Abstract
Myeloma patients may develop oligoclonal immunoglobulins, so-called abnormal protein bands (APB), after stem cell transplantation. APB do not correspond to the patient’s paraprotein and confer a good prognosis. We set out to investigate whether such APB represent a humoral anti-myeloma immune response by screening immunoglobulins of 15 myeloma patients after allogeneic stem cell transplantation and a control group of healthy donors for reactivity with myeloma protein extracts. While the immunoglobulins of healthy donors did not react with myeloma protein extracts, patient-derived immunoglobulins showed variable levels of interaction, depending on the presence of APB on immunofixation. Most commonly, we detected interactions with heat-shock proteins, followed by neutral alpha-glucosidase, alpha-enolase and vimentin, as well as proliferating cell nuclear antigen and MAGEA4. More than 80% of targets were upregulated in myeloma. Heat-shock protein 60 (HSP60) was subsequently evaluated as an exemplary antigen. We found that HSP60 was aberrantly displayed on the surface of primary myeloma cells. Indeed, patient-derived APB-containing immunoglobulins recognized surface HSP60 suggesting that this antigen becomes accessible to the immune system after aberrant membrane exposition. We conclude that immunoglobulin fractions with APB recognize recurrent myeloma antigens and that this humoral response may contribute to the more favorable prognosis in patients with APB.
Keywords: Multiple myeloma, Oligoclonal immunoglobulins, Abnormal protein bands, Immunofixation, Antigens, Anti-myeloma immune response
Introduction
Multiple myeloma (MM) is the second most frequent hematological malignancy accounting for over 10% of all hematological cancers worldwide [1, 2]. This currently incurable disease is characterized by the clonal expansion of malignant plasma cells in the bone marrow resulting in anemia, renal insufficiency and bone disease. Malignant plasma cells are engaged in the production of a characteristic monoclonal immunoglobulin, termed paraprotein, which is abundantly present in the patients’ sera and can be detected as a narrow band by immunofixation.
While the outcome of patients with MM treated with standard therapies has been disappointing with a historical median survival of about 3 years [3], novel highly active agents as well as autologous stem cell transplantation have significantly improved overall survival in this disease [4]. Also allogeneic stem cell transplantation can offer long-term remissions, especially for patients with high-risk disease [5]. During the immune reconstitution phase after stem cell transplantation, up to 70% of patients develop oligoclonal abnormal protein bands (APB) on immunofixation, most likely corresponding to a somewhat overshooting antibody production by different B-cell subclones [6–8]. In any case, APB do not correspond to the patient’s paraprotein secreted by the malignant plasma cell clone. Contrariwise, they have been found to confer a good prognosis although their function and antigen-specificity remain unclear [6–12]. In fact, APB could represent the regeneration of a limited immune response to foreign antigens, for example infectious agents, after transplant. Alternatively, they may mediate allo- or autoimmune reactions against ubiquitous antigens, which are barely suppressed during this phase of immune reconstitution. Yet, the better prognosis associated with the presence of APB suggests that these oligoclonal immunoglobulins could be involved in a specific anti-myeloma immune response.
Here, we set out to test whether APB may react with common myeloma antigens. We found that immunoglobulin fractions with APB recognize multiple recurring protein targets, many of which are upregulated in myeloma. Characterization of HSP60 as one such target revealed an aberrant, tumor-specific membrane display pattern of this antigen, apparently triggering a humoral immune response. These findings suggest that the better prognosis of myeloma patients developing APB may be due to a specific anti-myeloma activity of such immunoglobulins.
Materials and methods
Ethics statement
Patients consented to the use of residual serum after routine diagnostics for this investigation. Control serum was obtained from healthy donors after informed consent.
Immunofixation and purification of immunoglobulins from patients’ sera
Immunofixation was performed according to the Hydrasys protocol (Sebia, Paris, France). Immunoglobulins were purified from human sera by protein A chromatography (GE Healthcare, Buckinghamshire, UK) and positive/negative selection on KappaSelect medium (GE Healthcare, Buckinghamshire, UK) following the supplied protocol.
Preparation of protein extracts
Cell lines OPM-2 (DSMZ # ACC 50), IM-9 (ATCC #CCL-159) and primary myeloma or healthy donor peripheral mononuclear cells (PBMCs) were resuspended in 2D lysis buffer (40 mM Tris base, 8 M urea, 2 M thiourea, 2% CHAPS, 50 mM DTT with 0.5%v/v IPG buffer pH 4–7 [GE Healthcare, Buckinghamshire, UK]). After 30 min of incubation on ice, the protein extract was cleared from insoluble material by centrifugation.
One-dimensional and two-dimensional gel electrophoresis
For one-dimensional gel electrophoresis, protein extracts were resuspended in a reducing protein sample buffer and run on 10% SDS-PAGE gels (5 μg/lane). For two-dimensional gel electrophoresis, isoelectric focussing (IEF) was performed with 7 cm pH 4–7 IPG strips (GE Healthcare, Buckinghamshire, UK). Briefly, 50 μg of protein were diluted to 125 μl with rehydration buffer (8 M urea, 2 M thiourea, 2% CHAPS, 50 mM DTT with 0.5%v/v IPG buffer pH 4–7) and used to rehydrate each IPG strip overnight. The focussing was performed on PROTEAN IEF system (Bio-Rad, Hercules, CA; 250 V/30 min., 5,000 V/5,000 Vh, 5,000 V until 12 kVh). The IPG strips were equilibrated in DTT and iodoacetamide containing buffers, applied on 12.5% SDS-PAGE gels and subjected to electrophoresis as above. Gels were stained with colloidal coomassie (Serva, Heidelberg, Germany) or blotted.
Western blotting
After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes, blocked for 1 h, incubated overnight with the immunoglobulin fractions as primary antibody (100 μg/10 ml blocking buffer) followed by detection with goat anti-human kappa or lambda (Invitrogen Camarillo, CA) and a horseradish peroxidase-conjugated rabbit anti-goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For detection of HSP60 or Beta-actin, commercial antibodies were used (HSP60 clone 2E4, Acris, Herford, Germany, and Beta-actin, BioLegend, San Diego, CA). We used the ECL kit for immunodetection, followed by autoradiography on ECL Hyperfilms (GE Healthcare, Buckinghamshire, UK).
Mapping of protein spots
Spots on 2D western blots were overlayed with the coomassie-stained reference map by using Delta 2D software (Decodon), and matching spots were excised for protein identification by mass spectrometry (MS).
Protein identification by LC–ESI–ion trap analysis
Tryptic digestion of the gel spots was done according to Bertinetti et al. [13]. Identification was performed on an Agilent 1100 LC/MSD-trap XCT series system. The electrospray ionization system was the Chip Cube system using a ProtID-Chip-43 (Agilent Technologies, Santa Clara, CA). Sample loading from the microtiter plate onto the enrichment column was performed at a flow rate set to 2 μl/min with two mobile phases at a ratio of 98:2 (mobile phase A: 0.2% FA in H2O; mobile phase B: 100% ACN). LC gradient was delivered with a flow rate of 400 nl/min. Tryptic peptides were eluted from the reversed-phase column into the mass spectrometer using a linear gradient elution of 2–40% B in 40 min. For MS experiments, the following mode and tuning parameters were used: Scan range: 300–2,000 m/z, polarity: positive, capillary voltage: −1,800 V, flow and temperature of the drying gas were 4 l/min and 325°C. The MS/MS experiments were carried out in auto MS/MS mode using a 4 Da window for precursor ion selection. After 3 MS/MS spectra, the precursor ions were actively excluded from fragmentation for at least 1 min. The generic files for database searching were generated by Data Analysis software version 3.4, for precursor ion selection, a threshold of 5 S/N was applied and the absolute number of compounds was restricted to 1,000 per MS/MS experiment. Protein identification was performed with Mascot software [14] using the Swiss-Prot database [15].
