Abstract
Cancer vaccines have been developed to instruct the endogenous immune responses to autologous tumors and to generate durable clinical responses. However, the therapeutic benefits of cancer vaccines remain insufficient due to the multiple immunosuppressive signals delivered by tumors. Thus, to improve the clinical efficacy of cancer immunotherapy, it is important to develop new modalities to overcome immunosuppressive tumor microenvironments and elicit effective antitumor immune responses. In this study, we show that novel monoclonal antibodies (mAbs) specifically targeting either T cell immunoglobulin mucin protein-3 (TIM-3) or T cell immunoglobulin mucin protein-4 (TIM-4) enhance the therapeutic effects of vaccination against established B16 murine melanomas. This is true for vaccination with irradiated B16 melanoma cells engineered to express the flt3 ligand gene (FVAX). More importantly, combining anti-TIM-3 and anti-TIM-4 mAbs markedly increased vaccine-induced antitumor responses against established B16 melanoma. TIM-3 blockade mainly stimulated antitumor effector activities via natural killer cell-dependent mechanisms, while CD8+ T cells served as the main effectors induced by anti-TIM-4 mAb. Our findings reveal that therapeutic manipulation of TIM-3 and TIM-4 may provide a novel strategy for improving the clinical efficacy of cancer immunotherapy.
Keywords: TIM-3, TIM-4, Cancer vaccines, NK cells, CD8+ T cells, Melanoma
Introduction
Cancer vaccines have been developed with the aim of efficiently inducing tumor-specific cytotoxic lymphocytes and controlling tumor growth [1, 2]. However, the clinical efficacies of cancer vaccines remain unsatisfactory at present, as hostile tumor microenvironments exploit multiple strategies to counter antitumor immune responses induced by cancer vaccines [3, 4]. Thus, new strategies to overcome immunosuppressive barriers and improve the therapeutic effects of cancer vaccines are urgently needed.
Tumor microenvironments suppress tumor-specific immune responses and impair tumor immunosurveillance by coordinating with negative checkpoint regulators expressed on tumor-infiltrating lymphocytes [5, 6] such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), lymphocyte activation gene-3 (LAG-3), BTLA (B and T lymphocyte attenuator) or T cell immunoglobulin mucin protein-3 (TIM-3) [7–12]. Indeed, the recent clinical success of anti-CTLA-4 and anti-PD-1 monoclonal antibodies (mAbs) in vastly improving the prognosis and survival of patients with advanced cancers has substantiated the major impact of these inhibitory pathways in the negative regulation of antitumor immune responses and clinical prognosis [13–15]. Thus, it is critical to address whether therapeutic manipulation of immune checkpoint regulators other than CTLA-4 or PD-1 can control tumor growth and enhance antitumor immune responses.
TIM-3 is a key checkpoint receptor responsible for T cell exhaustion, which arises during the chronic phase of infections and cancer, and blockade of TIM-3 restores the antigen-specific effector activities of CD8+ T lymphocytes [16, 17]. Pharmacological targeting of TIM-3 increased tumor-specific immune responses and enhanced efficient control of tumor burden, implying that TIM-3 is a potential candidate for reversing immune tolerance and restoring antitumor immune responses within tumor microenvironments [12].
T cell immunoglobulin mucin protein-4 (TIM-4) is a phosphatidylserine receptor that promotes phagocytosis of apoptotic cells [18, 19]. Recent analysis of TIM-4-deficient mice has demonstrated that TIM-4 is critical for the repression of inflammation and maintenance of tolerance [20]. These findings raise the possibility that manipulation of TIM-3 and/or TIM-4 may have an impact on immune responses in tumor microenvironments.
In this study, we demonstrate that the pharmacological blockade of TIM-3 and/or TIM-4 using mAbs stimulates distinct antitumor effector cells within tumor microenvironments. Treatment with anti-TIM-3 mAb increased the numbers and activity of tumor-infiltrating natural killer (NK) cells, whereas anti-TIM-4 mAb recruited mainly CD8+ T cells as the source of antitumor activities. Moreover, a combined treatment with anti-TIM-3 and anti-TIM-4 mAbs further increases the efficacy of cancer vaccines as compared to either mAb alone by increasing the numbers and effector functions of both NK cells and CD8+ T cells in tumors. These findings imply that pharmacological targeting of TIM-3 and TIM-4 provides a new strategy for improving the antitumor efficacies of cancer vaccines.
