Abstract
Introduction
Ipilimumab, a cytotoxic T lymphocyte-associated antigen-4 blocking antibody, has improved overall survival (OS) in metastatic melanoma in phase III trials. However, about 80 % of patients fail to respond, and no predictive markers for benefit from therapy have been identified. We analysed a ‘real world’ population of patients treated with ipilimumab to identify markers for treatment benefit.
Methods
Patients with advanced cutaneous melanoma were treated in the Netherlands (NL) and the United Kingdom (UK) with ipilimumab at 3 mg/kg. Baseline characteristics and peripheral blood parameters were assessed, and patients were monitored for the occurrence of adverse events and outcomes.
Results
A total of 166 patients were treated in the Netherlands. Best overall response and disease control rates were 17 and 35 %, respectively. Median follow-up was 17.9 months, with a median progression-free survival of 2.9 months. Median OS was 7.5 months, and OS at 1 year was 37.8 % and at 2 years was 22.9 %. In a multivariate model, baseline serum lactate dehydrogenase (LDH) was demonstrated to be the strongest predictive factor for OS. These findings were validated in an independent cohort of 64 patients from the UK.
Conclusion
In both the NL and UK cohorts, long-term benefit of ipilimumab treatment was unlikely for patients with baseline serum LDH greater than twice the upper limit of normal. In the absence of prospective data, clinicians treating melanoma may wish to consider the data presented here to guide patient selection for ipilimumab therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1528-9) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Immunotherapy, Ipilimumab, Lactate dehydrogenase, Biomarker
Introduction
Ipilimumab is a fully human monoclonal IgG1 antibody that blocks the immune-checkpoint molecule cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) thereby reversing its immunosuppressive effect on T cells, although its exact mechanism of action is still under investigation [1].
Ipilimumab was approved for the treatment of advanced melanoma by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2011, on the basis of increased overall survival (OS) in two phase III studies [2, 3]. Treatment with ipilimumab at 3 mg/kg as a second and subsequent line of therapy resulted in a median OS of 10.1 months compared with 6.4 months in the vaccine control arm together with a one-year survival of 45.6 versus 25.3 %, respectively, in one of the studies [2]. Best overall response rate (BORR) was 11 %.
Subgroup analysis for OS revealed that the hazard ratio for ipilimumab in comparison with the control arm was only significant in patients with normal serum lactate dehydrogenase (LDH) levels [2]. Raised serum LDH is known to be a poor prognostic factor in a number of cancers including melanoma for reasons that remain unknown. This is reflected in the 2009 AJCC staging system for melanoma, which subdivides metastatic disease into: M1a, skin, lymph node or subcutaneous metastases only; M1b, lung metastases only; and M1c, visceral metastases or metastases at any site with a raised LDH [4].
Ipilimumab is currently under evaluation in several phase II trials for other malignancies including renal cell carcinoma, non-small cell lung cancer and metastatic castration-refractory prostate carcinoma [5–8]. While potentially effective, ipilimumab can be toxic as a consequence of reduction in peripheral tolerance to self-antigens, which is reflected in the induction of immune-related adverse events (irAEs) such as colitis, dermatitis, hepatitis and hypophysitis [2, 3].
Several clinical parameters, such as high absolute lymphocyte count (ALC) after two courses of ipilimumab, a rise of ALC over baseline and the occurrence of grade 3 or 4 irAEs have been suggested to correlate with OS and response to therapy [9–15]. A major drawback of these potential markers is that they become evident only during the course of treatment, thereby making them unsuitable for upfront patient selection. The aim of this study was, therefore, to identify baseline patient characteristics for response to ipilimumab, to assess their independent prognostic value and to compare them with the markers described above [16, 17].
Patients and methods
Patient inclusion and treatment plan
Patients with advanced melanoma and progression on systemic therapy were treated in expanded access programmes in the Netherlands (NL EAP) (NCT00495066) and in the United Kingdom after licensing. Consequently, the NL cohort included patients exclusively treated within the EAP, whereas the UK cohort comprised of both EAP and post-licensing patients. A modified World Health Organization (mWHO) performance status of 0, 1 or 2 was required. An mWHO performance status of 0 indicates that the patient has no restrictions in carrying out pre-disease activities; an mWHO performance status of 1 indicates that the patient has restrictions in more strenuous activities but is completely ambulatory and is able to carry out work of a light nature; an mWHO performance status of 2 indicates that the patient is in bed for less than 50 % during the day but is still ambulatory and capable of all self-care. Treatment protocols were approved according to local regulations and ethical committees. Data acquisition and subsequent analyses mentioned hereafter apply to the NL cohort unless stated otherwise. Data from the UK cohort were obtained to validate findings on baseline LDH and survival probability. All patients received four cycles of 3 mg/kg ipilimumab every 3 weeks, unless severe side effects or rapid disease progression occurred. M-stage was defined according to site of metastasis in combination with elevated LDH levels, as described previously [4].