Recombinant expression of HSP60
HSP60 was recombinantly expressed in a prokaryotic system (IMPACT kit, New England BioLabs, Ipswich, MA). In brief, RNA was extracted from human PBMCs (Roboklon Kit, Berlin, Germany), and cDNA was generated by reverse transcription (Omniscript, Qiagen, Hilden, Germany). The human HSP60 gene was amplified from this cDNA template by PCR, cloned into the pTXB1 vector and expressed in E. coli as C-terminal fusion to intein, which contains a chitin binding domain. E. coli cells were cultured, IPTG-induced and lysed. The crude lysate containing the HSP60-intein precursor was purified by chitin chromatography. HSP60 was cleaved from the affinity matrix by DTT and used for subsequent experiments.
Immunofluorescence and confocal microscopy
Primary bone marrow cells from myeloma patients and healthy controls were spun on slides (76 × 26 mm, Roth, Germany), fixed with ethanol and blocked. Stainings were performed using a mouse anti-human HSP60 antibody (Acris, Herford, Germany, clone 2E4) at 20 μg/ml and a secondary Fluorescein-isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or using patient-derived immunoglobulin fractions as primary antibody followed by secondary detection with a FITC-conjugated secondary mouse anti-human antibody (SouthernBiotech, Burmingham, Alabama). Images were obtained by confocal microscopy (Leika TCS SP2 AOBS; lens 63×, Wetzlar, Germany) and analyzed using Leika confocal software.
Results
Characteristics of myeloma patient cohort
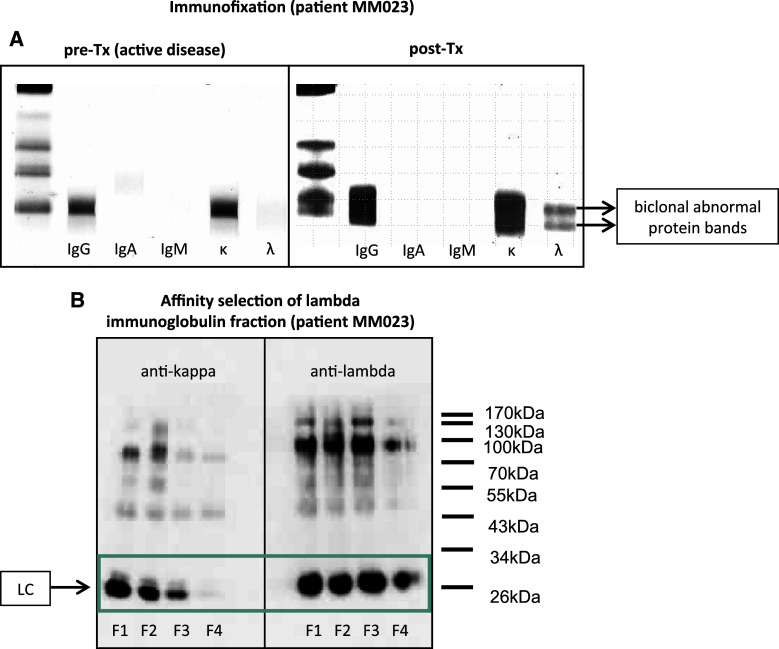
Consecutive myeloma patients visiting the outpatient unit within 18 months after allogeneic stem cell transplantation were screened for evidence of serum oligoclonal abnormal protein bands (APB) by means of immunofixation as a routine clinical testing. An example of prominent APB in the lambda immunoglobulin fraction of patient MM023 is shown in Fig. 1a. A total of eleven myeloma patients with APB after transplantation as well as a control group of 4 myeloma patients with comparable pre-transplant clinical features but without evidence of APB after transplant and 4 age-matched healthy control subjects were included in this study (Table 1). We clinically characterized the patient cohort by assessing the objective responses on day 100 as well as 12 months after allogeneic transplantation using the IMWG criteria (Table 1) [16, 17]. Patients who developed APB after transplant had higher chances of achieving complete remissions (CR) or very good partial remissions (vgPR) than patients without APB. Whereas there was only a statistical trend at day 100 after transplant (CR/vgPR rate: 72.7% in APB group versus 25% in non-APB group, p = 0.23; CR rate: 63.6% versus 0%, p = 0.077), the differences between patients with and without APB resulted statistically significant 12 months after transplant (CR/vgPR rate: 90% in APB group versus 25% in non-APB group, p = 0.041; CR rate: 80% versus 0%, p = 0.015). Although these data have to be interpreted with caution due to the small number of patients and the short post-transplant follow-up, it may underline the clinical significance of APB development in the allogeneic transplant setting.
Fig. 1.
Oligoclonal abnormal protein bands on immunofixation. a Immunofixation of serum immunoglobulins of patient MM023. Immunofixation of serum immunoglobulins was performed before (pre-Tx) and after (post-Tx) allogeneic stem cell transplantation as described by the manufacturer. b Western blot analysis determining the purity of an APB-containing lambda immunoglobulin fraction after positive selection of whole serum on protein A sepharose followed by negative selections on kappaSelect affinity chromatography medium over four selection rounds. The final flow-through (F4) was cleared from kappa immunoglobulins. F flow-through, LC (immunoglobulin) light chain
Table 1.
Characteristics of myeloma patients included in this study
| Pat. code | Paraprotein isotype | Allo-Tx date | APB after allo-Tx | Serum sample collection dates | Response at day 100/12 months after allo-Txa |
|---|---|---|---|---|---|
| MM023 | IgG kappa | 04/09 | + | 03/09 (pre-Tx), 06/09, 08/09, 11/09, 08/10 | CR/CR |
| MM025 | IgG kappa | 11/09 | + | 03/09 (pre-Tx), 03/10, 09/10, 05/11 | PR/CR |
| MM031 | IgG lambda | 05/10 | + | 03/10 (pre-Tx), 09/10, 02/11, 05/11 | CR/CR |
| MM040 | IgG kappa | 10/09 | + | 03/11 | CR/CR |
| MM041 | Lambda LC | 12/09 | + | 03/11 | PR/CR |
| MM043 | IgG kappa | 09/10 | + | 03/11 | CR/CR |
| MM045 | IgG lambda | 02/10 | + | 03/11 | CR/CR |
| MM046 | IgA lambda | 10/09 | + | 03/11 | CR/CR |
| MM047 | IgG lambda | 11/09 | + | 03/11 | vgPR/vgPR |
| MM051 | IgG kappa | 09/10 | + | 04/11 | PR/PD |
| MM054 | IgG kappa | 02/11 | + | 06/11 | CR/n.e. |
| MM033 | IgA kappa | 01/11 | − | 05/11 | PR/PR |
| MM037 | IgG lambda | 11/10 | − | 03/11 | PD/PD |
| MM038 | IgG kappa | 12/09 | − | 03/11 | vgPR/vgPR |
| MM039 | IgG lambda | 04/10 | − | 03/11 | SD/SD |
Pat. patient, MM multiple myeloma, APB abnormal protein bands, allo-Tx allogeneic stem cell transplantation, pre-Tx serum samples that had been collected prior to transplantation, LC light chain, CR complete remission, vgPR very good partial remission, PR partial remission, SD stable disease, PD progressive disease
aResponse to treatment was assessed using the IMWG uniform response criteria
Screening of serum kappa and lambda immunoglobulin fractions for anti-myeloma reactivity
To study their antigen-reactivity, immunoglobulins of the patient and donor cohort were purified and—depending on downstream applications—separated into kappa and lambda immunoglobulin fractions. The purity of the resulting fractions was assessed by western blot analysis (Fig. 1b).
