mod-5 | 20/20 | 26/13 | 30/15 | +++ | – | nt | nt |
| mod-6 | 60/28 | 26/13 | 30/15 | ++ | + | nt | nt |
| mod-7 | 17/8 | 12/6 | 10/5 | +++ | + | nt | nt |
| mod-8 | 30/14 | 20/11 | 23/11 | +++ | – | no | nt |
| mod-9 | 11/11 | – | – | nt | nt | nt | nt |
| mod-10 | 43/22 | 25/12 | 26/12 | +++ | + | – | nt |
| mod-11 | 17/9 | 15/8 | 15/7 | + | – | – | nt |
| mod-12 | 24/12 | 15/8 | 17/9 | +++ | + | no | nt |
i.v. intraveneously, i.d. intradermally, no no outgrowth or outgrowth of less than 1 × 105 DTH-infiltrating lymphocytes, n.t. not tested
aProliferative response to KLH is given as proliferation index (proliferation +KLH/proliferation –KLH). + Proliferation index (PI) between 2 and 10; ++ PI between 10 and 20; +++ PI higher than 20, at least one time point after vaccination
bAntibody titer, total IgG, + designates >1 in 400 times diluted serum
cPresence of functional tetramer positive T cells in DTH is marked as +:
(c1) Ag-recognition of DTH derived T cells after stimulation with T2 cells loaded with the gp100 or tyrosinase peptides (peptide recognition), BLM transfected with the antigens (protein recognition) or the gp100 and tyrosinase-expressing tumor cell line Mel624 (tumor recognition) was analyzed by IFNγ-production. Ag-recognition was analyzed 1 year after the third cycle of DC vaccinations. DTH derived T cells of patient wt-4 recognized tumor peptides (T2) and tumor (Mel624)
(c2) Ag-recognition by DTH derived T cells analyzed by cytotoxic activity (chromium release assay). Ag-recognition was analyzed after the first cycle of DC vaccinations. DTH derived T cells of patient wt-9 recognized tumor proteins gp100 and tyrosinase
(c3) Ag-recognition of DTH derived T cells after stimulation with gp100- or tyrosinase peptide-loaded T2 cells, BLM transfected with the antigens or Mel624 (tumor recognition) was analyzed by IFNγ-production. Ag-recognition was analyzed after the second cycle of DC vaccinations. DTH derived T cells of patient mod-4 recognized tumor peptides
Clinical outcome and toxicity
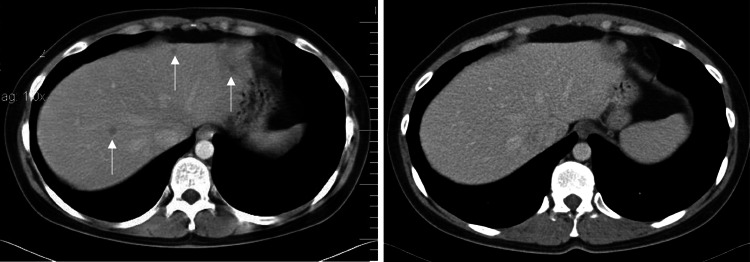
Toxicity was limited to grade I–II fever, fatigue, flu-like symptoms and injection site reactions, which always resolved within 2 days after vaccination. In the wild-type group one patient (wt-9) had a 27% reduction in diameter of a distant lymph node metastasis, which was resected after the first maintenance vaccination cycle. The resected tissue showed areas of necrosis, diffuse CD8+ T cell infiltrates and decreased expression of tyrosinase when compared with the primary cutaneous melanoma that had been resected years prior to vaccination. This patient completed treatment according the protocol (Supplementary Fig. 1), and is free of disease at 10 years of follow up. Six patients in the wild-type group had stable disease for at least 4 months (4, 4.5, 4.5, 6, 8, and 13 months). In the modified group one patient (mod-4) had multiple histological proven synchronous liver metastases that had been diagnosed 2 months prior to inclusion. The liver biopsies were revised by an academic dermal pathologist and showed unambiguous melanoma metastases. This patient had a complete response after the first vaccination cycle (Fig. 1), completed the full protocol treatment and is currently free of disease at 8 years of follow up. One patient had disease stabilization of 4 months duration.
