Abstract
The prognosis for pancreatic ductal adenocarcinoma (PDAC) remains extremely poor. Recent studies have focused on the role of lymphocytes in the PDAC microenvironment. Using immunohistochemistry, our study explored the clinical significance of intratumoral or peritumoral CD4+Foxp3+ regulatory T cells (Tregs) and CD8+ T cells in the tumor microenvironment and analyzed their relation to the prognosis of PDAC in a consecutive series of 92 patients after resection. CD8+ T cells were more frequently seen within peritumoral sites, while CD4+Foxp3+ Tregs were more frequent within intratumoral areas. Neither exhibited any relationship with other clinicopathologic factors. Patients with low levels of intratumoral Tregs had longer disease-free survival than those with higher levels (DFS 22.2 vs. 11.2 months, p < 0.001), and patients with higher levels of peritumoral CD8+ T cells had longer overall survival than those with lower levels (OS 31.0 vs. 14.2 months, p < 0.001). Multivariate analysis demonstrated that intratumoral Tregs (hazard ratio, HR 3.39, p = 0.010) and peritumoral CD8+ T cells (HR 0.10, p < 0.001) are related to DFS and OS, respectively. These results indicate that intratumoral Tregs are a negative predictor of DFS, while peritumoral CD8+ T cells are a positive predictor of OS for PDAC patients with pancreatectomy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1775-4) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic ductal adenocarcinoma, Pancreatectomy, Tumor microenvironment, Tregs, CD8+ T cells, Prognosis
Introduction
The prognosis of patients with pancreatic cancer remains extremely poor, with a 5-year survival rate of only 7 % [1]. For patients with resectable lesions, the 5-year survival rate following pancreatectomy is <20 %, despite improved diagnostic and treatment strategies [2–7]. In recent years, the microenvironment of pancreatic cancer that contributes to tumor initiation, progression, and metastasis has been investigated, and special focus has been on the role of tumor-infiltrating lymphocytes (TILs) in tumor progression [8, 9]. TILs are regarded as reflections of host antitumor immunity and have been studied in various tumors including pancreatic cancer; however, the results have been inconsistent. For example, high levels of CD4+ T cells are an independent prognosticator for patients undergoing resection for pancreatic cancer [8], which is somewhat different from another study that indicated that the ratio of tumor-infiltrating T helper type 2 (Th2)/T helper type 1 (Th1) cells is an independent predictor of survival [9]. Recent data showed that regulatory T cells (Tregs) could offer a new explanation of the pro- and antitumor implications of TILs. Tregs, a subpopulation of T lymphocytes with an immunophenotype of CD4+CD25+, are thought to hamper T cell immunity against cancer and participate in immune regulation. On the other hand, Foxp3, a transcription factor of the forkhead/winged-helix family, fulfills the criteria of being a Treg-specific marker [10]. In actuality, CD4+CD25+Foxp3− T cells do not show Tregs’ function [11] and nonregulatory T cells do not express Foxp3 [12]; therefore, Foxp3 is used as a more specific marker to identify functional Tregs, which prompted our investigation of the level of Tregs in pancreatic cancer. Nevertheless, the results were contradictory because some reports showed a beneficial role of Foxp3+ Tregs, while others suggested their adverse prognostic action. Some studies claim that the accumulation of Foxp3+ Tregs in pancreatic, liver, and ovarian cancer is generally associated with a poor prognosis because of their capacity to suppress antitumor immunity [13, 14], while other studies claim that high tumor infiltration of Foxp3+ Tregs in colorectal carcinoma and Hodgkin’s lymphoma shows a favorable prognosis [14–16].
In contrast to Tregs, CD8+ T cells can directly kill target cells, including cancer cells, through the perforin/granzymes cell death pathway or the Fas/Fas ligand (FasL) acquired immune response pathway, both of which are expected to take a high responsibility for antitumor immunity [17]. It was reported that a high level of infiltrating CD8+ T cells in cancer tissue is an indicator for favorable prognostic in ovarian cancer [18], colorectal cancer, [19], and some other cancers [10, 20].
It is reported that the proportion of CD8+ T cells to Tregs is correlated with survival [21, 22]. Several studies also identified the increased prevalence of CD8+ T cells, and a high ratio of CD8+/CD4+ or CD8+/Tregs is a significant predictor of better survival in many cancers [23–25]. Because of the complexity of the interaction of immune cells with tumor cells, the mechanisms by which regulatory factors hamper effective immune responses in the microenvironment of tumors are very complex and not completely known.
This study tried to analyze the possible impact of intratumoral or peritumoral Tregs and CD8+ T cells on clinicopathologic characteristics and survival of a cohort of patients undergoing pancreatectomy for pancreatic ductal adenocarcinoma (PDAC).
