Abstract
Reduced expression of HLA class I is an important immune escape mechanism from cytotoxic T cells described in various types of malignancy. It often correlates with poor prognosis and resistance to therapy. However, current knowledge about the frequency, underlying molecular mechanisms, and prognostic value of HLA class I and II alterations in prostate cancer (PC) is limited. Immunohistochemical analysis demonstrated that 88 % of the 42 studied cryopreserved prostate tumors have at least one type of HLA alteration as compared to adjacent normal prostate epithelium or benign hyperplasia. Total loss of HLA-I expression found in 50 % of tumors showed an association with increased incidence of tumor relapse, perineural invasion, and high D’Amico risk. The remaining HLA-I-positive tumors demonstrated locus and allelic losses detected in 26 and 12 % of samples, respectively. Loss of heterozygosity at chromosome 6 was detected in 32 % of the studied tumors. Molecular analysis revealed a reduced expression of B2M, TAP2, tapasin and NLRC5 mRNA in microdissected HLA-I-negative tumors. Analysis of twelve previously unreported cell lines derived from neoplastic and normal epithelium of cancerous prostate revealed different types of HLA-I aberration, ranging from locus and/or allelic downregulation to a total absence of HLA-I expression. The high incidence of HLA-I loss observed in PC, caused by both regulatory and structural defects, is associated with more aggressive disease development and may pose a real threat to patient health by increasing cancer progression and resistance to T-cell-based immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1774-5) contains supplementary material, which is available to authorized users.
Keywords: Human prostate cancer, HLA molecules, Beta-2-microglobulin, Antigen presentation, Tumor recurrence, Clinical prognosis
Introduction
Prostate cancer (PC) is the most frequently diagnosed cancer and one of the leading causes of cancer-related mortality among men in developed countries. The response to existing therapies is not satisfactory, and tumor recurrence is frequent. Therefore, it is important to identify molecules that improve the susceptibility of PC cells to an immune attack. Human leukocyte antigens class I and II (HLA-I and HLA-II) play a key role in the adaptive immune responses, including anti-tumor immunity. Cell surface HLA-I complex has a critical role in the recognition and lysis of tumor cells by presenting intracellular antigenic peptides to cytotoxic T lymphocytes (CTLs) [1, 2]. This complex consists of a membrane-anchored heavy chain (A, B, and C variants) and a non-covalently associated beta-2-microglobulin (B2M) chain. Antigenic peptide processing and presentation to T cells requires the participation of various molecules that regulate folding, intracellular assembly, and transport to the cell surface of HLA-I, including immunoproteasome components LMP2/7, peptide transporters TAP1/2, and the chaperones calreticulin, calnexin, ERP57, and tapasin. Different transcriptional factors, such as IFR-1 and NLRC5, control the expression of HLA-I-related genes [3, 4], and most HLA-associated molecules are inducible by interferon-gamma (IFN-γ).
At each step of antigen presentation process, both regulatory defects and genetic alterations can cause loss or downregulation of HLA-I complex leading to lack of CTL responses and cancer immune escape [5]. Aberrant HLA-I expression on cancer cells has been reported in a large percentage of tumors of different histological type and can be classified into distinct HLA-I phenotypes (Ph), ranging from a total loss of expression (PhI) to a partial loss of an HLA-I haplotype (PhII), locus (PhIII) or allele (PhIV) [5]. Total loss of HLA-I in some types of cancer, including melanoma and colorectal carcinoma, can be caused by the loss of B2M protein due to genetic structural defects (“hard” lesions) [6, 7]. Regulatory (“soft”) alterations in the HLA antigens or components of antigen presentation machinery (APM) have been also frequently detected in cancer [8, 9]. The clinical implications of structural defects can be more serious, because these defects cannot be recovered by cytokines or immunomodulating treatment and require gene therapy [10–14]. Thus, ongoing T-cell-mediated immunotherapy and novel cancer vaccines in PC treatment rely on tumor HLA-I expression.
Published data on human PCs are controversial with regard to tumor HLA-I expression and its predictive value in disease progression. Research into PC pathogenesis and immune escape has long been limited by the lack of studies using cryopreserved tumor tissues (where antigenic determinants of highly polymorphic HLA-I complex are better preserved) and new cell lines with well-characterized HLA expression. Only a few human PC cell lines with characterized HLA expression are available from ATCC, including Du145, LnCap and PC-3 [15], while the development of a cancer vaccine requires tumor cells expressing specific HLA-I alleles that selectively present a particular tumor-associated peptide to CTLs. In this paper, we characterized HLA-I alterations in PC using cryopreserved human prostate tumors and benign hyperplasia (BH) samples and immortalized cell lines established from PC patients.
Materials and methods
Patients and tumor samples
We analyzed 42 cryopreserved human prostate tumors and 12 prostatic BH samples collected from patients after radical prostatectomy between 1994 and 2006 in the Virgen de las Nieves University Hospital of Granada in Spain. We also analyzed a separate group of 6 samples collected between 2008 and 2010 (primary prostate tumors with adjacent normal epithelium). The research protocol was approved by the hospital institutional review board and ethical committee. Tumor Gleason grade and TNM were characterized according to the guidelines of the European Association of Urology. Tumors were also classified by disease progression risk according to the D’Amico criteria [16] based on known risk factors: clinical stage, prostate-specific antigen (PSA) level and Gleason tumor grade. Perineural invasion (PNI) was determined by histopathological examination. Clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Clinicopathological characteristics of the patients and tumors
| n | |
|---|---|
| Stage | |
| pT1-2c | 26 |
| pT3a-4 | 14 |
| n.a | 2 |
| PSA | |
| <10 | 18 |
| ≥10, <20 | 15 |
| ≥20 | 2 |
| n.a | 7 |
| Gleason | |
| <7 | 23 |
| =7 | 12 |
| >7 | 5 |
| n.a | 2 |
| Cancer risk | |
| Low | 5 |
| Medium | 28 |
| High | 7 |
| n.a | 2 |
| Perineural invasion | |
| Yes | 15 |
| No | 20 |
| n.a | 7 |
| Recurrence | |
| Yes | 25 |
| No | 15 |
| n.a | 2 |
n number of cases analyzed, n.a not analyzed
Cell lines
Twelve prostate cell lines derived from cancerous and/or normal prostate epithelia of eight different PC patients (cells immortalized by HPV16/18-E6/E7 gene transfer) were provided by our collaborators from Onyvax Limited (St George’s Hospital, UK) as a part of the European Union project ENACT (European Network for the identification and validation of antigens and biomarkers in Cancer and their application in clinical Tumor immunology, LSHC-CT-2004-503306). Malignant prostate cells in culture retained neoplastic phenotype and prostate-specific markers. Cells were cultured in keratinocyte medium with a growth factor (GIBCO, Life Technologies) supplemented with 2 % FCS, 1 % glutamine and 1 % penicillin/streptomycin. PC-3, DU-145, and LNCaP cell lines were purchased from ATCC (Manassas, VA, USA) and cultured in complete RPMI-1640 medium (Biochrom KG). Cell line identity was confirmed by HLA typing using genomic sequencing technique.