Fig. 1.
Computer tomography scanning images of the abdomen of patient mod-4. The images show multiple liver metastases (left, arrows), which regressed after the first cycle of DC vaccinations and showed complete remission after the peptide vaccinations (right)
Proliferation and cytokine production of PBMCs upon stimulation with KLH
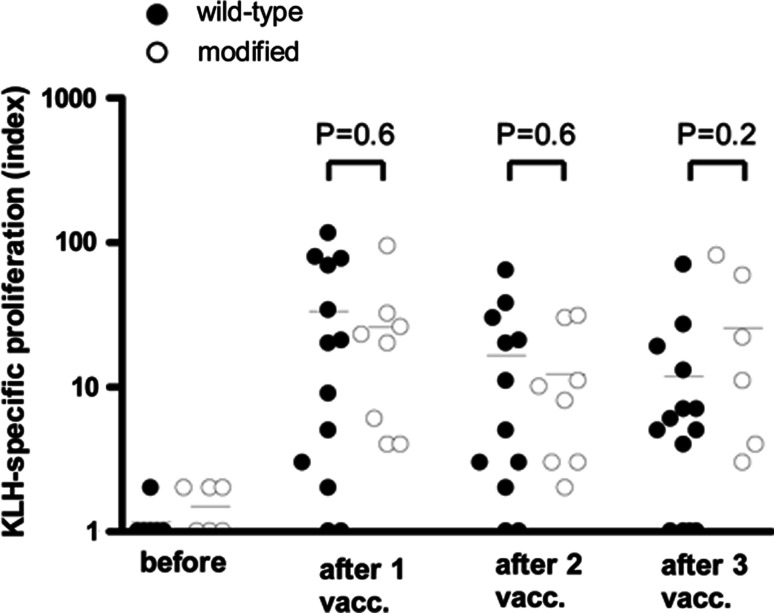
To investigate whether DC loaded with modified or wild-type gp100 peptides have similar capacity to activate the patient’s immune system in general, humoral and cellular responses to the control protein KLH were measured in the peripheral blood of patients after each cycle of vaccination. Most patients mounted a potent proliferative and antibody response against KLH (Table 2). KLH-specific IgG antibodies were induced after vaccination in 8 of 14 patients tested (57%) in the wild-type group and in 6 of 11 patients tested (55%) in the modified group. PBMC collected after each DC vaccination were analyzed for the presence of KLH-reactive T cells in a proliferation assay. In the wild-type group 13 out of 14 patients tested showed a cellular response to KLH, whereas in the modified group a cellular response was induced in all patients (Table 2). The magnitude of KLH-specific proliferation was similar in the wild type and modified group (Fig. 2). There were no significant differences between the groups at any time point after vaccination (P = 0.3 before vaccination; P = 0.6 after vaccination 1; P = 0.6 after vaccination 2; P = 0.2 after vaccination 3). Interestingly, the patient that had no proliferative response (patient wt-8) did show an antibody response to KLH. Thus, all patients developed a response to KLH, either cellular or humoral, and there are no differences in KLH-specific immune responses after vaccination with DC loaded with wild-type or modified gp100 peptides. These results indicate that the immunogenicity of the DC was comparable for both groups and therefore a possible difference in gp100 T cell reactivity upon vaccination between the two groups is unlikely to be the result of differences in capacity of DC to activate the patient’s immune system.
Fig. 2.