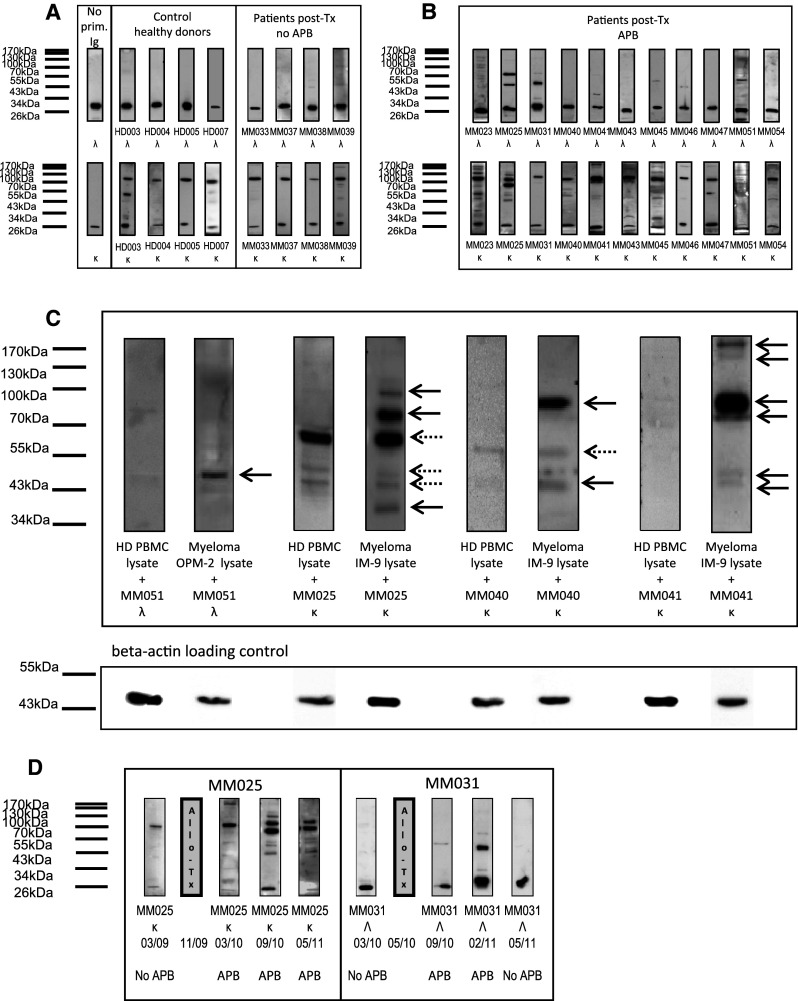
Purified immunoglobulin fractions were screened for reactivity with protein extracts from myeloma-derived cell lines by western blot analysis after one-dimensional gel-electrophoretic separation of the extracts. All kappa immunoglobulin fractions were screened on protein extracts from the IgG kappa expressing and secreting myeloma-derived IM-9 cell line, whereas all lambda immunoglobulin fractions were screened on protein extracts of the lambda expressing myeloma cell line OPM-2. This strategy was chosen as it provided an internal control in which the secondary anti-kappa or anti-lambda antibodies used to detect the primary immunoglobulin fractions produced a control band at 25 kDa in the cell lysate if the assay was performed correctly. Immunoglobulin fractions of healthy donors and myeloma patients without evidence of APB after transplantation revealed generally little or no reactivity with the myeloma protein extracts (apart from the 25 kDa kappa and lambda control bands detected invariably by the secondary antibodies) as shown in Fig. 2a. In contrast, kappa and lambda immunoglobulin fractions of myeloma patients who had developed APB after transplantation were more reactive, with some samples displaying high reactivity with a considerable number of proteins (Fig. 2b).
Fig. 2.
Screening of immunoglobulin fractions of multiple myeloma patients after allogeneic transplant for anti-myeloma protein reactivity. a Western blot analysis of immunoglobulin reactivity of healthy donors and myeloma patients after transplant without oligoclonal abnormal protein bands (APB). SDS gels were loaded with protein extracts from myeloma cell lines IM-9 (expressing kappa immunoglobulin) or OPM-2 (expressing lambda immunoglobulin) followed by electrophoresis and immunoblotting. Blots were incubated with patient-derived immunoglobulin purified from serum or immunoglobulin from healthy control serum, and target bands were visualized using secondary goat anti-kappa or anti-lambda and a tertiary anti-goat horseradish peroxidase-conjugated antibody. Control bands around 25 kDa representing kappa/lambda immunoglobulins from the myeloma cell lysates detected directly by the secondary antibodies indicated correct incubation conditions. HD followed by a number refers to the healthy donor sample code; Ig immunoglobulin, Tx transplantation, APB abnormal protein bands. b Western blot analysis of immunoglobulin reactivity of myeloma patients after transplant with APB. Western blotting of protein extracts was carried out as described in a. Blots were incubated with patient-derived immunoglobulin purified from serum after allogeneic transplant, and target bands were visualized using secondary goat anti-kappa or anti-lambda and a tertiary anti-goat horseradish peroxidase-conjugated antibody. MM followed by a number refers to the multiple myeloma patients’ sample code. c Selected myeloma patients’ immunoglobulin reactivity with myeloma protein extracts as compared to proteins from unrelated, non-malignant tissues. Western blotting of protein extracts (myeloma protein extracts and healthy donor PBMC extracts) was carried out as described in a. Blots were incubated with patient-derived immunoglobulin purified from serum after allogeneic transplant, and target bands were visualized using secondary goat anti-kappa or anti-lambda and a tertiary anti-goat horseradish peroxidase-conjugated antibody. Equal protein loading was ensured by beta-actin staining of all blots (lower panel). d Time course of APB and anti-myeloma protein reactivity of immunoglobulins. Serum samples of two myeloma patients were collected before and at different time points after allogeneic stem cell transplantation. Kappa and lambda immunoglobulin fractions were tested for anti-myeloma protein reactivity as described above. On the bottom, the presence of APB on immunofixation is indicated for the respective time points. Grey bars labelled “Allo-Tx” indicate timepoint of allogeneic stem cell transplantation
Next, we wished to find out whether such reactivities were myeloma-specific or the same immunoglobulin interactions could be found in protein extracts from unrelated, non-malignant tissues. Therefore, immunoglobulin fractions from patients MM025, MM040, MM041 and MM051, exhibiting distinct reactivity with proteins from myeloma extracts, were subjected to the same screening procedure using healthy donor PBMCs as a source of target protein. As shown in Fig. 2c, most of the reactivities were specifically found in the myeloma protein extracts, whereas there was no interaction with proteins from healthy donor PBMCs (12 out of 16 interactions, marked with black arrows). However, some of the interactions could also be detected when healthy donor PBMCs were used as protein source (4 out of 16 interactions, marked with dotted arrows). To ensure equal protein loading, all blots were subjected to beta-actin staining (Fig. 2c, lower panel).
