Abstract
Background
Antigen-presenting cells (APCs) play a crucial role in the induction of immune responses. However, the optimal administration route of tumor-specific APCs for inducing effective immunological responses via cancer immunotherapy remains to be elucidated. Human NKT cells are known to have strong anti-tumor activities and are activated by the specific ligand, namely, α-galactosylceramide (αGalCer).
Methods
Seventeen patients with head and neck squamous cell carcinoma (HNSCC) were enrolled in this study. Patients received an injection of αGalCer-pulsed APCs into the nasal, or the oral floor submucosa. Then total body image and single photon emission computed tomography (SPECT) images were examined. The immunological responses including the number of peripheral blood NKT cells, anti-tumor activities and the CD4+ CD25high Foxp3+ T cells (Tregs) induced following APCs were also compared.
Results
APCs injected into the nasal submucosa quickly migrated to the lateral lymph nodes and those injected into the oral floor submucosa dominantly migrated to the submandibular nodes rather than the lateral lymph nodes. An increase in the absolute number of NKT cells and the IFN-γ producing cells was observed in peripheral blood after injection of the APCs into the nasal submucosa, however, these anti-tumor activities were not detected and the increased frequency of Treg cells were observed after administration into oral floor.
Conclusions
These results indicate that a different administration route of APCs has the potential to bring a different immunological reaction. The submucosal administration of αGalCer into the oral submucosa tends to induce immunological suppression.
Keywords: Migration, Vaccine route, Nasal submucosa, Oral submucosa, Immunotherapy
Introduction
Antigen-presenting cells (APCs) such as dendritic cells (DCs) play a crucial role in the induction of immune responses, and APC-based cancer immunotherapy induces significant immunological responses as well as some clinical improvement in patients with various malignant diseases [1]. However, the desirable administration route for the antigen-pulsed APCs to induce an effective anti-tumor response still remains unknown. The migration of APCs, upon the uptake of antigens, from peripheral tissues to the regional draining lymph nodes is considered to be an immunological property.
Head and neck cancers arise from the mucosal surface of the upper respiratory or digestive tracts and the lymph nodes of the neck, which are normally defined to be regional. We previously demonstrated that radioactive isotope-labeled APCs administered into the nasal submucosa of patients with head and neck cancer quickly migrated to the regional neck lymph nodes and thereby induced a significant immunological response [2]. Human NKT cells are activated by the specific ligand, α-galactosylceramide (αGalCer), in a CD1d-dependent manner [3, 4], and have been shown to exert strong anti-tumor activity against various tumor types both in vitro and in vivo [5]. Recently, we conducted a phase I study examining the administration of αGalCer-pulsed APCs into the nasal submucosa of patients with HNSCC in order to evaluate the safety and feasibility of this treatment [6]. Nine patients with unresectable recurrent HNSCC received two doses of 1 × 108 αGalCer-pulsed autologous APCs into the nasal submucosa with an 1-week interval and the NK cell activation and anti-tumor activity were observed in some patients [7].
In the present study, we examined the migration pattern of isotope-labeled APCs which were administered into the oral floor submucosa, and compared it to APCs administered into the nasal mucosa. The immunological responses to each administration route were also investigated.
Materials and methods
Patients
Seventeen patients with head and neck squamous cell carcinoma (HNSCC) who were between 20 and 80 years of age were enrolled this study, as shown in Table 1. All patients received standard treatment, such as surgical resection, irradiation and/or chemotherapy, and had no evidence of a tumor for at least 3 months after undergoing the standard treatment at the time of enrollment in the study. Furthermore, the myelosuppressive findings such as myelosuppression or opportunistic infection were not achieved in all cases.
Table 1.
Patient profiles and administration site of APCs
| Case | Age/gender | Tumor lesion | Stage | Past treatment | Labelled APCs | Unlabelled APCs |
|---|---|---|---|---|---|---|
| 001 | 64/M | Larynx | T2N2c | CT | NS | |
| 002 | 63/M | Hypopharynx | T3N2b | RT | NS | |
| 003 | 65/M | Tongue | T2N1 | OP | NS | |
| 004 | 64/M | Hypopharynx | T3N1 | CT, RT | NS | |
| 005 | 48/M | Maxilla | T4N0 | CT, RT | NS | |
| 006 | 68/M | Hypopharynx | T4N0 | RT | NS | |
| 007 | 76/M | Larynx | T2N0 | RT | NS | |
| 008 | 57/M | Oral floor | T4N2b | OP | OF | |
| 009 | 71/F | Hypopharynx | T2N0 | CT, RT | OF | |
| 010 | 76/M | Larynx | T1aN0 | RT | OF | |
| 011 | 64/M | Tongue | T1N0 | OP | OF | |
| 012 | 64/M | Larynx | T4N0 | CT, RT, OP | OF | |
| 013 | 48/M | Oropharynx | T2N2b | CT, RT, OP | OF | |
| 014 | 40/F | Tongue | T2N2b | CT, RT, OP | OF | |
| 015 | 67/F | Maxilla | T4N0 | CT, RT, OP | OF | |
| 016 | 36/M | Larynx | T4N0 | CT, RT | OF | |
| 017 | 71/F | Tongue | T1N0 | OP | OF |
CT chemotherapy, RT radiation therapy, OP operation therapy NS nasal submucosa, OF oral floor submucosa
The additional criteria for enrollment included a performance status (PS) of 0, 1, or 2; an expected survival time of 6 months or more; and normal renal, hepatic, and hematopoietic functions. The exclusion criteria for exclusion from these studies included a positive antibody activity against HIV, hepatitis C virus, or human T cell lymphotrophic virus; the presence of hepatitis B surface antigen; an active inflammatory disease or active autoimmune disease; a history of hepatitis; pregnancy or infections; concurrent corticosteroid therapy; and the existence of another malignant neoplasm.
