Abstract
An ADP ribosylation factor-GTPase activating protein (ASAP1) is highly expressed in a variety of tumor cells and is involved in the cell motility, invasion, and metastasis. In order to elucidate the involvement of ASAP1 in lipopolysaccharide (LPS)-mediated inflammatory response, the effect of ASAP1 silencing on LPS-induced proinflammatory mediators production was examined by using RAW 264.7 macrophage-like cells. ASAP1 was constitutively expressed in the cells and the expression was augmented by LPS stimulation. Silencing of ASAP1 with small interfering RNA enhanced the production of tumor necrosis factor-α, interleukin 6, interferon-β, and nitric oxide in response to LPS. ASAP1 silencing augmented the activation of nuclear factor (NF)-κB and several mitogen-activated protein kinases (MAPKs). On the other hand, ASAP1 silencing did not affect the expression of IRAK4, TRAF6, and Akt as the upstream molecules of NF-κB signaling. A series of toll-like receptor ligands as well as LPS augmented the ASAP1 expression. Taken together, ASAP1 was suggested to negatively regulate LPS-induced proinflammatory mediators production through down-regulating LPS signaling. The feedback function of ASAP1 in LPS-mediated inflammatory response is discussed.
Keywords: ADP ribosylation factor-GTPase activating protein (ASAP1), Lipopolysaccharide (LPS), NF-κB, Toll-like receptor (TLR), RAW 264.7 cells
Introduction
An ADP ribosylation factor (Arf)-GTPase activating protein (ASAP1) functions on membrane surfaces to catalyze the hydrolysis of GTP bound to Arf. ASAP1 contains a tandem of BAR, pleckstrin homology, and Arf GAP domains, and contributes to the formation of invadopodia and podosomes [1]. Invadopodia (also called invasive feet) are frequently seen in metastatic cancer cells and required to invade surrounding tissues [2]. Structurally, podosomes are also simillar to invadopodia formed by normal cells that need to cross tissue barriers, such as macrophage and monocytes, or cells such as osteoclasts that remodel tissue [3]. Both podosomes and invadopodia are thought to contribute to cellular invasiveness in physiological and pathological situations [2]. ASAP1 also play a role in the regulation of membrane remodeling and cytoskeletal organization as well as cellular migration, and tumor invasion, and metastasis [4–6]. However, the function of ASAP1 is not yet characterized in other fields except oncology.
Lipopolysaccharide (LPS) is a component of the outer membrane of gram-negative bacteria that is recognized by toll-like receptor (TLR) 4, and triggers the activation of series of signaling pathways containing nuclear factor (NF)-κB, mitogen-activated protein kinases (MAPKs), and phosphoinositide 3 kinase (PI3K) [7]. Subsequently, LPS activates the responsive genes and leads to the production of a number of proinflammatory mediators, such as tumor necrosis factor-α (TNF-α), nitric oxide (NO), and interleukins (ILs) [8]. Excessive production of proinflammatory mediators causes systemic inflammatory response syndrome, endotoxic shock, and multiorgan failure. Recently, we have demonstrated that several tumor-associated genes are LPS-responsive and the products regulate LPS-induced inflammatory response [9, 10]. In the present study, we studied the involvement of ASAP1 in the regulation of LPS-induced proinflammatory mediators production in RAW 264.7 macrophage-like cells. Here, we report that ASAP1 is LPS-responsive and negatively regulates LPS-induced inflammatory mediators production through down-regulating the NF-κB and MAPKs activation.
Materials and methods
Materials
LPS from Escherichia coli O55: B5 was purchased from Sigma Chemicals (St Louis, MO, USA). A rabbit polyclonal antibody to ASAP1 was obtained from Abcam (Cambridge, UK). A series of antibodies to p65 NF-κB, p38, stress-activated protein kinase (SAPK)/JNK, extracellular signal-regulated kinase (ERK) 1/2, IκB kinase (IKK)-β, Akt and their phosphorylated forms, and horseradish peroxidase-conjugated anti-rabbit IgG were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies to IRAK4 and TRAF6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Pam3Cys was obtained from Calbiochem (San Diego, CA, USA). Poly I:C and imiquimod were purchased from Invivogen (San Diego, CA, USA). CpG DNA was synthesized by Rikaken (Nagoya, Japan).