This data may indicate that the majority of patient-derived immunoglobulins interact with proteins that are preferentially (or aberrantly) expressed by myeloma cells. It may suggest that the observed reactivities are myeloma-directed rather than representing general autoreactivity not sufficiently suppressed after allogeneic transplant.
Fluctuations of anti-myeloma reactivity and APB over time
Next, we studied the anti-myeloma protein reactivity with respect to APB presence or absence at different time points. Therefore, immunoglobulin fractions at different time points before and after allogeneic stem cell transplantation were screened for anti-myeloma reactivity as above. Interestingly, the ability of the immunoglobulin fractions to recognize myeloma proteins varied significantly over time. In patient MM025 and especially patient MM031, myeloma protein interactions even seemed to increase or decrease as a function of APB appearance or disappearance as demonstrated in Fig. 2d. The screenings at different time points demonstrate the fluctuations in anti-myeloma protein reactivity, with some proteins being constantly recognized, whereas others representing only transient targets of the humoral immune response.
Identification of APB targets
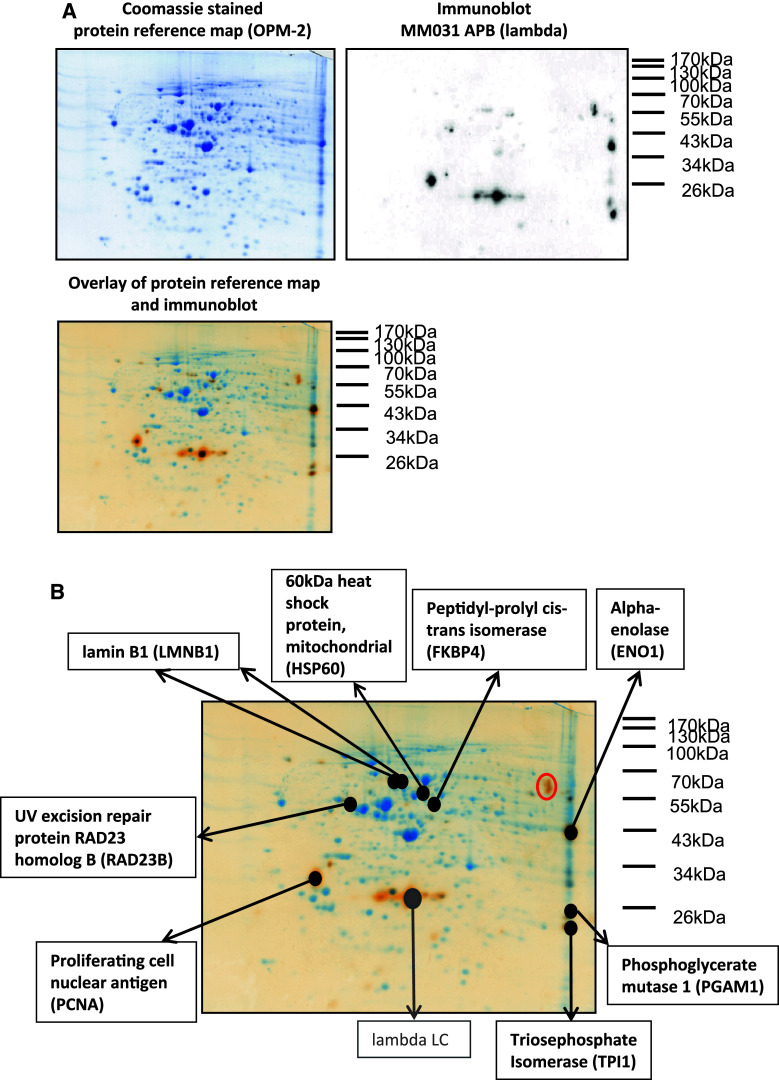
To identify the target proteins recognized by APB, myeloma protein extracts were subjected to two-dimensional electrophoretic separation followed by either coomassie staining or transfer to PVDF membranes for western blot analysis. All immunoglobulin fractions, which were reactive with myeloma proteins in the screening phase, were subsequently used to immunodetect two-dimensionally separated myeloma proteins. By overlaying immunoblots with the corresponding coomassie-stained protein reference map, relevant protein spots could be matched, excised and identified via mass spectrometry. An example of a protein reference map from the OPM-2 myeloma cell line, the corresponding immunoblot of patient MM031’s serum immunoglobulins and the overlay of both images are shown in Fig. 3a. In Fig. 3b, all matching spots were labeled with the respective protein IDs as analyzed by mass spectrometry. The lambda light chain spot was marked in gray as this protein is targeted by the secondary anti-human lambda detection antibody rather than being recognized by the serum immunoglobulins of patient MM031. Some of the immunodetected spots could not be matched with spots on the protein reference map (e.g. the protein spots designated with a red circle in upper right corner of Fig. 3b), most probably due to limited sensitivity of the coomassie staining method and relatively low concentration of the corresponding protein.
Fig. 3.
Identification of protein targets recognized by oligoclonal abnormal protein bands. a Serological proteome analysis (SERPA) of lambda immunoglobulin fraction of patient MM031. A protein extract was prepared from the myeloma cell line OPM-2 and subjected to two-dimensional gel electrophoresis with subsequent coomassie staining (left panel) or transfer to PVDF membrane for immunoblotting. Purified serum immunoglobulins of patient MM031 were used to detect protein targets on the PVDF membrane (secondary detection with goat anti-lambda and anti-goat horseradish peroxidase antibody) as shown in the right panel. The overlay of both images is shown in the lower panel. Delta 2D software was used for the analysis. b Identified protein targets of patient MM031’s lambda APB. The image represents the overlay of the protein reference map from OPM-2 myeloma cells with the MM031 lambda immunoblot (as described in a). Matching protein spots were excised and identified by mass spectrometry as indicated. The immunoblot signals designated with a red circle in the upper right corner could not be matched to any of the coomassie-stained protein spots
The number of recognized protein spots was highly variable across all tested immunoglobulin fractions, ranging from one to over 50 spots. An analysis of all identified protein targets recognized by APB showed that a number of recurring antigens were immunodetected by the immunoglobulin fractions of different patients. Table 2 summarizes all tested immunoglobulin fractions, screening results for APB target recognition and all subsequently identified proteins. Most commonly, patients’ samples reacted with heat-shock proteins such as HSP90, HSPA8, HSPA9 and HSP60. HSP reactivity was found in 45% of myeloma patients with APB, whereas immunoglobulin fractions of myeloma patients without APB as well as healthy donors did not show any reactivity with these proteins. In as much as 27% of patients, we found reactivity with neutral alpha-glucosidase or alpha-enolase. These reactivities also appeared to be specific for patients with APB as none of the control immunoglobulin fractions reacted with these protein targets. Moreover, several patient-derived immunoglobulin fractions recognized vimentin and heterogeneous nuclear ribonucleoprotein K, though these proteins were also targeted by the immunoglobulin fraction of one myeloma patient after allogeneic transplant without evidence of APB on immunofixation. Other biologically relevant proteins recognized by the immunoglobulin fractions of individual patients were the cancer-testis antigen MAGEA4, hematopoietic lineage cell-specific protein, proteasome activator complex subunit 1, proliferating cell nuclear antigen as well as different proteins involved in protein translation (data not shown).