Preparation of APCs
All procedures were conducted according to the Good Manufacturing Practice standards. Peripheral blood mononuclear cells (PBMCs) were collected from the peripheral blood samples from 17 patients and were separated by density gradient centrifugation (OptiPrep, Nycomed Amersham, Oslo, Norway). PBMCs were washed three times and resuspended in AIM-V (Invitrogen Corp., Carlsbad, CA, USA) with 800 units/ml of human granulocyte macrophage colony-stimulating factor (GeneTech Co., Ltd., China) and 100 Japanese reference units per milliliter of recombinant human IL-2 (Imunace, Shionogi, Osaka, Japan) as described [6, 7]. After 7 days of cultivation, the cells were harvested and washed three times and then resuspended in 0.2 ml of 2.5% albumin in saline. The cultured cells were pulsed with 100 ng/ml of αGalCer (KRN7000; Kirin Brewery, Gunma, Japan) on the day before administration. The cultured cells were then administered into the nasal submucosa or oral floor submucosa of each patient. The criteria for αGalCer-pulsed APCs administration included a negative bacterial culture 48 h prior to APCs administration, a cell viability of 60% or greater, and an endotoxin test with fewer than 0.7 Ehrlich units/ml, 48 h prior to APCs administration.
111In-labeled APC
Indium-111-oxine (111In) is widely used as a diagnostic radiopharmaceutical for radiolabeling autologous leukocytes (white blood cells). APCs labeled with 111In are routinely used to detect immunological events associated with antigen presentation. The labeling of APCs with 111In was performed according to the manufacturer’s protocol (Amersham Co. Ltd., UK). The αGalCer -pulsed APCs were resuspended in 10 ml phosphate-buffered saline (PBS) containing 37 Mbq 111In (T 1/2 = 2.8005 days). After incubation for 15 min, the APCs were washed with normal saline containing 5% autologous serum. The labeling efficacy was determined by measuring the radioactivity of the cellular fraction in comparison to that of the supernatant, and it was usually greater than 80%.
Administration of labeled/unlabelled APC
All patients received an injection of 1 × 108 APCs in 200 μl of PBS. Seven patients (001–007) were injected into the nasal submucosa, and three of these patients were injected with 111In-labeled APCs. Ten patients (008–017) were injected with APCs through the oral floor submucosa, and four of these patients were injected with 111In-labeled APCs (Table 1). The total body image and single photon emission computed tomography (SPECT) images were obtained 6, 24, and 48 h and 7 days after the administration of the labeled APCs to determine the APC migration kinetics. An image analysis was performed using a region-of-interest (ROI) analysis of the regional lymph nodes to obtain decay-corrected counts and geometric means for the anterior and posterior views.
Clinical protocol and study design
The present study, registered in the UMIN-Clinical Trial Registry as 000001933, was conducted at the Department of Head and Neck Surgery, Chiba University Hospital, Japan. Patients gave their written informed consent which was obtained from each of them before undergoing a screening evaluation to determine their eligibility for the study. The protocol was approved by the Institutional Ethics Committee. On day 0, 150 ml aliquots of peripheral blood were collected from each patient. The PBMCs were processed and pulsed with the antigen as described. The cells were resuspended in a final volume of 0.2 ml and injected into the nasal submucosa or oral submucosa. Each patient received an injection of 1 × 108 αGalCer-pulsed APCs. The injection site was the anterior portion of the unilateral inferior turbinate or the oral floor. A 27G needle and a 1 ml syringe were used for the injections. Extensive clinical and laboratory assessments were conducted weekly and included a complete physical examination and determination of standard laboratory values for 1 week. All patients underwent hematological assessments at the baseline time, and at 1 weeks after the first administration αGalCer-pulsed APCs.
APC phenotype evaluation
The APC phenotypes were determined using a FacsCaliber flow cytometer (BD Biosciences). The monoclonal antibodies (mAb) employed included FITC-labeled anti-HLA-DR, PE-labeled anti-CD86, APC-labeled anti-CD11c, FITC-labeled anti-CD83, and PE-labeled anti-CD1d (Becton–Dickinson, San Diego, CA, USA). Isotype-matched mAbs were used as negative controls. The phenotypes of the cultured cells containing APCs were analyzed by flow cytometry prior to each administration. The APC-rich population (large, granular lymphocyte fraction) was electronically gated by forward and side scatter parameters (FSC high SSC high).