Cell culture
The murine macrophage-like cell line, RAW 264.7, was obtained from the Riken Cell Bank (Tsukuba, Japan) and maintained in Eagle’s minimum essential medium (MEM) (Sigma–Aldrich, St. Louis, MO, USA) containing 5% decomplemented fetal calf serum (FCS, Gibco–BRL, Gaithersburg, MD, USA), non essential amino acid (Invitrogen, Carlsbad, CA, USA) and antibiotic cocktail from Sigma Chemicals (penicillin G, streptomycin and amphotericin B) at 37°C under 5% CO2.
Determination of TNF-α, IL-6, and interferon (IFN)-β
Transfected and untreated RAW 264.7 cells were stimulated with LPS (100 ng/ml) for 6 h and then concentrations of TNF-α, IL-6 (Invitrogen), and IFN-β (PBL Interferon Source, NJ, USA) in the culture supernatant were determined by enzyme-linked immunosorbent assay (ELISA) kit.
Determination of nitrite concentration
Nitrite, the end product of NO metabolism, was measured using the Griess reagent as described elsewhere [11]. Briefly, RAW 264.7 cells were transfected with ASAP1 or control siRNA and then transfected and untreated cells were stimulated with LPS (100 ng/ml) or Pam3Cys (10 μg/ml). The nitrite concentration in the culture supernatant was determined 24 h after the stimulation with reference to the standard curve.
Immunoblotting
Immunoblotting was performed as described previously [12]. Briefly, the cell lysates were extracted by the lysis buffer. The protein concentration of each sample was determined by the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA). Equal amounts of protein were subjected to analyze by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions. The proteins were electrically transferred to a membrane and the membranes were treated with a series of appropriately diluted antibodies. The immune complexes were detected with horseradish peroxidase-conjugated second antibody at 1 : 2,000 for 1 h. The protein bands were visualized using a chemiluminescence reagent (Pierce). The chemiluminescence was detected by a light capture system analyzer AE6955 (Atto, Tokyo, Japan). For reprobing, the membranes were stripped with the restore western blot striping buffer (Thermo Scientific, Rockford, IL, USA) for 15 min and treated with corresponding antibodies. The molecular sizes of the antigens were determined by comparison with a prestained protein size marker kit (Invitrogen). To quantify the expression of each molecule, an equal area of each band image on an immunoblotted membrane was gated and calculated as the intensity of band by ImageJ Software (NIH, USA).
Reverse transcription (RT)–polymerase chain reaction (PCR) analysis
The RT–PCR was performed as described previously [13]. Briefly, RNA was extracted from the cells with a RNeasy mini kit (Qiagen, Valencia, CA, USA). Semi-quantitative RT–PCR was carried out by using the Access Quick RT–PCR system (Promega, Madison, WI, USA). Primers were obtained from Invitrogen with the following sequences: ASAP1, forward 5′-CAGAGACGGAAGTGTGCGGT-3′ and reverse 5′-CCAGCAAGAGTTCGGAAGTCCCT-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-ATGGGGAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GCTGATGATCTTGAGGCTGTTGTC-3′. GAPDH was used as an equal loading control. Optimized RT–PCR conditions were 48° for 45 min followed by 95° for 2 min and 25 cycles at 95° for 45 s, 60° for 45 s, 72° for 60 s. The PCR products were analyzed by electrophoresis on 1.5% agarose gel. The gels were stained with CYBR safe DNA gel stain (Molecular Probe, Eugene, OR, USA) and visualized under an ultraviolet transilluminator. The 100 base pair DNA size marker (Invitrogen) was also run to determine the approximate size of the product.
Transfection of small interfering RNA (siRNA)
ASAP1-specific siGENOME SMART pool and a nontargeting siRNA were obtained from Dharmacon (Chicago, IL, USA). RAW 264.7 cells were seeded at a concentration of 2 × 105 cells/well in a 24-well culture plate in complete growth medium with FCS and antibiotics. After 3–4 h incubation under normal growth condition, the cells were transfected with Hiperfect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Briefly, ASAP1 or control siRNA (400 ng) was diluted in 100 μl/well OPTI–MEM medium (Gibco–BRL) and cationic lipid complexes were prepared by incubating 10–15 min with 6 μl/well Hiperfect transfection reagent. After 6–8 h of incubation, additional 400 μl culture medium containing FCS and antibiotics were added to each well and incubated for 48 h. Finally, transfected and untreated cells were stimulated with LPS (100 ng/ml) for 45 min and the efficiency of ASAP1 silencing was evaluated by immunoblotting and PCR, respectively.