KLH-specific proliferation of PBMCs before and after DC vaccination. Patients were vaccinated with DC loaded KLH together with tyrosinase peptide and either wild-type (filled circles) or modified (open circles) gp100 peptides. KLH-specific proliferation was measured in PBMCs isolated before the first vaccination and after one, two, or three vaccinations. Proliferative response to KLH is given as proliferation index (proliferation +KLH/proliferation –KLH)
Tumor antigen-specific T cell responses in post-treatment DTH
To investigate the immune response against tumor peptides generated in vaccinated patients, DTH challenges were performed with mature DC loaded with tyrosinase and/or wild-type gp100 peptides. For patients receiving a second and third cycle of vaccination (patients wt-4, wt-9, and mod-4), the best immunological response over all received cycles of vaccinations was scored (indicated in Table 2). In the group of patients vaccinated with wild-type peptide-loaded DC, 6 of 6 patients tested showed indurations against DC pulsed with KLH. In the group vaccinated with modified peptide-loaded DC, 4 of 6 patients tested showed indurations against KLH-loaded DC. Peptide-pulsed DC induced indurations in 11 of 13 wild-type patients and in 9 of 10 modified patients. DC only induced indurations up to 14 mm in 6 of 6 patients tested in the wild-type group and 4 of 6 patients tested in the modified group. Biopsies were taken from DTH induration sites, which were cultured in low amounts of IL-2 without the addition of antigen. Outgrowth of DTH-infiltrating lymphocytes, which was defined as outgrowth of at least 1 × 105 cells per biopsy, occurred in all the biopsies taken from patients treated with wild-type gp100, whereas only 63% of the biopsies in the modified group showed DTH-infiltrating lymphocytes outgrowth. The lack of outgrowth occurred in all different DTH, there was no preference for a DTH induced with a certain peptide.
From nine patients in the wild-type group sufficient DTH-infiltrating lymphocytes were available after short-time culture (i.e., 2–4 weeks) to perform tetramer staining and functional assays (Table 2). Biopsies of two patients (wt-4 and wt-9) contained gp100- and tyrosinase-tetramer positive CD8+ T cells that recognized gp100 or tyrosinase peptide and protein (previously shown in Ref. [4]). Already after the first cycle of DC vaccinations, DTH-infiltrating lymphocytes of patient wt-9 demonstrated specific lysis of gp100- and tyrosinase-transfected melanoma cells in a chromium release assay (Table 2; previously shown in Ref. [4]). Biopsies of patient wt-4 taken after the first cycle of DC vaccinations contained tetramer positive CD8+ T cells. Interestingly, 1 year after the third cycle of DC vaccinations, DTH-infiltrated lymphocytes of patient wt-4 still contained tetramer positive CD8+ T cells that produced IFNγ upon recognition of tumor peptides loaded on T2 cells and tumor proteins on Mel624 cells.
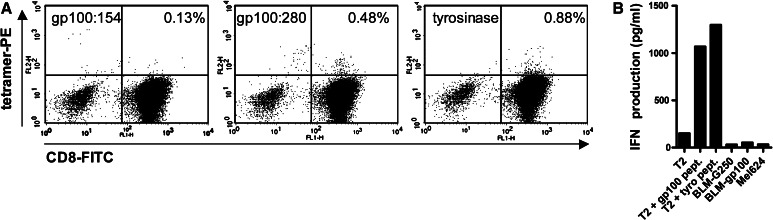
Tetramer staining and functional assays could be performed on DTH-infiltrating lymphocytes of five patients in the modified group. Only DTH-infiltrating lymphocytes derived from a biopsy of patient mod-4, taken after the second cycle of DC vaccinations, which was performed with wild-type peptide-pulsed DC, contained tetramer positive CD8+ T cells (Fig. 3a). These CD8+ T cells recognized gp100 or tyrosinase peptides loaded on T2 cells, as demonstrated by IFNγ production by these CD8+ T cells upon coculture with peptide-loaded T2 cells, however, they did not recognize the complete protein presented by BLM-gp100 or Mel624 cells (Fig. 3b). DC only and DC loaded with KLH induced induration in patient mod-4, but there was no outgrowth of DTH-infiltrating lymphocytes.
Fig. 3.