Safety measures
Patients underwent clinical evaluation prior to each ipilimumab infusion. irAEs were scored using the common terminology criteria for adverse events (CTCAE) version 4.02, and patients received standard of care accordingly. A list of irAEs was pre-specified in the protocol, and a serious adverse event was determined as grade 3 or 4. Haematological and serum parameters were determined at baseline, every 3 weeks during treatment, and every 3 months during follow-up. These included ALC, S100, erythrocyte sedimentation rate (ESR), LDH, liver, kidney and endocrine function. Upper limits of normal (ULN) were normalised for the reference range at each participating centre.
Response evaluation and follow-up
Radiologic evaluation (CT or PET/CT scanning) was performed at baseline and week 12 (i.e. 3 weeks after the fourth infusion) if patients had undergone all four cycles of ipilimumab. Responses were scored according to RECIST 1.1 criteria as well as immune-related response criteria (irRC), the latter to determine BORR [18]. Responses were confirmed at least 4 weeks later and during follow-up every 3 months thereafter or when disease progression was clinically suspected. Survival status from patients that withdrew from follow-up was obtained from primary care physicians.
Statistical analysis
Data were analysed using SPSS statistical software version 20.0 and R statistical software version 2.15.0. Univariate analysis for clinical and laboratory parameters was performed with respect to OS and progression-free survival (PFS). Survival curves of categorical variables were drawn using the Kaplan–Meier technique and compared by the log-rank test. Continuous variables in association with survival outcomes were explored by means of martingale residuals. LDH and S100 were log-transformed. Multivariate models of patient and tumour characteristics in association with PFS and OS were based on Cox proportional hazards regression analyses. PFS was defined as time from start of ipilimumab to the onset of progression or death. Patients without progression and still alive at time of analysis were censored. OS was defined as time from start of ipilimumab to death of any cause. Patients still alive at analysis were censored. OS analyses were identical for both cohorts. The association of parameters measured at 6 weeks with either PFS or OS were analysed by means of a landmark method (ignoring time and events before 6 weeks) or as time-dependent variable in a Cox proportional hazards model. Decision trees were generated using recursive partitioning.
Results
Patient characteristics
Hundred and sixty-six patients received ipilimumab between April 2010 and December 2011 within the Netherlands expanded access programmes (NL EAP). As of August 2012, 28 % of patients were still alive. The median follow-up of the cohort was 17.9 months; clinical characteristics are listed in Table 1. Median patient age at the time of study inclusion was 55 years, and 58 % were male. Modified WHO performance status 0, 1 and 2 was scored in 59, 36 and 5 % of patients respectively; 83 % of the patients had stage M1c disease. Baseline levels of LDH were elevated between the ULN and 2× ULN in 28 % of patients and >2× ULN in 16 % of patients. From the UK cohort, 64 patients were analysed. The median follow-up of this cohort was 19.0 months, and patient characteristics were similar as shown in Table 1. In this cohort, 39 % of patients had baseline levels of LDH elevated between ULN and 2× ULN, while 23 % of patients had elevated LDH > 2× ULN.
Table 1.