Patients and methods
Patients
Ethical approval was obtained from the research ethics committee of Zhongshan Hospital, Fudan University, China. Participants of this study were selected from all patients undergoing pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) for PDAC in our hospital between February 15, 2007, and September 7, 2011, with complete survival data. Other lesions, such as ampullary, duodenal, or distal bile duct adenocarcinomas; mucinous cyst adenocarcinomas, or intraductal papillary mucinous neoplasms, were excluded as were those patients with unresectable lesions, distant metastasis, peritoneal seeding or those requiring vascular reconstruction because of the invasion of portal vein, superior mesenteric vein, superior mesenteric artery, common hepatic artery, or celiac trunk. Patients with a gross residual tumor or microscopically positive resection margins, immunodeficiency disease, autoimmune disease, or any preoperative anticancer therapies were also excluded. The final study cohort comprised 92 patients. All patients underwent pancreatectomy for curative purpose. The clinical pathology of the tumors was determined according to the Union for International Cancer Control (UICC) staging classification of pancreatic cancer (6th edition).
The 92 eligible patients comprised 68 men and 24 women, with a median age of 61 years. The 72 patients (78.3 %) with lesions in the pancreatic head underwent pylorus-preserving pancreaticoduodenectomy (PPPD) or classic PD; the other 20 patients (21.7 %) with lesions in the pancreatic body or tail underwent DP with splenectomy. According to the UICC TNM system, 25 (27.2 %), 41 (44.5 %), and 26 patients (28.3 %) were diagnosed as stage I, stage II, and stage III, respectively. Tumors were identified as a histologic grading of 1 in 4 patients (4.3 %), 2 in 57 patients (62.0 %), and 3 in 31 patients (33.7 %). The demographics and clinicopathologic characteristics of the patients are summarized in Table 1.
Table 1.
Patient demographics and clinicopathologic factors (n = 92)
| No. of patients | % | |
|---|---|---|
| Age (years) | ||
| Median | 61 | |
| Range | 35–81 | |
| Sex | ||
| Male | 68 | 73.9 |
| Female | 24 | 26.1 |
| Tumor location | ||
| Head | 72 | 78.3 |
| Body or tail | 20 | 21.7 |
| UICC stage | ||
| I | 25 | 27.2 |
| II | 41 | 44.5 |
| III | 26 | 28.3 |
| Histologic grading | ||
| 1 | 4 | 4.3 |
| 2 | 57 | 62.0 |
| 3 | 31 | 33.7 |
| Perineural invasion | 72 | 78.3 |
| Vascular invasion | 9 | 9.8 |
| Lymphatic invasion | 32 | 34.8 |
UICC Union for International Cancer Control
Surgery
Patients underwent PPPD, classic PD, or DP. All operations were performed by one of the four surgeons (D.J., W.L., D.W. and W.W.). For the tumor in the pancreatic head, the standard surgical procedure was PPPD with lymphadenectomy on the right side of the portal vein. In patients with the tumor invading pylorus or duodenum, a Whipple’s procedure was performed. For cancer of the pancreatic body or tail, patients underwent DP plus splenectomy. If the carcinoma was close to (within 1 mm) or present at the final pancreatic neck, uncinate process, bile duct, duodenal or retroperitoneal soft tissue, the patient was not enrolled in this study.
Postoperative treatment and follow-up
Postoperatively, the patients remained in the recovery room for 1–2 h. In cases of ventilatory disorder or perioperative infection, patients were transported to the intensive care unit for 1–3 days, and then sent back to the surgical ward. The drainage tubes were removed 5–11 days after surgery.
After surgery, patients received a regimen of adjuvant chemotherapy with GEMOX for 6–12 cycles: gemcitabine 1000 mg/m2 and oxaliplatin 100 mg/m2 on day 1, and then one cycle every 14 days. Follow-up was completed on January 1, 2014. Postsurgery follow-up was performed in our hospital on an outpatient basis. Follow-up examinations comprised physical examinations, abdominal ultrasonography or computed tomography (CT) scans and thoracic CT scans, as well as routine blood analysis including serum CA 19–9 every 3–6 months. All patients received CT scans every 3 months in the first year after surgery and every 6 months in the second year. If the follow-up data suggested a recurrence or metastasis, CT and/or magnetic resonance imaging (MRI) was/were used to verify the condition. The treatment after relapse varied based on patients’ situations. Nine patients with recurrence gave up treatment because of poor liver function. Most common causes of death were recurrence, metastasis, or complications related to liver cirrhosis. Overall survival (OS) was defined as the interval between surgery and death or the last observation for surviving patients. Disease-free survival (DFS) was defined as the time between surgery and the first treatment failure (either recurrence or death). All patients who died of events unrelated to PDAC were also excluded.
Pathological examination
After tumor resection, pathology protocols were performed on all specimens to ensure consistent interpretation of tumor location, histology, resection margin, UICC stage, histologic grading, perineural invasion, vascular invasion, and lymphatic invasion.