HLA genomic typing
Genomic HLA typing of all cell lines and autologous PBMCs corresponding to prostate tumors was performed using Dynal RELI®SSO Typing Trays and Dynal RELI®SSO Strip Detection Reagent kit, following the manufacturer’s instructions.
Immunohistochemistry and antibodies
Tissue immunolabeling was done using a wide panel of anti-HLA monoclonal antibodies (mAb) (Supplementary Table 1), and the results were interpreted as described previously [17]. Immunolabeling was graded in accordance with the HLA and Cancer Component of the 12th International Histocompatibility Workshop (IHW) [18] as negative (when <25 % cells were stained), heterogeneous (when 25–75 % of cells were stained), or positive (when >75 % of cells were stained). In some cases tumors with negative and heterogeneous patterns of HLA-I labeling were grouped together as opposed to HLA-I-positive ones. We also analyzed HLA-I/B2M positive tumors to identify undetected partial locus and allelic alterations. Based on the obtained results we classified tumors into different phenotypes: positive, total loss (PhI), and partial losses—PhIII (locus loss) and PhIV (allelic loss).
Flow cytometry
FACS analysis using a panel of specific antibodies directed against HLA-I and II antigens was performed on a BD FACS Canto (Becton Dickinson) in baseline conditions and after 48-h incubation with IFN-γ (800 U/ml) as previously described [19]. FITC-conjugated goat anti-mouse Ab (Sigma) was used as a secondary Ab. Results are shown either as representative FACS plots or based on fluorescence intensity as follows: − negative (MFI 0–1), + weakly positive (MFI 1–10), ++ positive (MFI 10–100), +++ strongly positive (MFI > 100).
Tissue microdissection, DNA/RNA isolation, and reverse transcription
Tissue microdissection, DNA/RNA extraction, and cDNA synthesis were done as previously described [17]. In cell lines, total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen). Cells and tumor samples were analyzed for the expression of B2M, HLA-A, HLA-B, Tap1, Tap2, Tapasin, LMP2, LMP7, calnexin, calreticulin, IRF1, and NLRC5 mRNA by quantitative real-time PCR (Q-PCR) on Applied Biosystems Fast 7500 apparatus using TaqMan PCR master mix and target-specific primers/probes from Applied Biosystems (UK). Results are expressed relative to GUS expression to control for variations in amounts of mRNA. In some cases we included results obtained at high amplification cycles with CT cutoff of 39 due to the small RNA quantities obtained from microdissected tissue samples.
PCR of genomic DNA and B2M gene sequencing
DNA from microdissected HLA-I negative tumors was used to amplify specific “hot-spot” sequences of B2M gene, using the following primers: fw (5′-gtccctctctctaacctggc-3′) and bw (5′-cagagcgggagggtaggaga-3′) to amplify exon 1, and fw (5′-taccctggcaatattaatgtgtc-3′) and bw (5′-catacacaactttcagcagcttac-3′) to amplify exon 2, with annealing temperatures of 64 and 53 °C, respectively. DNA from OPCN3 cells was isolated with the QIAamp DNA Mini Kit (Qiagen) and was used to amplify B2M gene sequences with the following primers: fw (5´-aattgctatgtcccaggcac-3′) and bw (5′-acacaactttcagcagcttac-3′), at an annealing temperature of 53 °C. PCR products were amplified using Bigdye Terminator v1.1 cycle sequencing kit (Applied Biosystems), followed by sequence analysis in an Abi Prism 377 DNA sequencer.
Microsatellite analysis
To determine loss of heterozygosity (LOH) in chromosomes 6 and 15 (LOH-6 and LOH-15), DNA obtained from microdissected tumor specimens, peripheral blood, and cell lines was studied using eight short tandem repeats (STRs) mapping the HLA-I region of chromosome 6 and five markers flanking the B2M gene on chromosome 15. The STRs, PCR, electrophoresis methods and data interpretation were previously described [20, 21].
Statistical analysis
We used the Chi-square and Fisher’s exact test with a significance threshold of p < 0.05 to evaluate possible association of the tumor HLA expression with clinicopathological data. We used the Student’s t test with a significance threshold of p < 0.05 to evaluate possible differences in mRNA expression levels between BH controls and the following tumor groups: group 1—total HLA-I loss (including heterogeneous samples); and group 2, which includes other types of HLA alterations (locus or allelic losses). SPSS v17.0 software was used for all statistical analyses.
Results
Immunohistochemical analysis of HLA-I and HLA-II expression in PC
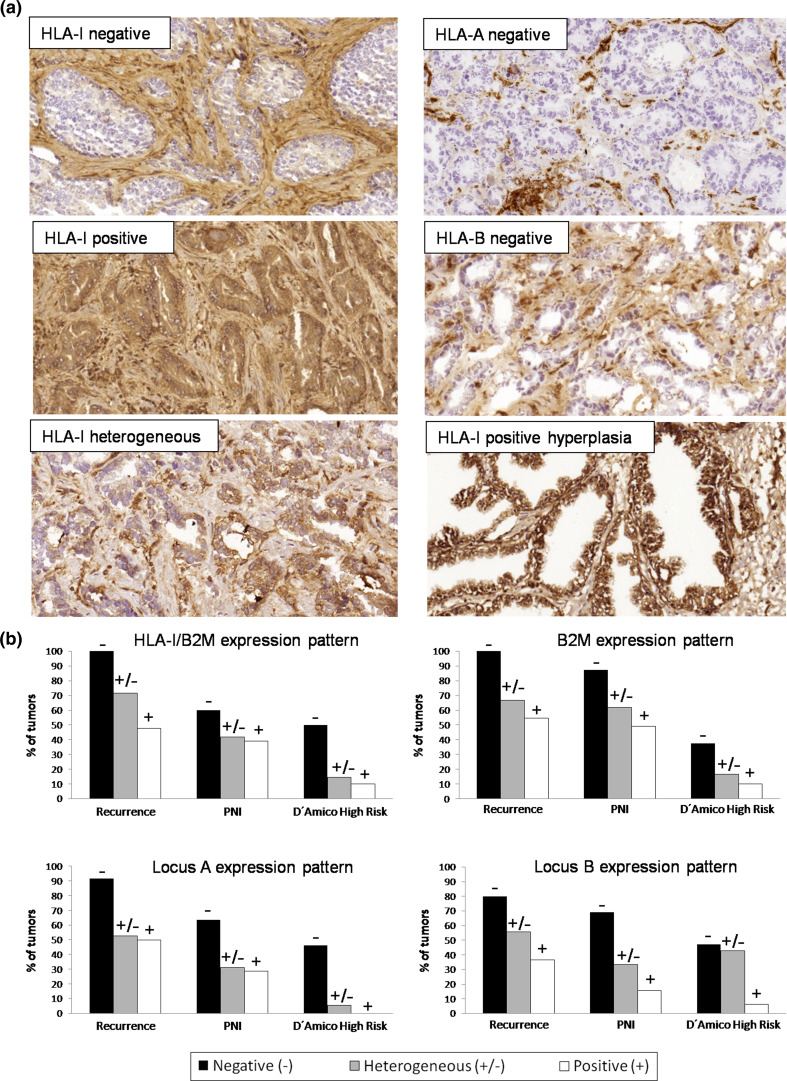
Around 88 % of studied tumors had at least one type of HLA-I alteration. Using anti-HLA-I/B2M antibody w6/32, we found that 50 % of the studied tumors were positive, while another 50 % had total loss of HLA-I/B2M complex expression with either complete (16.7 %) or heterogeneous (33.3 %) pattern. B2M loss was found in 50 % of all the studied samples, and loss of the free HLA-I heavy chain (HC-10 Ab) in 75 % of the cases (Table 2). HC-10 antibody detects free HLA heavy chains not associated with B2M protein, therefore, the percentage of HC-10 positive labeling can differ from that of the cell surface HLA-I/B2M complex. Based on the immunohistochemistry results using anti-locus-specific antibodies alone, we observed negative immunolabeling of locus-A and -B in 34.1 and 28.6 % of all the studied tumors, respectively (Table 2). In contrast, all BH samples were positive for class I expression.