Materials and methods
Mice
C57BL/6 mice (6–8-week-old females) were purchased from SCL. OT-I mice were kindly provided by Dr. Shigeo Koyasu (Keio University) and used as described previously [21]. All experiments were conducted under a protocol approved by the animal care committees of Hokkaido University.
Cell lines
B16-F10 melanoma cells were obtained from the American Tissue Culture Collection (ATCC). B16-Flt3L cells were kindly provided by Dr. James P. Allison (MSKKC, USA) and used as described previously [22, 23]. B16-OVA cells were kindly provided by Dr. Heiichiro Udono (Okayama University, Japan). The cell lines used in experiments were routinely authenticated by the Central Institute for Experimental Animals (Kawasaki, Japan) for interspecies and mycoplasma contamination by PCR.
Antibodies
Antimouse TIM-3 (RMT3-23) and antimouse TIM-4 (RMT4-53) mAbs were prepared as described previously [23, 24]. The antimouse NK1.1 (PK136), antimouse CD3ε (145-2C11), antimouse CD8α (53-6.7), antimouse CD69 (H1.2F3) and antimouse CD44 (IM7) mAbs were purchased from Biolegend.
In vivo antitumor activities of FVAX vaccines
Mice were injected in the flank subcutaneously at day 0 with 1 × 105 live B16-F10 melanoma cells and treated on days 3, 5 and 7 with subcutaneously 1 × 106 irradiated (150 Gy) B16-Flt3L cells in the contralateral flank and intraperitoneal injection of 250 μg of anti-TIM-3 and/or anti-TIM-4 mAbs. Where indicated, depletion of NK cells or CD8+ T cells was achieved by two 250-μg injections of anti-NK1.1 mAbs (PK136) or anti-CD8α mAbs (52–6.7) for CD8+ T cell depletion on days −2 and −4. Tumor size was measured on the indicated days.
Tumor infiltration/activation marker analysis
Tumor-infiltrating lymphocytes were isolated as described previously [21]. In brief, established tumors were excised from mice vaccinated with FVAX, and single-cell suspensions obtained from the excised tumors were stained with αNK1.1, αCD3ε and αCD69 for NK cells, or with αCD8α, αCD3ε and αCD44 for CD8+ T cells to assess the frequencies and activation status. Stained samples were analyzed on a flow cytometer (BD Bioscience).
Cytotoxic assay of intratumor lymphocytes
The established tumors (25 mm2) from B16-OVA cells inoculated subcutaneously into C57BL/6 wild-type mice were treated with FVAX in the presence of control Ig, anti-TIM-3 mAb, anti-TIM-4 mAb or anti-TIM-3 mAb and anti-TIM-4mAb. CD45+ lymphocytes were isolated from tumor tissues of the mice 4 days after final treatment. B16 or B16-OVA target cells were incubated with bulk intratumor lymphocytes or those depleted of NK1.1 or TCR-Vβ5 populations by flow cytometry for 6 h at 100:1 of effector to target ratios. Supernatants were collected and subjected to LDH release assay. The maximum or spontaneous release was defined as the counts that emerged from samples incubated in 5 % Triton-X or medium alone, respectively. Cytolysis was calculated with the following formula: % lysis = (release in experiment − spontaneous release) × 100/(maximum release − spontaneous release). The spontaneous release in all assays was less than 20 % of the maximum.
Statistical analyses
Statistical analyses were performed using the paired Student’s t test, and p < 0.05 was considered statistically significant.