Tumor antigen-specific T cell responses in post-treatment DTH of patient mod-4. a Tetramer analysis by flow cytometry of T cells derived from a biopsy of positive DTH reaction (DC loaded with KLH and peptides gp100:154, gp100:280, and tyrosinase) of patient mod-4. Tetramer staining of 1 log above the negative population was considered positive. The biopsy contains gp100:280- and tyrosinase-positive T cells. Since tetramer staining for gp100:154 was negative, we regard gp100:154 as a negative control, indicating that positive gp100:280- and tyrosinase staining is specific. b IFNγ production by T cells of patient mod-4 derived from a biopsy of a positive DTH site (DC loaded with peptides gp100:154, gp100:280, and tyrosinase) cocultured with T2 cell pulsed with gp100 peptides or tyrosinase peptides, with BLM cells expressing gp100 or the control protein G250, or with the melanoma cell line Mel624, which expresses both gp100 and tyrosinase. The DTH was performed after the second cycle of DC vaccinations, which were performed with wild-type peptide-pulsed DC
Thus, although the majority of patients in both the wild-type and modified group showed DTH-infiltrating lymphocytes at DTH sites, we could detect specific and functional CD8+ T cells in only one patient in the modified group and two patients in the wild-type group. Interestingly, these three patients also showed the best clinical outcome, with progression free survival of 13 (wt-4), >100 (mod-4), and >124 (wt-9) months. In contrast, the progression free survival of the patients without specific T cell reactivity in DTH sites ranged from 0 to 8 months.
Tumor antigen-specific T cell responses in peripheral blood
The presence of gp100- or tyrosinase-specific CD8+ T cells in blood was studied in eight patients in the wild-type group by tetramer staining (Table 2). Although we detected gp100- and tyrosinase-specific CD8+ T cells in patient wt-4 by tetramer analysis, these T cells did not recognize gp100 or tyrosinase peptides or protein as determined by IFNγ production upon coculture with gp100/tyrosinase-loaded target cells. We could not detect tumor antigen-specific T cells in blood of the other patients tested. The presence of tumor antigen-specific T cells was not analyzed for patients vaccinated with modified peptide-loaded DC.
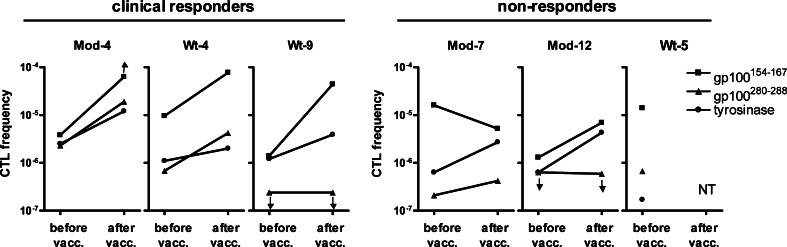
The frequencies of antigen-specific T cells in blood are generally very low, and often we were unable to detect antigen-specific T cells in peripheral blood by direct tetramer staining, while functional tumor antigen-specific T cells were present in biopsies taken from DTH challenges [6]. To further examine anti-gp100 and anti-tyrosinase CTL responses, we analyzed precursor frequencies of tumor antigen-specific CD8+ T cells in peripheral blood before and after one cycle of three bi-weekly DC vaccinations in mixed lymphocyte-peptide cultures at limiting dilution conditions [24]. These studies were performed in blood of the three clinically responding patients who had specific T cells in the DTH biopsies (wt-4, wt-9, and mod-4) and in blood of three non-responding patients (wt-5, mod-7, and mod-12). Before vaccination, CTLp frequencies ranged from 1.7 × 10−7 to 1.6 × 10−5 of total blood CD8+ T cells (Fig. 4). CD8+ T cells directed against gp100:154 showed the highest frequencies in both clinical responders and non-responders before vaccination. After DC vaccination, frequencies of gp100:154- and tyrosinase-specific CTLs increased in all three responders, up to 31-fold, whereas frequencies of gp100:280-specific CTLs increased only in patients mod-4 and wt-4. CTLp frequencies of non-responders increased only modestly after DC vaccination. Thus, the increase of peripheral blood T cell frequencies correlates with the presence of tumor antigen-specific T cells in DTH biopsies upon vaccination.