Immunological monitoring
The PBMC samples were obtained by extracting 50 ml of blood at least two times before the administration of APCs, and weekly up to 1 week after the final treatment. The number of Vα 24+ Vβ11+ NKT cells in PBMCs was assessed by flow cytometry. Mononuclear cells were stained with FITC-conjugated anti-T cell receptor (TCR) Vα24 mAb (C15; Immunotech, Marseilles, France), PE-conjugated anti-TCR Vβ11 mAb (C21, Immunotech), and APC-conjugated anti-CD3 mAb (UCTH1; PharMingen) as previously described [8]. The stained cells were subjected to flow cytometry and the percentages of Vα24+ Vβ11+ CD3+ cells among mononuclear cells was calculated. The peripheral NKT cell numbers (counts/ml) were subsequently estimated based on the PBMCs cell counts. The remaining PBMCs were suspended in 5 × 105/ml in Cell Banker 2, and stored at −80°C.
Elispot assay of IFN-γ producing cells in PBMCs
Frozen PBMCs from each patient were thawed and cultured for 6 h in AIM-V. The cultured cells (1 × 105) were washed and transferred to an ELISPOT assay kit (BD Pharmingen) in 96-well filtration plates coated with anti-IFN-γ capture antibody for 16 h and cells were stimulated conditions with 100 ng/ml of αGalCer in AIM-V. After extensive washing with PBS, a biotinylated anti-human IFN-γ antibody was added. Two hours later, spots were detected with an avidin–biotin peroxidase complex and an aminoethyl carbazole solution. The mean values of the spots in three wells were utilized for the subsequent analyses. According to the ELISPOT assay protocol, the majority of the IFN-γ producing cells detected after αGalCer stimulation for 16 h were determined to be NKT cells. The number of spot-forming cells (SFCs) generated by vehicle stimulation were subtracted from the SFCs following αGalCer stimulation to calculate the number of IFN-γ-producing SFCs in the NKT cell population.
Intracellular staining
To determine the frequency of CD4+ CD25high Foxp3+ T cells and the expression of Treg markers, PBMCs (at least 1 × 106 cells or greater per tube) were stained with the following mAbs for 15 min at 4°C.
Intracellular staining was performed as previously described [9, 10] with the following modifications as follows: PBMCs (at least 1 × 106 cells per tube) were stained with mAbs against cell surface markers PerCP-Cy5.5-conjugated CD4 and PE-conjugated CD25 (Becton–Dickinson, San Diego, CA, USA) for 15 min at 4°C. Cells were washed and fixed with 4% (v/v) paraformaldehyde in PBS for 10 min at room temperature, washed twice with cold PBS, permeabilized with PBS containing 5 mM EDTA, 0.02% NaN3 and 0.5% Triton X. Nonspecific antigen-binding sites were blocked with 3% (v/v) BSA in PBS and stained with Alexa488-conjugated Foxp3 (eBiosience) mAb for 30 min at 4°C. Cells were washed twice and resuspended in fluorescence-activated cell sorting (FACS) flow solution and immediately analyzed by flow cytometry. Appropriate isotype controls were also run for each sample.
Statistical analysis
The differences between groups were assessed using the Mann–Whitney U test. Two-tailed adjusted P values of less than 0.05 were considered to be statistically significant.
Results
Characteristics of APCs
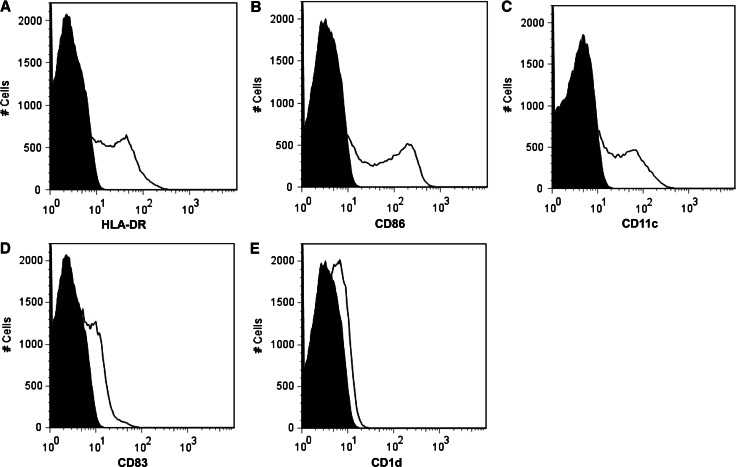
The surface phenotypes of αGalCer-pulsed APCs were analyzed by flow cytometry. The APCs profile of one representative case is shown in Fig. 1. In all preparations, the FSC-high, SSC-high cells exhibited a mature monocyte-derived APC phenotype, expressing HLA-DR, CD11c, CD86. The APC profiles of other patients are yielded similar results.