Table 2.
Immunohistological analysis of HLA expression patterns in prostate tumors
| PC (%) | HLA-/B2M | B2M | Free HLA-I heavy chain | Locus A | Locus B | HLA-DR |
|---|---|---|---|---|---|---|
| Negative | 16.7 % | 21.4 % | 35 % | 34.1 % | 28.6 % | 75.7 % |
| Heterogeneous | 33.3 % | 28.6 % | 40 % | 36.6 % | 30.9 % | 21.6 % |
| Positive | 50 % | 50 % | 25 % | 29.3 % | 40.5 % | 2.7 % |
| No. of samples | 42 | 42 | 40 | 42 | 42 | 37 |
Based on HLA-I genotyping results of each tumor sample, we selected locus- and allele-specific mAbs to detect unnoticed partial alterations in HLA-I/B2M positive tumors. We found that 26 % of HLA-I positive tumors have locus loss, while 12 % showed allelic losses. Based on the obtained results, we classified the studied PC tumors into different Ph with the following incidence: PhI (total loss) in 50 %, PhIII (locus loss) in 26 %, and PhIV (allelic loss) in 12 % (Table 3). There may have been additional undetected allelic losses, due to the lack of Abs against all possible allelic specificities. Representative images of different patterns of HLA-I immunolabeling in PC and BH are depicted in Fig. 1a. HLA-DR expression was negative in all BH samples and in around 75 % of the tumor samples. Approximately 22 % of tumors showed a heterogeneous HLA-DR labeling pattern, and only one tumor was graded as DR-positive.
Table 3.
Frequency of different HLA-I altered phenotypes (immunohistochemistry) and LOH-6/LOH-15 (microsatellite analysis) in prostate tumors
| Phenotype | Total loss (PhI) | Locus loss (PhIII) | Allelic loss (PhIV) | Positive | Total alterations |
|---|---|---|---|---|---|
| Tumors (n = 42) | 21 (50 %) | 11 (26 %) | 5 (12 %) | 5 (12 %) | 37 (88 %) |
| LOH-6 (n = 25) | 5/13 | 0/5 | 1/3 | 2/4 | 8/25 |
| LOH-15 (n = 25) | 1/13 | 0/5 | 0/3 | 0/4 | 1/25 |
Fig. 1.
a Representative immunohistochemistry images of HLA-I expression in cryopreserved PC and BH: negative, positive and heterogeneous expression patterns of HLA-I/B2M complex in PC (w6/32 mAb); loss of HLA-A (Tu155 mAb) and loss of HLA-B (421b5 mAb) in PC; and positive HLA-I expression in benign prostate hyperplasia (w6/32). b Correlation between the expression of HLA-I, B2M, HLA-A and HLA-B and clinicopathological characteristics: PC recurrence, PNI and D’Amico high cancer risk. Tumors with negative and heterogeneous expression of HLA-I/B2M, B2M, locus A, and locus B have a strong tendency to relapse and develop PNI. (−) negative, (+) positive, and (±) heterogeneous expression
From a separate set of six PC patients, we were able to compare cryopreserved tumors and matching normal prostate epithelium and stroma and observed a higher incidence of HLA alterations in cancerous samples (Table 4). Four tumors showed a loss of HLA-I expression (two totally negative and two heterogeneous, w6/32 mAb), and one tumor showed loss of locus B. Only one tumor had the same positive labeling pattern as the adjacent normal epithelium (Table 4).
Table 4.
Immunohistological analysis of HLA expression in tumors (T) versus matching normal epithelium (N)
| Sample | HLA-I | B2M | Heavy chain | Locus A | Locus B | HLA-DR |
|---|---|---|---|---|---|---|
| 12241 N | ++ | +++ | +++ | +++ | +++ | ++ |
| 12241 T | − | − | − | − | − | − |
| 8723 N | ++ | ++ | ++ | ++ | ± | − |
| 8723 T | − | − | + | − | − | − |
| 9238 N | ++ | ++ | ++ | ++ | ± | ± |
| 9238 T | ± | ± | ± | ± | ± | − |
| 8722 N | ++ | +++ | ++ | ++ | ++ | ± |
| 8722 T | ± | ± | ± | − | − | − |
| 2489 N | ++ | ++ | ++ | ++ | ++ | ++ |
| 2489 T | +++ | +++ | ++ | ± | − | ± |
| 10216 N | ++ | ++ | ++ | ++ | ++ | ± |
| 10216 T | ++ | ++ | ++ | ++ | ++ | ± |
+ weakly positive, ++ positive, +++ strongly positive, ± heterogeneous, − negative
Analysis of the association of HLA-I and HLA-II expression with tumor recurrence, PNI, and high D’Amico cancer risk
Tumors negative for HLA-I/B2M complex, B2M, locus A or locus B expression showed a strong tendency to relapse and develop PNI, and were also linked to high D’Amico risk (as compared to HLA-I positive lesions) (Fig. 1b, Supplementary Table 2), although the number of cases was too low for statistical significance to be reached. Interestingly, when we grouped together heterogeneous and positive samples versus negative samples, we observed a statistical significance for the correlation of locus A loss with high D’Amico risk (p = 0.005) (data not shown). When we further explored the relevance of HLA-I loss in correlation with different Gleason scores, we discovered that the percentage of HLA-I loss increases with higher Gleason grade. Tumors with Gleason 4 + 3 (known to be more aggressive) have higher incidence of HLA-I/B2M, B2M and locus losses as compared to 3 + 4 tumors (Supplementary Table 3). We did not find such correlation with tumor stage (Supplementary Table 4).