Fig. 4.
Frequencies of tumor antigen-specific CD8+ T cells in peripheral blood. CTLp frequencies were analyzed in peripheral blood of three DTH-responders (patients mod-4, wt-4, and wt-9) and three non-responders (patients mod-7, mod-12, and wt-5) before vaccination and after one cycle of three DC vaccinations by in vitro restimulation of PBMCs with antigenic peptides in limiting dilutions followed by tetramer analysis. CTLp frequencies could not be tested (NT) in patient wt-5 after vaccination due to lack of outgrowth after peptide stimulation
Discussion
In this study, we compared mature DC loaded with wild-type or modified gp100 peptides in their ability to induce immunological and clinical responses in metastatic melanoma patients. It has previously been suggested that wild-type peptides activate CTLs with strong tumor reactivity, whereas modified peptides induce strong expansion of antigen-specific CTLs [25]. Therefore, we treated the first group of metastatic HLA-A2.1+ melanoma patients with wild-type peptide-loaded DC in the first cycle of vaccination (to activate highly specific T cells) followed by a second cycle of modified peptide-loaded DC (to expand the highly specific T cells). The second group was treated with modified peptide-loaded DC in the first cycle of vaccination (to first expand antigen-specific CTLs) followed by a second cycle of wild-type peptide-loaded DC (to activate highly specific CTLs). Although the number of patients in our study is limited, we observed no obvious advantage for the use of modified versus wild-type peptides in either endpoint.
In a previous clinical study, we demonstrated that only mature KLH-loaded DC, and not immature KLH-loaded DC are able to induce a KLH-specific immune response in melanoma patients [4]. Since both groups of patients in this study were vaccinated with DC that were matured and loaded with KLH in a similar way, no differences in KLH responses were expected. Indeed, patients vaccinated with wild-type or modified gp100 peptides showed similar cellular and humoral responses to the control protein KLH. KLH responses did not correlate with immunological responses against tumor antigens or clinical responses, as all patients developed a KLH response, either cellular or humoral, and only three patients showed functional T cell responses against tumor antigens, which coincided with long-term stable disease. In addition to KLH responses, indurations in DTH challenges were comparable in both groups. As reported before, induration was also not predictive for clinical outcome [6]. Outgrowth of DTH biopsies occurred more often in the wild-type group than in the modified group. In both the wild-type group and the modified group DTH challenges were performed with wild-type gp100 peptides. Since also in DTH challenges performed with tyrosinase peptide lack of outgrowth occurred in the modified group, the lack of outgrowth is probably not due to the fact that DTH challenges were performed with wild-type gp100 peptides.
We observed tumor-specific T cells in DTH biopsies of only three patients, two vaccinated with wild-type peptides and one vaccinated with modified peptides. Interestingly, in the patient vaccinated with modified peptides during the first cycle, the best immunological response was found after the second cycle of DC vaccinations with wild-type peptide-loaded DC, which might suggest that modified peptide-pulsed DC were unable to induce tumor antigen-specific responses. However, this patient already had a complete response of multiple liver metastases after the first vaccination cycle (with modified peptide-pulsed DC and modified peptides), indicating that an immune response was already induced after the first vaccination cycle. Interestingly, the best clinical outcome was observed in these three patients in whom tumor-specific T cell responses of DTH-infiltrating lymphocytes were observed. This is in accordance with our previous findings that the presence of tumor-specific T cells in DTH biopsies correlates with clinical outcome [6]. Two of these patients, wt-9 and mod-4, are currently free of disease, at 10 and 8 years after the first DC vaccination, which strongly suggests that DC vaccination induced long lasting and functional anti-tumor responses in these patients.