Fig. 1.
The histograms of APC surface markers are shown. The isotype-match control is shown in black. The expression of HLA-DR, CD86, CD11c, CD83, CD1d are represented in a, b, c, d, and e, respectively
Migration of APCs after the administration into nasal submucosa
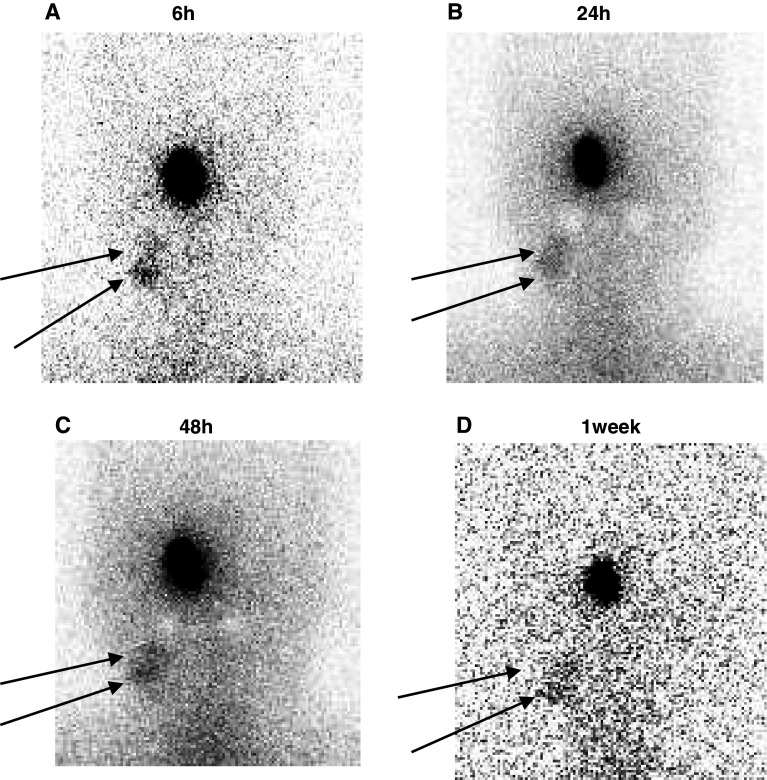
Three patients received an injection of radioactive labeled αGalCer-pulsed APCs into the nasal submucosa at the inferior turbinate. The representative anterior images of case 003 of the head and neck area of 6, 24, 48 h and 1 week after administration are shown in Fig. 2.
Fig. 2.
The patient received an injection of radioactively labeled αGalCer-pulsed APCs into the nasal submucosa. The anterior images of the head and neck area at 6, 24, 48 h and 1 week after administration are shown in a, b, c, and d, respectively
The spots were intensively confined to the neck region at the lateral (internal jugular) lymph nodes from 6 h until 1 week after administration. Once APCs arrived at the regional lymph node, they did not migrate to other lymph nodes. A similar pattern of migration was observed for all patients.
Migration of APCs after the administration into either the oral floor
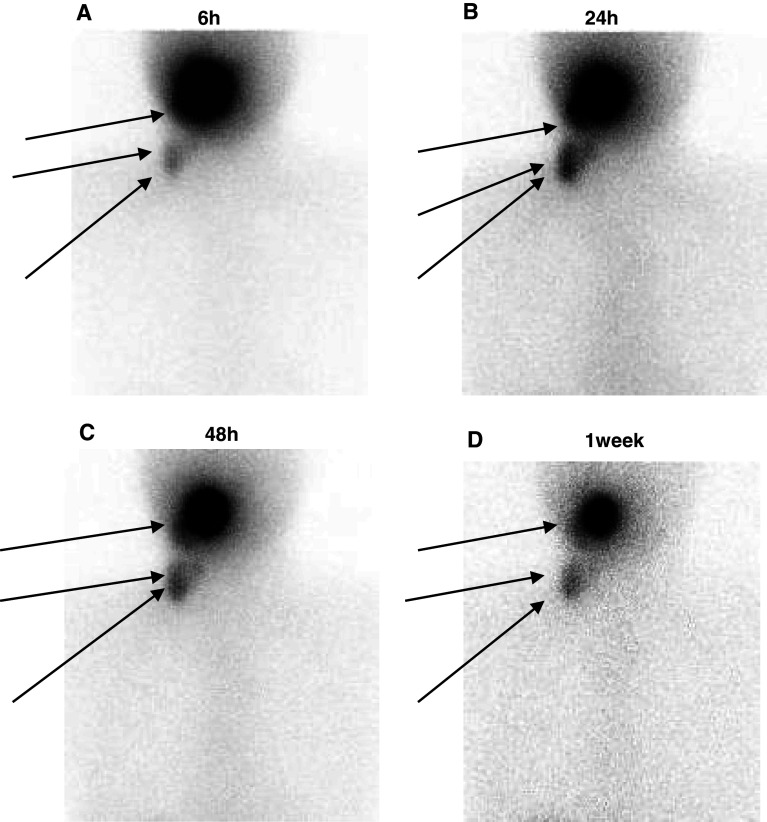
Four patients received an administration of radioactive labeled APCs into the oral floor submucosa. The anterior images from case 010 for the head and neck area 6, 24, 48 h and 1 week after administration are shown in Fig. 3.
