Abstract
Dendritic cell (DC) vaccine has been used to treat patients with advanced colorectal cancer (CRC). The results of vaccine-induced clinical responses have not always been satisfactory partially because of DC incompetence. In order to evaluate the feasibility of novel mature DCs for therapeutic adjuvants against CRC, we conducted clinical trials with carcinoembryonic antigen (CEA) peptide-loaded DC quickly generated with a combination of OK432 (Streptococcuspyogenes preparation), prostanoid, and interferon-α (OPA-DC). In the ten patients enrolled in this study, the OPA-DC vaccine was well tolerated and administered four times every 2 weeks except for two patients, who were switched to other treatments due to disease progression. Among the eight evaluable patients, one displayed stable disease (SD), while the remaining seven showed progressive disease (PD). In the SD patient, natural killer (NK) cell frequency and cytolytic activity were increased. In the same patient, the frequency of CEA-specific cytotoxic T cells (CTLs) increased stepwise with repetitive vaccinations; however, most of the CTLs exhibited central memory phenotype. In those with PD, NK cells proliferated well regardless of failure of response, whereas CTLs failed to do so. We concluded that the OPA-DC vaccine is well tolerated and has immune-stimulatory capacity in patients with CRC. Additional modulation is needed to attain significant clinical impact.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1051-1) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cell, Vaccine, Cancer, Clinical study
Introduction
Colorectal cancer (CRC) is one of the most intractable malignant diseases causing the death of 600 thousand people annually around the world. Recently, the development of new chemotherapeutic regimens including molecular-targeted drugs has significantly improved the clinical outcomes of patients with CRC [1]. However, the severe adverse effects of chemotherapeutic agents often deteriorate their quality of life (QOL), thus limiting continuation of therapy at full dose. The development of better therapies against CRC is needed.
Dendritic cells (DCs) are the most potent antigen-presenting cells that enhance innate and adaptive immune reactions. Previous studies have demonstrated that antigen-loaded DC is one of the most promising candidates for a therapeutic vaccine to induce tumor-specific immune reactions and preferable clinical responses in cancer patients with limited side effects. However, less than 10% of vaccinated patients showed favorable clinical responses [2, 3]. One of the primary reasons for such unsatisfactory results may be that the DCs have not been fully exploited to induce anti-tumor immunity.
Previous DC-vaccine studies have shown that mature DCs are better than immature ones for inducing anti-tumor responses in patients. The protocols of maturation stimuli are yet to be standardized. Although a combination stimulus using interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and prostaglandin (PG)-E2 is widely used as a monocyte-conditioned medium (MCM) mimic for monocyte-derived DCs (MoDCs), it lacks the ability to promote DCs to secrete IL-12p70 [4], a well-known enhancer of cytotoxic activity of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) [5, 6]. From the mechanistic point of view, DCs loaded with antigens migrate into draining lymph nodes, where they produce IL-12p70 and activate NK cells, prime type-1 helper-T (Th1) cells and CTLs [7, 8]. We have previously reported that novel mature DCs (OPA-DCs) more effectively exert such functions than MCM-mimic DCs, which can be generated even from monocytes of refractory cancer patients within 3 days by using a combination of OK432 (Streptococcus pyogenes preparation), low-dose prostanoid, and interferon (IFN)-α [9] (OPA). In order to evaluate the tolerability and the clinical or immunological responses of OPA-DC vaccine, we conducted a phase I/II clinical study of OPA-DC vaccine in patients with CRC, which shed light on the importance of NK cells and the limitations of CTL differentiation even with fully matured DC.
Subjects and methods
Subjects
Patients with advanced CRC (stage IV) who had been followed in or referred to Osaka University Hospital were enrolled as candidates in this clinical trial. The eligibility and exclusion criteria are summarized in Table 1. Among them, ten patients were selected. Their clinical backgrounds are given in Table 2.
Table 1.
Eligibility criteria for the enrollment of patients with colorectal cancer for receiving quickly matured dendritic cell vaccine
| (1) Eligibility criteria | (a) Patients with colon cancer (stage IV) |
| (b) Performance status: 0–2 | |
| (c) Age: 20–75 years | |
| (d) Tolerability for apheresis | |
|
(e) Stability in bone marrow, liver, and renal functions: 2,000 < WBC (/μl) < 15,000, Plt (/μl) > 75,000, AST and ALT (IU/I) < 150, T-bil (mg/dl) < 2.0, Crn < 2.0 | |
| (f) Acquired informed consent | |
| (g) Having metastatic lesions for the assessment of therapeutic efficacy | |
| (h) More than 4 weeks have passed from previous anti-cancer treatments | |
| (i) HLA-A*2402 positive | |
| (j) Increased serum CEA level: >5 ng/ml | |
| (k) Positivity for CEA in cancer tissues | |
| (2) Exclusion criteria | (a) Pregnant or lactating woman |
| (b) Severe bleeding tendency: PT < 50%, APTT > 60 s, fibrinogen < 100 mg/dl, FDP > 20 μg/ml | |
| (c) Patient with infectious diseases (HIV, HBV, HCV, HTLV, RPR) | |
| (e) Patient with autoimmune disorders | |
| (f) Patient who needs to take steroids or immunosuppressive drugs during treatment | |
| (g) Patient who has uncontrollable metastatic brain or intrathecal tumors | |
| (h) Patient whom the doctors define as inappropriate |
Table 2.