Table 2.
Identification of immunoglobulin protein targets
| Pat. code | Protein targets |
|---|---|
| MM pat. post-Tx with APB | |
| MM023 | Heat-shock protein HSP 90-beta (HSP90AB1), Heat-shock protein HSP 90-alpha (HSP90AA1), 40S Ribosomal protein SA (RPSA), Melanoma-associated antigen 4 (MAGEA4), 60 kDa Heat-shock protein, Mitochondrial (HSP60), Hematopoietic lineage cell-specific protein (HCLS1), Neutral alpha-glucosidase AB (GANAB) |
| MM025 | Alpha-enolase (ENO1), Neutral alpha-glucosidase AB (GANAB), Heat-shock cognate 71 kDa protein (HSPA8), Stress-70 protein, Mitochondrial (HSPA9), Zinc finger CCCH domain-containing protein 11A (ZC3H11A), Tryptophanyl-tRNA synthetase, Cytoplasmic (WARS), X-ray repair cross-complementing protein 5 (XRCC5), T-complex protein 1 subunit alpha (TCP1), Ezrin (EZR) |
| MM031 | Heat-shock protein HSP 90-beta (HSP90AB1), Heat-shock protein HSP 90-alpha (HSP90AA1), Alpha-enolase (ENO1), Proliferating cell nuclear antigen (PCNA), 60 kDa Heat-shock protein, Mitochondrial (HSP60), UV excision repair protein RAD23 homolog B (RAD23B), Lamin B1 (LMNB1), Phosphoglycerate mutase 1 (PGAM1), Triphosphate isomerase (TPI1), Peptidyl-prolyl cis–trans isomerase (FKBP4) |
| MM040 | Elongation factor 1-delta (EEF1D) |
| MM041 | Heat-shock protein HSP 90-beta (HSP90AB1), Heat-shock protein HSP 90-alpha (HSP90AA1), Vimentin (VIM), Heat-shock cognate 71 kDa protein (HSPA8) |
| MM043 | Protein disulfide-isomerase (P4HB) |
| MM045 | Vimentin (VIM) |
| MM046 | Neutral alpha-glucosidase AB (GANAB), Alpha-enolase (ENO1) |
| MM047 | Heat-shock protein HSP 90-beta (HSP90AB1) |
| MM051 | Tubulin beta chain (TUBB) |
| MM054 | Proteasome activator complex subunit 1 (PSME1) |
| MM pat. post-Tx without APB | |
| MM033 | – |
| MM037 | – |
| MM038 | – |
| MM039 | Heterogeneous nuclear ribonucleoprotein (HNRNPK), Vimentin (VIM), Eukaryotic initiation factor 4A-I (EIF4A1) |
| Healthy donors | |
| HD003 | – |
| HD004 | – |
| HD005 | – |
| HD007 | – |
Pat. patient, APB abnormal protein bands, MM multiple myeloma, post-Tx post-transplantation
A systematic search of the NCI-60 database [18] and the Human Protein Atlas [19] revealed that more than 80% of all identified protein targets are upregulated in myeloma tissue and cell lines, a lot of them being candidate cancer biomarkers and some showing aberrant expression in cancer cells. This analysis may suggest that most of the detected immunoglobulin reactivities correspond to a specific myeloma-directed immune response rather than simply representing increased and insufficiently suppressed autoimmunity after transplant.
Evaluation of HSP60 as APB target
After having analyzed a broad spectrum of immunoglobulin reactivities, we set out to evaluate HSP60 as an exemplary antigen involved in the presumed anti-myeloma immune response in more detail. This antigen was chosen for two reasons: First, heat-shock proteins were the most commonly detected APB targets in our cohort. Second, the lambda immunoglobulin fraction of patient MM023, which recognized this antigen, showed only two prominent bands on immunofixation (Fig. 1a), suggesting limited reactivity of this immunoglobulin fraction with other protein targets thereby facilitating the evaluation of HSP60 as an antigen. In fact, the lambda fraction of MM023 reacted only with one additional antigen—hematopoietic lineage cell-specific protein—when screened on a myeloma cell line (data not shown).
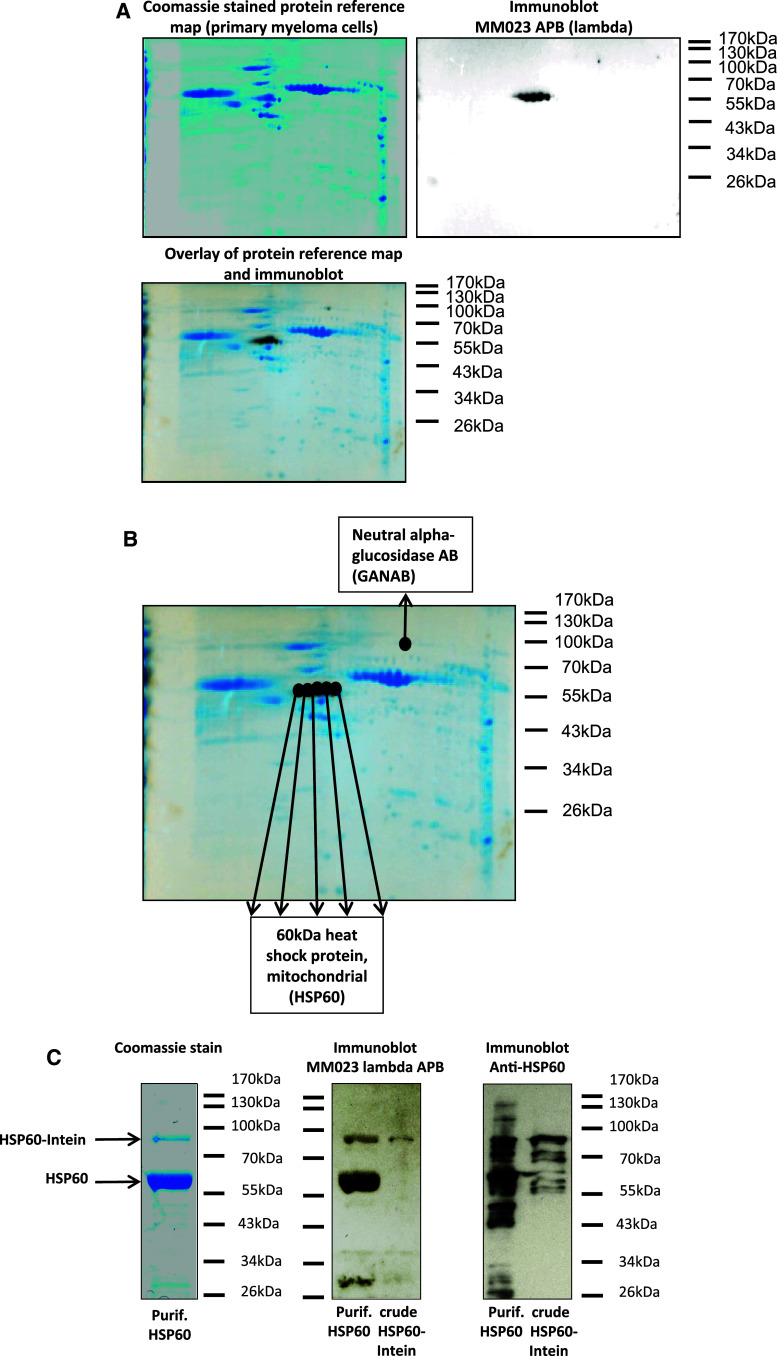
First, we evaluated the reactivity of the lambda immunoglobulin fraction of patient MM023 with protein extracts from primary myeloma cells. Therefore, a protein extract was prepared from a bone marrow aspirate with 95% infiltration by myeloma cells from an unrelated patient after informed consent. The percentage of infiltration was confirmed by cytology and immunophenotyping (data not shown). The lambda immunoglobulin fraction of patient MM023 showed a strong interaction with five major and one minor protein spots from the primary myeloma protein extract as shown in Fig. 4a. The five major spots corresponded to HSP60 (Fig. 4b), thus confirming the data from the myeloma cell line screening. The minor spot was identified as neutral alpha-glucosidase, whereas hematopoietic lineage cell-specific protein, which had been previously detected by this immunoglobulin fraction in the myeloma cell line protein extract, could not be detected in the primary myeloma protein extract. These findings underline the biological relevance of the HSP60 interaction.