Fig. 3.
The patient received an injection of radioactively labeled APCs into the oral floor submucosa. The anterior images of the head and neck area at 6, 24, 48 h and 1 week after administration are shown in Fig. 3 a, b, c, and d, respectively
APCs were detected in the submandibular and lateral lymph nodes. APCs in the submandibular lymph node were only detected in subjects who were injected via the oral floor submucosa. The migration to the submandibular lymph node therefore appeared to be a dramatic feature following APC administration into the oral floor submucosa. A similar pattern of migration was observed among all the patients.
ROI analysis
Figure 4 shows the isotope counts in the ROI analysis APC administration via the nose or oral floor among the neck lymph nodes. The total migration intensity from the oral floor submucosa to the regional lymph nodes was higher than migration that from the nasal submucosa.
Fig. 4.
The isotope counts in the region-of-interest analysis of the administration site and neck lymph nodes are shown. The isotope counts rapidly decreased, but some counts were observed in the neck lymph nodes at 6 h after administration, and the peak was observed after 48 h in the neck
Immunological monitoring
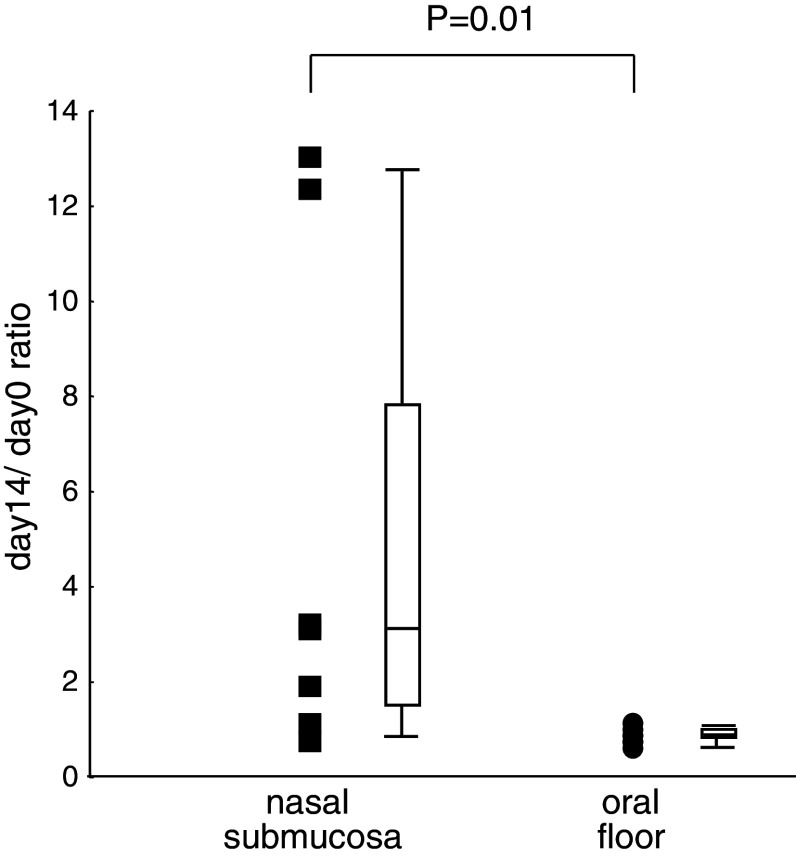
The day 14/day 0 ratio for the absolute numbers of peripheral circulating NKT cells in the patients who received APCs into the nasal submucosa or oral floor submucosa are shown in Fig. 5. The day 14/day 0 ratio for the number of peripheral blood NKT cells after the administration of αGalCer-pulsed APCs into the nasal submucosa was higher than that after the administration into the oral floor. A statistically significant difference was observed (P = 0.01).
Fig. 5.