In addition, tumor recurrence was more frequent among DR-negative tumors than in tumors with heterogeneous DR expression pattern (73 vs. 37 %, respectively) (Supplementary Table 2).
mRNA expression of B2M, APM, IRF-1, and NLRC5 in microdissected PC and BH tissues
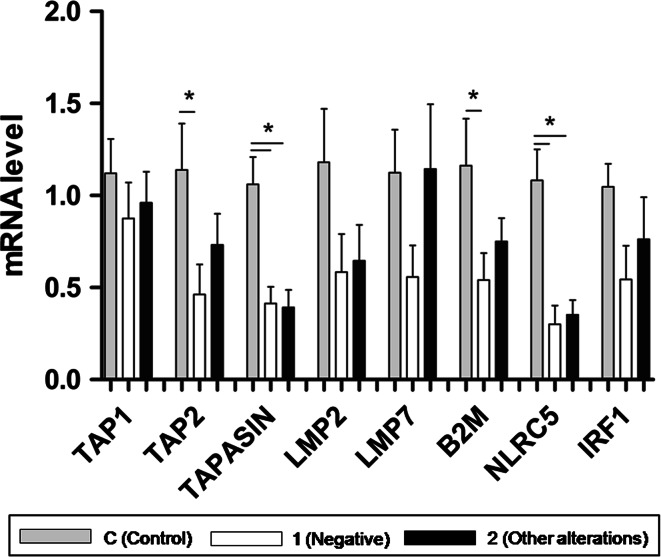
cDNA from microdissected tissues was analyzed for mRNA expression of B2M, Tap1, Tap2, tapasin, LMP2, LMP7, NLRC5 and IRF-1 genes. The results were compared among three groups: Control—benign hyperplasia; group 1—total HLA-I loss (combined negative and heterogeneous samples); and group 2—other types of HLA alterations (locus or allelic losses). A positive correlation was found between tumor HLA-I alterations and reduced transcriptional level of all studied genes in each group versus BH control. Notably, a statistically significant decrease in mRNA expression of B2M (p = 0.046), tapasin (p = 0.003), NLRC5 (p = 0.001) and TAP2 (p = 0.05) was found for tumors with total HLA-I loss (group 1) (Fig. 2), while transcriptional levels of NLRC5 (p = 0.003) and tapasin (p = 0.003) were significantly lower in group 2.
Fig. 2.
Correlation of the tumor HLA-I loss with the reduced mRNA expression of B2M, APM molecules, IRF-1 and NRLC5 in two groups of PC versus control BH (C). Tumor group 1—total HLA loss (combined negative and heterogeneous tumors); and group 2—other types of HLA alterations (locus or allelic losses). Statistically significant decrease in the expression of B2M (p = 0.046), tapasin (p = 0.003), NLRC5 (p = 0.001) and TAP2 (p = 0.05) was found in tumors with total HLA-I loss (group 1). In addition, mRNA expression levels of NLRC5 (p = 0.003) and tapasin (p = 0.003) were also significantly lower in group 2 than in control. *Statistically significant difference compared to control
Microsatellite analysis and B2M gene sequencing in prostate tumors
We performed microsatellite analysis of DNA isolated from 25 microdissected tumor samples to detect LOH-6 and LOH-15. LOH-6 was observed in 32 % (8/25) of the tumors, and only one showed LOH-15. Among 8 tumors with LOH-6, five had total HLA-I loss, one demonstrated allelic loss, while two tumors were positive for HLA-I (Table 3). We sequenced B2M gene in 11 selected microdissected tumors with total HLA-I loss (including tumors with heterogeneous expression pattern) but did not find aberrations in B2M gene (data not shown).
Analysis of HLA-I and HLA-II expression in cell lines derived from PC patients
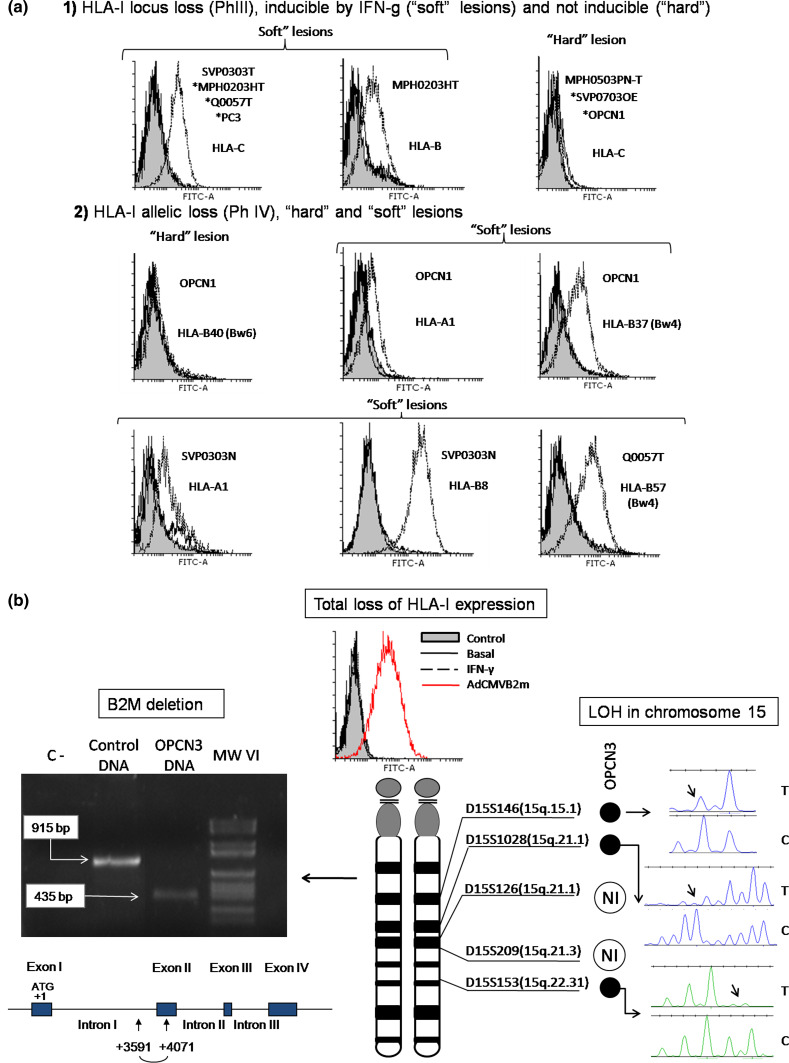
Using flow cytometry altered HLA-I and HLA-II expression phenotypes were observed in 12 previously unreported immortalized cell lines derived from tumor (T) and normal epithelium (N) of PC patients. Various types of HLA-I alterations were found in both types of cells, ranging from locus or allelic downregulation to a total loss of class I expression (Table 5), with both regulatory and structural molecular defects. HLA-I locus loss (PhIII) was found in six cell lines. MPH0203HT cells showed reversible downregulation of locus B and locus C (Fig. 3a1). IFN-inducible locus C loss was detected in SVP0303-T, Q0057T, MPH0203HT cells and in PC3 cells (ATCC), while locus C downregulation in MPH0503PN-T, SVPO703OE, and OPCN1 cells was resistant to IFN-γ, suggesting a structural defect. Three cell lines demonstrated allelic losses (PhIV) (Fig. 3a2). OPCN1 showed downregulation of both HLA alleles: HLA-B37 loss was inducible, while HLA-B40 loss was resistant to IFN-γ. SVP0303N (N) cells demonstrated reversible loss of HLA-A1 and HLA-B8. Inducible B-57 loss was detected in Q0057T cells. In OPCN3 cells, we found a total loss of HLA-I expression (PhI), not inducible by IFN-γ (Table 5; Fig. 3b).