Immunohistochemical staining
CD4+, Foxp3+, CD4+Foxp3+, and CD8+ T cells in the resectional regimen were analyzed through immunohistochemical (IHC) staining. The mouse monoclonal antihuman Foxp3, CD8 (Abcam Biotech Co, Cambridge, UK), CD4, and granzyme B (GrB, Novocastra Biotech Co, Cambridge, UK) antibodies were used. The Novolink Polymer Detection System (Leica Biosystems, Newcastle, UK) was used to allow the identification of these antigens following the manufacturer’s instructions. IHC of formalin-fixed, paraffin-embedded 3-μm sections was carried out using the Bond-max system automated IHC (Leica Biosystems, Newcastle, UK). IHC Protocol F program was used for single IHC staining. Double staining using IHC Protocol F DOUBLE program was done on CD4 and Foxp3. In brief, sections were deparaffinized with xylene and then hydrated with gradient ethanol. After heat-induced epitope retrieval in citrate buffer, tissues were blocked with 0.3 % H2O2 (RE7101) and protein block (RE7102). The slides were then serial incubated with primary antibodies, postprimary block (RE7111), and horseradish peroxidase-conjugated goat anti-mouse antibody (RE7112). Finally, the antigens were identified with diaminobenzidine solution (RE7105), and slides were counterstained with hematoxylin (RE7112, all the reagents above are included in the Novolink Polymer Detection System). Slides incubated without primary antibodies were used as the negative control, and the tissues from autoimmune pancreatitis were used as the positive control for GrB staining.
The number of T cells was counted by two authors (L.L. and Y.R.) who were blinded to the patient’s clinicopathologic information. Lymphocytes count was done with at least five independent 400× high-power fields (HPF). The results are shown as the mean ± SE number of cells in one field.
The influence of a low versus high level of intratumoral or peritumoral CD4+Foxp3+ Tregs and CD8+ T cells was evaluated. The median numbers were used as the cutoff, and patients were classified into either low group or high group.
Statistical analysis
Associations of the number of lymphocytes with various clinicopathologic factors were assessed by Chi-squared or Fisher’s exact tests. Survival rates were calculated by Kaplan–Meier method and analyzed using the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. p < 0.05 was considered statistically significant. All statistical analyses were performed with Stata 10.0 (Stata Corp LP, College Station, TX, USA).
Results
Characteristics of T lymphocytes in the tumor microenvironment
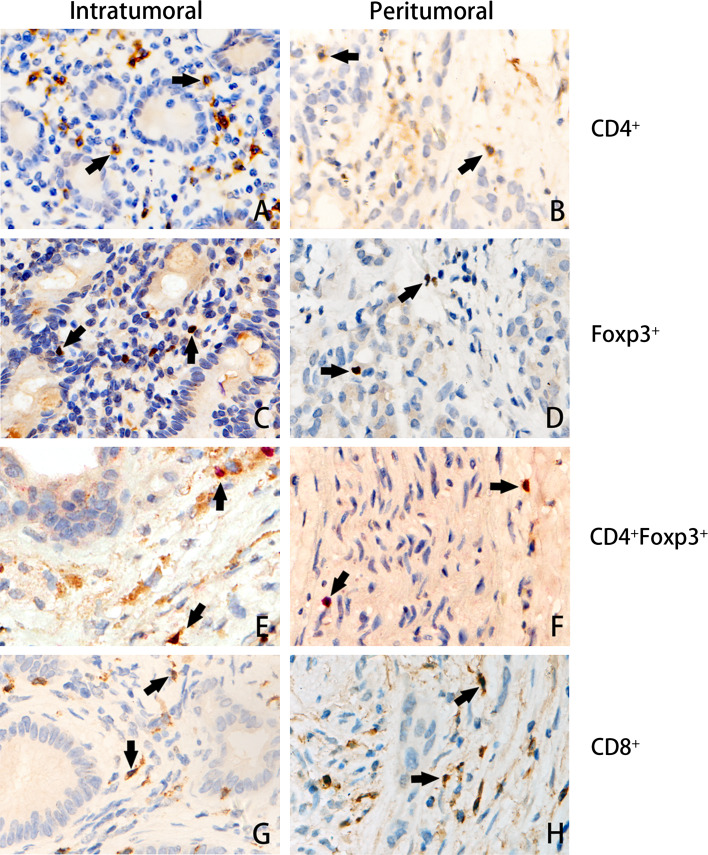
The T lymphocytes in the peritumoral and intratumoral stroma were investigated in patients with pancreatic ductal adenocarcinoma. CD4+ and CD8+ T cells displayed a cytoplasmic staining pattern despite being either intratumoral (Fig. 1a, g) or peritumoral (Fig. 1b, h). Foxp3+ cells displayed a nuclear staining, which reflects the function of Foxp3 in transcription (Fig. 1c, d). The CD4+ and Foxp3+ double-staining cells represented the Tregs (Fig. 1e, f). Either CD4+ (25.23 ± 8.21 vs. 16.25 ± 4.56 cells/HPF, p = 0.026) or CD8+ T cells (36.68 ± 9.45 vs. 16.07 ± 6.25 cells/HPF, p < 0.001) were more frequent in the peritumoral microenvironment than in the intratumoral microenvironment, respectively; CD4+Foxp3+ Tregs, although their numbers were far less than those of CD4+ or CD8+ T cells, were more frequent in the intratumoral than in the peritumoral microenvironment (4.05 ± 5.86 vs. 2.56 ± 2.31 cells/HPF, respectively; p < 0.001, Table 2). Furthermore, no GrB+ cells were found in either intratumoral or peritumoral tissues.