The absolute numbers of peripheral blood Vα24+ Vβ11+ NKT cells were calculated using flow cytometry and automated full blood counts. The day 14/day 0 ratio of the number of peripheral blood NKT cells after the administration of αGalCer-pulsed APCs was calculated. All cases from 001 to 007, who were administered APCs into the nasal submucosa, and cases 008 to 017, who were administered APCs into the oral floor are shown as closed boxes and closed circle, respectively. A summary box plot has been attached on the side of either the boxes or circles
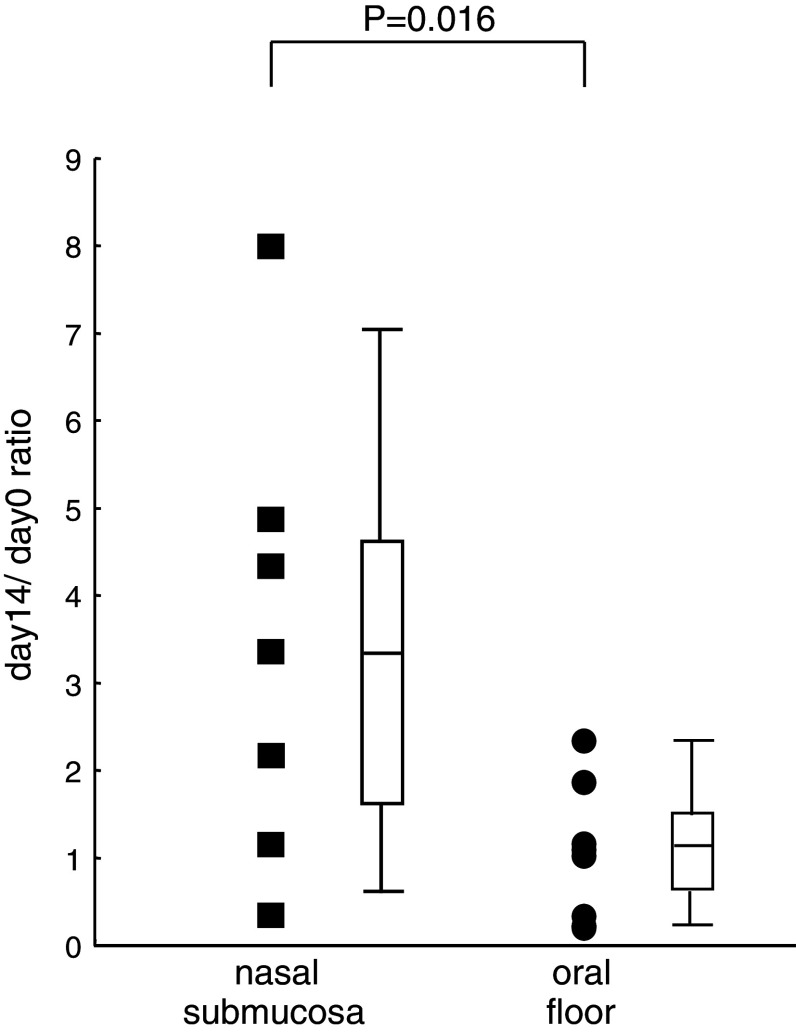
The activity of peripheral blood Vα24+ Vβ11+ NKT cells was measured as IFN-γ-spot-forming cells using the ELISPOT technique. The day 14/day 0 ratio for NKT cell function after the administration of αGalCer-pulsed APCs into the nasal submucosa was higher than that after the administration into the oral floor (Fig. 6). The differences in NKT activity were found to be statistically significant (P = 0.016).
Fig. 6.
The day 14/day 0 ratio of the number of IFN-γ-spot-forming cells after the administration of αGalCer-pulsed APCs is shown. All cases who were administered APCs into the nasal submucosa, and the cases who were administered APCs into the oral floor are shown as closed boxes and closed circle, respectively. A summary box plot has also been attached on the side of either the boxes or circles
The frequency of regulatory T cells in CD4
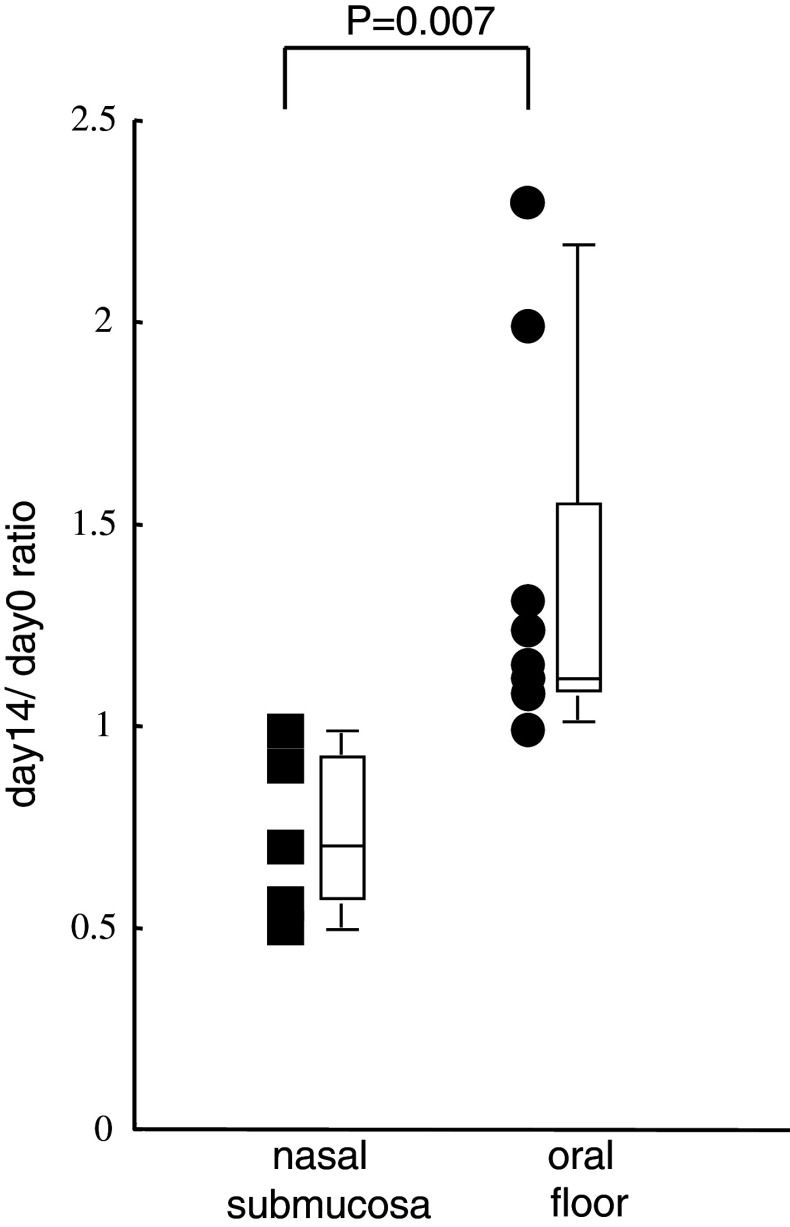
Flow cytometric analyses revealed the frequency of CD4+ CD25high Foxp3+ T cells (Tregs) to increase in the peripheral blood CD4+ T cell subset after APC administration into the oral floor submucosa in comparison to the nasal submucosa. (P = 0.007; Fig. 7). We quantified the surface expression of CD25high based on the percentage of Foxp3+, which was more than 96%. The percentage of Tregs in the whole lymphocytes ranged from 0.7 to 2.4%.
Fig. 7.
The day 14/day 0 ratio of the percentage of CD4+ CD25high Foxp3+ T cells (nTreg) from the CD4+ T cell subsets after administration into the nasal submucosa and oral floor submucosa is shown as closed boxes and closed circle, respectively. A summary box plot has also been attached on the side of either the boxes or circles
Discussion