Table 5.
Constitutive and IFN-γ inducible cell surface HLA expression and genomic typing of prostate cell lines
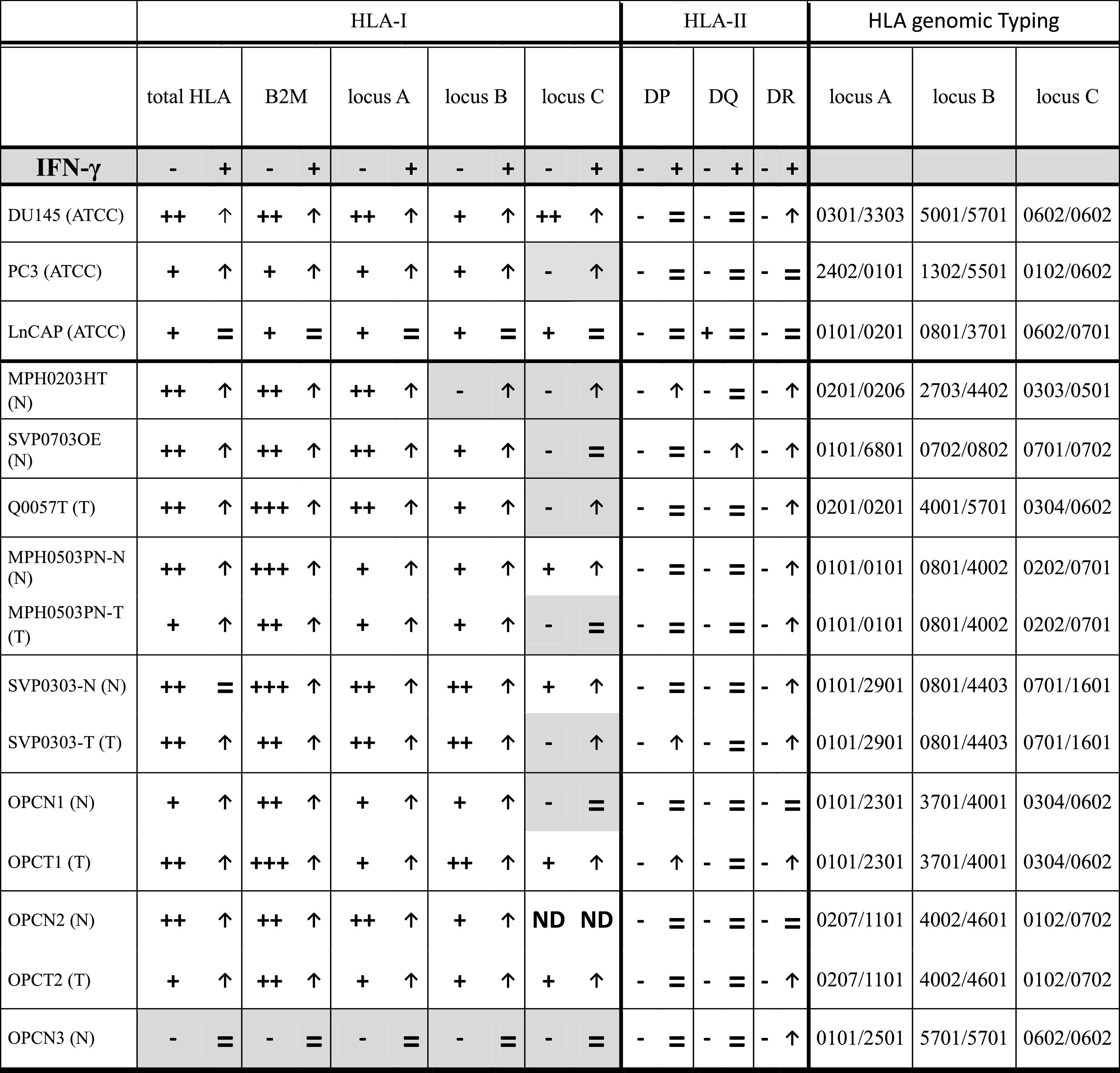
HLA expression was measured by flow cytometry in baseline conditions and after incubation with IFN-γ using several antibodies directed against different HLA specificities. MFI has been normalized using an isotype control
– no expression (MFI 0–1), + weak expression (MFI 1–10), ++ strong expression (MFI 10–100), +++ very strong expression (MFI > 100)
↑ IFN-γ-induced HLA upregulation, = resistance to IFN-γ, no HLA upregulation, ND not done
(N)—cells derived from normal prostatic epithelium of PC patients
(T)—cells derived from malignant prostatic epithelium of PC patients
Fig. 3.
a Different patterns of HLA-I loss resistant to IFN-γ (“hard” lesions) or inducible by IFN-γ (“soft” lesions) in PC cell lines determined by FACS. (1) Irreversible locus C loss and IFN-γ-inducible downregulation of locus C and B (PhIII); (2) irreversible loss of HLA-B40 allele and IFN-γ-inducible downregulation of A1, B37, B57 and B8 (Ph IV). Gray fill—only secondary Ab control, solid line—baseline conditions, dashed line—IFN-γ treatment. mAb used: w6/32 (HLA-I/B2M complex), 421B5 (locus B), Hb122 (HLA-A3), Marb3 (Bw4 specificities) and HB152 (Bw6 specificities). b Total loss of HLA-I expression in OPCN3 cells caused by a deletion in B2M gene and LOH-15. FACS histogram demonstrating loss of HLA-I cell surface expression (w6/32 mAb) not inducible by IFN-γ. Adenovirus vector coding for human B2M gene recovers HLA-I expression. Gray fill—control; solid line—baseline expression; dotted line—IFN-γ treatment; dashed line—after AdCMVB2M transfection. PCR amplification of B2M gene fragment (including intron 1 and exon 2) using DNA from OPCN3 cells. B2M amplicon from OPCN3 cells is smaller than in control; sequencing of the amplicon showed a 480 pb microdeletion that includes the first half of exon 2 (schematic representation). Loss of heterozygosity at chromosome 15 was determined by microsatellite analysis using five specific markers flanking B2M region. (NI not informative reading). *Representative FACS histogram for several cell lines
Constitutive HLA-DP, HLA-DQ and HLA-DR expression was negative in all studied cell lines. IFN-γ induced HLA-DR expression in seven cell lines, DR and DP in three cell lines, and DR and DQ in one cell line (Table 5).
HLA expression in three cell lines from ATCC, PC-3, DU-145 LnCAP, was previously analyzed by our group and others. These cell lines are widely used by many research groups and have been studied from various standpoints. In comparison with DU145 and PC3 cells, LnCAP showed a very low expression level with weak response to IFN-γ. In addition, DU145 cells lack HLA-A3 allele, due to a mutation [22].