NF-κB-dependent luciferase assay
RAW 264.7 cells were transfected with ASAP1 or control siRNA and incubated for 48 h. The cells were further transfected with 500 ng/well of NF-κB-Taluc luciferase reporter gene (Invitrogen) and an equal amount of pRL-TK plasmid (Promega) by FuGene HD transfection reagent (Roche Applied Science, Mannheim, Germany) and incubated for 48 h. The transfected and untreated cells were stimulated with LPS (100 ng/ml) for 6 h. After treatment with a lysis reagent, the luciferase activity was determined with the dual luciferase assay kit (Promega). The NF-κB-dependent luciferase activity in the cell lysates was determined with a luminometer. The fold increase was calculated based on the untreated control.
Statistical analysis
Experimental values are represented as the mean ± standard deviation from at least three independent experiments. The significance of differences between experimental and control groups was determined by the Student’s t test. A value of P < 0.01 was considered statistically significant.
Results
LPS augments a constitutive expression of ASAP1 in RAW 264.7 macrophage-like cells
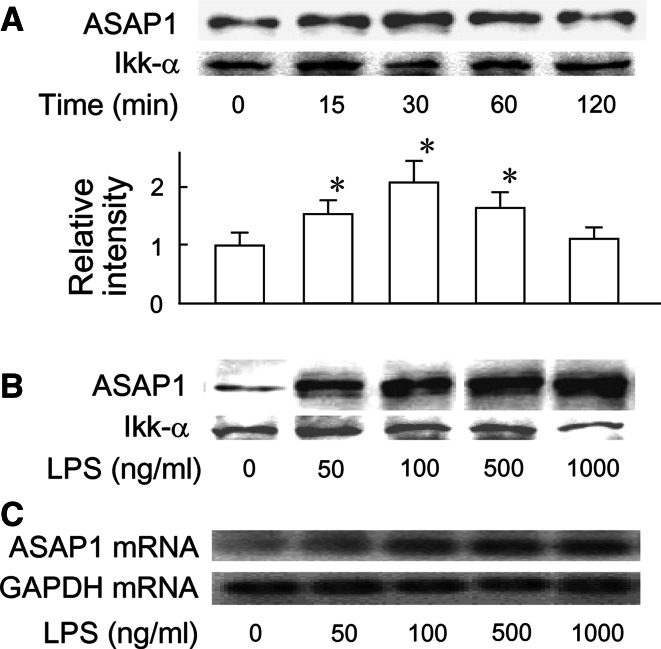
The expression of ASAP1 in RAW 264.7 macrophage-like cells and its responsiveness to LPS was examined (Fig. 1). The cells were incubated with LPS (100 ng/ml) in various time periods and the expression of ASAP1 protein was determined by immunoblotting. ASAP1 was detected in untreated control cells and was significantly augmented by LPS stimulation. The densitometric analysis demonstrated that the ASAP1 protein expression was approximately twice as high as untreated control 30 min after LPS treatment and declined at 120 min (Fig. 1a). Next, the cells were stimulated with various concentrations of LPS for 30 min. LPS-induced expression of ASAP1 protein and mRNA was analyzed by immunoblotting and RT–PCR, respectively. The expression of ASAP1 protein and mRNA was significantly upregulated by LPS (50 ng/ml) and further augmented by higher concentrations of LPS (Fig. 1b, c). The ASAP1 protein expression at LPS (100 ng/ml) was at an approximately threefold higher level of that in untreated control. Collectively, ASAP1 was constitutively expressed in RAW 264.7 cells and the expression was significantly augmented by LPS stimulation. In addition, ASAP1 was expressed in murine resident peritoneal macrophages as well as RAW 264.7 cells and it was also augmented by LPS (data not shown).
Fig. 1.