APCs were found to quickly migrate to the lateral neck (internal jugular) lymph nodes after the administration into the nasal submucosa of the present series of patients. These results were consistent with a previous report with the tumor-specific peptide pulsed APCs [2]. However, after the administration of APCs into the oral floor submucosa, APCs tended to migrate to the submandibular lymph nodes rather than to the lateral lymph nodes, although the ROI peak radioactivity was observed 48 h after the intranasal submucosal administration was similar. The differences in the migration sites may therefore reflect the different anatomical regions, and the submandibular lymph nodes are close to the oral floor submucosa and act as draining sites.
Interestingly, a significant increase in the number of peripheral blood NKT cells and IFN-γ producing cells, which reflected the NKT activity, was observed after the administration of αGalCer-pulsed APCs into the nasal submucosa as reported in a previous study [2]; however, these anti-tumor activities could not be detected after the administration of APCs into the oral floor submucosa. It is well known that the nasal mucosa can directly absorb various substances and thereby become an inductive site of effective immune responses. The intranasal administration of vaccines such as the influenza virus vaccine is effective for mediating protection [11–13]. However, the oral submucosa is known to be “rich” in Langerhans-like DCs. Subsequent to antigen capture, these DCs would migrate to the proximal draining lymph nodes including the submandibular node[14].
There are numerous arguments regarding the phenotypes of DCs and the regulatory function of effector cells, including NKT cells. However, the oral submucosa is known to be “rich” in Langerhans-like DCs. Subsequent to antigen capture, these DCs are considered to migrate to the proximal draining lymph nodes, including the submandibular node [14]. Langerhans-like DCs are known to provide specialized microenvironments favoring the induction of mucosal tolerance and the induction of T cells with suppressive functions such as regulatory T cells (Treg) [15, 16].
NKT cell produces various cytokines, not only IFN-γ and IL-4, but also GM-CSF, TNFα and IL 2, 3, 5, 6, 9, 10, 13, 17, and 22 [17]. It is therefore very difficult to control such a large number of cytokines, but either IFN-γ or IL-4 production had been regulated to DC provided microenvironments in recent studies. Interleukin-12 production by DCs up regulates IFN-γ production by NKT cells [18, 19]. CD40-CD40L interactions also regulate IFN-γ and IL-4 production by NKT cells [20]. The rich Langerhans-like DCs microenvironments in the submandibular lymph nodes may therefore affect both the APC–NKT interactions and the IFN-γ productive activity of NKT cells.
