Abstract
There have been several studies suggesting that cancer stem cells (CSCs) contribute to the high rates of recurrence and resistance to therapies observed in hepatocellular carcinoma (HCC). Epithelial cell adhesion molecule (EpCAM) has been demonstrated to be a biomarker of CSCs and a potential therapeutic target in HCC. Here, we prepared two anti-EpCAM monoclonal antibodies (1H8 and 2F2) and an anti-EpCAM bispecific T cell engager (BiTE) 1H8/CD3, which was derived from 1H8, and used them to treat HCC in vitro and in vivo. The results demonstrated that all of the developed anti-EpCAM antibodies specifically bound to EpCAM. Neither anti-EpCAM monoclonal antibody had obvious anti-HCC activities in vitro or in vivo. However, anti-EpCAM BiTE 1H8/CD3 induced strong peripheral blood mononuclear cell-dependent cellular cytotoxicity in Huh-7 and Hep3B cells but not EpCAM-negative SK-Hep-1 cells. Notably, 1H8/CD3 completely inhibited the growth of Huh-7 and Hep3B xenografts in vivo. Treatment of the Huh-7 HCC xenografts with 1H8/CD3 significantly suppressed tumor proliferation and reduced the expression of most CSC biomarkers. Intriguingly, galectin-1 (Gal-1) overexpression inhibited 1H8/CD3-induced lymphocytotoxicity in HCCs while knockdown of Gal-1 increased the lymphocytotoxicity. Collectively, these results indicate that anti-EpCAM BiTE 1H8/CD3 is a promising therapeutic agent for HCC treatment. Gal-1 may contribute to the resistance of HCC cells to 1H8/CD3-induced lysis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1497-4) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Epithelial cell adhesion molecule, Bispecific T cell engager, Cancer stem cells, Galectin-1
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide [1]. This cancer is characterized by resistance to adjuvant chemotherapy and high recurrence after curative liver resection [2, 3]. In recent years, there have been several studies demonstrating that one of the likely causes for the high resistance to chemotherapy and high recurrence of HCC is the presence of cancer stem cells (CSCs) [4–7]. The CSC hypothesis states that CSCs are one cause of tumor initiation, metastasis and local recurrence after therapy [8, 9]. Conventional anticancer therapies such as chemotherapy and radiation merely kill rapidly growing differentiated tumor cells but not CSCs. So, successful eradication of cancer requires an anticancer therapy that can kill both differentiated cancer cells and CSCs [10, 11].
Epithelial cell adhesion molecule (EpCAM; CD326) is a type I transmembrane glycoprotein that functions as an epithelial-specific cell adhesion molecule and is involved in cell migration, proliferation and differentiation [12–17]. Recently, EpCAM was recognized as a CSC marker in HCC [18, 19]. Additionally, EpCAM+AFP+ (alpha fetoprotein) HCC was shown to be correlated with poor prognosis [20], and EpCAM-positive circulating stem-like cells were associated with poor prognosis of HCC after curative resection [7]. Silencing EpCAM expression significantly decreased the proliferative activity and invasiveness of HCC cells [21].
Epithelial cell adhesion molecule on normal epithelial tissues is mostly sequestered in intercellular boundaries while becoming accessible on the surface of cancer cells [22]. So, EpCAM may represent a promising target for therapeutic antibodies. Several anti-EpCAM monoclonal antibodies have been developed for cancer treatment with promising therapeutic efficacy [23, 24]. Additionally, a bispecific T cell engager (BiTE) antibody recognizing EpCAM has also been developed for cancer treatment [25, 26]. BiTEs are fusion proteins consisting of two single-chain variable fragments (scFvs) on a single peptide chain. One of the scFvs binds to CD3 on T cells and the other to a tumor-specific molecule on tumor cells. BiTE can efficiently gear up the potential of CD8+ and CD4+ T cells to lyse cancer cells predominantly through perforin and pro-apoptotic components of cytotoxic T-cell granzyme B [27]. Apart from this, activated CD8+ T cells can secrete cytokines such as TNF-α (tumor necrosis factorα) and IFN-γ (interferon γ), which can induce cancer cell apoptosis via the Fas/CD95 pathway [28].
Although EpCAM has been regarded as a HCC CSC biomarker and a potential therapeutic target for HCC treatment, EpCAM-directed immunotherapy has never been investigated in HCC models. Thus, in this study, we developed monoclonal antibodies and a BiTE directed at EpCAM and explored their potential for treating HCC.
Materials and methods
Cells
The human HCC cell lines Huh-7, Hep3B, SK-Hep-1, PLC/PRF/5 (ATCC), Huh7-EGFRvIII (Huh-7 cells with exogenous epidermal growth factor receptor variant III overexpression) [29] and SMMC-7721 (Chinese Academy of Sciences, Shanghai, China) were used. All of the cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum and antibiotics in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C. Peripheral blood mononuclear cells (PBMCs) were obtained from Shanghai Red Cross Blood Center.
Antibody-dependent cell-mediated cytotoxicity
Antibody-dependent cell-mediated cytotoxicity (ADCC) was performed as following: Huh-7, Huh-7-EGFRvIII and SMMC-7721 cells were plated at a density of 5,000 cells/well with varying amounts of 1H8 or 2F2 (0.005–50 μg/mL) in 96-well plates. Effector cells (mouse thymus and abdominal cavity mononucleocytes) were freshly prepared and added to the target cells to achieve an effector cell: target cell ratio of 50:1. After incubation at 37 °C for 48 h, the percent cytotoxicity of the antibody was calculated using the CytoTox 96® Non-Radioactive Cytotoxicity Assay according to the manufacturer’s instructions (Promega, Madison, WI).