Fig. 1.
Tumor-infiltrating CD4+, Foxp3+, CD4+Foxp3+, and CD8+ cells. Consecutive sections were used for immunohistochemical study. CD4+ and CD8+ T cells displayed a cytoplasmic staining pattern despite intratumoral (a, g) or peritumoral (b, h). Foxp3+ cells displayed a nuclear staining, which reflects the function of Foxp3 in transcription (c, d). The CD4+ and Foxp3+ double staining representing the Tregs are shown in e, f (original ×400 magnification)
Table 2.
Intratumoral and peritumoral T lymphocytes in patients undergoing pancreatectomy for PDAC (n = 92)/high-power field (×400 magnification)
| Mean | SE | Median | Range | p value | |
|---|---|---|---|---|---|
| CD4+ T | 0.026* | ||||
| Intratumoral | 16.25 | 4.56 | 8.20 | 4–125 | |
| Peritumoral | 25.23 | 8.21 | 15.34 | 10–189 | |
| CD8+ T | <0.001* | ||||
| Intratumoral | 16.07 | 6.25 | 10.23 | 0–95 | |
| Peritumoral | 36.68 | 9.45 | 25.45 | 5–136 | |
| CD4+Foxp3+ Tregs | <0.001* | ||||
| Intratumoral | 4.05 | 5.86 | 3.15 | 0–75 | |
| Peritumoral | 2.56 | 2.31 | 2.02 | 0–55 |
Tregs regulatory T cells, SE standard error
* p < 0.05
Correlation between CD8+ T cells and CD4+Foxp3+ Tregs and clinicopathologic factors
The relationship between clinicopathologic factors and CD8+ T cells as well as between clinicopathologic factors and Tregs in either intratumoral or peritumoral tissues is shown in Supplementary Table S1. There was no significant relationship to age, sex, tumor location, UICC stage, histologic grading, perineural invasion, vascular invasion, and lymphatic invasion of CD8+ T cells or Tregs in the tumor environment in either intratumoral or peritumoral tissue (p > 0.05).
Survival and prognostic factors
The OS and DFS rates of all 92 patients who underwent pancreatectomy for PDAC were 73.7 and 63.9 % at 1 year and 50.0 and 24.1 % at 2 years, respectively.
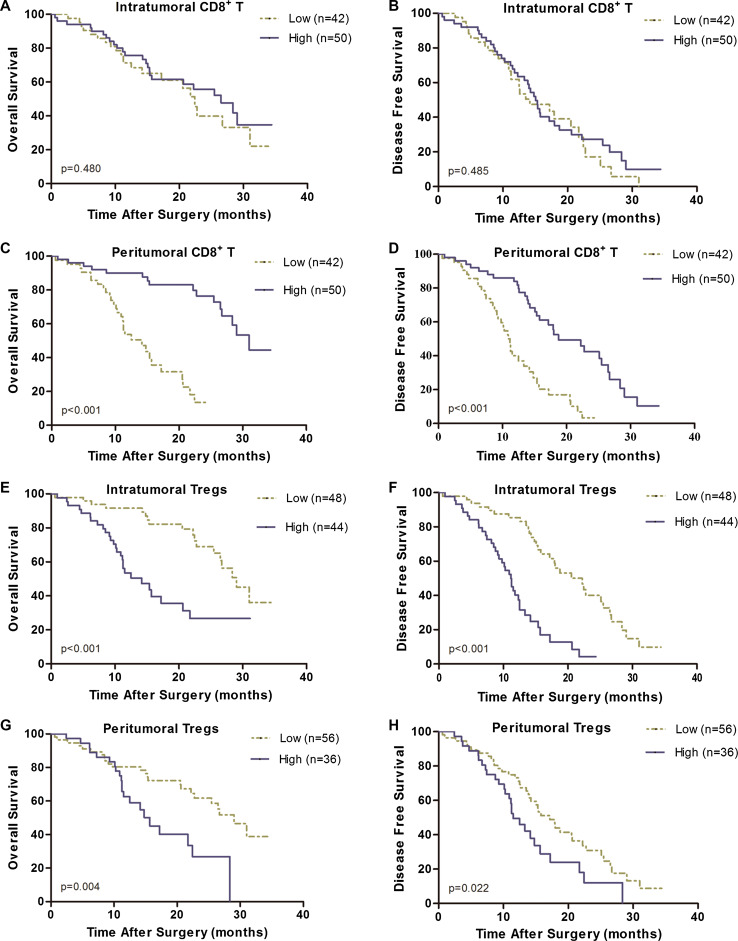
Among the 92 patients, the prognostic impact of intratumoral or peritumoral CD4+Foxp3+ Tregs and CD8+ T cells was investigated by evaluating the factors associated with OS and DFS using univariate and multivariate analyses. By univariate analysis (Table 3), age, sex, tumor location, perineural invasion, and intratumoral CD8+ T showed no prognostic significance for OS or DFS. There were six prognostic factors significantly correlated with OS as follows: histologic grading (p = 0.045), UICC stage (p < 0.001), lymphatic invasion (p = 0.041), peritumoral CD8+ T cells (Fig. 2c, p < 0.001), intratumoral Tregs (Fig. 2e, p < 0.001), and peritumoral Tregs (Fig. 2g, p = 0.004). Meanwhile, the following five factors were correlated with DFS: UICC stage (p = 0.013), vascular invasion (p = 0.027), peritumoral CD8+ T cells (Fig. 2d, p < 0.001), intratumoral Tregs (Fig. 2f, p < 0.001), and peritumoral Tregs (Fig. 2h, p = 0.022).