While antigen-specific T cells were readily detected in the DTH biopsies of three clinical responders, we found tetramer positive CD8+ cells in the blood of only one patient (wt-4) and these CTLs did not recognize tumor antigens in a coculture with gp100 or tyrosinase-loaded target cells. Antigenic stimulation of blood lymphocytes at limiting dilution conditions demonstrated that antigen-specific T cells were detected in blood at low frequencies in our clinically responding patients. Coulie et al. [26] also reported low T cell frequencies in blood and implied that even these low numbers of CTL in blood correlated with the rejection of a large volume tumor. The fact that in our studies the presence of antigen-specific T cells in DTH biopsies correlates stronger with clinical outcome than the presence of these cells in blood may be due to the more stringent conditions to obtain vaccine-specific DTH-infiltrating lymphocytes, as they have to migrate and proliferate in vivo after antigen recognition.
For other modified peptides (gp100:209–217 and MART:26–35) it was described that only a minority (~15–25%) of T cells raised against modified peptides cross-reacts with the wild-type epitope [27, 28]. However, for modified gp100:154 and gp100:280 we and others have demonstrated that in vitro and in HLA-A2.1 transgenic mice that CTLs raised against modified peptides recognized wild-type peptides [12, 13]. Our CTLp frequency analysis, which was performed with wild-type gp100 peptides, showed no differences in T cell expansion between the wild-type and modified group in the DTH responders. All three patients showed increased precursor frequencies of gp100:154-specific CTLs. Two of these patients, one vaccinated with wild-type and one with modified peptides, showed a comparable increase in gp100:280-specific CTLs after one round of DC vaccinations. This suggests that vaccination with modified peptide raised CTLs cross-reacting with wild-type tumor antigens.
Recently, Speiser et al. [25] demonstrated that, although vaccination with modified peptides resulted in higher T cell frequencies than vaccination with natural wild-type peptides, these T cells have lower tumor reactivity as demonstrated by lower avidity for the natural antigen and decreased T cell activation and effector function. This is in contrast with previous in vitro and mouse studies demonstrating that CTLs raised against modified peptides could recognize both wild-type and modified peptides as well as gp100-expressing melanoma cells [12, 13]. However, based on the results of Speiser et al., it may be beneficial to first vaccinate patients with mature DC loaded wild-type peptides, which activates CTLs with strong tumor reactivity, followed by injection with mature DC loaded with modified peptides in the second round of vaccination to improve expansion of these CTLs, thus inducing high number of functional tumor antigen-specific CTLs.
In conclusion, vaccination with peptide-loaded DC results in clinically meaningful responses in a minority of metastatic melanoma patients, however, the use of modified as compared to wild-type gp100 peptides for DC loading does not result in relevant enhanced immune responses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the Netherlands Organization for Scientific Research (920-03-250), the Dutch Cancer Society (AZN/KUN 95/910 and 99/1950), the EU (Cancer immunotherapy LSHC-CT-2006-518234 and DC-THERA LSHB-CT-2004-512074), and the TIL-foundation.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Lesterhuis WJ, Aarntzen EHJG, Vries IJM, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJA. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol. 2008;66:118–134. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 4.de Vries IJM, Lesterhuis WJ, Scharenborg NM, Engelen LPH, Ruiter DJ, Gerritsen MJP, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJA. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 5.de Vries IJM, Krooshoop DJEB, Scharenborg NM, Lesterhuis WJ, Diepstra JHS, van Muijen GNP, Strijk SP, Ruers TJ, Boerman OC, Oyen WJG, Adema GJ, Punt CJA, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 6.de Vries IJM, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJP, Ruiter DJ, Figdor CG, Punt CJA, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 7.de Vries IJM, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJG, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JWM, Scheenen TWJ, Punt CJA, Heerschap A, Figdor CG. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 8.Lesterhuis WJ, de Vries IJM, Schuurhuis DH, Boullart ACI, Jacobs JFM, de Boer AJ, Scharenborg NM, Brouwer HMH, van de Rakt MWMM, Figdor CG, Ruers TJ, Adema GJ, Punt CJA. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol. 2006;17:974–980. doi: 10.1093/annonc/mdl072. [DOI] [PubMed] [Google Scholar]