Expression and purification of anti-EpCAM monoclonal antibodies and BiTE
To prepare monoclonal antibody against human EpCAM, BALB/c mice were immunized with EpCAM ectodomain recombinant protein. Fusion of splenocytes was performed with SP2/0 myeloma cells, from which hybridomas of the desired characteristics were subsequently selected. To prepare anti-EpCAM BiTE, the 1H8 scFv derived from anti-EpCAM monoclonal antibody 1H8 was obtained by 5′-race kit (Takara, Dalian, China) and overlap PCR. The BiTE 1H8/CD3 was constructed by standard DNA recombination technologies. The EpCAM-specific scFv fragment was fused by a flexible peptide linker to the CD3 scFv fragment, which specifically recognizes the human T-cell receptor/CD3 complex (United States Patent, Patent No.: 7, 112, 324, B1). The BiTE DNA sequence was then cloned into the pIH expression vector. Recombinant 1H8/CD3 was expressed in CHO-DG44 cells, affinity-purified through a C-terminal 6 × His tag and isolated by Ni Sepharose TM 6 Fast Flow. The purity of 1H8/CD3 was estimated by SDS-PAGE and SEC-HPLC (size exclusion chromatography high performance liquid chromatography). CH12 is an anti-EGFRvIII monoclonal antibody developed in our laboratory, which can preferentially bind to EGFRvIII and significantly inhibit the growth of EGFRvIII-positive HCC xenografts [29, 30].
Enzyme-linked immunosorbent assay (ELISA)
Cell culture supernatants were harvested and analyzed for cytokines by ELISA Kit according to the manufacturer’s protocol (Peprotech, Hangzhou, China).
Cytotoxicity assay
hepatocellular carcinoma cells SMMC-7721, Huh-7, Hep3B and SK-Hep-1 were used as target cells. Effector cells (PBMC) were freshly prepared and added to the target cells to achieve effector cell: target cell ratios of 10:1. For the cytotoxicity assay, Huh-7, Huh-7 CD133+EpCAM+ and SMMC-7721 cells were plated at a density of ~7,000 cells/well or Hep3B, Hep3B CD133+EpCAM+ and SK-Hep-1 cells were plated at a density of ~5,000 cells/well, with varying amounts of the 1H8/CD3 in 96 wells plates. Cytotoxicity analysis was performed using the CytoTox 96® Non-Radioactive Cytotoxicity Assay according to the manufacturer’s instructions (Promega).
CD133+EpCAM+ cells sorting
CD133+EpCAM+ and CD133−EpCAM− cells derived from Huh-7 and Hep3B were sorted using the Pan anti-Mouse Kit (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. First, anti-CD133 antibody (Miltenyi Biotec, Köln, Germany) was incubated with magnetic beads for 1 h at 4 °C with gentle tilting and rotation. Then, cells were incubated with anti-CD133 antibody-coated magnetic beads for 20 min at 4 °C with gentle tilting and rotation. CD133+ and CD133− cells were separated by a Dynal magnet. CD133+ bead-bound cells were incubated with DNaseI to release the cells from the beads via DNA linker cleavage. This procedure was repeated using anti-EpCAM antibodies to separate EpCAM+ cells from CD133+ or CD133− cells to obtain CD133+EpCAM+ and CD133−EpCAM− cells.
Western blot analysis
Cell lysates were collected and centrifuged for 15 min at 13,000 rpm, 4 °C. The supernatant was transferred to a clean tube, and proteins were quantified using the BCA Kit (Pierce, Rockford, IL, USA). Proteins were separated on 10 or 12 % SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked with 5 % skim milk and incubated overnight at 4 °C with primary antibodies. Immune complexes were detected by incubating the nitrocellulose membranes with HRP-conjugated goat anti-mouse antibody for 2 h at room temperature and subsequent exposure of the membrane to enhanced chemiluminescence reagents.
Real-time PCR
Total RNAs were extracted with the TRIzol Kit (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was carried out using the SYBR Green PCR kit (Takara), according to the manufacturer’s instructions. Primers for Nanog, Oct3/4 and Klf4 were used as Ref. [25]; the primers of EpCAM as Ref. [31] and primers for β-actin served as an internal control.
CCK-8 assay
Cells were plated in 96-well plates at 2 × 103 cells per well. After 24 h, cells were placed in complete medium containing the indicated concentrations of doxorubicin (Hisunpharm, Taizhou, China), 5-FU (Jinghua, Nantong, China) or vehicle control. Then, the plates were incubated at 37 °C. After 72 h, CCK-8 (Dojindo, Kumamoto, Japan) solution (10 μL) was added to 100 μL of culture media, and the optical density was measured at 450 nm.
Colony formation assay
CD133+EpCAM+ or CD133−EpCAM− Huh-7 and Hep3B cells were seeded into cell culture plates at 1,000 cells/plate. 10 days later, cells were washed with PBS and fixed with 4 % paraformaldehyde. Then, the cells were stained with crystal violet and clones were counted.
In vivo model for human HCC cancer
Single-cell suspensions of HCC Huh-7 (2 × 106) or Hep3B (4 × 106) cells together with or without freshly isolated donor-derived PBMCs at a ratio of 1:1 (Cancer cells: PBMC) were injected subcutaneously into the flanks of 6- to 8-week-old NOD/SCID (non-obese diabetic/severe combined immunodeficiency disease) mice. Mice were manipulated and housed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission. For the treatment model, six animals per group were i.v. treated with 1H8, PBS or 1H8/CD3 starting 1 h after cancer cell/PBMC inoculation at the indicated doses and treatment was repeated for 10 consecutive days. Tumor volumes were measured as described previously [29].
Statistical analysis
All data are presented as the mean ± SD and were analyzed by the Student’s t test. p < 0.05 was considered statistically significant.