Augmentation of ASAP1 expression by LPS. a RAW 264.7 cells were incubated with LPS (100 ng/ml) for various time periods. b and c The cells were incubated with different concentrations of LPS for 30 min. The expression of ASAP1 protein and mRNA was analyzed by immunoblotting (a, b) and RT–PCR (c), respectively. A typical result of three independent experiments is shown
Silencing of ASAP1 with siRNA augments the production of proinflammatory mediators in response to LPS
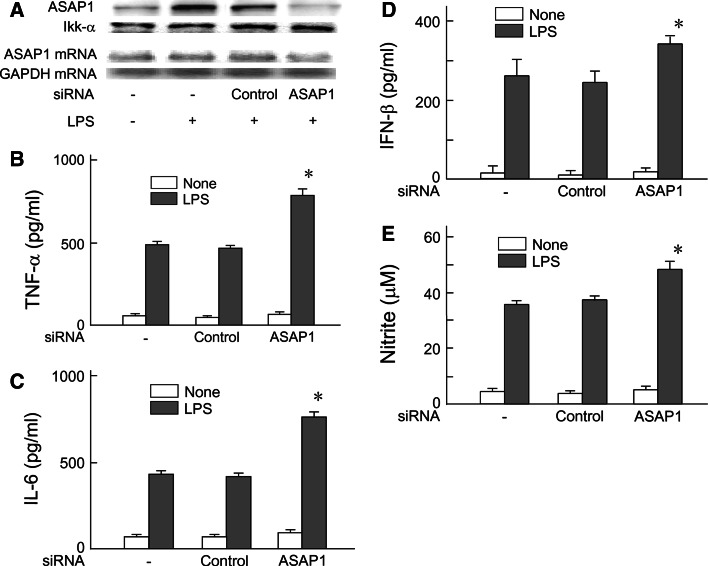
The effect of ASAP1 silencing on LPS-induced proinflammatory mediators production was examined (Fig. 2). First, we confirmed the effect of ASAP1 siRNA on the expression of ASAP1 protein and mRNA. As shown in Fig. 2a, the ASAP1 expression was almost completely abolished by ASAP1 siRNA. On the other hand, control siRNA did not affect the expression of ASAP1 protein and mRNA. ASAP1-specific siRNA was shown to be effective for ASAP1 silencing.
Fig. 2.
Augmentation of LPS-induced proinflammatory mediators production by ASAP1 silencing. a RAW 264.7 cells were transfected with ASAP1 siRNA or control siRNA for 48 h and then stimulated with LPS (100 ng/ml) for 45 min. The expression of ASAP1 protein and mRNA was analyzed with immunoblotting (top) and RT–PCR (bottom), respectively. A typical result of three independent experiments is shown. b, c and d ASAP1 or control siRNA-transfected cells were stimulated with LPS (100 ng/ml) for 6 h. The levels of TNF-α, (b) IL-6, (c) and IFN-β (d) in the culture supernatant were determined with ELISA. e ASAP1 or control siRNA-transfected cells were stimulated with LPS (100 ng/ml) for 24 h. The level of nitrite in the culture supernatant was determined by Griess reagent. *P < 0.01 versus control siRNA
ASAP1 or control siRNA-transfected and untreated cells were stimulated with LPS (100 ng/ml) for 6 h and the levels of TNF-α and IL-6 in the culture supernatants were determined by ELISA. Silencing of ASAP1 markedly augmented the production of both TNF-α (Fig. 2b) and IL-6 (Fig. 2c) in response to LPS although control siRNA failed to do so. The effect of ASAP1 silencing on LPS-induced IFN-β and NO production was also determined to elucidate the involvement of MyD88-independent pathway. The transfected and untreated cells were stimulated with LPS (100 ng/ml) for 6 and 24 h to determine IFN-β and NO production, respectively. LPS-induced IFN-β and NO production were augmented by ASAP1 silencing (Fig. 2d, e). ASAP1 was suggested to negatively regulate LPS-induced proinflammatory mediators production because of the augmentation by ASAP1 silencing.
Silencing of ASAP1 augments the activation of NF-κB in response to LPS
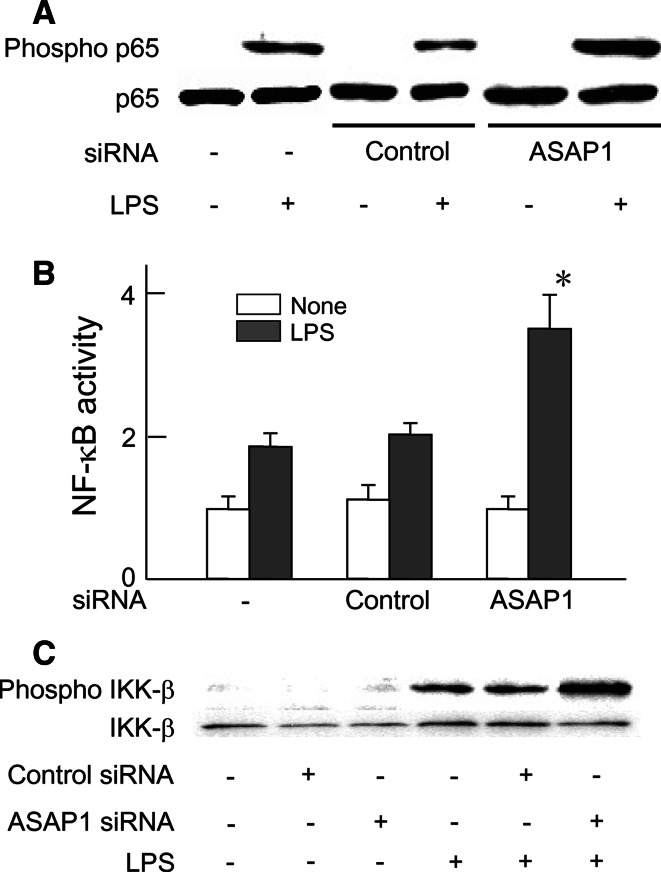