Table 3.
Univariate overall survival and disease-free survival analysis of prognostic factors for patients undergoing pancreatectomy for PDAC (n = 92)
| OS | DFS | |||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p value | HR | 95 % CI | p value | |
| Age (years) (≤60 vs. >60) | 1.53 | 0.84–2.80 | 0.160 | 1.11 | 0.69–1.80 | 0.666 |
| Sex (female vs. male) | 1.02 | 0.50–2.06 | 0.959 | 0.90 | 0.51–1.59 | 0.721 |
| Tumor location (head vs. body or tail) | 1.65 | 0.69–3.92 | 0.253 | 0.69 | 0.38–1.27 | 0.236 |
| Histologic grading (1 + 2 vs. 3) | 1.87 | 1.01–3.45 | 0.045* | 1.11 | 0.66–1.88 | 0.679 |
| UICC stage (I + II vs. III) | 1.30 | 0.68–2.49 | 0.424 | 1.37 | 0.80–2.34 | 0.250 |
| Perineural invasion (yes vs. no) | 1.83 | 0.77–4.32 | 0.168 | 1.67 | 0.85–3.28 | 0.135 |
| Vascular invasion (yes vs. no) | 0.59 | 0.18–1.92 | 0.383 | 2.23 | 1.09–4.52 | 0.027* |
| Lymphatic invasion (yes vs. no) | 1.87 | 1.02–3.41 | 0.041* | 1.64 | 0.99–2.71 | 0.056 |
| Intratumoral CD8+ t (low vs. high)a | 0.88 | 0.49–1.59 | 0.672 | 0.89 | 0.55–1.45 | 0.634 |
| Peritumoral CD8+ T (low vs. high)a | 0.15 | 0.07–0.32 | <0.001* | 0.28 | 0.17–0.49 | <0.001* |
| Intratumoral Tregs (low vs. high)a | 3.27 | 1.76–6.07 | <0.001* | 3.97 | 2.29–6.87 | <0.001* |
| Peritumoral Tregs (low vs. high)a | 2.47 | 1.31–4.62 | 0.005* | 1.79 | 1.08–2.96 | 0.024* |
OS overall survival, DFS disease-free survival
* p < 0.05
aUsing median values as cutoff
Fig. 2.
Kaplan–Meier analysis of overall survival (OS) and disease-free survival (DFS) for tumor-infiltrating Tregs and CD8+ T cells. Intratumoral CD8+ T cells showed no prognostic significance for OS (a) and DFS (b). High peritumoral CD8+ T cells showed benefit of OS (c) and DFS (d). Neither high intratumoral (e, f) nor peritumoral Tregs (g, h) correlated with OS and DFS
All of these factors (p < 0.1) were enrolled into multivariate analysis using a Cox proportional hazards model (Table 4). Peritumoral CD8+ T cells (p < 0.001) remained independently associated with overall survival. The median OS of patients with high peritumoral CD8+ T cells was significantly longer than that of patients with low peritumoral CD8+ T cells (31.0 vs. 14.2 months, respectively; p < 0.001, Fig. 2c). Vascular invasion (p < 0.001) and intratumoral Tregs (p = 0.024) also remained independently associated with DFS. The median DFS of patients with low intratumoral Tregs was significantly longer than that of patients with high intratumoral Tregs (22.2 vs. 11.2 months, respectively; p < 0.001, Fig. 2f).
Table 4.