Fig. 4.
HSP60 as exemplary target of an anti-myeloma immune response. a Serological proteome analysis (SERPA) of lambda immunoglobulin fraction of patient MM023. A protein extract was prepared from bone marrow primary myeloma cells and subjected to two-dimensional gel electrophoresis with subsequent coomassie staining (left panel) or transfer to PVDF membranes for immunoblotting with lambda immunoglobulins of patient MM023 (secondary detection with goat anti-lambda and anti-goat horseradish peroxidase antibody) as shown in the right panel. The overlay of both images is shown in the lower panel. Delta 2D software (Decodon) was used for the analysis. b Identified protein targets of patient MM023’s lambda APB. The image represents the overlay of the protein reference map from primary myeloma cells with the MM023 lambda immunoblot (as described in a). Matching protein spots were excised and identified by mass spectrometry as indicated. c Confirmation of HSP60 as target of patient MM023’s lambda APB. HSP60 was recombinantly expressed in a prokaryotic system. The left panel shows the purified HSP60 after cleavage from its intein tag (coomassie stain, 5 μg/lane). Purified HSP60 (60 kDa) as well as the crude lysate containing HSP60-intein precursor protein (88 kDa) were subjected to western blot analysis using a HSP60 antibody and the lambda immunoglobulin fraction of patient MM023 for detection. Secondary detection was performed with either a horseradish peroxidase-conjugated anti-mouse IgG antibody or a goat anti-lambda and anti-goat horseradish peroxidase antibody
Next, we investigated immunoglobulin MM023 lambda reactivity with recombinantly expressed HSP60. Therefore, HSP60 was expressed as C-terminal fusion to intein (28 kDa) in a prokaryotic system. The recombinant expression yielded high amounts of purified HSP60 as shown in Fig. 4c (left panel). Purified HSP60 and the crude lysate containing HSP60-intein precursor protein were subjected to western blot analysis using a HSP60 antibody and the lambda immunoglobulin fraction of patient MM023 for detection. Anti-HSP60 and MM023 lambda detected HSP60 as purified protein as well as in the crude bacterial lysate as shown in Fig. 4c (middle and right panel) confirming the reactivity of patient MM023’s lambda APB with this antigen.
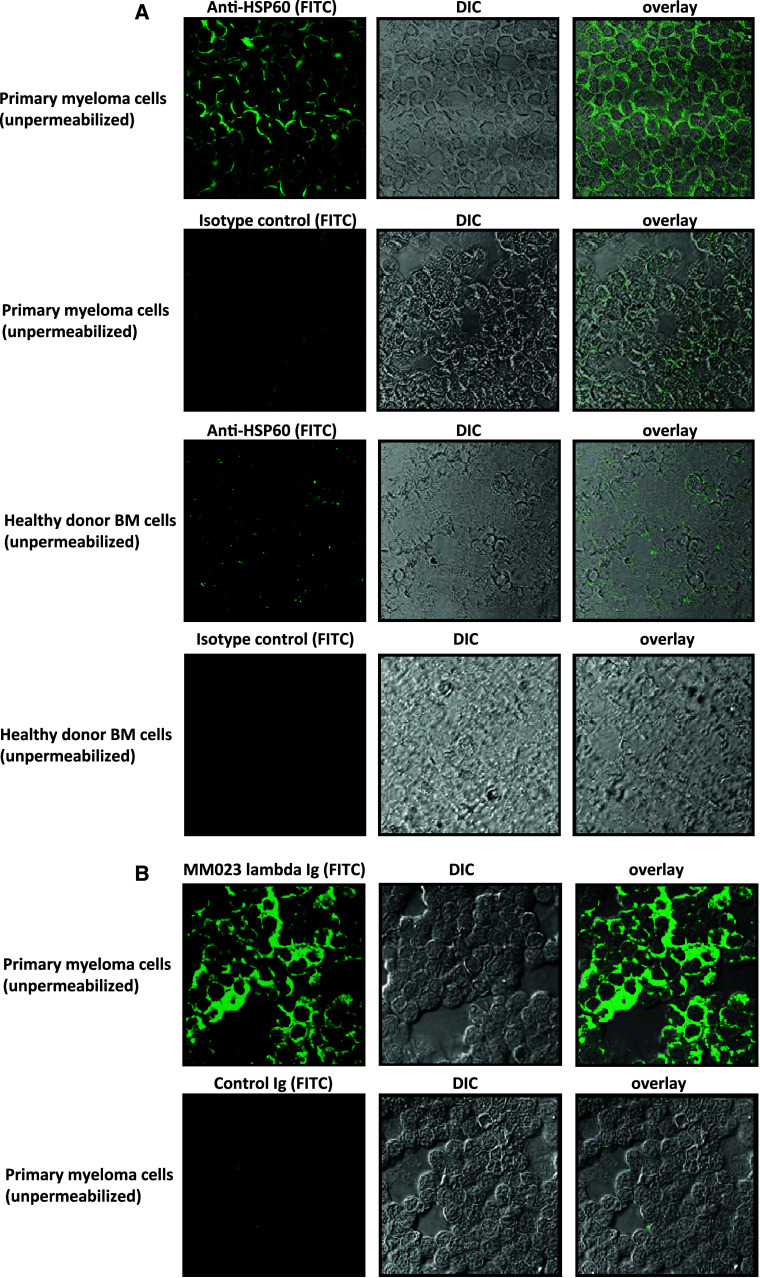
Whereas HSP60 is known for its intracellular functions, most notably as a mitochondrial chaperonin that is responsible for the transportation and refolding of proteins from the cytoplasm into the mitochondrial matrix, some studies suggest that HSP60 can be aberrantly expressed on the surface of virally infected and tumor cells [20, 21]. We, therefore, asked whether HSP60 is also displayed on multiple myeloma cells, as such cell surface exposition would render this protein accessible to the immune system. To this end, we stained unpermeabilized primary multiple myeloma cells with a HSP60 antibody (exemplarily shown in Fig. 5a). Strong HSP60 membrane expression could be detected by laser scanning microscopy, while control bone marrow cells of healthy donors showed, if at all, only faint membrane staining. To investigate whether the lambda immunoglobulin fraction of patient MM023 indeed recognizes membrane-bound HSP60, we performed additional stainings using MM023 lambda as primary antibody followed by fluorescein-based secondary detection. This immunoglobulin fraction equally stained the membrane of primary myeloma cells indicating that surface-exposed HSP60 is recognized by the myeloma patient’s antibody immune response (Fig. 5b).