Results
Two anti-EpCAM monoclonal antibodies 1H8 and 2F2 displayed no obvious anti-tumor effect on HCC cells in vitro and in vivo
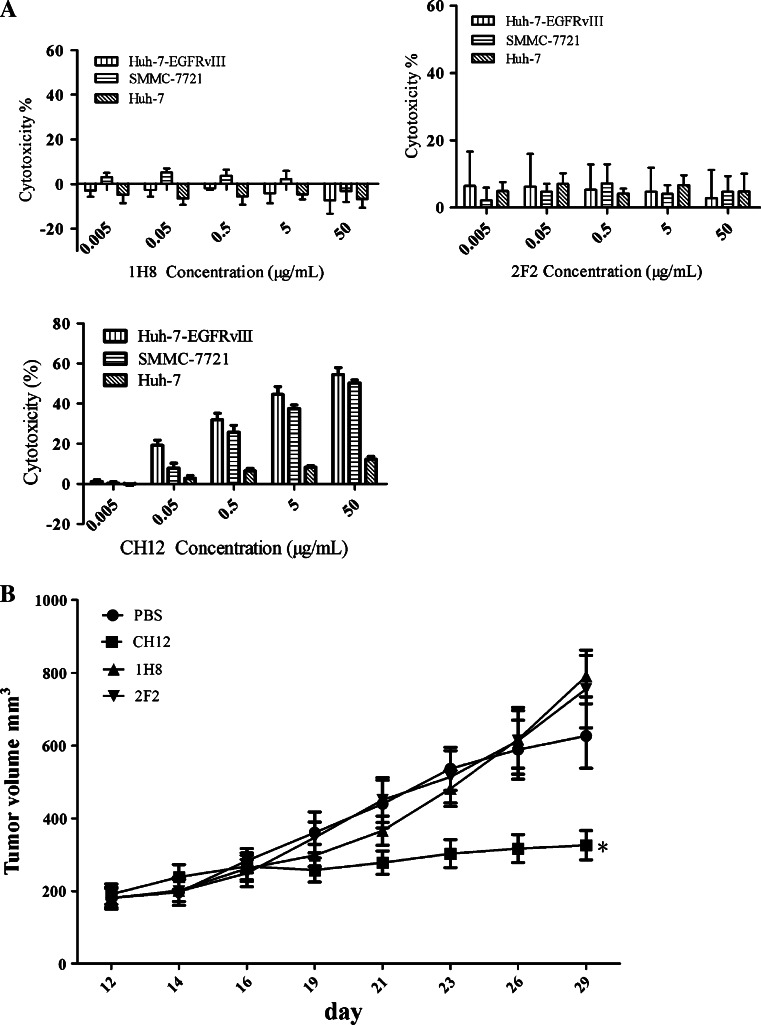
Two anti-EpCAM monoclonal antibodies, 1H8 and 2F2, were shown by FACS analysis to efficiently bind Huh-7, SMMC-7721 and Huh7-EGFRvШ cells but not to PLC/PRF/5 cells (Supplementary Figure S1). As shown in Figure S1B, 1H8 and 2F2 are effectively bound to recombinant EpCAM protein as C-10. Binding studies on CHO-K1 cells expressing human/mouse chimeric proteins showed that 1H8 but not 2F2 bound to the N-terminal portion of human EpCAM encoded by exon 2 (Supplementary Figure S2) [32]. Unfortunately, no obvious ADCC effect induced by 1H8 or 2F2 was observed on Huh-7, SMMC-7721 or Huh7-EGFRvIII cells even at a concentration of 50 μg/mL (Fig. 1a). Additionally, CH12, an anit-EGFRvIII monoclonal antibody, displayed obvious anti-tumor effects on Huh7-EGFRvIII xenografts in vivo, but the two anti-EpCAM monoclonal antibodies did not (Fig. 1b).
Fig. 1.
Anti-tumor effects of anti-EpCAM monoclonal antibodies 1H8 and 2F2 on HCC in vitro and in vivo. a Antibody-dependent cellular cytotoxicity function of monoclonal antibodies 1H8, 2F2 and CH12. Hepatocellular carcinoma cells Huh7-EGFRvIII, Huh-7 and SMMC-7721 were used as target cells. Effector cells (mouse thymus and abdominal cavity mononucleocytes) were freshly prepared and added to the target cells to achieve an effector cell: target cell ratio of 50:1. The antibody concentrations ranged from 0.005 to 50 μg/mL. Data are represented as mean ± SD. b Huh7-EGFRvIII cells (3 × 106) were subcutaneously injected into 4–6-week-old nude mice. When tumors had reached a mean tumor volume of 200 mm3, the mice were randomly allocated into four groups (n = 6) and treated with sterile PBS or 25 mg/kg of monoclonal antibodies (1H8, 2F2 or CH12) in sterile PBS. Injections were administered intraperitoneally three times per week for 2 weeks. Data are represented as mean ± SD, n = 6
Expression and purification of 1H8/CD3 BiTE
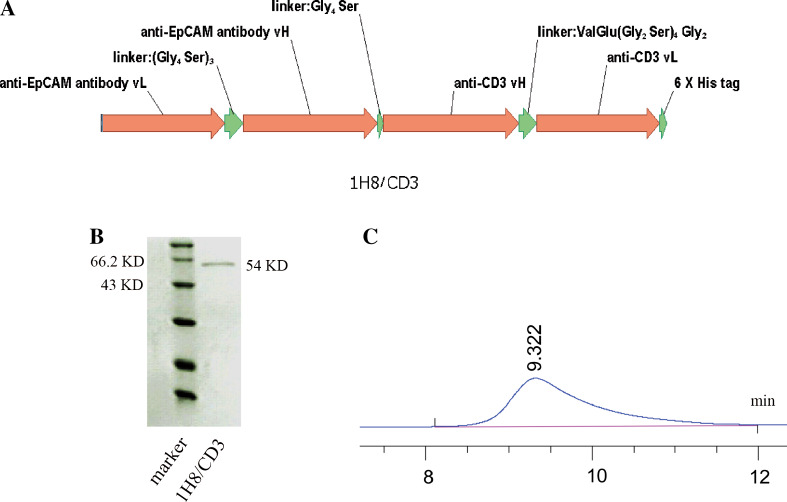
Since the anti-EpCAM monoclonal antibodies could not effectively suppress the growth of EpCAM-positive HCC, we resorted to BiTE. An anti-EpCAM BiTE was generated by genetically fusing a scFv derived from mAb 1H8 and a scFv against CD3 (Fig. 2a). As shown in Fig. 2b, the molecular mass of anti-EpCAM BiTE 1H8/CD3 is about 54 KD. The purity of 1H8/CD3 monomer isolated by molecular sieve approached 100 % as assessed by SEC-HPLC (Fig. 2c).
Fig. 2.
Purification and characterization of anti-EpCAM BiTE 1H8/CD3. a The structure of 1H8/CD3. b Coomassie blue-stained SDS-PAGE gel of purified 1H8/CD3. c The SEC-HPLC result indicated that the purity of 1H8/CD3 monomer segregation by molecular sieve is about 100 %
1H8/CD3 effectively binds to PBMC and EpCAM+ HCC cells
To test the binding specificity of 1H8/CD3, FACS assay was performed. The results indicated that 1H8/CD3 bound to PBMCs efficiently as CD3scFv. FACS analysis revealed that 1H8/CD3 obviously bound to Hep3B, SMMC-7721 and Huh-7 cells, barely bound to PLC/PRF/5 cells and weakly bound to SK-Hep-1 cells (Supplementary Figure S3).