Clinical characteristics and outcomes of ten patients enrolled in this study
| Case | Gender | Age | Metastases | CEA (ng/ml)a | Total tumor volume (cm3)a | Administrated DCs (mean) (×106/injection) | DC injection time | CEA decline | RECIST |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 62 | Lung | 111 | 4.7 | 106.7 | 8 | + | SD |
| 2 | Female | 64 | Lung, liver | 647 | 590.8 | 11.2 | 4 | − | PD |
| 3 | Female | 63 | Lung, liver | 987 | 603.3 | 19.2 | 4 | − | PD |
| 4 | Male | 60 | Lung, liver | 631 | 694.4 | 121.1 | 4 | − | PD |
| 5 | Female | 48 | Lung, liver, peritoneal dissemination | 482 | Difficult to measureb | Withdrawn | 2 | Withdrawn | |
| 6 | Male | 65 | Liver | 925 | 64.7 | 51.6 | 4 | + | PD |
| 7 | Female | 59 | Liver, pancreas | 692 | 543.7 | 115.0 | 4 | − | PD |
| 8 | Female | 42 | Lung, liver | 12 | 110.0 | 36.6 | 4 | − | PD |
| 9 | Female | 32 | Lung, liver, abdominal wall, LN | 402 | 163.7 | Withdrawn | 2 | Withdrawn | |
| 10 | Male | 49 | Lung, abdominal LN | 612 | 19.1 | 35.225 | 4 | − | PD |
a Before vaccination
b Peritoneal disseminations are too small to measure their diameter
Study design
The current clinical trial was performed to evaluate tolerability as well as immune and clinical responses; it has been registered with the University-hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), under the code of UMIN000000743. The protocol was approved by the ethical committee of the Osaka University Graduate School of Medicine and also reviewed by the Translational Research Review Board, Osaka University Hospital. Standard operating procedures for manufacturing OPA-DCs were reviewed by the Medical center for Translational Research (MTR). Every detailed procedure described in the Manufacturing Instructions and Records was programmed to automatic process-control software to avoid operational errors. All patients gave written informed consent prior to the treatment.
The patients were vaccinated four times every 2 weeks in conformity with the regulation in previous DC vaccine [10–12]. In order to collect peripheral blood mononuclear cells (PBMCs) as a DC source, they underwent leukocyte apheresis at the first day of each session. After OPA-DCs had been generated as described in the next paragraph, they were administered subcutaneously at bilateral groin sites. Blood samples were collected before and 2 weeks after DC injection for the purpose of immunological and biochemical tests. Serum carcinoembryonic antigen (CEA) levels were determined on the day of blood collection. Two weeks after the last vaccination, the clinical response was evaluated by the changes in tumor size measured on computerized tomography (CT) scans, based on the Response-Evaluation Criteria In Solid Tumors (RECIST) [13]. Toxicity was scored occasionally according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v. 3.0. If severe toxicities of grade 3 or 4 developed, the patient was withdrawn from the study and switched to other appropriate treatment. A successive series of vaccinations could be applied to the patients evaluated as achieving a partial response (PR) or a stable disease (SD) condition based on the RECIST criteria.
Preparation of quickly inducible mature dendritic cells (OPA-DCs)
The OPA-DCs were generated as reported previously with some modifications [9]. At least 5 × 108 PBMCs were recovered via leukapheresis (Amicus) (Baxter, Deerfield, IL) from the patients. The PBMCs were incubated at a concentration of at least 5 × 106 cells/ml with serum-free AIM-V in 225 cm2 culture flasks (Iwaki, Japan) for 2–3 h at 37°C in 5% CO2. Subsequently, these flasks were washed with saline in order to separate monocytes by means of their preferential adherence to plastic [9]. Briefly, monocytes were cultured with 50 ng/ml GM-CSF and 20 ng/ml IL-4 for 3 days at 37°C in 5% CO2. During the final 24 h, the cells were matured with 0.1 KE/ml OK432, 500 IU/ml IFN-α, and 50 ng/ml PG-E1. Concomitantly, 20 μg/ml CEA.652(9) was loaded to the cells. On day 3 of culture, non-adherent cells (OPA-DCs) were harvested. These procedures were performed in the cell-processing center affiliated with MTR under a clean condition according to the guidelines for translational research using human stem cells by Japanese Ministry of Health, Welfare and Labor.
Quality assessment of DC injections
During the DC-preparation process, samples of the culture supernatant were collected. No bacterial contamination was confirmed by means of their endotoxin measurement. Before handling the OPA-DC injections, we examined their viability and cell number using 0.3% trypan-blue staining. Viability was assessed as the percentage of viable cells among all countable cells. All the final products were confirmed as having at least 70% viability and containing at least 3.0 × 106 viable cells in conformity with the regulation in previous DC vaccine [10–12]. The purity of DCs was also examined. Briefly, the cells sampled from each final product were stained with fluorescent-material-conjugated anti-CD14 monoclonal antibody (mAb), anti-CD11c mAb, and anti-HLA-DR mAb. They were analyzed on a FACS Caliber (BD, Franklin Lakes, NJ). The live cells, except for lymphoid cells, were gated at forward scatter versus side scatter plot. Subsequently, the percentage of the CD14-/CD11c+/HLA-DR+ cells among the gated cells was analyzed as the purity. All final products were confirmed as having at least 70% purity.