Patient characteristics
| Variable | NL (N = 166) | UK (N = 64) |
|---|---|---|
| Age | ||
| Median (range) | 55 (22–88) | 58 (18–84) |
| Sex | ||
| Female | 69 (42 %) | 27 (42 %) |
| Male | 97 (58 %) | 37 (58 %) |
| WHO status | ||
| WHO 0 | 98 (59 %) | 21 (33 %) |
| WHO 1 | 60 (36 %) | 37 (58 %) |
| WHO 2 | 8 (5 %) | 6 (9 %) |
| Breslow | ||
| <1 mm | 10 (6 %) | 5 (8 %) |
| 1–2 mm | 23 (14 %) | 14 (22 %) |
| 2–4 mm | 50 (30 %) | 14 (22 %) |
| >4 mm | 24 (14 %) | 7 (11 %) |
| NA | 59 (36 %) | 24 (38 %) |
| M-stage | ||
| M1a | 8 (5 %) | NA |
| M1b | 20 (12 %) | NA |
| M1c | 138 (83 %) | NA |
| Site metastasis | ||
| Single | 31 (19 %) | 5 (8 %) |
| Multiple | 135 (81 %) | 59 (92 %) |
| Cycles of ipilimumab | ||
| 1 | 17 (10 %) | 17 (27 %) |
| 2 | 19 (11 %) | 11 (17 %) |
| 3 | 22 (13 %) | 11 (17 %) |
| 4 | 114 (66 %) | 25 (39 %) |
| Responders | 17 (11 %) | 7 (11 %) |
| Non-responders | 149 (89 %) | 57 (89 %) |
| Immune-related adverse events (grade III/IV) | ||
| Responders | 3/17 (18 %) | 3/7 (43 %) |
| Non-responders | 25/149 (17 %) | 2/57 (4 %) |
| Baseline LDH | ||
| <1× ULN | 87 (52 %) | 24 (38 %) |
| 1–2× ULN | 46 (28 %) | 25 (39 %) |
| >2× ULN | 27 (16 %) | 15 (23 %) |
| NA | 6 (4 %) | 0 (0 %) |
WHO World Health Organization, NA not available, LDH lactate dehydrogenase, ULN upper limit of normal
Prior and post-ipilimumab treatment modalities
All patients in the NL cohort had received prior systemic treatment of which 80 % received only dacarbazine (DTIC), while 20 % underwent one other systemic treatment or had more than one prior treatment including DTIC (Supplementary Table 1). Most patients (75 %) did not receive any other treatment after ipilimumab therapy. Post-ipilimumab treatment modalities comprised BRAF inhibitors (13 %) or reinduction therapy with ipilimumab (3 %). One out of five patients experienced disease stabilisation upon reinduction with ipilimumab.
Toxicities
The frequencies of irAEs in the NL cohort are listed in Supplementary Table 2. We observed serious (grade 3 or 4) adverse events in 28/166 (16 %) of the patients. The majority (75 %) comprised dermatitis, hypophysitis and colitis, of which only the latter two led to discontinuation of treatment with ipilimumab in 8 % of patients. There was one treatment-related death (1 %) due to severe colitis resulting in bowel perforation. Grade 1 and 2 immune-related toxicity events mainly comprised dermatitis for which topical intervention was sufficient.
Response to therapy
The clinical responses in the NL cohort measured using RECIST or irRC (including BORR) are listed in Supplementary Table 3. Response rates at 12 weeks were 10 % by RECIST and 11 % by irRC, indicating no initial difference between the two response evaluation methods. However, when looking at the BORR, which takes into account the late development of anti-tumour immune effects, we observed an increase in the RR to 17 %. The median time to achieve BORR was 3.7 months (range 2.5–18.6), which is beyond the standard time point for response evaluation at week 12 and underscores the relevance of a week-16 response re-evaluation. Four patients eventually became complete responders after initially being scored as SD or PR, and one patient was scored with progressive disease (PD) at 12 weeks but eventually became a complete responder 18 months after treatment initiation. A substantial number of patients (27 %) were not evaluable (NE) for response (Supplementary Table 3), due to rapid deterioration and death (39/166) before week 12 or serious adverse events (5/166) resulting in withdrawal from ipilimumab treatment. There was no correlation between the occurrences of grade 3 and 4 irAEs and response to therapy (P = 0.62).
Survival analysis and biomarker assessment
Data on all patients were available for survival analysis. The median PFS in the NL cohort was 2.9 months (95 % CI 2.8–3.2, Fig. 1a). Median OS of the patients in the NL cohort was 7.5 months (95 % CI 6.1–10.5, Fig. 1b). The one-year survival was 37.8 % (95 % CI 31.1–46.0), and two-year survival 22.9 % (95 % CI 16.4–32.1). The median OS in the UK cohort was 4.1 months (95 % CI 3.6–5.1, Fig. 1c). The one-year survival was 15.6 % (95 % CI 8.8–27.6) and two-year survival 14.1 % (95 % CI 7.67–25.8).
Fig. 1.