In order to elucidate augmented cytokines production by ASAP1 silencing, the effect of ASAP1 silencing on LPS-induced NF-κB activation was examined (Fig. 3). The cells were transfected with ASAP1 or control siRNA, and then transfected and untreated cells were stimulated with LPS (100 ng/ml) for 45 min. The phosphorylation of p65 NF-κB was determined with immunoblotting. ASAP1 silencing significantly augmented the phosphorylation of p65 NF-κB (Fig. 3a). The phosphorylated p65 expression in ASAP1 silencing was at an approximately twofold higher level of that in control siRNA. The effect of ASAP1 siRNA on LPS-induced NF-κB activation was also examined with the luciferase activity. ASAP1 or control siRNA-transfected and untreated RAW 264.7 cells were further transfected with NF-κB luciferase reporter gene with internal control palsmid and then stimulated with LPS (100 ng/ml) for 6 h. LPS significantly enhanced the NF-κB-dependent luciferase activity in untreated normal cells and control siRNA-transfected cells. In ASAP1 siRNA-transfected cells, LPS augmented the NF-κB-dependent luciferase activity more than that in control siRNA-transfected cells or untreated control cells (Fig. 3b). Since ASAP1 silencing enhanced LPS-induced NF-κB activation, the effect of ASAP1 silencing on the activation of IKK-β, an upstream kinase of IκB in the NF-κB activation was examined with immunoblotting. The cells were transfected with control or ASAP1 siRNA and then stimulated with LPS (100 ng/ml) for 45 min. LPS-induced IKK-β phosphorylation in ASAP1 siRNA-transfected cells was approximately twice as high as that in control siRNA-transfected cells (Fig. 3c).
Fig. 3.
Augmentation of LPS-induced NF-κB activation by ASAP1 silencing. a and c ASAP1 or control siRNA-transfected cells were treated with LPS (100 ng/ml) for 45 min and the phosphorylation of p65 NF-κB or IKK-β in whole cell extract was analyzed by immunoblotting with anti-phosphorylated form antibody. A typical result of three independent experiments is shown. b ASAP1 or control siRNA-transfected RAW 264.7 cells were further transfected with a luciferase reporter gene and then stimulated with LPS (100 ng/ml) for 6 h and the luciferase activity was determined using a luminometer. *P < 0.01 versus control siRNA
Silencing of ASAP1 enhances LPS-induced phosphorylation of p38 and SAPK but not ERK1/2 MAPKs
The activation of MAPKs, such as p38, SAPK, and ERK/1/2, is also involved in LPS-induced cytokines production [14–16]. Therefore, the effect of ASAP1 silencing on LPS-induced activation of a series of MAPKs was examined. The cells were transfected with control or ASAP1 siRNA and then stimulated with LPS (100 ng/ml) for 45 min. The phosphorylation of a series of MAPKs were determined by immunoblotting. ASAP1 silencing augmented LPS-induced phosphorylation of p38 and SAPK, but not ERK1/2 compared with control siRNA (Fig. 4). The expression of phosphorylated p38 and SAPK in ASAP1 siRNA-transfected cells was at an approximately three and twofold higher level of that in control siRNA-transfected cells, respectively.
Fig. 4.