1H8/CD3 binds to an Exon 2-encoded Sequence of human EpCAM
The binding epitope of 1H8/CD3 on human EpCAM was determined with the help of mouse EpCAM, which is not recognized by 1H8/CD3 (Supplementary Figure S4). A crude mapping of binding domains on EpCAM was done for 1H8/CD3 by using CHO-K1 cells expressing either human, mouse or two human/mouse chimeric EpCAM proteins (Supplementary Figure S2A). The two chimeric proteins exchanged human and mouse sequences in the junction of exon 2 and exon 3. Binding studies with CHO-K1 cells expressing human/mouse chimeric proteins showed that 1H8/CD3 bound to the N-terminal portion of human EpCAM encoded by exon 2 (Supplementary Figure S4).
Target-cell-dependent T-cell activation and lymphocytotoxicity mediated by 1H8/CD3
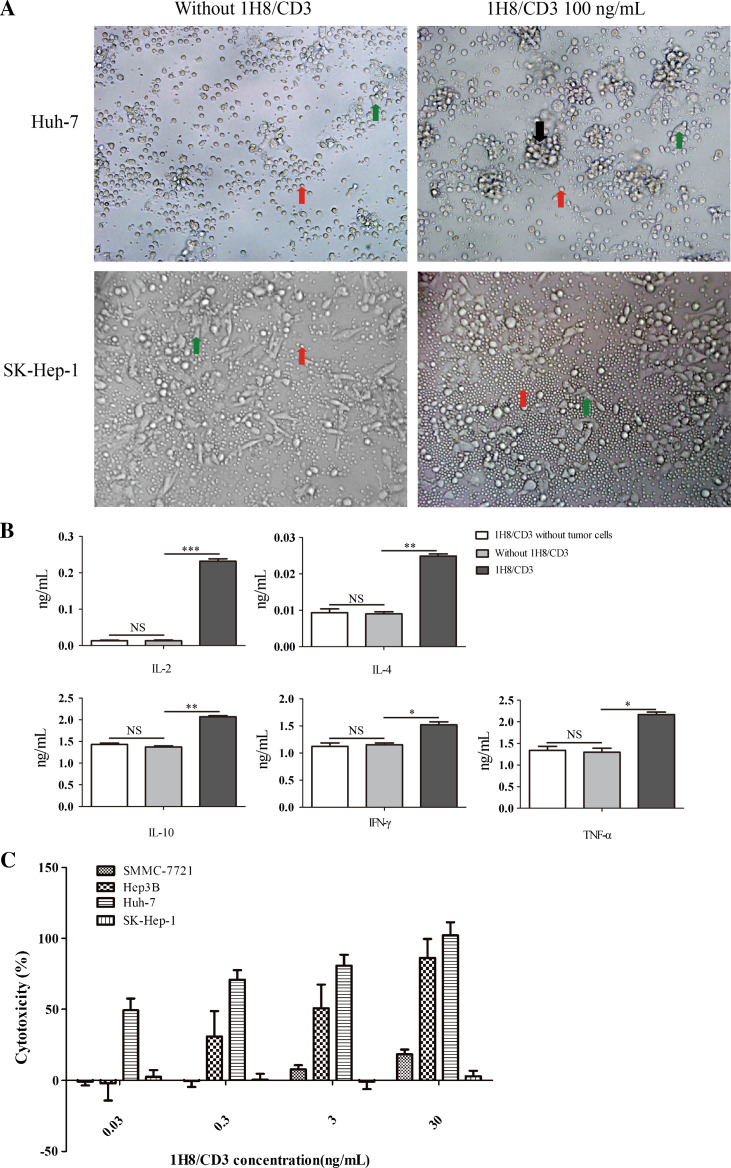
Production of cytokines is a character of T-cell activation. To analyze the effect of 1H8/CD3 on T-cell activation, cytokine release was studied with PBMC as the effector cells using 100 ng/mL of 1H8/CD3. In the presence of Huh-7 cells and 1H8/CD3, human PBMCs secreted significantly higher concentrations of IL-2, IL-4, IL-10, TNF-α and IFN-γ in the cell culture supernatant than PBMCs incubated with Huh-7 cells in the absence of 1H8/CD3 (Fig. 3b).
Fig. 3.
Cytotoxic activity of 1H8/CD3-engaged T cells against HCC cells. a Phase-contrast micrographs were taken 24 h after co-culture of PBMCs with a confluent monolayer of HCC Huh-7 or SK-Hep-1 cells in the presence or absence of 100 ng/ml of 1H8/CD3. Red arrows mark the position of PBMCs, green arrows mark the position of cancer cells and black arrows mark the position of PBMCs engaged around a cancer cell. b Cytokine concentrations measured by ELISA 48 h after co-culturing effector and target cells with or without 1H8/CD3, 1H8/CD3 co-culturing with PBMCs without target cells are shown. (*p < 0.05; **p < 0.01; ***p < 0.001) Data are represented as mean ± SD, n = 3. c A 1H8/CD3 dose–response assay for redirected lysis of HCC Huh-7, Hep3B, SMMC-7721 and SK-Hep-1 cells. Data are represented as mean ± SD, n = 3
The in vitro lymphocytotoxicity of 1H8/CD3 was tested in a lactate dehydrogenase (LDH)-based cytotoxicity assay using human PBMCs of healthy donors as effector cells and various hepatocellular carcinoma cell lines as target cells. Effector cells were mixed with target cells at an effector-to-target ratio of 10:1 and incubated with tenfold serial dilutions (0.03–30 ng/mL) of 1H8/CD3 for 48 h. The activity of 1H8/CD3 was dose dependent on EpCAM-positive Huh-7, Hep3B and SMMC-7721 (Fig. 3c). The EC50 values of 1H8/CD3 on Huh-7 and Hep3B cells were 71 ± 5.7 pg/mL and 4.8 ± 1.5 ng/mL, respectively. However, the EC50 value of 1H8/CD3 on SMMC-7721 cells was not reached even with 30 ng/mL of 1H8/CD3. Additionally, 1H8/CD3 did not show obvious lytic activity on EpCAM-negative HCC SK-Hep-1 cells (Fig. 3a, c). These results demonstrated that 1H8/CD3 could mediate indirect lysis in an antigen-dependent manner.