Kaplan–Meier curves for progression-free survival and OS of NL and UK cohort. Progression-free survival curve for a the NL cohort and OS curves for b the NL and c UK patient cohorts are shown
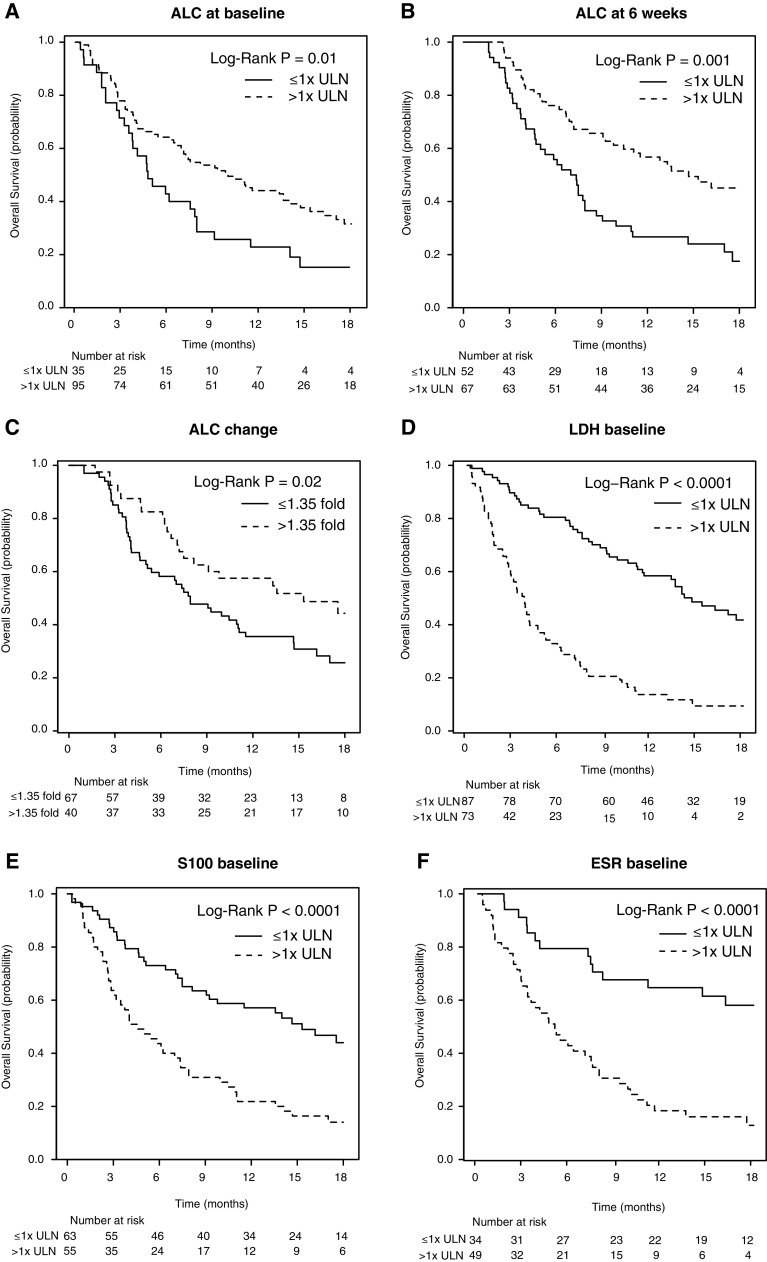
Univariate analysis of the NL cohort for patient and disease characteristics such as gender, age, Breslow thickness and location of primary tumour, as well as prior treatment revealed no significant association with OS. Both mWHO performance score 0 and disease stage M1a/b, univariately correlated with a favourable outcome for OS (P = 0.001 and P = 0.04, respectively). Baseline ALC and week 6 ALC measurements showed a significant difference in survival when stratifying high versus low (P = 0.01 and P = 0.001; Fig. 2a, b). When assessing the slope in ALC as identified by Martingale residuals, we found that patients with greater than or equal to 1.35-fold higher value compared with baseline after two cycles of treatment, determined 6 weeks after the start of therapy, had a significantly higher OS than patients with a lower increase (P = 0.02; Fig. 2c). Low baseline levels of, respectively, LDH, S100 and ESR were also significantly associated with improved OS (all P < 0.0001) (Fig. 2d–f).
Fig. 2.