Results
Targeting TIM-3 or TIM-4 improves the antitumor effects of cancer vaccines against established B16 melanomas
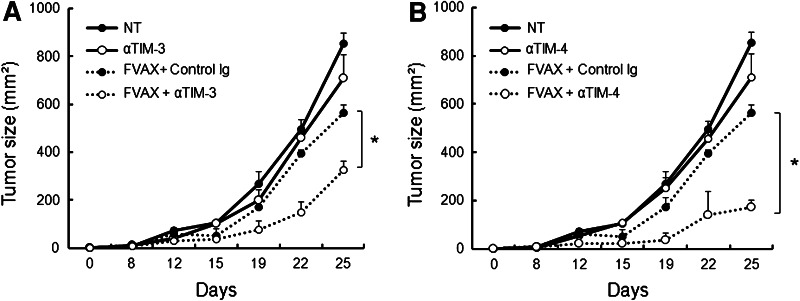
We examined whether therapeutic manipulation of TIM-3 and/or TIM-4 impacts the antitumor responses induced by cancer vaccines against B16 melanomas. To do so, B16 melanoma cells were inoculated subcutaneously into C57BL/6 mice. After the establishment of tumors (~25 mm2), mice were vaccinated with irradiated Flt3L-secreting B16 cells (FVAX) in the presence of isotype-matched control Ig, anti-TIM-3 mAb (RMT3-23) or anti-TIM-4 mAb (RMT4-53). Vaccination with FVAX partially reduced tumor burden when combined with control Ig. On the other hand, co-administration of anti-TIM-3 or anti-TIM-4 mAb with FVAX significantly suppressed tumor growth to a greater extent than FVAX alone (Fig. 1a, b). Monotherapy with anti-TIM-3 or anti-TIM-4 mAb had little impact on B16 melanoma growth (Fig. 1a, b). The treatment with anti-TIM-3 mAb or anti-TIM-4 mAb also augmented antitumor activities of plasmid DNA encoding melanoma target gp100 or TRP2, suggesting that targeting TIM-3 or TIM-4 improves antitumor activities of other forms of cancer vaccines (data not shown).
Fig. 1.
Blockade of TIM-3 or TIM-4 enhances the therapeutic responses elicited by cancer vaccines. C57BL/6 mice (n = 3 per group) were inoculated subcutaneously in the flank with 1 × 105 B16-F10 melanoma cells on day 0. a, b The tumor-bearing mice were treated with subcutaneous injection of 1 × 106 irradiated (150 Gy) flt3L-expressing B16 melanoma cells (FVAX) and intraperitoneal injection of anti-TIM-3 mAb (αTIM-3) (a) or anti-TIM-4 mAb (αTIM-4) on days 3, 5 and 7 (b). Tumor growth was measured on the indicated days. Similar results were obtained in three independent experiments. *p < 0.05
These results indicate that treatment with either anti-TIM-3 or TIM-4 mAb improves the antitumor effect of cancer vaccines similarly.
Combined blockade of TIM-3 and TIM-4 elicits potent antitumor responses in cooperation with cancer vaccines
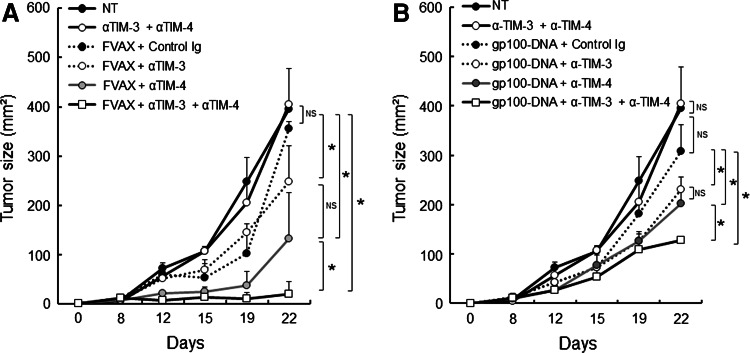
We next examined whether combined blockade of TIM-3 and TIM-4 could further enhance antitumor responses elicited by cancer vaccines against B16 melanoma. Treatment with anti-TIM-3 plus anti-TIM-4 mAbs markedly augmented FVAX to a greater level than with either mAb alone (Fig. 2a). In contrast, the combination of anti-TIM-3 and anti-TIM-4 mAbs had little impact on controlling B16 melanoma growth in the absence of FVAX (Fig. 2a). Furthermore, the combined regimens prevented further tumor growth in the vaccinated mice during the follow-up periods (more than 50 days), while all of the vaccinated group were succumbed to death when treated with anti-TIM-4 mAb or anti-TIM-4 mAb alone (data not shown). The combined treatment with anti-TIM-3 mAb and anti-TIM-4 mAb also augmented antitumor activities of vaccination with plasmid DNA encoding melanoma target gp100 or TRP2 compared to either mAb alone (Fig. 2b and data not shown).