Reagents
Recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 were purchased from Primmune Inc., Japan. OK432 (Picibanil®) was purchased from Chugai, Japan. The amount of OK432 is expressed in units designated as KE (Klinische Einheit [clinical unit]). One KE OK432 is equivalent to 0.1 mg dry streptococci. Natural human IFN-α (OIF®) was purchased from Otsuka, Japan. PG-E1 (Prostandin®) was purchased from Ono, Japan. The 9-mer peptide (CEA.652(9): TYACFVSNL), reported to be a human leukocyte antigen (HLA)-A*2402 restricted CTL epitope in CEA [9], was purchased from TaKaRa Bio (Japan) or Mimotope-Genzyme Pharmaceuticals (Switzerland). Therapeutic grade medium (AIM-V) was purchased from Invitrogen (Carlsbad, CA).
Fluorescent antibodies and HLA/peptide-pentamer
Fluorescein-isothiocyanate (FITC)-conjugated LineageCocktail 1, anti-CD14 mAb (M5E2), anti-CD3 mAb (UCHT1), anti-CD4 mAb (RPA-T4), anti-CD27 mAb (M-T271), and anti-CD45RO mAb (UCHL1) were purchased from Becton–Dickinson (BD), Franklin Lakes, NJ. FITC-conjugated anti-CCR7 mAb (150503) was purchased from R&D Systems, McKinley Place, NE. Phycoerythrin (PE)-conjugated anti-CCR4 mAb (1G1), anti-CD4 mAb (RPA-T4), anti-CD45RA mAb (HI100), and anti-CD69 mAb (FN50) were obtained from BD. PE-conjugated anti-FoxP3 mAb (PCH101) was obtained from eBiosciences, San Diego, CA. PE-conjugated anti-CXCR3 mAb (49801) was purchased from R&D Systems. Peridinin-chlorophyll-protein-complex (PerCP)-conjugated anti-CD4 mAb (SK3), anti-CD8 mAb (SK1), and anti-HLA-DR mAb were purchased from BD. Allophycocyanin (APC)-conjugated anti-CD11c mAb (B-ly6) and anti-CD56 mAb (B159) were obtained from BD. APC-conjugated anti-CD4 mAb (13B8.2) was purchased from Beckman Coulter Inc., Fullerton, CA. PE-conjugated HLA-A*2402/CEA.652(9)-pentamer (CEA(24)-pentamer) and HLA-A*2402/EBV peptide (HLA-A*2402 restricted peptide derived from Epstein-Bar virus (EBV) LMP2: TYGPVFMSL)-pentamer were purchased from ProImmune Ltd., Bradenton, FL.
Cell lines
The NK-cell-sensitive cell line (K562) was obtained from ATCC (Manassas, VA). T2-A24 is a transporter associated with an antigen processing (TAP) deficient cell line (T2) transfected with HLA-A*2402 gene. This cell line expresses a high level of HLA-A24 protein and is used for targets in cytotoxicity assay. K562 and T2-A24 were cultured in RPMI-1640 containing 10% fetal calf serum (FCS), 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2.
Calculation of total tumor volume
We calculated the total tumor volume of each patient before vaccination. Maximum diameters (D1, D2, …) were measured for all visible tumors in CT images from the neck to the perineum, and total tumor volume was calculated as follows:
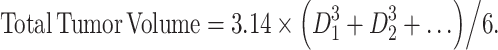 |
Assessment of immune responses
Frequency analyses of immune cells
PBMCs derived from blood samples were stained with fluorescent-material conjugated antibodies or a CEA(24)-pentamer. We analyzed the frequency of NK, Th1, Th2, FoxP3+ regulatory CD4+ T cells (Treg), and CEA.652(9)-specific CTLs on a FACS canto II (BD). For the analysis of Tregs, the cells were permeabilized with the Human FoxP3 Buffer Set (BD). Th1 and Th2 cells were detected as CD4+/CD45RO+/CXCR3+ and CD4+/CD45RO+/CCR4+ cells, respectively [14]. NK cells were characterized as CD3−/CD56+ cells and their active phenotypes were assessed by the expression of CD69. The ratios of CD69+ NK-cell frequency before and 2 weeks after every DC injection were calculated as the CD69+ NK variation rate (VR). We analyzed FoxP3+ Tregs as CD4+/CD45RO+/CD25high/Foxp3+ cells. Highly avid CTLs for CEA.652(9) were identified as CD8+/CEA(24)-pentamer+ cells. Additionally, the CTLs were subdivided into CCR7+/CD45RA- central memory cells, CCR7−/CD4RA− effector memory cells, and CCR7−/CD45RA+ terminal differentiated effector memory cells [15].
-
(2)
Analysis of NK cell activity
The cytotoxic ability of NK cells during vaccination was analyzed by flow-cytometric methods [16]. NK cells were separated from PBMCs using CD56 microbeads (Miltenyi Biotec). K562 cells were labeled with carboxyfluorescein succinimidyl ester (Invitrogen) and cultured with or without NK cells in 24-well culture plates (Falcon) for 12 h at 37°C in 5% CO2 (E/T ratio: 24/1). At the end of the incubation, the samples were put on ice and 50 μg/ml propidium iodide (SIGMA) was added for DNA labeling of the dead cells. The samples were then incubated for 10 min on ice and analyzed within 60 min using FACS Canto II (BD). The percentage of specific-target-cell death (cytotoxicity) was calculated as:
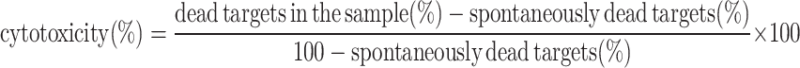 |
The representative plots of flow-cytometric analyses are given in Online Resource 1.