Redirected lysis of CD133+EpCAM+ HCC CSCs by 1H8/CD3-engaged T cells
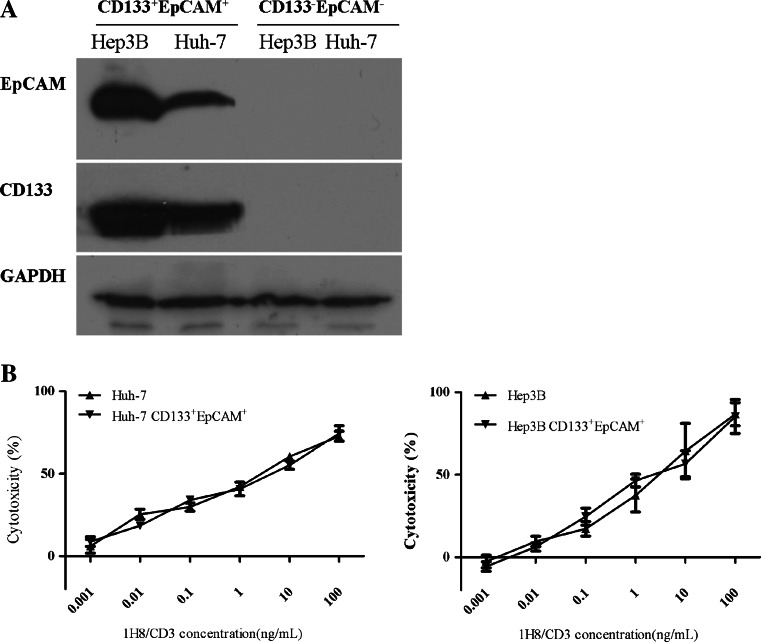
It has been reported that CD133+EpCAM+ HCC cells possesses characteristics of CSCs [33]. Therefore, CD133+EpCAM+ HCC cells (Huh-7 and Hep3B) were isolated, and the expression of CD133 and EpCAM in the isolated cells was confirmed by Western blot (Fig. 4a). High potential for colony formation and drug resistance are regarded as two features of CSCs. To demonstrate that the CD133+EpCAM+ HCC cells carried features of CSCs, colony formation and drug resistance assays were performed. The results showed that CD133+EpCAM+ Huh-7 and CD133+EpCAM+ Hep3B cells formed more colonies than CD133−EpCAM− Huh-7 and CD133−EpCAM− Hep3B cells, respectively (Supplementary Figure S5A). Additionally, CD133+EpCAM+ Huh-7 or Hep3B cells were relatively resistant to the anti-growth effects of doxorubicin and 5-FU compared with CD133−EpCAM− Huh-7 or Hep3B cells, respectively (Supplementary Figure S5B). Since our experiment in the HCC cell lines demonstrated that BiTE-mediated indirect lysis was in an EpCAM-dependent manner, we compared the sensitivity of CD133+EpCAM+ Huh-7 and Hep3B cells to 1H8/CD3-induced redirected lysis with that of their parental cells. The results showed that redirected lysis of CD133+EpCAM+ Huh-7 and Hep3B cells by 1H8/CD3 was almost the same as that observed in their parental cells (Fig. 4b).
Fig. 4.
Redirected lysis of HCC CSCs by 1H8/CD3-engaged T cells. a Western blot analysis of tumor cell lysates. Antibodies specifically recognizing CD133 and EpCAM were used. GAPDH was used as an internal control. b PBMC-dependent cellular cytotoxicity on CD133+EpCAM+ or parental Huh-7 and Hep3B cells at an effector: target cell ratio of 10:1 in the presence of various concentration of 1H8/CD3. Data are represented as mean ± SD, n = 3
Gal-1 expression level has a reverse correlation with the sensitivity of HCC to 1H8/CD3-induced redirected lysis
To understand the molecular mechanism underlying the relative resistance of SMMC-7721 to the 1H8/CD3-induced indirect lysis, several molecules involved in the T-cell immunity were examined. PD-L1 (programmed death 1 ligand) is a molecule participated in immune evasion and its expression is induced by IFN-γ [34]; overexpression of c-FLIP (cellular FLICE-inhibitory protein) and PI-9 (serine protease 9) in cancer cells can lead to their escape from T-cell immunity [35, 36]. Gal-1, a 14.5-kD protein, has been reported to often contribute to tumor immune privilege by modulating survival of T-cell subsets [37]. RT-PCR was used to detect the PD-L1, PI-9 and c-FLIP mRNA expression in HCC cell lines. The results shown in Figure S6A indicate that PD-L1 and PI-9 mRNA expression in SMMC-7721 cells were obviously lower than their expression in Huh-7 and Hep3B cells while strong c-FLIP mRNA expression was expressed in all three cell lines. Additionally, in the presence of IFN-γ, more PD-L1 expression was induced in Huh-7 cells than that in SMMC-7721 cells (Supplementary Figure S6A). Real-time PCR was performed to further compare the c-FLIP mRNA expression in these three cell lines. The results showed that SMMC-7721 cells expressed lower levels of c-FLIP mRNA than Huh-7 and Hep3B cells (Supplementary Figure S6B). Thus, PD-L1, PI-9 and c-FLIP might not be the factors involved in SMMC-7721 cells resistant to 1H8/CD3-induced indirect lysis.