Fig. 2.
Combined blockade of TIM-3 and TIM-4 maximizes the antitumor effect of cancer vaccines. a, b C57BL/6 mice (n = 3 per group) were challenged in the flank with B16-F10 melanoma cells and were treated with subcutaneous injection of irradiated FVAX or plasmid DNA encoding gp100 (b), and intraperitoneal injection of anti-TIM-3 mAb (αTIM-3), anti-TIM-4 mAb (αTIM-4) or both (αTIM-3 + αTIM-4). Tumor growth was measured on the indicated days. Similar results were obtained in three independent experiments. *p < 0.05, NS not significant
These results indicate that combined administration of anti-TIM-3 and anti-TIM-4 mAbs further augments the antitumor effects of cancer vaccines compared to monotherapy.
Blockade of TIM-3 and TIM-4 stimulates distinct antitumor effector mechanisms
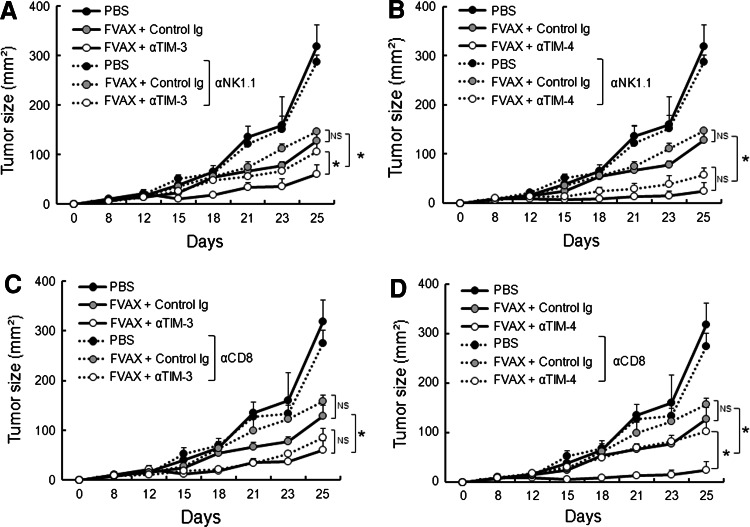
We next examined the mechanism by which anti-TIM-3 or anti-TIM-4 mAb triggers effector functions within tumor microenvironments. To do so, we repeated the above experiments in mice that had been treated with depleting mAb for NK1.1 (PK136) or CD8 (53–6.7) 2 days prior to tumor inoculation, as NK cells and CD8+ T cells serve as the major innate and adaptive effectors controlling tumor immunosurveillance, respectively [25, 26]. Treatment with anti-TIM-3 mAb did not augment the antitumor effects elicited by FVAX in mice depleted of NK1.1+ cells (Fig. 3a). In contrast, TIM-4 blockade augmented the therapeutic effects of FVAX in mice depleted of NK1.1+ cells at a similar level to that in control mice (Fig. 3b).
Fig. 3.
Blockade of TIM-3 and TIM-4 enhances the antitumor responses elicited by FVAX via distinct effector mechanisms. C57BL/6 mice (n = 3 per group) were treated with depleting Ab for NK1.1 (a, b) or CD8 (c, d) 2 days prior to B16-F10 melanoma inoculation. The tumor-bearing mice were then treated with FVAX and anti-TIM-3 mAb (αTIM-3: a, c) or anti-TIM-4 mAb (αTIM-4: b, d) as described in Fig. 1. Tumor growth was measured on the indicated days. Similar results were obtained in two independent experiments. *p < 0.05, NS not significant
On the other hand, the depletion of CD8+ cells abrogated the immunostimulatory effect of anti-TIM-4 mAb, whereas it had little impact on the effect of anti-TIM-3 mAb (Fig. 3c, d).