Statistical analysis
For the vaccinated patients, a comparison was made between plasma cytokine level and total tumor volume before vaccination using the Pearson’s product-moment correlation coefficient (r), which was analyzed with Prism 5 software (Graph Pad Software, San Diego, CA). A P value is the probability that an r value is zero. Therefore, a P value of less than 0.05 indicates that the two variables are correlated.
Results
Quality of the OPA-DC vaccine
The mean DC number administered at each vaccination is shown in Table 2. The viability and purity of DC from all patients were 91.1 ± 2.6% (mean ± SD) and 88.8 ± 4.28%, respectively. All products met the quality assessment of the OPA-DC vaccine except for one form Case 3 due to its lesser number.
Clinical outcomes
Eight of the ten patients enrolled in this study were able to receive at least one series of vaccinations. Two patients (Case 5 and 9) were withdrawn from the study because of ileus, respiratory distress, or obstructive jaundice of grade 3 accompanied by cancer progression. One patient (Case 1) displayed a clinical response of SD lasting 2 months from the initiation of vaccination. Two weeks after the first session, the diameter of the patient’s maximum lung metastasis increased by less than 20% and cavity formation was observed at the core of the lesion (Fig. 1a). Based on such clinical responses to the first session, this patient received the second series of vaccinations. Seven other patients had PD after the therapy.
Fig. 1.
Changes in CT images of metastatic lesion and serum CEA levels in patients after receiving the OPA-DC vaccines. a CT images of the patient with stable disease after OPA-DC vaccination (Case 1) are shown. The diameters of the maximum metastatic lesion in the lung are shown before (day 0) and after (day 59) the vaccination period. b The changes in serum CEA levels during vaccine treatment in the patients (Case 1 and 6) are shown. Downward arrows indicate the time points of OPA-DCs injections
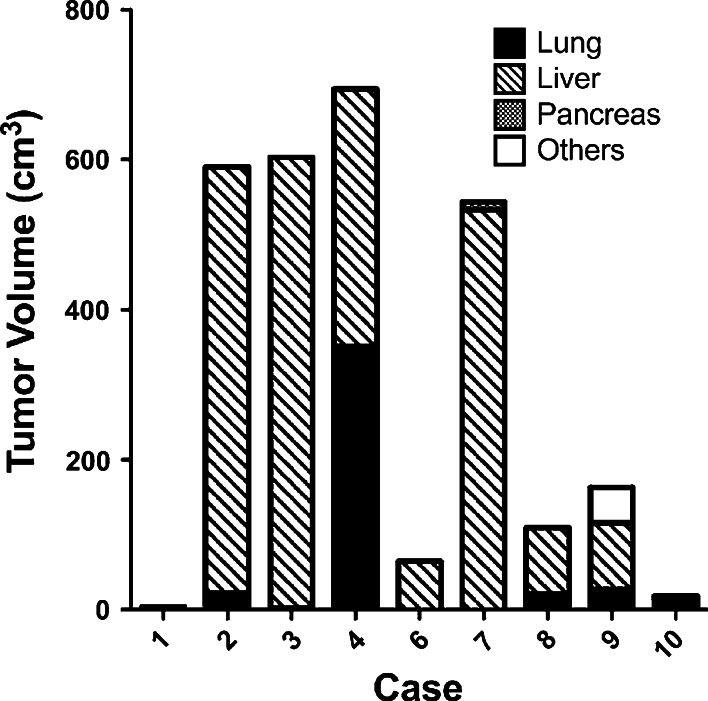
Decline in the serum CEA level during the vaccination period was observed in two patients (Case 1 and 6) (Fig. 1b). They had clearly smaller total tumor volumes in the body before vaccination than the other patients (Fig. 2).
Fig. 2.