Augmentation of LPS-induced MAPKs activation by ASAP1 silencing. ASAP1 or control siRNA-transfected RAW 264.7 cells were stimulated with LPS (100 ng/ml) for 45 min and the phosphorylation of p38, SAPK, and ERK1/2 was analyzed with immunoblotting. A typical result of three independent experiments is shown
Silencing of ASAP1 does not affect the expression of IRAK4, TRAF6, and the activation of Akt
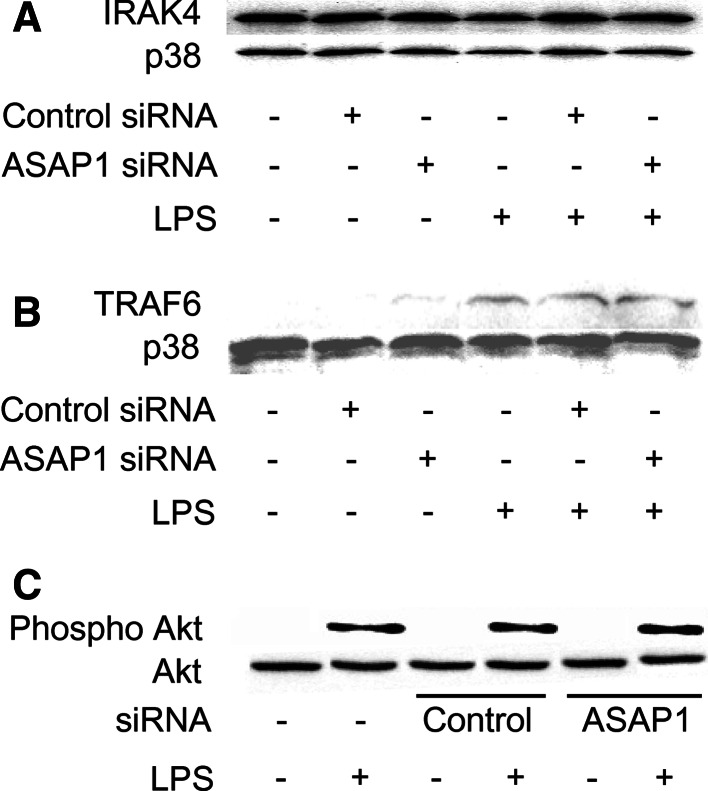
Since ASAP1 silencing augmented the activation of NF-κB and MAPKs in response to LPS, the effect of ASAP1 siRNA on the activation of their upstream signaling molecules, such as IRAK4 and TRAF6 [17], was examined. The cells were transfected with ASAP1 or control siRNA, and then stimulated with LPS (100 ng/ml) for 45 min. The expression of IRAK4 and TRAF6 was examined by immunoblotting. Silencing of ASAP1 did not affect in the expression of IRAK4 and TRAF6 (Fig. 5a, b). Moreover, PI3K triggers LPS-induced NF-κB activation via Akt [18]. Therefore, the effect of ASAP1 silencing on LPS-induced Akt phosphorylation was examined with immunoblotting (Fig. 5c). There was no significant difference in the Akt phosphorylation between the cells transfected with ASAP1 siRNA and control siRNA, suggesting that ASAP1 might not affect the LPS signaling from the cell surface receptors to TRAF6.
Fig. 5.
Effect of ASAP1 silencing on LPS-induced expression of IRAK4, TRAF6, and activation of Akt. ASAP1 or control siRNA-transfected RAW 264.7 cells were stimulated with LPS (100 ng/ml) for 45 min, and the expression of IRAK4 (a), TRAF6 (b), and the phosphorylation of Akt (c) were analyzed with immunoblotting. A typical result of three independent experiments is shown
Various TLR ligands augment the expression of ASAP1
A series of toll-like receptor (TLR) ligands, such as TLR2, TLR7, and TLR9 ligands, as well as LPS as a TLR4 ligand utilize MyD88-dependent signaling pathway to activate NF-κB in the early stage whereas TLR3 ligand utilizes TRIF-mediated signaling pathway and activates NF-κB in the late stage [19]. The effect of a series of TLR ligands on the expression of ASAP1 was examined. The cells were stimulated with various TLR ligands for 30 min, and then expression of ASAP1 protein was determined by immunoblotting. A series of TLR ligands enhanced the ASAP1 expression (Fig. 6a). The ASAP1 expression on Pam3 stimulation was approximately twice as high as that in untreated control. Next, the effect of Pam3cys as a TLR2 ligand on the NO production was examined in ASAP1-silenced cells (Fig. 6b). Pam3cys augmented the NO production in ASAP1 siRNA-transfected cells more than that in control siRNA-transfected cells.
Fig. 6.