Kaplan–Meier curves for OS stratified by blood parameters. OS is shown when stratifying for blood values of a baseline absolute lymphocyte count (ALC), b ALC at week 6, c slope of ALC after two infusions, d baseline lactate dehydrogenase (LDH), e S100 and f baseline erythrocyte sedimentation rate (ESR). ULN upper limit of normal. P < 0.05 is statistically significant
When patient characteristics (M-stage and mWHO performance status), baseline LDH, S100, ESR, and ALC were examined in a multivariate model, only ESR and LDH remained as significant independent negative prognostic factors (P < 0.01). Using this approach, we identified a subset of patients with a significantly higher OS. The majority of patients with low OS were identified when separating the cohort by elevated baseline levels of LDH (median OS 14.7 months for LDH normal versus 3.7 months for LDH > ULN; P < 0.001), with some further discrimination after stratification by baseline ESR (Supplementary Figure 1). Of note, only 17/44 patients not evaluable for response were available for baseline ESR assessment, while 40/44 were available for baseline LDH assessment. Within the poor prognosis group (elevated LDH above ULN), a small number of patients, including four with a partial response and one complete response (5/73), survived beyond the first year. The response rate in this subgroup was 9 % compared to 23 % in the ≤1× ULN group, indicating some potential predictive value of this marker. When stratifying by 2× ULN of baseline LDH values, only one patient survived beyond 12 months in the LDH-high group who had a partial response and was censored at 12.9 months follow-up. In total, only two partial responses occurred in this subgroup, whereas all other responses occurred in the LDH-low group. Median OS was 2.9 versus 10.0 months in the LDH greater than twice ULN versus less than twice ULN group, respectively, (P < 0.001; Fig. 3).
Fig. 3.

Patient selection based on baseline LDH for NL and UK cohort. Upper part of panel a shows the stratification of patients based on their upper limit of normal (ULN) baseline values of serum lactate dehydrogenase (LDH) in the NL cohort. For six patients, baseline LDH values were not available and these were excluded from further analyses. Lower part shows patients grouped by their response to therapy and the occurrence of toxicities. A statistically significant difference in OS was observed when stratifying by 1× ULN baseline LDH. However, seven patients with a clinical response upon ipilimumab treatment would have been missed when implementing this cut-off. Only two patients with a clinical response remained in the LDH-high subgroup when stratifying by 2× ULN of baseline LDH. Toxicities in the high and low LDH subgroups were similar. Panel b shows the stratification by baseline LDH values of patients from the UK cohort. At a cut-off of 1× ULN of baseline LDH, there is no significant influence on OS. Stratification by 2× ULN baseline LDH does significantly increase OS, and no patients in the LDH-high subgroup survive beyond the first year after treatment initiation. Percentages may not add up to 100 due to rounding. HR hazard ratio, CR complete response, PR partial response, SD stable disease, PD progressive disease, NE non-evaluable
To investigate the broader value of these findings, we analysed an independent cohort of 64 patients treated in the UK at two academic centres. Only baseline LDH values were assessed in this cohort and, unlike in the Dutch cohort, stratification on 1× ULN baseline LDH did not significantly influence OS. However, a significant difference in survival was observed when stratifying patients by their 2× ULN baseline LDH values (Fig. 3). Median OS in the LDH-high group was 3.2 months compared with 5.0 months in the LDH-low group (P = 0.004). Only within the LDH ≤ 2× ULN group survivors were observed beyond a year.
Overall, one- and two-year survival rates of the combined NL and UK cohorts in the LDH-low group (≤2× ULN; N = 182) were 51.2 and 34.6 %, respectively, whereas they were 4.8 and 0 % in the LDH-high group (>2× ULN; N = 42) (Supplementary Table 4). We found that 66 % of patients in the LDH-low group received all four cycles, whereas in the LDH-high group only 24 % of patients completed the full treatment (data not shown).
Discussion and conclusions
The aim of our analysis was to identify, from a ‘real world’ melanoma patient cohort, parameters used in routine clinical practice that may identify patients most likely to benefit from ipilimumab therapy prior to the start of treatment. We found that a high ESR and, in particular, a high LDH at baseline are statistically significant independent prognostic factors. The use of LDH alone at the cut-off at 2× ULN, as a negative prognosticator, was confirmed in an external cohort of patients. Patient characteristics such as M-stage and performance status did not have an independent significant effect on OS.