Multivariate overall survival and disease-free survival analysis of prognostic factors for patients undergoing pancreatectomy for PDAC (n = 92)
| Median (months) | HR | 95 % CI | p value | ||
|---|---|---|---|---|---|
| OS | |||||
| Histologic grading (1 + 2 vs. 3) | 26.7 | 15.7 | 1.72 | 0.92–3.22 | 0.093 |
| Lymphatic invasion (yes vs. no) | 15.3 | 26.5 | 2.29 | 1.21–4.33 | 0.011* |
| Peritumoral CD8+ T (low vs. high)a | 14.2 | 31.0 | 0.10 | 0.03–0.31 | <0.001* |
| Intratumoral Tregs (low vs. high)a | 29.0 | 14.2 | 0.96 | 0.38–2.44 | 0.929 |
| Peritumoral Tregs (low vs. high)a | 29.0 | 15.7 | 0.63 | 0.29–1.38 | 0.249 |
| DFS | |||||
| Peritumoral CD8+ T (low vs. high)a | 11.2 | 18.8 | 0.41 | 0.16–1.04 | 0.061 |
| Intratumoral Tregs (low vs. high)a | 22.2 | 11.2 | 3.39 | 1.33–8.61 | 0.010* |
| Peritumoral Tregs (low vs. high)a | 17.2 | 11.5 | 0.58 | 0.30–1.11 | 0.099 |
| Vascular invasion (yes vs. no) | 8.6 | 15.4 | 3.94 | 1.85–8.44 | <0.001* |
OS overall survival, DFS disease-free survival, Tregs regulatory T cells, HR hazard ratio, CI confidence interval
* p < 0.05
aUsing median values as cutoff
Discussion
Recent researches paid attention to the immune cells in the PDAC microenvironment and their prognostic value through IHC or flow cytometry [8, 13, 26], but none of these studies discussed immune cells in intratumoral or peritumoral sites as a prognostic indicator, respectively. There is much evidence indicating that high T cell infiltration leads to a favorable outcome in a variety of human cancers [14, 27]. Although flow cytometry might provide more accurate data for the prevalence of TILs [26], it cannot be used to show the location of cells in tumor tissues.
There were some limitations in our study. First, we excluded patients with immunodeficiency disease, autoimmune disease, or any preoperative anticancer therapies to avoid changes in the immune microenvironment unrelated to cancer. Nevertheless, some comorbidities such as diabetes that can alter immunity were not completely excluded in this study, which might influence the results. A recent study showed that neoadjuvant chemoradiotherapy can significantly increase the number of CD4+ and CD8+ cells [28], but it is not known whether the results would be influenced if this kind of patients had been enrolled in our study. In addition, only resectable patients were included in our study, and this might cause bias because these patients have relatively favorable prognoses. Otherwise, patients with malignance developed from chronic pancreatitis or other diseases which may alter the immune microenvironment of the pancreas have not been analyzed here. Taken together, this study comprising 92 patients draws attention to the importance of the immune system in the pancreatic cancer microenvironment and provokes speculation about the roles and mechanisms involved.
Tregs play a crucial role in impeding immune surveillance and preventing overactive antitumor immunity [29]. In this study, both the intratumoral and peritumoral areas were assessed, and more Tregs were found in the intratumoral areas than in the peritumoral areas (4.05 vs. 2.56 cells/HPF, respectively, p < 0.001, Table 2) which was determined by another study as well [8]. This might suggest that tumor cells can mobilize Tregs to evade antitumor immunity. Our study also showed that higher intratumoral Tregs were significantly correlated with poor DFS, which could be an independent prognostic factor in multivariate analysis. In fact, this result is somewhat different from that of the study mentioned above, which showed that higher Treg levels were associated with not only shorter DFS but also shorter OS [8]. In addition, a recent study showed that the intratumoral density of Foxp3+ cells is an independent prognostic factor for OS of the PDAC patient, but the relationship with DFS has not been determined [26]. A high incidence of Tregs in the tumor microenvironment, representing the dominance of immunosuppression, might help cancer cells successfully escape from immune surveillance [30] and into distant organs or lymph nodes through the vascular or lymphatic pathway; therefore, intratumoral Tregs might be related to the cancer’s “ability to escape.” At the point of resection, patients with a high number of intratumoral Tregs should have a higher number of metastatic cancer cells, remaining in drained lymph nodes, residual organs, or circulating blood, which leads to an earlier relapse with shorter DFS; however, the relationship between intratumoral Tregs and OS remains undetermined, it is highly possible that because most patient in this study are relatively early stages (71.7 % patients are UICC stage I or II).