Together, these results suggest that the blockade of TIM-3 or TIM-4 induces different effector arms and that NK1.1+ or CD8+ effector cells are responsible for the augmented antitumor effect of FVAX by anti-TIM-3 mAb or anti-TIM-4 mAb, respectively.
Blockade of TIM-3 and TIM-4 activates distinct sets of tumor-infiltrating effector cells
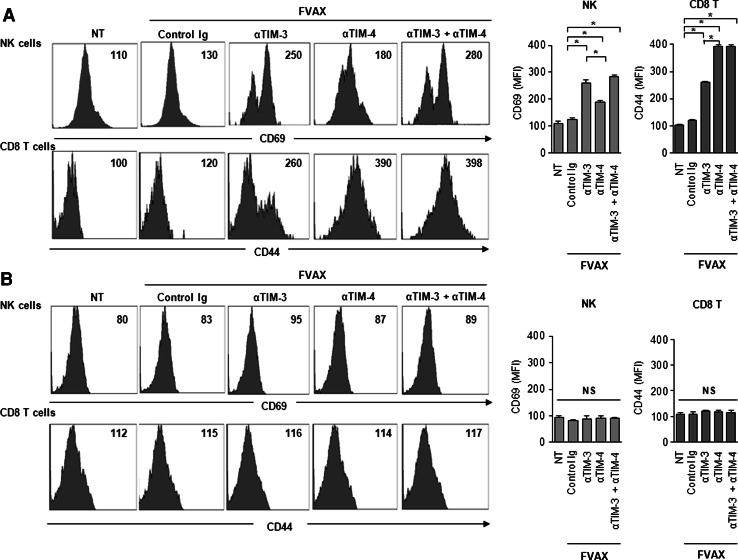
Finally, we examined the mechanisms by which anti-TIM-3 and anti-TIM-4 mAbs modulate the immune responses in tumor microenvironments. To do so, we isolated tumor-infiltrating lymphocytes from established B16 tumors and analyzed the frequencies and activation state of NK cells and CD8+ T cells in the tumors and spleens of the treated mice. We found that the frequency of NK1.1+ CD3− cells was much higher in mice treated with anti-TIM-3 mAb and FVAX compared to those treated with FVAX alone, whereas the treatment with anti-TIM-4 mAb and FVAX did not significantly alter the frequency of NK cells (Fig. 4a). In contrast, TIM-4 blockade resulted in a marked increase in CD8+ T cells infiltrating the tumors, while the TIM-3 blockade had no significant effect on CD8+ T cells (Fig. 4a). Importantly, a combined blockade of TIM-3 and TIM-4 resulted in increased frequencies of both NK cells and CD8+ T cells in tumors (Fig. 4a). Treatment with anti-TIM-3 mAb, anti-TIM-4 mAb or both had little impact on the frequencies of NK cells or CD8+ T cells in spleens of the same tumor-bearing mice (Fig. 4b).
Fig. 4.