Total loss of HLA-I expression in OPCN3 cells caused by B2M gene alterations
Total loss of HLA-I found in OPCN3 cells derived from normal prostatic epithelium of a PC patient was not inducible by IFN-γ, but was recovered after adenovirus-mediated B2M gene transfer (Fig. 3b). Amplification of the DNA isolated from these cells produced a short fragment, approximately 500 pb smaller than in the control (Fig. 3b). Sequencing of the fragment showed a 480 pb microdeletion between nucleotides +3591 and +4071, including first half of exon 2 (Fig. 3b). This deletion affects exon 2 and generates a premature stop codon producing a truncated protein. In addition, microsatellite analysis showed LOH-15 for three specific markers in the long arm (q) of chromosome 15, pointing to a macrodeletion in this region (Fig. 3b). Hence, the total loss of HLA-I expression in this cell line is caused by two structural defects in B2M gene, a microdeletion in one gene copy and a chromosomal loss (LOH-15) affecting another copy.
HLA-I cell surface expression on cell lines correlates with transcriptional levels of HLA-I and APM genes
We analyzed the mRNA expression of the following genes: HLA-I heavy chain, B2M, TAP1, TAP2, tapasin, LMP2, LMP7, calreticulin, calnexin, IRF-1 and NLRC5. Protein and mRNA expression patterns of HLA-I loci coincided in the majority of the studied cell lines with frequently observed increased expression of calreticulin. Interestingly, LnCAP cells showed a coordinated downregulation of all studied APM and HLA-I genes, as well as of the transcriptional factors NLRC5 and IRF-1 (Supplementary Table 5).
Discussion
In the present study using tumor immunohistochemistry, we observed a high percentage (88 %) of HLA-I alterations in cryopreserved PC tumors as compared to HLA-I-positive BH or normal matching prostate epithelium. Half of the studied tumors showed total loss of HLA-I expression (PhI) associated with a tendency to increased tumor recurrence, high D’Amico cancer risk, and PNI. Interestingly, tumors with Gleason 4 + 3 (known to be more aggressive) showed higher incidence of HLA-I/B2M, B2M and locus losses as compared to 3 + 4 tumors. HLA-I downregulation linked to a higher tumor grade was previously reported in different types of cancer [23, 24]. Reduced HLA-I heavy chain expression in PC has been previously reported to correlate with tumor relapse, while loss of calnexin and tapasin has been linked to early recurrence and aggressive tumor phenotype [9]. In our study, we also observed a significant association between total HLA-I loss and reduced mRNA expression of APM molecules, including tapasin, TAP2, B2M, and NLRC5, while tumors with other types of HLA-I alteration showed only a strong tendency toward this correlation. Reduced expression of NLRC5, a transcription factor known to regulate HLA expression [25], suggests a possible defect in the coordinated regulation of HLA expression.
Total loss of HLA-I expression associated with structural genetic aberrations, such as B2M mutations, has been reported in melanoma and in colorectal carcinoma with microsatellite instability [6, 7, 21, 26]. However, we did not find any defects in B2M gene in the microdissected tumors. Even though 50 % of the tumors positively expressed cell surface HLA-I/B2M complex, many of them demonstrated locus or allelic HLA-I losses. In addition, we detected LOH-6 in about 32 % of the studied tumors, suggesting an accumulation of chromosomal loss in HLA-I genes linked to HLA haplotype loss. A similar frequency of LOH-6 was previously reported in other types of malignancy [27]. Loss of a single functional HLA haplotype, locus, or allele required to present a particular antigen to CTLs may be responsible for the failure of immune surveillance to detect tumor cells.
The overall frequency of HLA-I aberrations and of total loss of expression in this study is similar to that found in other types of malignancy [28] and is higher than in earlier reports on PC. The published incidence of HLA-I loss in prostate tumors varies depending on the tissue processing method and antibodies used. Thus, HLA-I heavy chain expression in paraffin-embedded tumors was found to be retained in some studies [9], whereas others reported loss of expression in 50 % [29], 74 % [30], and 90 % [31] of cases. Analysis of HLA-I expression in cryopreserved prostate tumors allows more detailed analysis; thus, when individual allelic expression was assessed, 85 % of the primary PCs and 100 % of the metastases have shown either a complete lack or a reduced expression of class I molecules [32]. In contrast, Nanda and colleagues reported that all 27 studied cryopreserved human prostate tumors were HLA-I-positive, although they used a different primary antibody [33].
HLA-DR expression was negative in all BH and majority of prostate tumors. Approximately 22 % of tumors showed a heterogeneous HLA-DR labeling pattern, which appeared to be associated with decreased relapse and lesser PNI. De novo class II expression has been associated with a more aggressive phenotype in melanoma [34], but with a better prognosis in colorectal [35] and laryngeal carcinoma.
In the present study, loss of B2M expression was observed in 50 % of the HLA-I negative tumors versus 65 % reported by Seliger et al. and 24.8 % by Kitamura and co-authors and was correlated with poor prognosis. Moreover, we found that B2M mRNA expression was significantly lower in tumors totally negative for HLA-I. However, the role of B2M expression in PC is controversial. Some groups associate B2M downregulation with bone metastasis [36], whereas others correlated elevated B2M expression with clinically aggressive PC [37]. It has also been proposed that the downregulation of prostate tumor HLA-I increases the secretion of free soluble B2M, leading to increased serum and urine levels [38], which have been correlated with clinical stage, Gleason grade, PSA, distant metastasis, and therapeutic efficacy [39]. Some reports suggest that soluble B2M is a growth-promoting factor in hematological malignancy and PC, and anti-B2M blocking antibody has been proposed as a treatment for myeloma and PC and has been evaluated in in vivo models [40]. However, this approach could impair anti-tumor T cell activity, because HLA-positive tumor cells are usually successfully eliminated by CTLs, while HLA-negative cells can escape immune surveillance and disseminate. Although we did not see any B2M gene mutations/deletions in microdissected primary prostate tumors and only one tumor had LOH-15, we found a B2M mutation and LOH-15 causing a total loss of HLA-I expression in one prostate cell line, OPCN3, derived from normal prostatic epithelium of a PC patient. Mutation/deletions in B2M gene linked to total HLA-I loss have been described in many types of cancer (especially melanoma and colorectal cancer) with a mutational “hot-spot” detected in the CT repeat region of exon 1 reflecting an increased genetic instability in this region [6, 7, 21, 27]. The presence of irreversible “hard” HLA lesions resistant to cytokines could represent a serious obstacle to the efficacy of PC immunotherapy [41]. The studied cell lines also displayed various allele- and locus-specific HLA-I losses, in some cases resistant to IFN-γ (mostly locus C). Constitutive HLA-DP, HLA-DQ and HLA-DR expressions were negative in all studied cell lines but were inducible by IFN-γ in some cases. Expression of HLA-II has been shown to be lacking on human PC cells, while upregulation of class II molecules on prostate tumor cells have been reported by others [42]. Interestingly, we detected HLA alterations both in malignant cells and in cells derived from normal epithelium of PC patients, which could be attributable to the immortalization of the cells with HPV, since it has been described that E6/E7 immortalization is able to cause chromosomal instability. Interestingly, other reports also provide an evidence suggesting that HLA-I expression in melanoma cells can be higher than in normal epidermal keratinocytes [43], although a very high percentage of melanoma primary tumors and metastatic lesions are known to have altered HLA-I expression.