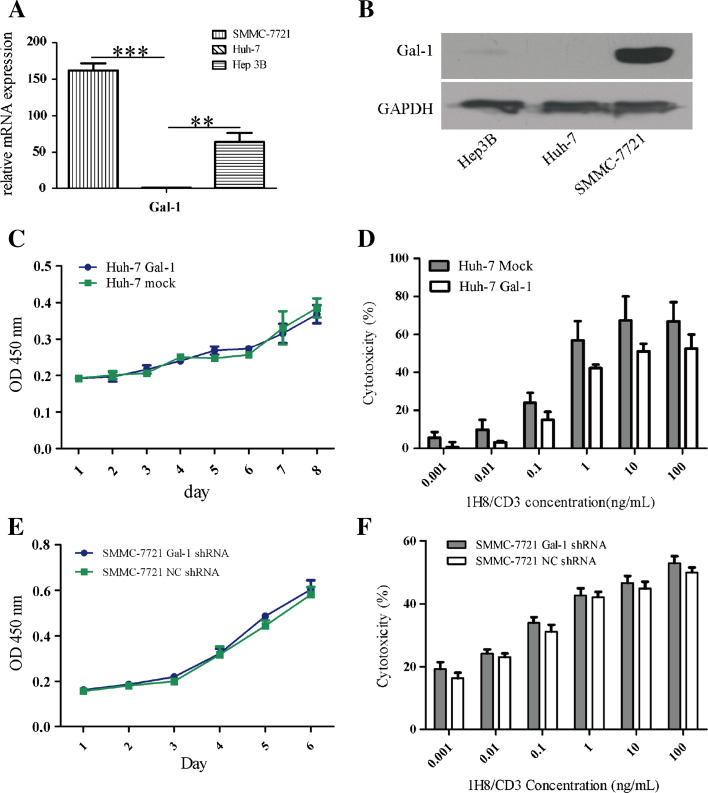
Intriguingly, in comparison to SMMC-7721 and Hep3B, Huh-7 had almost no Gal-1 expression while SMMC-7721 had the most Gal-1 expression in these three cell lines (Fig. 5a, b). To examine the role of Gal-1 in the 1H8/CD3-induced indirect lysis response of HCC cells, Huh-7 cells with Gal-1 overexpression were established (Supplementary Figure S6C). The Gal-1 overexpression in Huh-7 cells did not promote cell growth (Fig. 5c). The EC50 values of 1H8/CD3 on Huh-7 Gal-1 and Huh-7 mock cells were 17.81 ± 7.1 and 4.38 ± 3.48 ng/mL, respectively (p < 0.05) (Fig. 5d), suggesting that Gal-1 overexpression can lead to relative resistance of the HCC cells to 1H8/CD3. To further confirm the activities of Gal-1, SMMC-7721 cells with Gal-1 knockdown were established (Supplementary Figure S6D). The knockdown of Gal-1 in SMMC-7721 did not change the growth of the cells (Fig. 5e). The EC50 values of 1H8/CD3 on SMMC-7721 Gal-1 shRNA or SMMC-7721 NC shRNA cells were 25.4 ± 14.0 or 41.3 ± 17.3 ng/mL, respectively (p < 0.05) (Fig. 5f).
Fig. 5.
Gal-1 reduces the sensitivity of HCC cells to BiTE-induced indirect lysis. a Gal-1 mRNA expression in HCC cell lines was determined by real-time PCR. b Gal-1 expression in HCC cell lines was determined by Western blot. mean ± SD, n = 3. (**p < 0.01; ***p < 0.001). c Overexpression of Gal-1 in Huh-7 cells did not affect cell proliferation. Data are represented as mean ± SD, n = 3. d Overexpression of Gal-1 reduced sensitivity of Huh-7 cells to BiTE-induced indirect cytotoxicity. Data are represented as mean ± SD, n = 5. e Knockdown of Gal-1 expression in SMMC-7721 cells did not affect cell proliferation. Data are represented as mean ± SD, n = 3. f Knockdown of Gal-1 increased sensitivity of SMMC-7721 cells to BiTE-induced indirect cytotoxicity. Data are represented as mean ± SD, n = 5. NC negative control
1H8/CD3 effectively eliminates HCC cells in mouse models
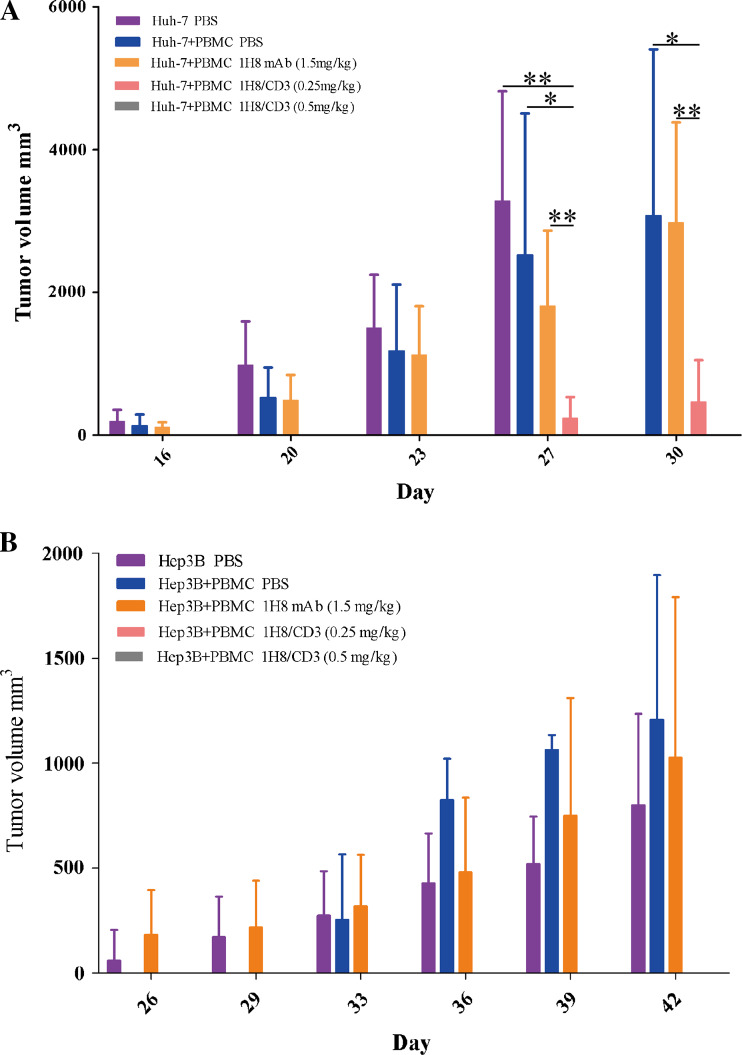
To further demonstrate the anti-tumor activities of 1H8/CD3, 2 × 106 Huh-7 or 4 × 106 Hep3B tumor cells as target cells were mixed with human PBMCs as effector cells at a ratio of 1:1 (target: effector) and inoculated subcutaneously into NOD/SCD mice. Tumor model animals were then given daily doses of 1H8/CD3 or an equimolar injection of the 1H8 control antibody for 10 days. The results in Fig. 6 indicate that 1H8/CD3 displayed potent growth suppression in both HCC models while 1H8 displayed no obvious anti-tumor effect. For Huh-7 tumor xenograft mouse models, doses of 0.5 mg/kg 1H8/CD3 prevented tumor outgrowth in all of the mice for the entire observation period of 30 days. At a dose of 0.25 mg/kg 1H8/CD3 intravenous injection for 10 days, 3 out of 6 mice were free of tumor (Fig. 6a). Doses of 0.5 mg/kg and 0.25 mg/kg 1H8/CD3 intravenous injection for 10 days prevented Hep3B tumor outgrowth in all of the mice for the entire observation period of 39 days (Fig. 6b).