Anti-TIM-3 and anti-TIM-4 mAb alter the frequency of distinct effector cells in tumors. C57BL/6 mice bearing B16-F10 tumor were treated with FVAX and anti-TIM-3 mAb (αTIM-3), anti-TIM-4 mAb (αTIM-4) or both (αTIM-3 + αTIM-4) on day 3 and 5. Seven days after tumor inoculation, lymphocytes were isolated from the tumors and spleens of tumor-bearing mice. The frequencies of NK1.1+CD3− (NK) cells or CD3+CD8+ (CD8+ T) cells in tumors (a) or spleens (b) were analyzed by flow cytometry. Similar results were obtained in two independent experiments. *p < 0.05, NS not significant
We also evaluated the activation state of NK cells and CD8+ T cells in the tumors and spleens by measuring the levels of CD69 and CD44, respectively. Neither NK1.1+ nor CD8+ T cells within tumors displayed increased CD69 or CD44 expression in mice treated with FVAX alone as compared to those in untreated mice. Treatment with anti-TIM-3 mAb was more effective in increasing CD69 expression on NK cells isolated from the B16 tumor sites of FVAX-immunized mice compared to those treated with anti-TIM-4 mAb (Fig. 5a). On the contrary, TIM-4 blockade increased CD44 expression on tumor-infiltrating CD8+ cells to a greater extent than did TIM-3 blockade (Fig. 5a). Importantly, a combined treatment with anti-TIM-3 and anti-TIM-4 mAbs maximized the numbers and activation state of NK1.1+ cells and CD8+ T cells infiltrating FVAX-immunized B16 tumors (Fig. 5a). Again, treatment with anti-TIM-3 mAb, anti-TIM-4 mAb or both had little impact on the activation status of NK cells or CD8+ T cells obtained from the spleens of tumor-bearing mice (Fig. 5b).
Fig. 5.
Anti-TIM-3 and anti-TIM-4 mAbs activate distinct sets of effector cells in tumors. C57BL/6 mice bearing B16-F10 tumor were treated with FVAX and anti-TIM-3 mAb (αTIM-3), anti-TIM-4 mAb (αTIM-4) or both (αTIM-3 + αTIM-4) on days 3 and 5. Seven days after tumor inoculation, lymphocytes were isolated from the tumors and spleens of tumor-bearing mice. NK cells and CD8+ T cells were gated as the NK1.1+CD3− and CD3+CD8+ populations, respectively, and the frequencies of CD69 among NK cells or CD44 among CD8+ T cells in tumors (a) or spleens (b) were analyzed by flow cytometry. The mean fluorescence intensity (MFI) was shown in the right-upper panel in the histogram plot. Similar results were obtained in two independent experiments. *p < 0.05, NS not significant
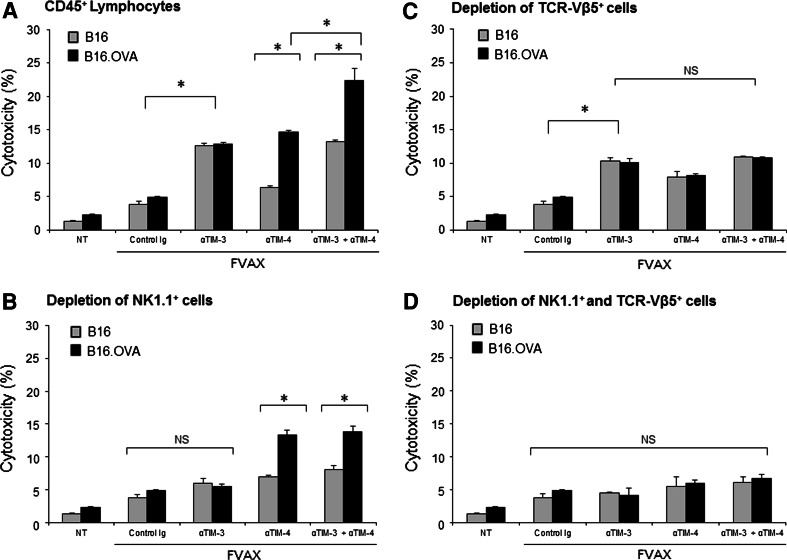
To further define whether TIM-3 and TIM-4 distinctly regulate antitumor effector functions, B16-OVA cells and TCR-transgenic mice recognizing H-2Kb-restricted OVA sequence (SIINFEKL: OT-I) were utilized for dissecting tumor antigen-specific responses and antigen-nonspecific innate responses in tumors. In this system, B16-OVA cells were inoculated subcutaneously into C57BL/6 wild-type mice 5 days after intravenous transfer of OVA-specific TCR-Vβ5+ OT-I cells. The established tumors then received vaccination with FVAX in the presence of anti-TIM-3 mAb, anti-TIM-4 mAb or both. Bulk CD45+ lymphocytes isolated from the established tumors or those depleted of NK1.1+ cells or OVA-specific TCR-Vβ5+ populations were used for analyzing OVA-specific cytotoxic activities against B16-OVA cells and OVA-independent cytotoxic activities against B16 cells. The FVAX alone induced greater cytotoxic activities of intratumor lymphocytes against B16 or B16-OVA targets compared to nontreatment. The treatment with anti-TIM-3 mAb and FVAX enhanced cytotoxic activities of intratumor lymphocytes against either B16 or B16-OVA targets at greater levels than FVAX alone, which were largely abolished by the NK1.1+ cell depletion. In marked contrast, the treatment with anti-TIM-4 mAb and FVAX elicited greater cytotoxic responses against B16-OVA targets compared to FVAX alone, which were completely abrogated by the TCR-Vβ5+ cell depletion.