Characterization of the nature of molecular defects causing HLA altered expression is important in patient selection for immunotherapy. However, current knowledge on HLA-I expression in human PC cell lines is very incomplete, partially due to a limited availability. In addition, novel human PC cell lines with characterized HLA-I expression can be useful in research related to HLA loss in cancer immune escape, providing an in vitro model for cancer immunotherapy or gene therapy aimed to recover normal HLA expression. Many studies have used LNCaP and DU145 as target cells for T-cell immune response analysis, despite the fact that LNCaP has HLA-I downregulation caused by intracellular defect in the assembly of the HLA-I complex [44], while DU145 has HLA-A3 allelic loss due to a mutation [22]. Altered expression of HLA-I molecules can also be caused by reduced expression of APM proteins, but we did not see it in the studied cell lines (even in immortalized ones), and this expression was inducible by IFN-γ (Supplementary Table 5).
In conclusion, our findings demonstrate that HLA-I altered expression is a very frequent event in human PC, ranging from total loss to reduced expression of single loci and alleles, and it is associated with more aggressive clinical course. Notably, a downregulation of the mRNA levels of HLA-I and some APM molecules was the most frequent molecular mechanism responsible for these alterations, although a significant number of the studied tumors (32 %) displayed “hard” lesions in a form of LOH-6, while one tumor had LOH-15. Our group previously reported a coordinated downregulation of HLA-I and APM molecules to be responsible for HLA-I loss in bladder carcinoma [45]. The present findings suggest that PC has a similar regulatory molecular mechanism of HLA loss. We could speculate that structural genetic aberrations are likely to be continuously accumulating during cancer progression and, together with the existing regulatory defects, may generate tumor escape variants with lower immunogenicity. Our findings suggest that an effective immunotherapy needs to bypass the selection and outgrowth of HLA-I-negative tumor cells. In addition, the marked differences in clinical responses among cancer patients receiving adoptive TILs treatment, peptide-based immunotherapy or antibodies blocking immune checkpoint inhibitors, highlight the need for further research into the role of HLA molecules in the resistance to therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Svitlana Zinchenko for technical assistance. This work was supported by grants co-financed by FEDER (Fondo Europeo de Desarollo Regional/European Regional Development Fund, European Union) and by ISCIII (Instituto de Salud Carlos III)-Subdireccion General de Evaluacion y Fomento de la Investigacion grants as a part of the Plan Nacional de I + D + I 2008–2011 (CP03/0111, PI12/02031, PI08/1265, PI11/01022, PI11/01386, RETIC RD 06/020, RD09/0076/00165) and Plan Nacional de I + D + I 2013–2016 (PT13/0010/0039, PI 14/01978); as well as by Junta de Andalucía (Groups CTS-143, CTS-695, CTS-3952, CVI-4740, and PI 09/0382 Grant); and by the European Union project ENACT (European Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumor immunology, LSHC-CT-2004-503306). N. Aptsiauri was supported by Miguel Servet and I3 SNS contracts from Fundación Progreso y Salud of the Junta de Andalucia and Instituto de Salud Carlos III. FJ. Carretero was supported by a pre-doctoral fellowship of the FPU program (Formación de profesorado Universitario) from the Ministry of Education of Spain.
Abbreviations
- APM
Antigen presentation machinery
- B2M
Beta-2-microglobulin
- BH
Benign prostate hyperplasia
- cDNA
Complementary DNA
- CTL
Cytotoxic T lymphocyte
- DNA
Deoxyribonucleic acid
- FACS
Fluorescence-activated cell sorter/sorting
- FCS
Fetal calf serum
- FITC
Fluorescein isothiocyanate
- HLA
Human histocompatibility leukocyte Ag
- HPV
Human papillomavirus
- HRP
Horseradish peroxidase
- IFN
Interferon
- IHC
Immunohistochemistry
- LOH
Loss of heterozygosity
- mAb
Monoclonal Ab
- MFI
Mean fluorescence intensity
- PC
Prostate cancer
- Ph
Phenotype
- PNI
Perineural invasion
- PSA
Prostate-specific antigen
- RT-PCR
Reverse transcriptase polymerase chain reaction
Compliance with ethical standards
Conflict of interest
The authors declare that they have no financial or commercial conflict of interest.
References
- 1.Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL. Natural history of HLA expression during tumour development. Immunol Today. 1993;14:491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 2.Aptsiauri N, Cabrera T, Garcia-Lora A, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol. 2007;256:139–189. doi: 10.1016/S0074-7696(07)56005-5. [DOI] [PubMed] [Google Scholar]
- 3.Shen Y, Xia M, Zhang J, Xu L, Yang J, Chen A, Miao F, Ferrone S, Xie W. IRF-1 and p65 mediate upregulation of constitutive HLA-A antigen expression by hepatocellular carcinoma cells. Mol Immunol. 2009;46:2045–2053. doi: 10.1016/j.molimm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- 5.Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar JJ, López-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/S0167-5699(96)10075-X. [DOI] [PubMed] [Google Scholar]
- 6.Paschen A, Méndez RM, Jimenez P, Sucker A, Ruiz-Cabello F, Song M, Garrido F, Schadendorf D. Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer. 2003;103:759–767. doi: 10.1002/ijc.10906. [DOI] [PubMed] [Google Scholar]
- 7.Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61:1359–1371. doi: 10.1007/s00262-012-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitale M, Rezzani R, Rodella L, Zauli G, Grigolato P, Cadei M, Hicklin DJ, Ferrone S. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–742. [PubMed] [Google Scholar]
- 9.Seliger B, Stoehr R, Handke D, Mueller A, Ferrone S, Wullich B, Tannapfel A, Hofstaedter F, Hartmann A. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother. 2010;59:529–540. doi: 10.1007/s00262-009-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feenstra M, Veltkamp M, van Kuik J, Wiertsema S, Slootweg P, van den Tweel J, de Weger R, Tilanus M. HLA class I expression and chromosomal deletions at 6p and 15q in head and neck squamous cell carcinomas. Tissue Antigens. 1999;54:235–245. doi: 10.1034/j.1399-0039.1999.540304.x. [DOI] [PubMed] [Google Scholar]
- 11.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 12.del Campo AB, Aptsiauri N, Méndez R, Zinchenko S, Vales A, Paschen A, Ward S, Ruiz-Cabello F, González-Aseguinolaza G, Garrido F. Efficient recovery of HLA class I expression in human tumor cells after beta2-microglobulin gene transfer using adenoviral vector: implications for cancer immunotherapy. Scand J Immunol. 2009;70:125–135. doi: 10.1111/j.1365-3083.2009.02276.x. [DOI] [PubMed] [Google Scholar]
- 13.del Campo AB, Carretero J, Aptsiauri N, Garrido F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue Antigens. 2012;79:147–154. doi: 10.1111/j.1399-0039.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 14.Del Campo AB, Carretero J, Muñoz JA, Zinchenko S, Ruiz-Cabello F, González-Aseguinolaza G, Garrido F, Aptsiauri N. Adenovirus expressing β2-microglobulin recovers HLA class I expression and antitumor immunity by increasing T-cell recognition. Cancer Gene Ther. 2014;21:317–332. doi: 10.1038/cgt.2014.32. [DOI] [PubMed] [Google Scholar]