In contrast, CD8+ T cells are the most prominent lymphocytes acting against cancer cells and are the potential targets for Tregs. Infiltration of CD8+ T cells decreased from the peritumoral (mean 36.68 cells/HPF) to intratumoral area (mean 16.07 cells/HPF) (Table 2), which was confirmed by Ino et al. [8]. Because activated Tregs can suppress cytotoxic T cells (CTLs) in both an antigen-independent and a suppressive cytokine-dependent manner [12], it is not unusual that less CD8+ T cells are found in intratumoral sites where more Tregs exist. Our study showed that the peritumoral CD8+ T cells were significantly correlated with only OS in the multivariate survival analysis. It can be reasonably speculated that peritumoral CD8+ T cells are related to the “ability to prevent” tumor progression of the immune system; therefore, patients with high peritumoral CD8+ T cells could more effectively suppress tumor growth, whether more or less tumor burden remained just after resection, and then prolong OS. Ino et al. [8] claimed that their detailed analysis indicated that the infiltration of the tumor by CD8+ T alone is not sufficiently associated with longer survival because this is also positively related to the infiltration of CD4+ T cells. Because CD8+ T cells and CD4+ T cells are differentiated from CD3+ T cells, we speculate that there is a positive relationship between these two kinds of different T cells and the total number of CD3+ T cells which reflect the immune status not just the tumor microenvironment; therefore, we could still regard the infiltrated CD8+ T cells as an indicator for antitumor immunity in the PDAC microenvironment. In fact, melanoma patients with a higher number of CD8+ T cells in both the tumor and the invasive margin responded better to therapeutic PD-1 blockade [31], which indicates infiltrating CD8+ T cells might also be a predictor for immunotherapy.
In addition, a specific type of CD8+ cells, activated CD8+ CTLs, could be positively correlated with survival [21]. GrB expression might serve as a marker of CTL activation and elevated as an indicator for disease activity, providing additional evidence for immune activation [32, 33]. Higher GrB expression or GrB+/Foxp3+ has also been regarded as a predictor of longer survival in colorectal cancer, lung cancer, and ovarian cancer [34–36]. A decreased expression of GrB has been identified in lung cancer, breast cancer, and colorectal cancer [34, 35, 37]. Protease inhibitor-9 (PI-9, serpin 9), a natural inhibitor of GrB, plays an important role in the suppression of GrB activity by prostate cancer and leukemia cells [38, 39]. The suppression of GrB might also refer to prostaglandin E2/cyclooxygenase 2 pathways and some other mechanisms [34, 40]. However, no GrB+ cells were found in the intratumoral or peritumoral tissues, which is absolutely out of our exception. The exact reason still has not been determined, but the GrB+ cells was found in our positive control shown in the Supplementary Figure S1, which may exclude any suspected technical problems. Our findings raised the question of why the GrB+ CD8+ CTLs disappear and how the Tregs affect CD8+ T cells in PDAC. It could be speculated that the PDAC cells in our study exhibited such malignant biologic behavior that GrB+ cells were too few to be detected, or that Tregs in the tumor microenvironment profoundly suppress CD8+ CTLs [22]. As a result, the perforin pathway was somewhat blocked, and CTLs may kill pancreatic cancer cells mainly through the Fas/FasL pathway [17]. Additional studies must be conducted to confirm this.
In conclusion, a high number of intratumoral Tregs, which is related to the ability of cancer cells to escape, were a predictor of the poor prognosis for patients with PDAC. Under these conditions, Tregs could be a mediator of tumor cells’ inhibitory aggression on the immune system, suppressing its antitumor function. Tregs would determine the decrease in the number of the activated antitumor lymphocytes including activated CD8+ T cells [41]. The cell infiltration of peritumoral CD8+ T cells, which is related to the ability of immune surveillance, was positively correlated with patient OS. These results might provide new independent predictors of prognosis and suggest that the depletion of Tregs might be an effective strategy in pancreatic cancer immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the following grants: the National Natural Science Foundation of China (Nos. 30801104, 81272731), the Science and Technology Commission of Shanghai Municipality (No. 11JC1402502), and the Public Welfare Industry of Health (No. 201202007).
Abbreviations
- CT
Computed tomography
- CTLs
Cytotoxic T cells
- DFS
Disease-free survival
- DP
Distal pancreatectomy
- FasL
Fas ligand
- GrB
Granzyme B
- HPF
High-power fields
- HR
Hazard ratio
- IHC
Immunohistochemistry
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PD
Pancreaticoduodenectomy
- PDAC
Pancreatic ductal adenocarcinoma
- PPPD
Pylorus-preserving pancreaticoduodenectomy
- Th1
T helper type 1
- Th2
T helper type 2
- TILs
Tumor-infiltrating lymphocytes
- Tregs
Regulatory T cells
- UICC
Union for International Cancer Control
Compliance with ethical standards
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Footnotes
Li Liu and Guochao Zhao have contributed equally to this work.
Contributor Information
Wenchuan Wu, Phone: +8621- 64041990, Email: wu.wenchuan@zs-hospital.sh.cn, Email: wu.wenchuan@fudan.edu.cn.