Fig. 6.
Anti-tumor effects of 1H8/CD3 on HCC xenografts. a Huh-7. b Hep3B. Data are represented as mean ± SD, n = 6, *p < 0.05; **p < 0.01
The most definite feature of CSCs is their ability to form tumors following transplantation into immunocompromised mice. As the results showed that 1H8/CD3 effectively eradicated HCC cells in mouse models, we further investigated whether 1H8/CD3 effectively eliminated CSCs in Huh-7 xenograft mouse models. Huh-7 xenograft tumor tissues were analyzed by real-time PCR for expression of mRNA coding for CSC marker proteins. As shown in supplementary Figure S7, levels of EpCAM, CD133, Nanog, Sox-2 and Oct-3/4 mRNA expression in tumor tissues treated with 0.25 mg/kg of 1H8/CD3 were remarkably lower than those treated with 1H8 or PBS. These results suggested that 1H8/CD3 could effectively eliminate CSCs in Huh-7 and Hep3B xenograft mouse models.
Discussion
Given that HCC is characterized by adjuvant chemotherapy resistance and high rates of recurrence after a curative liver resection [2, 3], it is urgently necessary to develop novel therapeutic strategies to eventually achieve better prognosis. Previous studies have shown that EpCAM-positive HCC cells exhibit the characteristics of CSCs [18, 19] and are correlated with poor prognosis of HCC [20]. Therefore, EpCAM may represent a promising target for HCC treatment.
Several EpCAM-targeting antibodies are in clinical development, for example, catumaxomab, adecatumumab, chimeric edrecolomab and ING-1 [32, 38]. Clinical trials have been carried out in various cancers, including colon cancers, malignant ascites, ovarian cancer and breast cancer [38–41]. Among them, Catumaxomab has been approved for treating malignant ascites in Europe. However, an anti-HCC effect of EpCAM-directed antibodies has not yet been reported in preclinical or clinical studies. It is therefore necessary to explore their potential in HCC treatment.
In this study, we developed two anti-EpCAM monoclonal antibodies 1H8 and 2F2 and examined their growth suppression effect on HCC cells. Unfortunately, the two monoclonal antibodies did not display obvious anti-tumor effects on HCC cells in vitro or in vivo.
There is increasing evidence that cytotoxic T cells are able to inhibit tumor growth and survival both in early and late stages of the disease [42]. BiTEs can induce potent anti-tumor activity at a very low dose. More importantly, BiTEs have the potential to overcome mutations (for instance, KRAS and BRAF mutation) that classically lead to resistance to monoclonal antibody therapy [43]. Additionally, a phase 2 clinical study of blinatumomab, an anti-CD19 BiTE could cause prolongation of leukemia-free survival in B-lineage acute lymphoblastic leukemia patients, which further supports that BiTE is a promising way for eliminating cancer cells [44]. Two recent studies have demonstrated the highly efficient elimination of colorectal and pancreatic CSCs by anti-EpCAM BiTE [25, 26]. Our data showed that anti-EpCAM BiTE 1H8/CD3 could activate PBMCs and redirect lysis EpCAM-positive HCC cells as well as CSCs of HCC. Additionally, the cancer cell killing capability of 1H8/CD3 was produced in an EpCAM-specific manner.
Interestingly, SMMC-7721 cells, although efficiently bound by 1H8/CD3, displayed resistance to redirected lysis induced by 1H8/CD3. By checking several factors that may contribute to this resistance, we observed that Gal-1 expression level had an inverse relation with the sensitivities of the cells to anti-EpCAM BiTE. Gal-1 is overexpressed not only by a variety of cancer cells including HCC cells [45] but also by activated regulatory T (Treg) cells [46]. Recently, it has been demonstrated that Gal-1 plays a pivotal role in promoting escape from T-cell-dependent immunity [47]. Thus, we propose that Gal-1 may contribute to the resistance of HCC cells to 1H8/CD3-induced lysis indicating the anti-HCC efficacy of anti-EpCAM BiTE and also a potential therapeutic target.