- 15.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama J Am Med Assoc. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 17.Carretero R, Cabrera T, Gil H, Saenz-Lopez P, Maleno I, Aptsiauri N, Cozar JM, Garrido F. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int J Cancer. 2011;129:839–846. doi: 10.1002/ijc.25733. [DOI] [PubMed] [Google Scholar]
- 18.Garrido FCT, Accolla RS, Bensa JC, Bodmer W, Dohr G, Drouet M, Fauchet RFG, Ferrone S, Giacomini P, Kageshita T, Koopman L, Maio MMF, Mazzilli C, Morel PA, Murray A, Crh Papasteriades, Salvaneschi L, Stern PL, Ziegler A. 12th International Histocompatibility Conference. Genetic diversity of HLA: functional and medical implications. Hum Immunol. 1996;47:1–184. doi: 10.1016/S0198-8859(96)85602-3. [DOI] [PubMed] [Google Scholar]
- 19.Méndez R, Rodríguez T, Del Campo A, Monge E, Maleno I, Aptsiauri N, Jiménez P, Pedrinaci S, Pawelec G, Ruiz-Cabello F, Garrido F. Characterization of HLA class I altered phenotypes in a panel of human melanoma cell lines. Cancer Immunol Immunother. 2008;57:719–729. doi: 10.1007/s00262-007-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maleno I, Romero JM, Cabrera T, Paco L, Aptsiauri N, Cozar JM, Tallada M, López-Nevot MA, Garrido F. LOH at 6p21.3 region and HLA class I altered phenotypes in bladder carcinomas. Immunogenetics. 2006;58:503–510. doi: 10.1007/s00251-006-0111-8. [DOI] [PubMed] [Google Scholar]
- 21.del Campo AB, Kyte JA, Carretero J, Zinchencko S, Méndez R, González-Aseguinolaza G, Ruiz-Cabello F, Aamdal S, Gaudernack G, Garrido F, Aptsiauri N. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int J Cancer. 2014;134:102–113. doi: 10.1002/ijc.28338. [DOI] [PubMed] [Google Scholar]
- 22.Jiménez P, Cabrera T, Méndez R, Esparza C, Cozar JM, Tallada M, López-Nevot MA, Ruiz-Cabello F, Garrido F. A nucleotide insertion in exon 4 is responsible for the absence of expression of an HLA-A*0301 allele in a prostate carcinoma cell line. Immunogenetics. 2001;53:606–610. doi: 10.1007/s002510100371. [DOI] [PubMed] [Google Scholar]
- 23.Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83(83):117–158. doi: 10.1016/S0065-230X(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 24.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neerincx A, Rodriguez GM, Steimle V, Kufer TA. NLRC5 controls basal MHC class I gene expression in an MHC enhanceosome-dependent manner. J Immunol. 2012;188:4940–4950. doi: 10.4049/jimmunol.1103136. [DOI] [PubMed] [Google Scholar]
- 26.Kloor M, Michel S, Buckowitz B, Rüschoff J, Büttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, Schwitalle Y, Kuchenbuch B, von Knebel Doeberitz M. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 27.Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, López-Nevot MA, Garrido F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63:65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 28.Aptsiauri N, García-Lora AM, Garrido F. ‘Hard’ and ‘soft’ loss of MHC class I expression in cancer cells. In: Rees R, editor. Tumor immunology and immunotherapy. Oxford: Oxford University Press; 2014. pp. 63–78. [Google Scholar]
- 29.Zhang H, Melamed J, Wei P, Cox K, Frankel W, Bahnson RR, Robinson N, Pyka R, Liu Y, Zheng P. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003;3:2. [PubMed] [Google Scholar]
- 30.Kitamura H, Torigoe T, Asanuma H, Honma I, Sato N, Tsukamoto T. Down-regulation of HLA class I antigens in prostate cancer tissues and up-regulation by histone deacetylase inhibition. J Urol. 2007;178:692–696. doi: 10.1016/j.juro.2007.03.109. [DOI] [PubMed] [Google Scholar]
- 31.Lu QL, Abel P, Mitchell S, Foster C, Lalani EN. Decreased HLA-A expression in prostate cancer is associated with normal allele dosage in the majority of cases. J Pathol. 2000;190:169–176. doi: 10.1002/(SICI)1096-9896(200002)190:2<169::AID-PATH517>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL (1995) Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology 46: 681–686; discussion 686–687 [DOI] [PubMed]
- 33.Nanda NK, Birch L, Greenberg NM, Prins GS. MHC class I and class II molecules are expressed in both human and mouse prostate tumor microenvironment. Prostate. 2006;66:1275–1284. doi: 10.1002/pros.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, Reali UM, Giannotti B. In situ expression of transforming growth factor beta is associated with melanoma progression and correlates with Ki67, HLA-DR and beta 3 integrin expression. Melanoma Res. 1997;7:313–321. doi: 10.1097/00008390-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S, Spagnoli GC, Lauro D, Lugli A, Han J, Iezzi G, Ferrone C, Ferlosio A, Tornillo L, Droeser R, Rossi P, Attanasio A, Ferrone S, Terracciano L. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014;16:31–42. doi: 10.1593/neo.131568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, Pohl J, Hsieh CL, Weitzmann MN, Farach-Carson MC, Chung LW. Beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 37.Mink SR, Hodge A, Agus DB, Jain A, Gross ME. Beta-2-microglobulin expression correlates with high-grade prostate cancer and specific defects in androgen signaling. Prostate. 2010;70:1201–1210. doi: 10.1002/pros.21155. [DOI] [PubMed] [Google Scholar]
- 38.Abdul M, Hoosein N. Changes in beta-2 microglobulin expression in prostate cancer. Urol Oncol. 2000;5:168–172. doi: 10.1016/S1078-1439(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YX, Wang L, Ji PY, Zhao GG, Zhong GP, Wang ZP. Correlation of serum β2-microglobulin levels with prostate-specific antigen, Gleason score, clinical stage, tumor metastasis and therapy efficacy in prostate cancer. Arch Med Res. 2013;44:259–265. doi: 10.1016/j.arcmed.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Yi Q. Killing tumor cells through their surface beta(2)-microglobulin or major histocompatibility complex class I molecules. Cancer. 2010;116(7):1638–1645. doi: 10.1002/cncr.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aptsiauri N, Carretero R, Garcia-Lora A, Real LM, Cabrera T, Garrido F. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol Immunother. 2008;57:1727–1733. doi: 10.1007/s00262-008-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, Moy P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate. 1997;33:233–239. doi: 10.1002/(SICI)1097-0045(19971201)33:4<233::AID-PROS2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Boegel S, Löwer M, Bukur T, Sahin U, Castle JC. A catalog of HLA type, HLA expression, and neo-epitope candidates in human cancer cell lines. Oncoimmunology. 2014;3(8):e954893. doi: 10.4161/21624011.2014.954893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlsson B, Tötterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigen TARP. Prostate. 2004;61:161–170. doi: 10.1002/pros.20091. [DOI] [PubMed] [Google Scholar]
- 45.Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta 2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–610. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.