Abstract
Inflammation has been implicated in the initiation and progression of ovarian cancer (OC), the underlying mechanisms of which are still unclear. We hypothesized that the abnormal expression of Toll-like receptors (TLRs), which were potential activators of nuclear factor-kappa B p65 (NF-κB p65), could promote inflammation and tumorigenesis in OC. In this study, we characterized the expression of TLRs in peripheral blood mononuclear cells (PBMCs) and found TLR2 and TLR6 mRNAs levels to be higher in PBMCs from OC patients than in those from benign disease (BC) or healthy normal controls (NC). Flow cytometry analysis showed that TLR1, TLR2 and TLR6 were highly expressed in monocytes from OC patients, but not in those from control subjects. Consistently, inflammatory cytokines interleukin (IL)-1β and IL-6 were up-regulated in PBMCs from OC patients upon stimulation with Pam3CSK4 (TLR1 ligand) and HKLM (TLR2 ligand), compared with unstimulated PBMCs. Stimulation of PBMCs with TLR ligands led to activation of downstream signaling molecules in TLRs (MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65). We also discovered that SK-OV-3-secreted factors were potent PBMCs activators, leading to the production of IL-1β, IL-6 and IL-8 through activation of TLRs and downstream signaling molecules in PBMCs. Before coculturing with SK-OV-3, pretreatment of THP-1 cells or PBMCs with monoclonal antibodies against TLR1, TLR2 or TLR6 inhibited the production of IL-1β and IL-6 and activation of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65. Our results provided new evidence that TLR1, TLR2 and TLR6 signaling was linked with inflammation in OC microenvironment.
Keywords: Toll-like receptor, Ovarian cancer, Inflammation, Cytokine
Introduction
Epithelial ovarian cancer (EOC) accounts for 25 % of all malignant tumors and has, for a long time, been the most fatal gynecologic malignancy affecting female reproductive health. Its high mortality rate is atypical, with only 45 % of women who are diagnosed surviving beyond 5 years [1].
Chronic infection and inflammation are usually thought to be two of the most important epigenetic and environmental factors leading to tumorigenesis and tumor progression [2, 3]. Individuals with inflammation are more likely to develop cancer progression [3, 4]. For instance, inflammatory bowel disease has been connected to colon cancer [5], infection with Helicobacter pylori is associated with gastric carcinoma [6], chronic viral hepatitis is linked to liver cancer [7], infection with some strains of human papillomavirus have been associated with cervical cancer, and infection with Epstein–Barr virus has been linked with Burkitt lymphoma and nasopharyngeal carcinoma [8, 9]. Ovarian endometriosis can promote and induce a proinflammatory environment within the ovary on a cyclic basis, predisposing the individual to tumorigenesis associated with specific types of epithelial ovarian carcinoma [10, 11]. Such observations suggest that chronic inflammation is concerned with tumor initiation, promotion and progression.
Toll-like receptors (TLRs) are pattern recognition receptors that play a significant role in innate immunity [12, 13]. TLRs can recognize microbe- or pathogen-associated molecular patterns (PAMPs) expressed by bacteria, viruses and host-derived PAMPs such as stress proteins [14]. Stimulation and activation of TLRs can recruit different signaling molecules, which contribute to the activation of different downstream mediators and targets. TLRs signals are generally transduced in a MyD88-dependent manner, leading to a proinflammatory response. MyD88 signaling is involved in the early activation of nuclear factor-κB (NF-κB) p65, which leads to the expression of proinflammatory cytokines [15, 16]. TLRs, as potential activators of NF-κB p65, are considered to be the gateway to inflammation and tumorigenesis.
The expression and function of TLRs in cancer cells and their relationship with tumorigenesis and tumor progression have been widely researched in recent years [17, 18]. Through MyD88 signaling and potentially NF-κB p65 activation, TLR2/6 ligands induce cell proliferation and promote the expression of proinflammatory cytokines, including interleukin (IL)-6, tumor necrosis factorα (TNFα) and IL-12 [19]. Kim isolated a factor that induced cytokine production by macrophages [19], which was recognized by both TLR2 and TLR6. Another recent study showed that Listeria monocytogenes promoted tumor growth through TLR2 [20]. Similarly, He et al., had a description about the expression of TLR4 in human lung cancer cells. TLR4 ligand in the cancer cells could induce the secretion of immunosuppressive cytokines and promote resistance to TNFα- and TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis [21].
In the present study, we described the expression of TLR1-9 in peripheral blood mononuclear cells (PBMCs) and the function of TLR1, TLR2 and TLR6, which were expressed prominently in the PBMCs of ovarian cancer (OC) patients. We also found that up-regulated TLR1, TLR2 and TLR6 constituted major signaling pathways in the activation of proinflammatory cytokines, which generated an inflammatory microenvironment that was beneficial to tumor growth. Our results demonstrated the link between TLR1/2/6-MyD88 signaling, inflammation and tumor immunology. Characterization of TLRs and their role in OC may lead to the development of novel therapeutic targets for treatments that can reduce the high mortality rate associated with OC.