In summary, in this study, we provide the first experimental evidence of the anti-tumor effect of anti-EpCAM monoclonal antibodies and BiTE on HCC models. Our results indicate that anti-EpCAM BiTE 1H8/CD3 is capable of redirecting T cells to eradicate HCC cells as well as CSCs of HCC in vitro and in vivo. Additionally, we revealed that anti-EpCAM BiTE has lower lymphocytotoxicity on HCC cells with Gal-1 overexpression than on HCC cells with limited Gal-1 expression. Thus, we propose here that anti-EpCAM BiTE 1H8/CD3 is a promising agent for treating HCC with limited Gal-1 expression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by the Supporting Program of the “Twelfth Five-year Plan” for Science and Technology Research of China (Grant No. 2012ZX09103-301-005 and 2012ZX10002014-006), the National Natural Science Foundation of China (No. 81071746) and the National Basic Research Program (Grant No. 2010CB529902).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Pengfei Zhang and Bizhi Shi have contributed equally to this work.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004;127:S218–S224. doi: 10.1053/j.gastro.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]
- 4.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Padura I, Marighetti P, Agliano A, Colombo F, Larzabal L, Redrado M, Bleau AM, Prior C, Bertolini F, Calvo A. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Investig. 2012;92:952–966. doi: 10.1038/labinvest.2012.65. [DOI] [PubMed] [Google Scholar]
- 6.Xu XL, Xing BC, Han HB, Zhao W, Hu MH, Xu ZL, Li JY, Xie Y, Gu J, Wang Y, Zhang ZQ. The properties of tumor-initiating cells from a hepatocellular carcinoma patient’s primary and recurrent tumor. Carcinogenesis. 2010;31:167–174. doi: 10.1093/carcin/bgp232. [DOI] [PubMed] [Google Scholar]
- 7.Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 8.Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol. 2010;222:1–15. doi: 10.1002/path.2727. [DOI] [PubMed] [Google Scholar]
- 9.Yao Z, Mishra L. Cancer stem cells and hepatocellular carcinoma. Cancer Biol Ther. 2009;8:1691–1698. doi: 10.4161/cbt.8.18.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers—therapeutic implications. Trends Mol Med. 2008;14:450–460. doi: 10.1016/j.molmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Dingli D, Michor F. Successful therapy must eradicate cancer stem cells. Stem Cells. 2006;24:2603–2610. doi: 10.1634/stemcells.2006-0136. [DOI] [PubMed] [Google Scholar]
- 12.Balzar M, Briaire-de Bruijn IH, Rees-Bakker HA, Prins FA, Helfrich W, de Leij L, Riethmuller G, Alberti S, Warnaar SO, Fleuren GJ, Litvinov SV. Epidermal growth factor-like repeats mediate lateral and reciprocal interactions of Ep-CAM molecules in homophilic adhesions. Mol Cell Biol. 2001;21:2570–2580. doi: 10.1128/MCB.21.7.2570-2580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 16.Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–5758. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- 17.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, Tang ZY, Wang XW. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 21.Bae JS, Noh SJ, Jang KY, Park HS, Chung MJ, Park CK, Moon WS. Expression and role of epithelial cell adhesion molecule in dysplastic nodule and hepatocellular carcinoma. Int J Oncol. 2012;41:2150–2158. doi: 10.3892/ijo.2012.1631. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin PM, Harmsen MC, Dokter WH, Kroesen BJ, van der Molen H, Brinker MG, Hollema H, Ruiters MH, Buys CH, de Leij LF. The epithelial glycoprotein 2 (EGP-2) promoter-driven epithelial-specific expression of EGP-2 in transgenic mice: a new model to study carcinoma-directed immunotherapy. Cancer Res. 2001;61:4105–4111. [PubMed] [Google Scholar]
- 23.Armstrong A, Eck SL. EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2:320–326. doi: 10.4161/cbt.2.4.451. [DOI] [PubMed] [Google Scholar]
- 24.Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer. 2007;96:1013–1019. doi: 10.1038/sj.bjc.6603505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin Cancer Res. 2012;18:465–474. doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann I, Baeuerle PA, Friedrich M, Murr A, Filusch S, Ruttinger D, Majdoub MW, Sharma S, Kufer P, Raum T, Munz M. Highly efficient elimination of colorectal tumor-initiating cells by an EpCAM/CD3-bispecific antibody engaging human T cells. PLoS One. 2010;5:e13474. doi: 10.1371/journal.pone.0013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbuse R, Schlereth B, Kufer P, Baeuerle PA. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, Kleindienst P, Wimberger P, Kimmig R, Fichtner I, Kufer P, Hofmeister R, da Silva AJ, Baeuerle PA. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Wang H, Tan Z, Hu S, Shi B, Yang L, Li P, Gu J, Li Z. Growth suppression of human hepatocellular carcinoma xenografts by a monoclonal antibody CH12 directed to epidermal growth factor receptor variant III. J Biol Chem. 2011;286:5913–5920. doi: 10.1074/jbc.M110.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Jiang H, Gao H, Kong J, Zhang P, Hu S, Shi B, Yao M, Li Z. The monoclonal antibody CH12 enhances the sorafenib-mediated growth inhibition of hepatocellular carcinoma xenografts expressing epidermal growth factor receptor variant III. Neoplasia. 2012;14:509–518. doi: 10.1593/neo.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, Cole DJ, Gillanders WE. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 32.Munz M, Murr A, Kvesic M, Rau D, Mangold S, Pflanz S, Lumsden J, Volkland J, Fagerberg J, Riethmuller G, Ruttinger D, Kufer P, Baeuerle PA, Raum T. Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies. Cancer Cell Int. 2010;10:44. doi: 10.1186/1475-2867-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Yu D, Zhang H, He H, Zhang C, Zhao W, Shao RG. CD133(+)EpCAM(+) phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells. Int J Biol Sci. 2012;8:992–1004. doi: 10.7150/ijbs.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review) Int J Oncol. 2010;37:1361–1378. doi: 10.3892/ijo_00000788. [DOI] [PubMed] [Google Scholar]
- 36.Medema JP, de Jong J, van Hall T, Melief CJ, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med. 1999;190:1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142:107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wimberger P, Gilet H, Gonschior AK, Heiss MM, Moehler M, Oskay-Oezcelik G, Al-Batran SE, Schmalfeldt B, Schmittel A, Schulze E, Parsons SL. Deterioration in quality of life (QoL) in patients with malignant ascites: results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol. 2012;23:1979–1985. doi: 10.1093/annonc/mds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumann K, Pfisterer J, Wimberger P, Burchardi N, Kurzeder C, du Bois A, Loibl S, Sehouli J, Huober J, Schmalfeldt B, Vergote I, Luck HJ, Wagner U. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecol Oncol. 2011;123:27–32. doi: 10.1016/j.ygyno.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt M, Scheulen ME, Dittrich C, Obrist P, Marschner N, Dirix L, Ruttinger D, Schuler M, Reinhardt C, Awada A. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2010;21:275–282. doi: 10.1093/annonc/mdp314. [DOI] [PubMed] [Google Scholar]
- 41.Strohlein MA, Lordick F, Ruttinger D, Grutzner KU, Schemanski OC, Jager M, Lindhofer H, Hennig M, Jauch KW, Peschel C, Heiss MM. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie. 2011;34:101–108. doi: 10.1159/000324667. [DOI] [PubMed] [Google Scholar]
- 42.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 43.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Kohne-Volland R, Bruggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele H, Riethmuller G, Hoelzer D, Zugmaier G, Bargou RC. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 45.Espelt MV, Croci DO, Bacigalupo ML, Carabias P, Manzi M, Elola MT, Munoz MC, Dominici FP, Wolfenstein-Todel C, Rabinovich GA, Troncoso MF. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology. 2011;53:2097–2106. doi: 10.1002/hep.24294. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Stannard K, Gabutero E, Clark AM, Neo SY, Onturk S, Blanchard H, Ralph SJ. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev. 2012;31:763–778. doi: 10.1007/s10555-012-9388-2. [DOI] [PubMed] [Google Scholar]
- 47.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: a potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.