Augmentation of ASAP1 expression and NO production by various TLR ligands. a RAW 264.7 cells were stimulated with polyI:C (TLR3 ligand, 10 μg/ml), pam3cys (TLR2 ligand, 10 μg/ml), imiquimoid (TLR7 ligand, 10 μg/ml) or CpG-DNA (TLR9 ligand, 2.5 μg/ml) for 30 min. The ASAP1 expression was analyzed with immunoblotting. A typical result of three independent experiments is shown. b ASAP1 or control siRNA-transfected RAW 264.7 cells were stimulated with Pam3cys (10 μg/ml) for 24 h. The level of nitrite in the culture supernatant was determined by Griess reagent. *P < 0.01 versus control siRNA
Discussion
In the present study, we have demonstrated that ASAP1 negatively regulates LPS-induced proinflammatory mediators production in macrophage-like cells via down-regulation of NF-κB and MAPKs activation and further that ASAP1 is constitutively expressed and augmented by LPS stimulation. We for the first time demonstrate that ASAP1 may be involved in LPS-mediated inflammatory response as well as tumor progression. Moreover, a series of TLR ligands as well as LPS augments the expression of ASAP1 and the silencing of ASAP1 augments the production of proinflammatory mediators in response to LPS or Pam3cys. Therefore, ASAP1 is suggested to negatively regulate inflammatory responses in macrophages in response to a variety of stimuli including TLR ligands. Once again, ASAP1 might be an important negative regulator in LPS-mediated inflammatory response.
The present study demonstrates that ASAP1 inhibits NF-κB activation and further IKK-β as an upstream molecule of NF-κB. Moreover, ASAP1 inhibits the activation of p38 and SAPK/JNK in response to LPS. In LPS signaling, NF-κB and MAPKs are activated via TRAF6, which is triggered by IRAK4 [17]. However, ASAP1 silencing affects the expression of neither TRAF6 nor IRAK4. Therefore, ASAP1 seems to down-regulate the function of TRAF6 regulating NF-κB and MAPKs without affecting TRAF6 expression. The NF-κB activation is also mediated by Akt via PI3K [18]. However, ASAP1 silencing does not affect the activation of Akt, excluding involvement of Akt. It is still unclear the precise mechanism how ASAP1 inhibits the activation of NF-κB and MAPKs without affecting the TRAF6 expression.
The present study suggests that ASAP1 may inhibit both MyD88-dependent and MyD88-independent pathway in LPS signaling. LPS-induced TNF-α and IL-6 production is mainly MyD88-dependent whereas the IFN-β and NO production is mainly MyD88-independent [20, 21]. ASAP1 silencing significantly augments their production. If ASAP1 acts on both MyD88-dependent and MyD88-independent pathways at the common site, ASAP1 might inhibit LPS signaling just under TLR4.
Activation of NF-κB has been found to control multiple cellular processes in cancer including inflammation, transformation, proliferation, angiogenesis, invasion, metastasis, chemoresistance, and radioresistance [22]. NF-κB is constitutively active in most tumor cells and its suppression inhibits the growth of tumor cells via apoptosis [23]. A hypothetical idea is raised that ASAP1 may down-regulate persistent NF-κB activation in tumor cells for regulation of cell growth and that additionally it may inhibit the NF-κB activation for proinflammatory mediators production in response to LPS. In fact, we for the first time demonstrate the inhibition of the NF-κB activation in inflammatory response by ASAP1. ASAP1 might be an important NF-κB regulator in inflammatory response as well as tumor progression.
ASAP1 is reported to be related to the differentiation of monocytes [24]. The expression of ASAP1 is upregulated when monocyte-like U937 cells differentiate into macrophage-like cells upon phorbol ester [24]. The present study demonstrates that ASAP1 is expressed in RAW 264.7 macrophage-like cells and is augmented in their activation by LPS. Moreover, ASAP1 is expressed in physiologic peritoneal macrophages. ASAP1 might be involved in the differentiation and activation of macrophage-lineage cells. The role of ASAP1 in the differentiation and activation of macrophages is still a matter for speculation.
ASAP1 functions on membrane surfaces to catalyze the hydrolysis of GTP bound to Arf. ASAP1 contains a tandem of BAR, pleckstrin homology, and Arf GAP domains and contribute to cellular invasiveness through the formation of invadopodia or podosomes in physiological and pathological situations [1, 2]. Further, ASAP1 has been shown to play a role in the regulation of membrane remodeling and cytoskeletal organization, as well as cellular migration and tumor invasion and metastasis [4–6]. However, it is unclear how the inhibitory action of ASAP1 against inflammatory response is related to the invasiveness and metastasis of cancer cells, or the membrane remodeling and cytoskeletal organization. ASAP1 might be involved in a close crosstalk among various cellular functions.
In summary, ASAP1 is suggested to negatively regulate an excessive production of proinflammatory mediators via downregulation of LPS signaling. LPS-induced ASAP1 expression might be a feedback mechanism in LPS-induced inflammatory response. ASAP1 might be a putative target for prevention of LPS-mediated cell injury or tissue damages.