Absence of correlation between total tumor volume and serum immunosuppressive cytokines in the vaccinated patients. Total tumor masses of each patient before vaccination were measured as described in “Subjects and methods”. Localizations and volumes of metastatic tumors are shown
Immunological responses
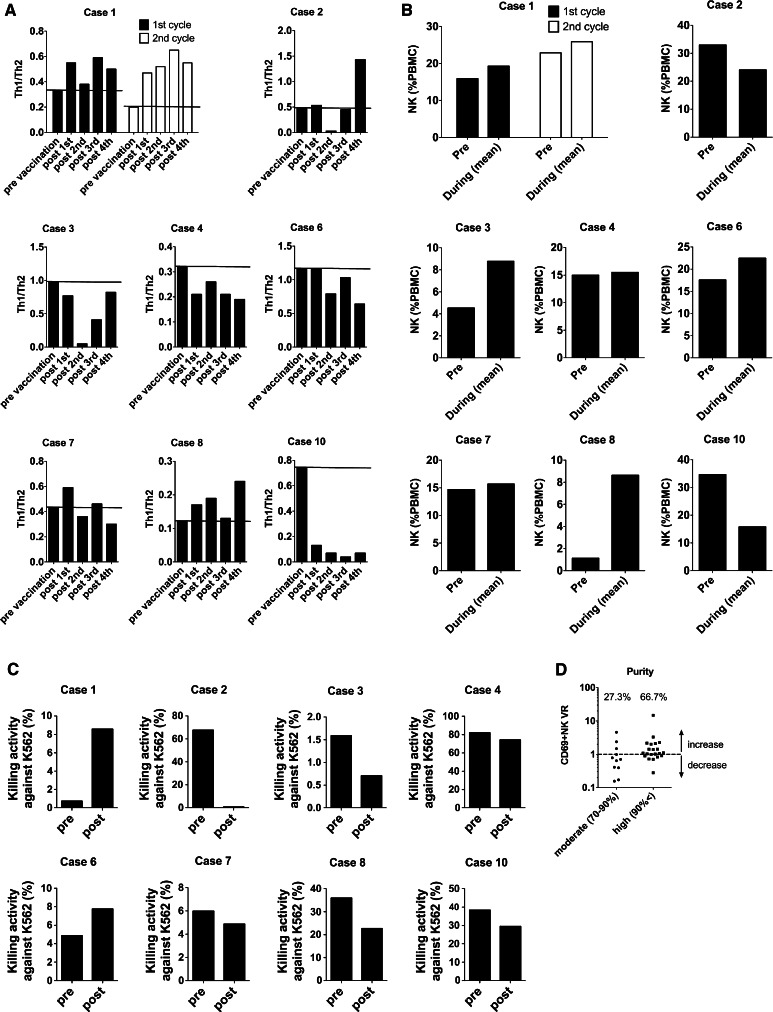
We previously reported that OPA-DC has potent Th1 priming ability in vitro [9]. Therefore, we analyzed the frequency of peripheral Th1 and Th2 cells during the vaccination period (Fig. 3a). In Cases 1 and 8, the Th1/Th2 ratio remained high during vaccination compared with the pre-vaccination value. In Case 1, the patient with SD continued to show increment of the Th1/Th2 ratio after vaccination during the two series of sessions.
Fig. 3.
Changes in Th1/Th2 ratio and NK cells before and during/after the OPA-DC vaccines. a Ratios between Th1 and Th2 frequency (Th1/Th2) were determined before and 2 weeks after every vaccination as described in “Subjects and methods”. In Case 1, four additional injections of DCs were performed (empty bars). Horizontal lines in each graph indicate the Th1/Th2 value before vaccination. b NK cell frequency was examined before and during the vaccinations. The values are shown as the mean of the NK cell frequencies between the first and the last DC injections. c NK activities are evaluated by the killing of NK-sensitive K562 cells before and 2 weeks after the first DC injection. d CD69+ NK variation rates (VR) are shown for all DC injections of all patients in the first session. VR values above 1 mean that CD69+ NK cells increased with that single injection of DC. Percentages in the graphs depict the rate of CD69+ NK increment induced by DCs with quality
OPA-DCs have potent ability to activate NK cells and antigen-specific CTLs in vitro [9]. Therefore, we analyzed the frequency of NK cells before and during vaccination (Fig. 3b). In all except Cases 2 and 10, the mean frequency of NK cells during vaccination increased compared with the pre-vaccination value. Moreover, in Cases 1 and 6 demonstrating a CEA decline, the NK cells displayed strong lytic activity against K562 cells after vaccination (Fig. 3c).
We and other investigators have found that DCs primed with OK432 can activate NK cells within 48 h [9, 17]. Such observations support the possibility of vaccine-dependent activation of NK cells with each DC injection. Therefore, we analyzed the increment of CD69 + NK cells after every DC injection with respect to its relationship with DC purity. In total, 32 injections were performed with eight patients in their first sessions. Among such vaccinations, 21 injections were done with highly pure DCs (purity: >90%) and the remaining 11 with moderately pure DCs (purity: 70–90%). As shown in Fig. 3d, regardless of the differences in clinical backgrounds of the patients, a post-vaccine increment of CD69 + NK cells was observed in 14 out of the 21 given highly pure DC injections (66.7%). In contrast, such an increment was detected only in 3 out of the 11 given moderately pure DC injections (27.3%). Therefore, highly pure DC injections resulted in a higher rate of NK activation compared with moderately pure DC injections.
Next, to assess the frequency of antigen-specific CTLs induced with OPA-DC vaccine, we analyzed PBMCs from vaccinated patients with CEA(24)-pentamer staining (Fig. 4a). In Case 1 with the SD response, the frequency of the pentamer-positive CTLs increased with vaccination. Of particular interest, during the second sessions, the frequency increased much more compared with those in the first sessions (Fig. 4a). In contrast, the frequency of specific CTLs against control peptide derived from Epstein-Bar virus (EBV) kept under 1% of all CD8+ T cells during all sessions (Online Resource 2). According to the differentiation stages, human CD8+ T cells have been subdivided into different populations based on their CD45RA and CCR7 expressions [15]. Therefore, we analyzed the frequencies of CEA.652(9)-specific central memory T (Tcm), effector memory T (Tem) and terminally differentiated Tem (Tem/td) cells during vaccination in Case 1 (Fig. 4b). The CEA.652(9)-specific Tcm cells increased gradually with OPA-DC vaccination; however, antigen-specific Tem or Tem/td cells were not induced. In other cases, including Case 6 with CEA decline, we did not observe such increment of antigen-specific CTLs after the vaccinations.