Previous evidence has revealed that the administration of allergen into oral floor submucosa, known as sublingual immunotherapy (SLIT) for allergic diseases, can induce CD4+ CD25high Foxp3+ regulatory T cells (Tregs) and peripheral immunological tolerance, also induces apoptosis and the depletion of peripheral effector T cells [21–23]. The oral floor submucosa may be exploited as a region of immune tolerance [24–27], and a regulatory mechanism, such as Tregs may influence this type of these immunological modulation.
In the present study, αGalCer-pulsed APCs injected into the oral floor submucosa migrated to the submandibular lymph node, and no NKT cell upregulation was observed in the peripheral blood. This finding might due to the unique microenvironment of the submandibular lymph node. IL-2 produced by NKT cells induced in the submandibular lymph nodes following the migration of αGalCer-pulsed APCs might accelerate Treg activity [23, 28–30].
In conclusion, these results indicate that different administration routes of APCs have the potential to induce distinct immunological reactions. Nasal submucosal administration was able to induce immunological activation, but the submucosal administration of APCs into the oral mucosa tends to induce immunological suppression. As a result, further studies are necessary to clarify these important issues.
References
- 1.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 2.Horiguchi S, Matsuoka T, Okamoto Y, Sakurai D, Kobayashi K, Chazono H, Hanazawa T, Tanaka Y. Migration of tumor antigen-pulsed dendritic cells after mucosal administration in the human upper respiratory tract. J Clin Immunol. 2007;27(6):598–604. doi: 10.1007/s10875-007-9112-0. [DOI] [PubMed] [Google Scholar]
- 3.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 4.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human Va24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- 6.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, Taniguchi M, Nakayama T, Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Science. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motohashi S, Kobayashi S, Ito T, Magara KK, Mikuni O, Kamada N, Iizasa T, Nakayama T, Fujisawa T, Taniguchi M. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2003;102(2):159–165. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 9.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+ CD25+ Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178(1):320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Putnam AL, Zhou X, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St. Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illum L, Davis SS. Nasal vaccination: a non-invasive vaccine delivery method that holds great promise for the future. Adv Drug Deliv Rev. 2001;51(1–3):1–3. [PubMed] [Google Scholar]
- 12.Mendelman PM, Cordova J, Cho I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine. 2001;19(17–19):2221–2226. doi: 10.1016/S0264-410X(00)00449-7. [DOI] [PubMed] [Google Scholar]
- 13.Belshe RB, Nichol KL, Black SB, Shinefield H, Cordova J, Walker R, Hessel C, Cho I, Mendelman PM. Safety, efficacy, and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004;39(7):920–927. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- 14.Moingeon P, Batard T, Fadel R, Frati F, Sieber J, Van Overtvelt L. Immune mechanisms of allergen-specific sublingual immunotherapy. Allergy. 2006;61(2):151–165. doi: 10.1111/j.1398-9995.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 15.Aoyama-Kondo T, Yoshida T, Tsobe K, Nakayama A, Asai J, Oka T, Nakashima I. Characterization of antibody responses of local lymph nodes to antigen given under the oral submucosa. Immunobiology. 1992;184(4–5):372–383. doi: 10.1016/s0171-2985(11)80594-2. [DOI] [PubMed] [Google Scholar]
- 16.van Helvoort JM, Samsom JN, Chantry D, Jansen W, Schadee-Eestermans I, Thepen T, Mebius RE, Kraal G. Preferential expression of IgG2b in nose draining cervical lymph nodes and its putative role in mucosal tolerance induction. Allergy. 2004;59(11):1211–1218. doi: 10.1111/j.1398-9995.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 17.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA. 2008;105(32):11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 19.Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T. Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol. 2009;254(2):81–84. doi: 10.1016/j.cellimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M, Yahata T, Meng H, Koda T, Nishimura S, Kawano T, Taniguchi M, Nishimura T. alpha-Galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199(1):37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 21.Sun JB, Cuburu N, Blomquist M, Li BL, Czerkinsky C, Holmgren J. Sublingual tolerance induction with antigen conjugated to cholera toxin B subunit induces Foxp3+ CD25+ CD4+ regulatory T cells and suppresses delayed-type hypersensitivity reactions. Scand J Immunol. 2006;64(3):251–259. doi: 10.1111/j.1365-3083.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 22.Sun JB, Czerkinsky C, Holmgren J. Sublingual ‘oral tolerance’ induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand J Immunol. 2007;66(2–3):278–286. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 23.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+ CD25+ Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 24.Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, Betbeder D, Balazuc AM, Van Overtvelt L, Moingeon P. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122(3):603–609. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120(3):707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci USA. 2008;105(5):1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy and immunotherapy: implications for future treatment. J Allergy Clin Immunol. 2008;121(6):1344–1350. doi: 10.1016/j.jaci.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 28.La Cava A, Van Kaer L, Fu-Dong-Shi (2006) CD4+ CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol 27(7):322–327 [DOI] [PubMed]
- 29.Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+ CD25+ regulatory T cells? Eur J Immunol. 2005;35(4):1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- 30.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63(15):4516–4520. [PubMed] [Google Scholar]