Fig. 5.
Surface exposition of HSP60 in primary myeloma cells. a HSP60 is displayed on the surface of non-permeabilized primary myeloma cells as visualized by confocal microscopy. Primary myeloma cells from bone marrow as well as bone marrow cells from healthy controls were spun on a slide and fixed with ethanol without further permeabilization. In the middle, the myeloma cells are shown in differential interference contrast (DIC). A HSP60 antibody or a murine isotype control antibody were used to stain the outer surface of the cells followed by secondary detection with an anti-mouse FITC-conjugated antibody (green) as shown in the left panels. The right panels display the overlay of both images. Images were obtained by confocal microscopy (Leika TCS SP2 AOBS; lens × 63) and analyzed using Leika confocal software. b Patient-derived HSP60-specific serum immunoglobulins recognize surface HSP60 on primary myeloma cells. Primary myeloma cells from bone marrow were spun on slides and fixed as described in a. The panels show (from left to right) primary myeloma cells stained with the lambda immunoglobulin fraction from patient MM023 or control IgG immunoglobulin followed by secondary detection with an anti-human FITC-conjugated antibody (green), myeloma cells in differential interference contrast and the overlay of both images. Images were obtained by confocal microscopy (Leika TCS SP2 AOBS; lens × 63) and analyzed using Leika confocal software
Discussion
After stem cell transplantation 30–70% of myeloma patients develop oligoclonal abnormal protein bands (APB) as evidenced by routinely performed immunofixation [6, 8]. These antibodies differ from the paraprotein secreted by the malignant plasma cells, and thus do not represent relapsed disease—except for rare isotype switched cases [10]. Although the development of APB has been reported across the spectrum of hematological disorders treated with autologous or allogeneic stem cell transplantation as well as after myeloma treatment with novel agents [9, 22], the significance and biological role of these oligoclonal antibodies remains largely unclear. Recent studies suggest that myeloma patients with APB have a better prognosis than patients with polyclonal reconstitution of their immunoglobulin repertoire [6–12]. These studies have been conducted primarily in the context of autologous stem cell transplantation, but also included a number of patients after allogeneic stem cell transplantation, suggesting a prognostic significance of APB also in this subset of patients. In addition, our own observations point in this direction, as cases with APB, were more likely to achieve CR or vgPR after allogeneic transplant as compared to patients who did not develop APB after transplant (Table 1).
This interesting correlation prompted us to hypothesize that APB may be produced by regenerating B-cells (from donor and/or host) as part of a humoral anti-myeloma immune response. This response may be facilitated by the release of large amounts of tumor antigens after highly effective therapies such as autologous or allogeneic stem cell transplantation and treatment with immunomodulatory novel agents, but the exact mechanisms by which cancer antigens are able to trigger humoral immune responses are still controversial. An emerging body of evidence suggests that most of these proteins are expressed in a tumor-specific or ectopic way rendering them immunogenic [23, 24]. Humoral immune responses have been observed against overexpressed proteins, mutations and splice variants, misfolded, degraded or post-translationally modified proteins as well as aberrantly localized proteins [23–26]. In lymphatic neoplasias, a number of antigens have been established as targets of spontaneous B-cell responses by reverse immunology (serological identification of antigens by recombinant expression cloning, SEREX) [27–31]. In myeloma, particularly cancer-testis antigens seem to be of importance as they are expressed with high specificity in myeloma cells (for a review, see Meklat et al.) [32].
In this study, we globally assessed serum immunoglobulin reactivities with myeloma proteins in myeloma patients within the first year after allogeneic stem cell transplantation by serological proteome analysis (SERPA). We found that immunoglobulin fractions of myeloma patients with APB reacted more with myeloma proteins than those of patients without APB. Interestingly, a number of recurring antigens or classes of proteins were recognized by immunoglobulin fractions with APB, many of which are overexpressed and/or differentially modified in myeloma. Heat-shock proteins were among the most frequently recognized antigens, as 45% of APB immunoglobulin fractions were reactive with proteins from this family. This was an interesting finding, as heat-shock proteins are not only generally upregulated in myeloma [33], but show aberrant expression profiles in various cancer cells. Moreover, heat-shock proteins have been implicated in cancer development and can favor or even be essential for tumor cell survival [20, 21]. HSP90—specifically recognized by four out of eleven immunoglobulin fractions with APB—forms cancer-cell-specific activated complexes that render these cells sensitive to a targeted treatment with novel HSP90 inhibitors [34]. When evaluating HSP60 as an exemplary antigen in myeloma, we found selective recognition of this protein in myeloma protein extracts, as recombinantly expressed and purified protein and as aberrantly exposed protein on the plasma membrane of primary myeloma cells. This latter finding is in line with data from the literature indicating that HSP60 (as well as heat-shock proteins of 70 and 90 kDa) [21] can be aberrantly displayed on the cell surface of various solid tumor cells as well as lymphomas [20, 35]. Such aberrant expression patterns of primarily intracellular antigens may help to understand the potential functional implications of our findings, as antibody immune responses directed against surface-exposed antigens could eventually lead to tumor cell killing by complement-mediated or cellular cytotoxicity. Additionally, antibody responses even against strictly intracellular antigens could trigger T cell responses by opsonization of post-chemotherapy tumor cell debris, facilitation of uptake by antigen-presenting cells followed by presentation to and priming of T cells as it has been suggested for autoimmune diseases and exemplary cancer antigens [36, 37]. The phenomenon of upregulation and aberrant cell surface display on tumor cells has also been described for alpha-enolase, an antigen targeted by the immunoglobulin fractions of almost one-third of our patients with APB [38]. Cancer cells express alpha-enolase with more post-translational modifications, such as acetylation, methylation and phosphorylation, and the patterns of such modifications are specific for tumor cells [38]. Due to the cancer-cell-specific expression and modification patterns, it is not surprising that humoral as well as cellular immune responses against this antigen have been found in various tumors [38]. Moreover, the above-mentioned integration of a humoral immune response facilitating T cell activation has been suggested to occur in alpha-enolase expressing pancreatic adenocarcinoma patients [37]. T cells of patients with anti-alpha-enolase IgG are more easily activated in response to the protein as compared to T cells of patients without such antibodies, suggesting the induction of a T cell and B cell integrated anti-tumor immune response [37].