Fig. 4.
Differentiation of CTLs was impaired regardless of the increased frequency of CEA-pentamer-positive CD8 T cells after DC vaccinations. a CEA-specific CTLs, as judged by CEA-pentamer-positive CD8+ T cells, were counted as described in “Subjects and methods”. The horizontal lines in each graph indicate the frequency of CEA-pentamer-positive cells before vaccination. b In the patient who showed an SD response (Case 1), the frequency of CEA-pentamer-positive CD8+ central memory T cells (Tcm), effector memory T cells (Tem), and terminal differentiated effector memory T cells (Tem/td) was analyzed as described in “Subjects and methods”
We performed ELISPOT assay in order to enumerate IFN-γ-producing CD8 cells reacting to the CEA peptide. Also, we had intended to measure the antigen-specific lytic activity of CTLs against CEA.652(9) pulsed T2-A24 cells by 51Cr-releasing assay, but in all cases, no such responses were observed (data not shown).
Regulatory T cells (Treg) play an active role in the suppression of anti-tumor immune responses. Many investigators reported that the accumulation of Tregs is observed in various types of cancers including CRC [18–21]. Therefore, we analyzed the frequency of Tregs during vaccination. In Cases 2, 3, 6, and 7, Tregs decreased after the vaccinations (Fig. 5). It is noteworthy that in Case 6, the reduction in Tregs was maintained throughout the vaccination period. Delayed-type hypersensitivity (DTH) against CEA.652(9) peptide was not observed in any of the cases (data not shown).
Fig. 5.
Changes in FoxP3+ regulatory T cells varied during the OPA-DC vaccination period. Frequency of FoxP3+ CD4+ CD25+ T cells (depicted as Tregs) during the vaccination period is shown. Horizontal lines in each graph indicate the frequency of Tregs before vaccination
Toxicity
All patients experienced grade-1 fever after every vaccination, but this could be controlled with anti-inflammatory drugs. In addition, induration of the vaccinated groin sites was observed about 2 weeks after the first vaccination with all patients. They were followed without treatment, except for one patient (Case 8) who received temporal antibiotics for abscess formation of the induration. OPA-DC vaccine could be performed without severe toxicity for all patients.
Discussion
Dendritic cells pulsed with CEA peptide are one of the feasible vaccines to induce anti-tumor immunity in patients with CRC [22]. We have previously reported that novel mature DCs (OPA-DCs) can be generated from monocytes using OK-432, low-dose prostanoid, and IFN-α (OPA) by a short-term process. OPA-DCs possess potent migrating ability and stimulating activity for Th1, CTL, and NK cells, which are desirable for DC vaccines using peptide antigen against cancers [9]. In this phase I/II clinical study, we evaluated the safety and efficacy of vaccination with OPA-DCs pulsed with CEA.652(9) peptide in ten patients with metastatic CRC. We chose the peptide as a target antigen because it has been reported as a tumor-associated antigen achieving preferable responses in previous cancer vaccine studies [23, 24]. OPA-DCs offer several advantages in clinical settings [25]. First, even with serum-free media, OPA-DCs are likely to possess better functional abilities with large-scale yield. Second, they can avoid the possibility of contamination and can save costs with the generation of clinical-grade DCs. Alternate strategies using short-term cultured DCs have been reported elsewhere [26–28]; however, this is the first clinical study of anti-tumor vaccine using quickly generated DCs.
Regarding toxicity, the vaccination with OPA-DCs was well tolerated in patients with advanced CRC. The most common adverse events were grade-1 fever and indurations at the injection sites. Such toxicities were comparable to findings reported for previous DC-vaccine trials [29–32]. Two patients were withdrawn from the study because of grade-3 ileus, respiratory distress, or obstructive jaundice; however, these problems were caused by exacerbation of preexisting peritoneal disseminations, lymphangitic carcinomatosis, or liver metastases.
In this clinical study, we observed an SD response in one patient (Case 1). Interestingly, several tumor lesions in this patient formed a central cavity, which was probably attributed to immunological tumor necrosis triggered by the OPA-DC vaccine. Histological analysis could have offered support for this, but we could not perform biopsies of the lesions for ethical reasons. Instead, some immunological events that could have contributed to her clinical outcomes were observed in the peripheral blood during vaccination. First, Th1 cells were dominant over Th2 cells throughout her vaccination period. Second, NK cells had increased and were activated, and their in vitro lytic activity was enhanced after vaccination. In addition, specific CTLs that possess strong avidity to HLA-A*2402/CEA.652(9)-pentamer (CEA(24)-pentamer) were increased gradually with OPA-DC injections. These findings demonstrate that OPA-DCs can have significant immunological impact on patients even in refractory stages.
As for clinical outcomes, we observed decline in CEA in only 2 of 8 patients (Case 1 and 6) and stable disease in one (Case 1). Such results are comparable with those of previous reports regarding DC vaccine against CRC [31, 33, 34], which implies that additional modifications, other than highly active DC, are required to improve clinical responses with DC vaccine. In our study, the responders had lesser tumor volume than non-responders at the beginning of the vaccination period. In addition, regulatory T cells (Tregs) were reduced after OPA-DC vaccine in half of the treated patients. In a patient with CEA decline (Case 6), a sustained reduction in Tregs was observed throughout the vaccination period. It is not clear whether OPA-DCs directly reduce Tregs; however, Treg reduction may exert a favorable impact on the clinical outcome. Even if such inhibitory factors could be removed, a sizable number of cancer cells could not be completely eliminated by a limited number of effector cells educated by DC vaccine. Therefore, initiating vaccination at earlier stages of the disease could be a key to success for DC therapy.