While ESR was also identified as an independent prognostic factor, only a small subgroup of twelve patients would additionally be excluded when implementing this cut-off, three of which had a partial response upon ipilimumab treatment. Therefore, we chose to focus on the more clinically relevant stratification based on baseline LDH alone. However, it cannot be excluded based on these data, whether the combination of low LDH/low ESR is of superior value to low LDH alone. Also, ESR data were not available for the UK cohort to confirm these findings. A planned meta-analysis on all EAP-treated patients in Europe should give more insight into the prognostic value of an inflammatory marker such as ESR combined with LDH.
The data presented here indicate that patients with elevated LDH, for the NL cohort at 1× ULN and for both cohorts at 2× ULN, are unlikely to benefit from ipilimumab therapy. High disease load and cell turnover, resulting in high serum levels of LDH, may negatively influence the potency of an immune response at the tumour site. Rapidly growing tumours are often poorly vascularized, resulting in anaerobic glycolysis as an alternative energy source, which in turn is mediated by activity of LDH that converts pyruvate into lactate [19, 20]. This switch of tumour cells to a glycolytic phenotype, which can even occur under normoxic conditions, gives rise to lactate accumulation and a subsequent decrease in extracellular pH, negatively affecting the function of lymphocytes present in the tumour microenvironment [21, 22]. Alternatively, elevated LDH may simply form a measure of disease state and thereby be correlated with likelihood of response.
We observed that the median OS in the >2× ULN group (hereafter referred to as LDH-high) was significantly lower than in the ≤2× ULN group (hereafter referred to as LDH-low): 2.9 versus 10.0 months in the NL cohort and 3.2 versus 5.0 months in the UK cohort. Interestingly, in both phase III trials leading to FDA and EMA approval of ipilimumab, a non-elevated LDH was associated with an increased OS [2, 3]. This fact and the observation that in two other ipilimumab EAPs with smaller patient numbers, reported by Wilgenhof et al. [13] and Delyon et al. [14], elevated LDH showed a negative association with survival further support our findings. OS in our cohorts was lower than what was reported in the phase III studies. This is not surprising since the inclusion criteria were less strict for the expanded access programmes than for the phase III studies. Furthermore, patients in the UK cohort benefited less from therapy than those in the NL cohort, potentially because fewer patients had a performance status of 0: 33 versus 59 %, respectively.
The one- and two-year survival rates of 51.2 and 34.6 % for the LDH-low patients of the combined NL and UK cohort indicate that the survival rates are comparable with the phase III study cohorts [2, 3]. In the LDH-high group, however, we found one- and two-year survival rates of 4.8 and 0 %, respectively, indicating no long-term benefit at all; except for one patient censored at 12.9 months. Taken together, our data show that treatment benefit from ipilimumab is almost exclusively concentrated in the LDH-low group. Patients in the high-LDH group, which is 19 % of patients from both cohorts combined, that carry the BRAF V600E mutation might benefit more from targeted therapies such as BRAF- and MEK-inhibitors that are capable of inducing rapid anti-tumour responses. Recent data from an open label multicentre safety study of vemurafenib showed that patients with elevated LDH at baseline were on therapy for a median duration 4.1 months, which is longer than the median OS for the ipilimumab patients with elevated LDH here [23]. The efficacy of sequential treatment with BRAF inhibitors and ipilimumab should be evaluated in a randomized trial. Sequencing targeted therapies with ipilimumab might be especially helpful to patients with more aggressive disease where the delayed tumour responses generally observed with immunotherapies take too long for the patient to benefit [18, 23–26].
Our analysis clearly has a number of limitations of which the most obvious is its retrospective nature and consequent possibility of bias. A strength of our data set, however, is that it involves a ‘real world’ population as opposed to the more selected group of patients that enter phase III registration trials. Furthermore, the observation of a similarly strong predictive value of elevated LDH in two independent cohorts lends further credibility to our observations. Ideally, our data would stimulate a prospective analysis of the value of elevated LDH as a patient selection criterion. However, in view of the rapidly changing landscape in melanoma immunotherapy, with the likely registration of agents targeting programmed cell death 1 (PD1) and programmed cell death ligand 1 (PDL1) [27–29], we consider such prospective studies unlikely to happen. Without such data, we feel that is important to carry out similar analyses on other large cohorts of ipilimumab-treated melanoma patients treated in routine clinical practice.