Again, the combined blockade of TIM-3 and TIM-4 further increased cytotoxic activities of intratumor lymphocytes against B16 and B16-OVA target cells, and these effects were largely abolished by combined depletion of NK- and TCR-Vβ5+-positive cells. Thus, these results further suggest that the therapeutic benefit by combined TIM-3 and TIM-4 blockade relied mainly upon the coordinated actions of OVA-specific responses as well as OVA-independent innate responses from the vaccinated tumors (Fig. 6).
Fig. 6.
B16-OVA cells were inoculated subcutaneously into C57BL/6 wild-type mice 5 days after intravenous transfer of OVA-specific TCR-Vβ5+ cells isolated from OT-I mice. The established tumors then received vaccination with FVAX in the presence of control Ig, anti-TIM-3 mAb (αTIM-3), anti-TIM-4 mAb (αTIM-4) or both (αTIM-3 + αTIM-4). Four days after final treatment, bulk CD45+ lymphocytes isolated from the established tumors (a), those depleted of NK1.1+ cells (b), TCR-Vβ5+ cells (c) or both (d) were cocultured with B16 or B16-OVA cells at the 100/1 of effector/target ratios for 6 h. The cytotoxic activities were measured by LDH release assay. Similar results were obtained in two independent experiments. *p < 0.05, NS not significant
Together, these findings provide a direct proof that the FVAX vaccines combined with TIM-3 blockade trigger NK cell-mediated innate effector responses in a tumor antigen-independent way, whereas TIM-4 blockade induces tumor antigen-specific cytotoxic activities of CD8+ T cells in synergy with the autologous tumor cell vaccines in tumor microenvironments.
In summary, these results suggest that the blockade of TIM-3 or TIM-4 induces distinct sets of effector arms within tumor microenvironments, and thus, the combined blockade can exert a synergistic effect.
Discussion
The antitumor efficacy of immunotherapy remains insufficient to achieve durable clinical responses in patients with advanced stage cancers. In this study, we demonstrate that the combined administration of anti-TIM-3 and anti-TIM-4 mAbs augmented the therapeutic efficacies of cancer vaccines by stimulating different effector arms within tumor microenvironments. These findings provide new evidence that targeting of TIM-3 and TIM-4 may serve as a promising option to improve therapeutic efficacy of cancer immunotherapy.
The antitumor effects exerted by anti-TIM-3 mAb were derived mainly from the activation of NK cell-mediated innate immune systems within tumor microenvironments. These findings contradict a recent report in which the administration of anti-TIM-3 mAb had an inhibitory effect on chemical carcinogenesis through T cell- and IFN-γ-dependent mechanisms [16, 27]. This discrepancy may arise from the unique properties of TIM-3 in modulating distinct sets of immune cells and the related signaling pathways that exist under different microenvironments and treatments. Consistent with this assumption, we recently demonstrated that TIM-3 expressed on tumor-infiltrating dendritic cells was responsible for suppressing the antitumor responses elicited by a chemotherapeutic agent cisplatin and that this effect relied mainly on the impairment of innate immune responses to the nucleic acids released from tumor cells upon chemotherapy [28]. Thus, TIM-3 blockade may serve as a useful strategy for overcoming tolerogenic tumor environments by triggering both innate and adaptive antitumor immunity when appropriate immunogenic adjuvants such as FVAX are co-administered. In this regard, it is of great interest to examine whether TIM-3 inhibition could co-opt other immunotherapies or cytotoxic chemotherapies to create antitumor environments in which endogenous immune systems are capable of antagonizing nascent tumors.