Natural killer (NK) cells are potent anti-tumor effectors that reciprocally interact with DCs [35]. Many previous reports about DC vaccine have regarded CTLs as a principal effector providing anti-tumor immunity. However, there are few studies reporting NK cell activation in response to DC vaccine. Osada et al. [36] reported that NK number increased in 5 of 9 patients (55.6%) vaccinated with CEA-gene-transfected MoDCs, of which the clinical outcomes were correlated with NK activity rather than with T-cell responses. Our study showed that NK cell frequency was increased in 6 of 8 OPA-DC-vaccinated patients (75%). Such a high response rate shows that OPA-DCs possess potent ability to stimulate NK cells in vivo. In addition, our results showed that highly pure DC could activate NK cells compared with moderately pure one, which indicate that such NK activation is dependent on specific action of administrated DCs. Of interest, in two patients (Case 1 and 6), NK cells gained strong lytic activity after vaccination. Interestingly, only these two patients showed decline in serum CEA or a preferable clinical outcome as SD. In support of this possibility, Shimizu and Fujii [37] have reported that mice immunized with DC vaccine acquired “primed” NK cells, which could be quickly re-activated in response to tumor-cell challenge even at 6–12 months after the vaccination. Such an observation suggests that NK cells primed with DC vaccine may contribute to long-term tumor rejection. Further exploration is needed to disclose how NK cells work in DC vaccine therapies.
In the patient displaying an SD response (Case 1), CEA(24)-pentamer-positive cells increased gradually with the injection of OPA-DCs. Interestingly, despite expansion of CTLs, we detected no IFN-γ-producing or antigen-specific-lytic ability of CTLs against antigen-loaded T2-A24 cells. One of the reasons for such a discrepancy may be the impaired differentiation of CTLs, exhibited by the predominance of Tcm phenotype as antigen-specific CTLs in this study. Sallusto et al. [15] identified different populations of memory CD8+ T cells in the peripheral blood of humans. The Tcm cells are reported to attain potent proliferative or IL-2-producing ability critical for maintenance of memory-T-cell pools, whereas Tem or Tem/td cells possess potent effector function such as IFN-γ/perforin production or cytotoxicity [15]. It is still uncertain which cells, Tcm or Tem, are more advantageous in providing protective immunity against cancer [38]. Mornarini et al. [39] reported that although most melanoma patients displayed antigen-specific CTLs belonging to Tcm subsets in tumor-invaded lymph nodes, few perforin-producing Tem or Tem/td cells infiltrated in the neoplastic tissues, and they found no evidence for tumor regression. Although the factors that regulate CTL differentiation are still unclear, further understanding of such regulators could provide clues to developing effective vaccines.
In summary, quickly inducible OPA-DC vaccine is tolerated and could induce preferable immunological responses in patients with advanced-stage CRC. OPA-DC vaccine exerted significant NK-activating ability, and such a response was partially linked to favorable clinical outcomes in the patients. Most antigen-specific CTLs induced with the OPA-DC vaccine belonged to a Tcm subset. In order to develop more practically effective DC vaccine against CRC, further investigation is necessary to explore the modality to induce coordinated and durable activation of both NK cells and CTLs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to the members of MTR, especially to the following Drs. Toshiki Yoshimine (Director), Yoshiki Sawa (Director), Akira Myoui (Vice Director), ChunMan Lee, Junji Kawada, Haruki Ide, and Masao Umegaki (Project Managers).
References
- 1.Hwang J, Marshall JL. Targeted therapy for colorectal cancer. Curr Opin Investig Drugs. 2006;7(12):1062–1066. [PubMed] [Google Scholar]
- 2.de Vries IJM, Lesterhuis WJ, Scharenborg NM, Engelen LPH, Ruiter DJ, Gerritsen M-JP, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJA. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9(14):5091–5100. [PubMed] [Google Scholar]
- 3.Vilella R, Benítez D, Milà J, Lozano M, Vilana R, Pomes J, Tomas X, Costa J, Vilalta A, Malvehy J, Puig S, Mellado B, Martí R, Castel T. Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother. 2004;53(7):651–658. doi: 10.1007/s00262-003-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AW, Truong T, Bickham K, Fonteneau J-F, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/S0264-410X(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 5.Mbawuike IN, Fujihashi K, DiFabio S, Kawabata S, McGhee JR, Couch RB, Kiyono H. Human interleukin-12 enhances interferon-gamma-producing influenza-specific memory CD8+ cytotoxic T lymphocytes. J Infect Dis. 1999;180(5):1477–1486. doi: 10.1086/315090. [DOI] [PubMed] [Google Scholar]
- 6.Alli RS, Khar A. Interleukin-12 secreted by mature dendritic cells mediates activation of NK cell function. FEBS Lett. 2004;559(1–3):71–76. doi: 10.1016/S0014-5793(04)00026-2. [DOI] [PubMed] [Google Scholar]
- 7.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother. 2002;51(10):521–531. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toes RE, Offringa R, Feltkamp MC, Visseren MJ, Schoenberger SP, Melief CJ, Kast WM (1994) Tumor rejection antigens and tumor specific cytotoxic T lymphocytes. Behring Inst Mitt Jul(94):72–86 [PubMed]