In November 2013, the EMA approved ipilimumab as a first-line therapy in metastatic melanoma at a 3 mg/kg dosing schedule [30]. This registration will extend the availability of ipilimumab to previously untreated patients throughout the European Union. After decades of limited and mostly failing therapies, treating oncologists are now faced with the difficulty of having to decide patient eligibility at both earlier and later stages of the disease. This will consequently have an effect on the number of responding patients as well as on OS. Related to this, the expected administration of ipilimumab to patients at earlier disease stages, due to its recent approval as a first-line treatment, warrants continued assessment of the prognostic value of the markers proposed here.
In conclusion, our results show efficacy of ipilimumab comparable with that reported in the phase III studies. Furthermore, we propose that elevated levels of baseline LDH at a 2× ULN cut-off may help physicians to select patients before treatment initiation. Serum LDH has been established for some time as a negative prognostic factor in advanced melanoma [16, 17]. From our data, in the absence of a control arm, we are unable to determine whether LDH forms either a predictive or a prognostic marker for benefit from ipilimumab. However, we would assert that in routine clinical practice the difference is not relevant and that using serum LDH to stratify patients for ipilimumab treatment should simply be considered pragmatic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funds were received to conduct this study. The authors want to acknowledge the members of the Dutch Working Group on Immunotherapy of Oncology (WIN-O) for their assistance in obtaining the data and their constructive discussion concerning the outcomes of this study.
Conflict of interest
G. A. P. Hospers, A. J. M. van den Eertwegh, E. W. Kapiteijn, J. W. de Groot, P. Lorigan, M. E. Gore, J. B. A. G. Haanen, J. M. G. Larkin and C. U. Blank have participated in advisory board meetings of Bristol-Myers Squibb for which the faculty has received compensation. P Lorigan has received support for travel and compensation for educational and speaker bureau activities. J. M. G. Larkin and M. E. Gore acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden Hospital. J. B. A. G. Haanen and T. N. M. Schumacher are members of the Bristol-Meyers Squibb Immuno-Oncology network and have furthermore received a grant for translational research from Bristol-Meyers Squibb. C. U. Blank receives funding for an investigator-initiated study from Bristol-Myers Squibb. All other authors declared that they have no conflict of interest.
Footnotes
Bianca Heemskerk and Harm van Tinteren have contributed equally to this work.
Contributor Information
James M. G. Larkin, Phone: +44-207-8082198, Email: James.Larkin@rmh.nhs.uk
Christian U. Blank, Phone: +31-20-5122960, Email: c.blank@nki.nl
References
- 1.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasini P, Khobta N, Greillier L, Barlesi F. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol. 2012;4(2):43–50. doi: 10.1177/1758834011431718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, Beer TM. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JA, Hamid O, Minor D, Amin A, Ron IG, Ridolfi R, Assi H, Berman D, Siegel J, Weber JS. Ipilimumab in treatment-naive and previously treated patients with metastatic melanoma: retrospective analysis of efficacy and safety data from a phase II trial. J Immunother. 2012;35(1):73–77. doi: 10.1097/CJI.0b013e31823735d6. [DOI] [PubMed] [Google Scholar]
- 10.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol Immunother. 2011;60(4):467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilgenhof S, Du Four S, Vandenbroucke F, Everaert H, Salmon I, Lienard D, Marmol VD, Neyns B. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013;36(3):215–222. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- 14.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24(6):1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 15.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, Biagioli M, Altomonte M, Maio M. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62(6):1021–1028. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide B, Elsasser M, Buttner P, Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F, Garbe C. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer. 2012;107(3):422–428. doi: 10.1038/bjc.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 18.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 19.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 21.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 22.Ratner S. Motility of IL-2-stimulated lymphocytes in neutral and acidified extracellular matrix. Cell Immunol. 1992;139(2):399–410. doi: 10.1016/0008-8749(92)90081-Y. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J, Del VM, Ascierto PA, Schachter J, Garbe C, Neyns B (2013) Open-label, multicenter safety study of vemurafenib in patients with BRAFV600E mutation-positive metastatic melanoma: interim analysis. In: 2013 ASCO Annual Meeting, Chicago, Illinois
- 24.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med. 2012;10:107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R, Trefzer U, Larkin JM, Utikal J, Dreno B, Nyakas M, Middleton MR, Becker JC, Casey M, Sherman LJ, Wu FS, Ouellet D, Martin AM, Patel K, Schadendorf D, Group MS. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Commission - Community Register of Medicinal Products (2011) http://ec.europa.eu/health/documents/community-register/html/h698.htm. Last updated November 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.