Wenhui Lou, Phone: +8621- 64041990, Email: lou.wenhui@zs-hospital.sh.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579. doi: 10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 3.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, Sasaki H, Sueda T. Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol. 2013;71(2):419–429. doi: 10.1007/s00280-012-2029-1. [DOI] [PubMed] [Google Scholar]
- 4.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, Robinson R, Laheru DA, Jaffee E, Hruban RH, Campbell KA, Wolfgang CL, Asrari F, Donehower R, Hidalgo M, Diaz LA, Jr, Yeo C, Cameron JL, Schulick RD, Abrams R. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26(21):3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL, Laheru DA, Farnell MB, Swartz MJ, Gunderson LL, Miller RC. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital—Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, Yuasa Y, Kondo N, Ohge H, Sueda T. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J Am Coll Surg. 2010;211(2):196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, Schulick RD, Choti MA, Lillemoe KD, Yeo CJ. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12(11 Pt 1):3355–3360. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Linehan DC, Goedegebuure PS. CD25+CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32(1–3):155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 14.Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33(4):335–340. doi: 10.1007/s00281-011-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60(7):909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 17.Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15(6):544–551. doi: 10.1007/s10147-010-0130-1. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann T, Moehler M, Gockel I, Sgourakis GG, Biesterfeld S, Muller M, Berger MR, Lang H, Galle PR, Schimanski CC. Low expression of chemokine receptor CCR5 in human colorectal cancer correlates with lymphatic dissemination and reduced CD8+ T-cell infiltration. Int J Colorectal Dis. 2010;25(4):417–424. doi: 10.1007/s00384-009-0868-y. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda S, Funakoshi N, Inagaki M, Shibata T. Clinicopathologic roles of tumor-infiltrating lymphocytes and CD8-positive lymphocytes in lung cancer imprint smears in squamous cell carcinoma and adenocarcinoma. Acta Cytol. 2006;50(4):423–429. doi: 10.1159/000325986. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936. [PubMed] [Google Scholar]
- 22.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 23.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67(1):354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 25.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One. 2014;9(3):e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26(3–4):373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 28.Homma Y, Taniguchi K, Murakami T, Nakagawa K, Nakazawa M, Matsuyama R, Mori R, Takeda K, Ueda M, Ichikawa Y, Tanaka K, Endo I. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2014;21(2):670–676. doi: 10.1245/s10434-013-3390-y. [DOI] [PubMed] [Google Scholar]
- 29.Wang RF. Regulatory T cells and innate immune regulation in tumor immunity. Semin Immunopathol. 2006;28(1):17–23. doi: 10.1007/s00281-006-0022-7. [DOI] [PubMed] [Google Scholar]
- 30.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115(10):2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellor-Heineke S, Villanueva J, Jordan MB, Marsh R, Zhang K, Bleesing JJ, Filipovich AH, Risma KA. Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front Immunol. 2013;4:72. doi: 10.3389/fimmu.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowacki TM, Kuerten S, Zhang W, Shive CL, Kreher CR, Boehm BO, Lehmann PV, Tary-Lehmann M. Granzyme B production distinguishes recently activated CD8(+) memory cells from resting memory cells. Cell Immunol. 2007;247(1):36–48. doi: 10.1016/j.cellimm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge G, Barnawi J, Jurisevic C, Moffat D, Holmes M, Reynolds PN, Jersmann H, Hodge S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-gamma by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin Exp Immunol. 2014;178(1):79–85. doi: 10.1111/cei.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salama P, Phillips M, Platell C, Iacopetta B. Low expression of granzyme B in colorectal cancer is associated with signs of early metastastic invasion. Histopathology. 2011;59(2):207–215. doi: 10.1111/j.1365-2559.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 36.Polcher M, Braun M, Friedrichs N, Rudlowski C, Bercht E, Fimmers R, Sauerwald A, Keyver-Paik MD, Kubler K, Buttner R, Kuhn WC, Hernando JJ. Foxp3(+) cell infiltration and granzyme B(+)/Foxp3(+) cell ratio are associated with outcome in neoadjuvant chemotherapy-treated ovarian carcinoma. Cancer Immunol Immunother. 2010;59(6):909–919. doi: 10.1007/s00262-010-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontani K, Sawai S, Hanaoka J, Tezuka N, Inoue S, Fujino S. Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. Eur J Surg Oncol. 2001;27(2):180–186. doi: 10.1053/ejso.2000.1060. [DOI] [PubMed] [Google Scholar]
- 38.Fritsch K, Finke J, Grullich C. Suppression of granzyme B activity and caspase-3 activation in leukaemia cells constitutively expressing the protease inhibitor 9. Ann Hematol. 2013;92(12):1603–1609. doi: 10.1007/s00277-013-1846-6. [DOI] [PubMed] [Google Scholar]
- 39.Ray M, Hostetter DR, Loeb CR, Simko J, Craik CS. Inhibition of granzyme B by PI-9 protects prostate cancer cells from apoptosis. Prostate. 2012;72(8):846–855. doi: 10.1002/pros.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D, Meneses-Flores M, Lopez-Gonzalez JS. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin Dev Immunol. 2012;2012:741741. doi: 10.1155/2012/741741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172(11):6558–6567. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.