Taken together, we conclude that immunoglobulin fractions with APB target recurrent myeloma antigens thus constituting an anti-myeloma immune response rather than representing general autoreactivity not sufficiently suppressed after allogeneic transplant. In linking APB to a myeloma-directed immune response, our results may suggest a biological mechanism explaining the more favorable prognosis of patients with APB. Future studies are needed to elucidate the clinical determinants of effective humoral immune responses as well as the functional B cell–T cell interplay potentially underlying anti-myeloma immunity. Such studies should also address the question, if highly aberrant tumor protein expression may be an intrinsic feature of myeloma cases, which are more likely to develop effective anti-tumor immune responses.
Acknowledgments
We thank Martin Trepel for helpful comments and Victoria Martens and Lisa Schindler for technical support. This work was supported by the Wilhelm Sander-Stiftung (grant #2009.035.1 to M.B.) and the Hamburger Krebsgesellschaft (scholarship to M.B.).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28:830–834. doi: 10.1200/JCO.2009.25.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Zhang MJ, Li P, Dispenzieri A, Milone GA, et al. Trends in allogeneic stem cell transplantation for multiple myeloma: a center for international blood and marrow transplant research (CIBMTR) analysis. Blood. 2011;118(7):1979–1988. doi: 10.1182/blood-2011-02-337329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall SL, Tate J, Gill D, Mollee P. Significance of abnormal protein bands in patients with multiple myeloma following autologous stem cell transplantation. Clin Biochem Rev. 2009;30:113–118. [PMC free article] [PubMed] [Google Scholar]
- 7.Sucak G, Suyani E, Ozkurt ZN, Yegin ZA, Aki Z, et al. Abnormal protein bands in patients with multiple myeloma after haematopoietic stem cell transplantation: does it have a prognostic significance? Hematol Oncol. 2010;28:180–184. doi: 10.1002/hon.936. [DOI] [PubMed] [Google Scholar]
- 8.Maisnar V, Tichy M, Smolej L, Zak P, Radocha J, et al. Isotype class switching after transplantation in multiple myeloma. Neoplasma. 2007;54:225–228. [PubMed] [Google Scholar]
- 9.Mark T, Jayabalan D, Coleman M, Pearse RN, Wang YL, et al. Atypical serum immunofixation patterns frequently emerge in immunomodulatory therapy and are associated with a high degree of response in multiple myeloma. Br J Haematol. 2008;143:654–660. doi: 10.1111/j.1365-2141.2008.07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zent CS, Wilson CS, Tricot G, Jagannath S, Siegel D, et al. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood. 1998;91:3518–3523. [PubMed] [Google Scholar]
- 11.de Larrea CF, Cibeira MT, Elena M, Arostegui JI, Rosinol L, et al. Abnormal serum free light chain ratio in patients with multiple myeloma in complete remission has strong association with the presence of oligoclonal bands: implications for stringent complete remission definition. Blood. 2009;114:4954–4956. doi: 10.1182/blood-2009-06-224832. [DOI] [PubMed] [Google Scholar]
- 12.Alejandre ME, Madalena LB, Pavlovsky MA, Facio ML, Corrado C, et al. Oligoclonal bands and immunoglobulin isotype switch during monitoring of patients with multiple myeloma and autologous hematopoietic cell transplantation: a 16-year experience. Clin Chem Lab Med. 2010;48:727–731. doi: 10.1515/cclm.2010.050. [DOI] [PubMed] [Google Scholar]
- 13.Bertinetti D, Schweinsberg S, Hanke SE, Schwede F, Bertinetti O, et al. Chemical tools selectively target components of the PKA system. BMC Chem Biol. 2009;9:3. doi: 10.1186/1472-6769-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, et al. A gene expression database for the molecular pharmacology of cancer. Nat Genet. 2000;24:236–244. doi: 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- 19.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 20.Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- 21.Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez de Larrea C, Tovar N, Cibeira MT, Arostegui JI, Rosinol L, et al. Emergence of oligoclonal bands in patients with multiple myeloma in complete remission after induction chemotherapy: association with the use of novel agents. Haematologica. 2011;96:171–173. doi: 10.3324/haematol.2010.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteom. 2007;6:1115–1122. doi: 10.1074/mcp.R600016-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009;276:6880–6904. doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- 26.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 27.Preuss KD, Zwick C, Bormann C, Neumann F, Pfreundschuh M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol Rev. 2002;188:43–50. doi: 10.1034/j.1600-065X.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou FL, Zhang WG, Chen G, Zhao WH, Cao XM, et al. Serological identification and bioinformatics analysis of immunogenic antigens in multiple myeloma. Cancer Immunol Immunother. 2006;55:910–917. doi: 10.1007/s00262-005-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwick C, Preuss KD, Kubuschok B, Held G, Ahlgrimm M, et al. Analysis of the antibody repertoire of patients with mantle cell lymphoma directed against mantle cell lymphoma-associated antigens. Ann Hematol. 2009;88:999–1003. doi: 10.1007/s00277-009-0711-0. [DOI] [PubMed] [Google Scholar]
- 30.Zwick C, Held G, Hammermeister V, Alahmad A, Kubuschok B, et al. Spontaneous high-titered IgG antibody responses against BCL-2 in patients with aggressive lymphomas. J Cancer Res Clin Oncol. 2009;135:1207–1213. doi: 10.1007/s00432-009-0561-0. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Preuss KD, Xie X, Regitz E, Pfreundschuh M. Analysis of the antibody repertoire of lymphoma patients. Cancer Immunol Immunother. 2002;51:655–662. doi: 10.1007/s00262-002-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meklat F, Li Z, Wang Z, Zhang Y, Zhang J, et al. Cancer-testis antigens in haematological malignancies. Br J Haematol. 2007;136:769–776. doi: 10.1111/j.1365-2141.2006.06484.x. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, et al. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- 34.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 35.Cicconi R, Delpino A, Piselli P, Castelli M, Vismara D. Expression of 60 kDa heat shock protein (Hsp60) on plasma membrane of Daudi cells. Mol Cell Biochem. 2004;259:1–7. doi: 10.1023/B:MCBI.0000021335.52162.51. [DOI] [PubMed] [Google Scholar]
- 36.Frisoni L, McPhie L, Colonna L, Sriram U, Monestier M, et al. Nuclear autoantigen translocation and autoantibody opsonization lead to increased dendritic cell phagocytosis and presentation of nuclear antigens: a novel pathogenic pathway for autoimmunity? J Immunol. 2005;175:2692–2701. doi: 10.4049/jimmunol.175.4.2692. [DOI] [PubMed] [Google Scholar]
- 37.Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer. 2009;125:639–648. doi: 10.1002/ijc.24355. [DOI] [PubMed] [Google Scholar]
- 38.Capello M, Ferri-Borgogno S, Cappello P, Novelli F. alpha-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]