- 9.Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-alpha as potent immune enhancers. J Immunother. 2006;29(1):67–77. doi: 10.1097/01.cji.0000183093.77687.46. [DOI] [PubMed] [Google Scholar]
- 10.Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94(3):412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 11.Babatz J, Röllig C, Löbel B, Folprecht G, Haack M, Günther H, Köhne C-H, Ehninger G, Schmitz M, Bornhäuser M. Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer immunology, immunotherapy CII. 2006;55(3):268–276. doi: 10.1007/s00262-005-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K-J, Wang C–C, Chen L-T, Cheng A-L, Lin D-T, Wu Y-C, Yu W-L, Hung Y-M, Yang H-Y, Juang S-H, Whang-Peng J. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res. 2004;10(8):2645–2651. doi: 10.1158/1078-0432.ccr-03-0430. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol. 1999;21(3):263–285. doi: 10.1007/BF00812257. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Marcusson-Stahl M, Cederbrant K. A flow-cytometric NK-cytotoxicity assay adapted for use in rat repeated dose toxicity studies. Toxicology. 2003;193(3):269–279. doi: 10.1016/S0300-483X(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 17.West E, Morgan R, Scott K, Merrick A, Lubenko A, Pawson D, Selby P, Hatfield P, Prestwich R, Fraser S, Eves D, Anthoney A, Twelves C, Beirne D, Patel P, O’Donnell D, Watt S, Waller M, Dietz A, Robinson P, Melcher A. Clinical grade OK432-activated dendritic cells: in vitro characterization and tracking during intralymphatic delivery. J Immunother. 2009;32(1):66–78. doi: 10.1097/CJI.0b013e31818be071. [DOI] [PubMed] [Google Scholar]
- 18.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+ CD25+ FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33(4):435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 22.Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30(12):5091–5097. [PubMed] [Google Scholar]
- 23.Ueda Y, Itoh T, Fuji N, Harada S, Fujiki H, Shimizu K, Shiozaki A, Iwamoto A, Shimizu T, Mazda O, Kimura T, Sonoda Y, Taniwaki M, Yamagishi H. Successful induction of clinically competent dendritic cells from granulocyte colony-stimulating factor-mobilized monocytes for cancer vaccine therapy. Cancer Immunol Immunother CII. 2007;56(3):381–389. doi: 10.1007/s00262-006-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda K, Tsunoda T, Tanaka H, Umano Y, Tanimura H, Nukaya I, Takesako K, Yamaue H. Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells. Cancer Immunol Immunother CII. 2004;53(7):609–616. doi: 10.1007/s00262-003-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 26.Dauer M, Lam V, Arnold H, Junkmann J, Kiefl R, Bauer C, Schnurr M, Endres S, Eigler A. Combined use of toll-like receptor agonists and prostaglandin E(2) in the FastDC model: rapid generation of human monocyte-derived dendritic cells capable of migration and IL-12p70 production. J Immunol Methods. 2008;337(2):97–105. doi: 10.1016/j.jim.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170(8):4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 28.Anguille S, Smits ELJM, Cools N, Goossens H, Berneman ZN, Van Tendeloo VFI. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med. 2009;7:109. doi: 10.1186/1479-5876-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avigan DE, Vasir B, George DJ, Oh WK, Atkins MB, McDermott DF, Kantoff PW, Figlin RA, Vasconcelles MJ, Xu Y, Kufe D, Bukowski RM. Phase I/II study of vaccination with electrofused allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother. 2007;30(7):749–761. doi: 10.1097/CJI.0b013e3180de4ce8. [DOI] [PubMed] [Google Scholar]
- 30.Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31(8):771–780. doi: 10.1097/CJI.0b013e3181833818. [DOI] [PubMed] [Google Scholar]
- 31.Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca M-B, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008;20(6):1305–1311. [PubMed] [Google Scholar]
- 32.Trepiakas R, Berntsen A, Hadrup SR, Bjørn J, Geertsen PF, Straten PT, Andersen MH, Pedersen AE, Soleimani A, Lorentzen T, Johansen JS, Svane IM (2010) Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. doi:10.3109/14653241003774045 [DOI] [PubMed]
- 33.Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30(7):762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 34.Babatz J, Röllig C, Löbel B, Folprecht G, Haack M, Günther H, Köhne C-H, Ehninger G, Schmitz M, Bornhäuser M. Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother. 2006;55(3):268–276. doi: 10.1007/s00262-005-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinushi M, Takehara T, Kanto T, Tatsumi T, Groh V, Spies T, Miyagi T, Suzuki T, Sasaki Y, Hayashi N. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170(3):1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 36.Osada T, Clay T, Hobeika A, Lyerly HK, Morse MA. NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol Immunother. 2006;55(9):1122–1131. doi: 10.1007/s00262-005-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu K, Fujii S. DC therapy induces long-term NK reactivity to tumors via host DC. Eur J Immunol. 2009;39(2):457–468. doi: 10.1002/eji.200838794. [DOI] [PubMed] [Google Scholar]
- 38.Perret R, Ronchese F. Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens. 2008;72(3):187–194. doi: 10.1111/j.1399-0039.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 39.Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, Cascinelli N, Parmiani G, Anichini A. Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res. 2003;63(10):2535–2545. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.