Furthermore, we demonstrated herein that the administration of TIM-4 mAb augmented the antitumor effects of FVAX by CD8+ T cell-dependent, but NK cell-independent, mechanisms. Since TIM-4 expression is highly restricted to myeloid cell lineages [20], TIM-4 might modulate the interaction between myeloid cells and antigen-specific cytotoxic T lymphocytes within tumor microenvironments. Indeed, accumulating evidence reveals that tolerogenic microenvironments formed by myeloid cells are critical to the tumor progression and the induction of resistance to anticancer drugs [29, 30]. Thus, it is of great interest to clarify the role of TIM-4 on tumor-associated myeloid cells in the regulation of antitumor immune responses and its affect on the clinical prognosis of cancer patients.
We also demonstrated that the combined administration of anti-TIM-3 and anti-TIM-4 mAbs augments the antitumor responses elicited by cancer vaccines against established B16 melanoma more efficiently than administration of anti-TIM-3 or anti-TIM-4 mAb alone. These findings further imply that the coordinated and/or distinct functions of TIM-3 and TIM-4 are responsible for creating tolerogenic conditions within tumor microenvironments. Since the appropriate stimulation of innate immune signals determines the direction and quality of adaptive immune responses [31, 32], the concurrent administration of anti-TIM-3 and anti-TIM-4 mAbs may co-opt immunogenic adjuvants to initiate sequential activation of innate and adaptive antitumor immunity. Further clarification of the molecular mechanisms by which TIM-3 and TIM-4 differentially regulate innate and adaptive immune responses in defined microenvironments will improve the therapeutic efficacies of immunotherapies against advanced cancers.
In summary, we have unveiled distinct roles for TIM-3 and TIM-4 in the regulation of innate and adaptive antitumor immunity. The molecular targeting of TIM-3 and TIM-4 provides a new therapeutic strategy, in combination with vaccination protocols or modalities to induce immunogenic cell death [33], to eradicate tumors through the coordinated activation of innate and adaptive antitumor immune responses in tumor microenvironments.
Acknowledgments
We thank Professor James P. Allison and Dr. Michael A. Curran (Memorial Sloan-Kettering Cancer Center) for the B16-Flt3L cell line, Dr. Heiichiro Udono (Okayama University) for the B16-OVA cell line, Dr. Jedd Wolchok (Memorial Sloan-Kettering Cancer Center) for DNA plasmids and Dr. Shigeo Koyasu (Keio University) for the OT-I mice. In addition, we wish to extend appreciation to Mr. Tsunaki Yamashina for assistance with animal care. This study is partially supported by a Grant-in-Aid for Scientific Research and Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Takeda Science Foundation, the Sumitomo Foundation and Terumo Life Science Foundation (M. J.); Grant-in-Aid from MEXT and the National Cancer Center Research and Development Fund (H.Ya.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 2.Andrews DM, Maraskovsky E, Smyth MJ. Cancer vaccines for established cancer: how to make them better? Immunol Rev. 2008;222:242–255. doi: 10.1111/j.1600-065X.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KS, Segal NH, Allison JP. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192–199. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo S-R, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fourcade J, Sun Z, Pagliano O, et al. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2012;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–5244. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71:6567–6571. doi: 10.1158/0008-5472.CAN-11-1487. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Karisola P, Peña-Cruz V, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinushi M, Sato M, Kanamoto A, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–7755. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama M, Akiba H, Takeda K, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 25.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 27.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 28.Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating dendritic cells suppress nucleic acids-mediated innate immune response through TIM-3-HMGB1 interactions. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2272–2559. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jinushi M. The role of innate immune signals in antitumor immunity. Oncoimmunology. 2012;1:189–194. doi: 10.4161/onci.1.2.18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]