- 9.Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 11.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/S0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 12.Bakker ABH, van der Burg SH, Huijbens RJF, Drijfhout JW, Melief CJM, Adema GJ, Figdor CG. Analogues of CTL epitopes with improved MHC class-I binding capacity elicit anti-melanoma CTL recognizing the wild-type epitope. Int J Cancer. 1997;70:302–309. doi: 10.1002/(SICI)1097-0215(19970127)70:3<302::AID-IJC10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 14.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 15.Keilholz U, Goey SH, Punt CJ, Proebstle TM, Salzmann R, Scheibenbogen C, Schadendorf D, Liénard D, Enk A, Dummer R, Hantich B, Geueke AM, Eggermont AM. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol. 1997;15:2579–2588. doi: 10.1200/JCO.1997.15.7.2579. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 17.de Vries IJM, Eggert AAO, Scharenborg NM, Vissers JLM, Lesterhuis WJ, Boerman OC, Punt CJA, Adema GJ, Figdor CG. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25:429–438. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Thurner B, Roder C, Dieckmann D, Heuer H, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/S0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Bakker ABH, Schreurs MWJ, Tafazzul G, de Boer AJ, Kawakami Y, Adema GJ, Figdor CG. Identification of a novel peptide derived from the melanocyte-specific Gp100 antigen as the dominant epitope recognized by an Hla-A2.1-restricted antimelanoma Ctl line. Int J Cancer. 1995;62:97–102. doi: 10.1002/ijc.2910620118. [DOI] [PubMed] [Google Scholar]
- 20.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL. Identification of a peptide recognized by 5 melanoma-specific human cytotoxic T-cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 21.Wolfel T, Schneider J, Zumbuschenfelde KHM, Rammensee HG, Rotzschke O, Falk K. Isolation of naturally processed peptides recognized by cytolytic T-lymphocytes (Ctl) on human-melanoma cells in association with Hla-A2.1. Int J Cancer. 1994;57:413–418. doi: 10.1002/ijc.2910570320. [DOI] [PubMed] [Google Scholar]
- 22.Haanen JBAG, Toebes M, Cordaro TA, Wolkers MC, Kruisbeek AM, Schumacher TNM. Systemic T cell expansion during localized viral infection. Eur J Immunol. 1999;29:1168–1174. doi: 10.1002/(SICI)1521-4141(199904)29:04<1168::AID-IMMU1168>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Bakker ABH, Schreurs MWJ, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, Figdor CG. Melanocyte lineage-specific antigen Gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanikas V, Lurquin C, Colau D, van Baren N, de Smet C, Lethe B, Connerotte T, Corbiere V, Demoitie MA, Lienard D, Dreno B, Velu T, Boon T, Coulie PG. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J Immunol. 2003;171:4898–4904. doi: 10.4049/jimmunol.171.9.4898. [DOI] [PubMed] [Google Scholar]
- 25.Speiser DE, Baumgaertner P, Voelter V, Devevre E, Barbey C, Rufer N, Romero P. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc Natl Acad Sci USA. 2008;105:3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coulie PG, Karanikas V, Colau D, Lurquin C, Landry C, Marchand M, Dorval T, Brichard V, Boon T. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290–10295. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuge TB, Holmes SP, Saharan S, Tuettenberg A, Roederer M, Weber JS, Lee PP. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 2004;1:149–160. doi: 10.1371/journal.pmed.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clay TM, Custer MC, Mckee MD, Parkhurst M, Robbins PF, Kerstann K, Wunderlich J, Rosenberg SA, Nishimura MI. Changes in the fine specificity of gp100(209–217)-reactive T cells in patients following vaccination with a peptide modified at an HLA-A2.1 anchor residue. J Immunol. 1999;162:1749–1755. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.