Acknowledgments
This work was supported by in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We are grateful to K. Takahashi and A. Morikawa for the technical assistance.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
- ASAP1
ADP ribosylation factor (Arf)-GTPase activating protein
- ERK1/2
Extracellular signal-regulated kinase 1/2
- ELISA
Enzyme-linked immunosorbent assay
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- IFN-β
Interferon-β
- IKK
IκB kinase
- IRAK4
Interleukin-1 receptor-associated kinase-4
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MyD88
Myeloid differentiation factor 88
- NF-κB
Nuclear factor-κB
- NO
Nitric oxide
- RT–PCR
Reverse transcription-polymerase chain reaction
- SAPK
Stress-activated protein kinase
- siRNA
Small interfering RNA
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-α
- TRAF6
TNF receptor-associated factor 6
References
- 1.Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem. 2009;284:1652–1663. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Linder S, Aepfelbacher M. Podosomes: adhesion hotspots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 4.Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Yerushalmi GM, Grigera PR, Parsons JT. Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J Biol Chem. 2005;280:8884–8892. doi: 10.1074/jbc.M412200200. [DOI] [PubMed] [Google Scholar]
- 6.Muller T, Stein U, Poletti A, Garzia L, Rothley M, Plaumann D, Thiele W, Bauer M, Galasso A, Schlag P, Pankratz M, Zollo M, Sleeman JP. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene. 2010;29:2393–2403. doi: 10.1038/onc.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Noman AS, Koide N, Iftakhar-E-Khuda I, Dagvadorj J, Tumurkhuu G, Naiki Y, Komatsu T, Yoshida T, Yokochi T. Retinoblastoma protein-interacting zinc finger 1, a tumor suppressor, augments lipopolysaccharide-induced proinflammatory cytokine production via enhancing nuclear factor-κB activation. Cell Immunol. 2010;264:114–118. doi: 10.1016/j.cellimm.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Khuda II, Koide N, Noman AS, Dagvadorj J, Tumurkhuu G, Naiki Y, Komatsu T, Yoshida T, Yokochi T. Astrocyte elevated gene-1 (AEG-1) is induced by lipopolysaccharide as toll-like receptor 4 (TLR4) ligand and regulates TLR4 signalling. Immunology. 2009;128:700–706. doi: 10.1111/j.1365-2567.2009.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 12.Haque A, Koide N, Iftakhar-E-Khuda I, Noman AS, Odkhuu E, Badamtseren B, Naiki Y, Komatsu T, Yoshida T, Yokochi T. Flavopiridol inhibits lipopolysaccharide-induced tumor necrosis factor-α production through inactivation of nuclear factor-κB and mitogen-activated protein kinases in the MyD88-dependent pathway. Microbiol Immunol. 2011;55:160–167. doi: 10.1111/j.1348-0421.2010.00304.x. [DOI] [PubMed] [Google Scholar]
- 13.Hassan F, Islam S, Tumurkhuu G, Naiki Y, Koide N, Mori I, Yoshida T, Yokochi T. Intracellular expression of toll-like receptor 4 in neuroblastoma cells and their unresponsiveness to lipopolysaccharide. BMC Cancer. 2006;8:281–288. doi: 10.1186/1471-2407-6-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saklatvala J, Davis W, Guesdon F. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond B Biol Sci. 1996;351:151–157. doi: 10.1098/rstb.1996.0011. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 16.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki N, Saito T. IRAK-4—a shared NF-κB activator in innate and acquired immunity. Trends Immunol. 2006;27:566–572. doi: 10.1016/j.it.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill LA, Bowie AG. The family of five: TIR-domaincontaining adaptors in Toll-like receptor signaling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 20.Kamijo R, Harada H, Matsuyama T, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 21.Perrella MA, Patterson C, Tan L, Yet SF, Hsieh CM, Yoshizumi M, Lee ME. Suppression of interleukin-1beta-induced nitric-oxide synthase promoter/enhancer activity by transforming growth factor-beta1 in vascular smooth muscle cells. Evidence for mechanisms other than NF-kappaB. J Biol Chem. 1996;271:13776–13780. doi: 10.1074/jbc.271.23.13776. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staudt LM. Oncogenic activation of NF-κB. Cold Spring Harb Perspect Biol. 2010;2:1–30. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata M, Raven JF, Baltzis D, Koromilas AE, Sabe H. IRES-mediated translational control of AMAP1 expression during differentiation of monocyte U937 cells. Cell Cycle. 2008;7:3273–3281. doi: 10.4161/cc.7.20.6883. [DOI] [PubMed] [Google Scholar]