Materials and methods
Preparation, isolation and culture of cells
PBMCs of OC patients were provided by the First Affiliated Hospital of Nanjing Medical University. Diagnosis of OC was established according to the diagnostic criteria of the Gynecologic Oncology Group. Blood donors were healthy women aged between 41- and 51-year old and women with uterine fibroids who tested negative for HIV, hepatitis B and hepatitis C aged between 39- and 50-year old. PBMCs were prepared from buffy coats using Ficoll–Hypaque density gradient centrifugation (TBD, Tianjin, China).
Human OC cell line (SK-OV-3) and human monocyte cell line (THP-1) were purchased from ATCC (Manassas, VA). Cells were grown in 5 % CO2 at 37 °C in McCoy’s 5A (Invitrogen, Carlsbad, CA) or RPMI 1640 (Gibco, Gaithersburg, MD) containing 10 % fetal bovine serum (FBS).
TLRs expression levels in PBMCs by RT-PCR
RNA was isolated using the miRNeasy Mini Kit (Qiagen, Valencia, CA) in accordance with the manufacturer’s instructions. The RNA template was qualitatively assessed by UV absorbance measurement at 260 and 280 nm (A260/280 ratio) and by gel electrophoresis. A fixed volume (3 μl) of total RNA was added to 20 μl of reverse transcriptase reaction mix (PrimeScript RT Master Mix Perfect Real Time) (TaKaRa Bio, Japan) and incubated at 37 °C for 15 min in ABI 2720 thermal cycler (Applied Biosystems/Life Technologies, Grand Island, NY), followed by a further 5 s at 85 °C to denature the enzyme. The resulting cDNA was used for the subsequent PCR analysis. The mRNA for β-actin was used as a normalization control in real-time PCR (RT-PCR) analysis and as a loading control in conventional PCR analysis. RT-PCR was performed in ABI 7500 system (Applied Biosystems/Life Technologies) using human TLRs primers (Table 1) and SYBR Green. Each analysis was repeated at least twice to ensure repeatability. To ensure a detailed analysis, samples were run on 4 % agarose gels to confirm correct band size, and melt curves were analyzed after each RT-PCR assay. The relative quantitation of target gene expression was carried out using the comparative C T method as described by Ross et al. [22].
Table 1.
PCR primers used for the detection of human TLRs and proinflammatory cytokines
| Primers | Sequence (5′ > < 3′) | Length (bp) | MW (bp) | |
|---|---|---|---|---|
| TLR1 | Forward | GGAGGCAATGCTGCTGTT | 20 | 120 |
| Reverse | GCCCAATATGCCTTTGTT ATCCTG | 24 | ||
| TLR2 | Forward | TGTTGCAAGCAGGATCCAAAG | 21 | 157 |
| Reverse | CACAAAGTATGTGGCATTGTCCAG | 24 | ||
| TLR3 | Forward | GGACTTTGAGGCGGGTGTT | 19 | 141 |
| Reverse | TGTTGAACTGCATGATGTACCTTG A | 25 | ||
| TLR4 | Forward | AGGATGATGCCAGCATGATGTC | 22 | 198 |
| Reverse | TCAGGTCCAGGTTCTTGGTTGAG | 23 | ||
| TLR5 | Forward | AAGATGTCGGAGCCTCAGATG | 21 | 181 |
| Reverse | GGGTCCCTGGTTGTTTAAAGACTTC | 25 | ||
| TLR6 | Forward | CAGAGTGAGTGGTGCCATTACGA | 23 | 138 |
| Reverse | AGCCTTCAGCTTGTGGTACTTGTTG | 25 | ||
| TLR7 | Forward | TCTTCAACCAGACCTCTACATTCCA | 25 | 172 |
| Reverse | GGAACATCCAGAGTGACATCACAG | 24 | ||
| TLR8 | Forward | GCGCTGCTGCAAGTTACGGA | 20 | 203 |
| Reverse | TCGACGATTGCTGCACTCTG | 20 | ||
| TLR9 | Forward | GGGACCTCGAGTGTGAAGCA | 20 | 258 |
| Reverse | CTGGAGCTCACAGGGTAGGAA | 21 | ||
| IL-1β | Forward | CACGATGCACCT GTACGATCA | 21 | 120 |
| Reverse | GTTGCTCCATATCCTGTCCCT | 21 | ||
| IL-6 | Forward | AACCTGAACCTTCCAAAGATGG | 22 | 865 |
| Reverse | TCTGGCTTGTTCCTCACTACT | 21 | ||
| IL-8 | Forward | GCCTTCCTGATTTCTGCAGCT | 21 | 868 |
| Reverse | TGCACTGACATCTAAGTTCTTTAGCAC | 27 | ||
| TNFα | Forward | ATGAGCACTGAAAGCATGATCC | 22 | 822 |
| Reverse | GAGGGCTGATTAGAGAGAGGTC | 22 | ||
| β- actin | Forward | TGGCACCCAGCACAATGAA | 19 | 186 |
| Reverse | CTAAGTCATAGTCCGCCTAGAAGCA | 25 | ||
TLRs expression levels in PBMCs subsets by flow cytometry
As soon as whole bloods were obtained, the cells were incubated away from light at room temperature with antihuman CD281 (TLR1) PE, antihuman CD282 (TLR2) FITC, antihuman CD286 (TLR6) PE and monoclonal antihuman CD4-FITC/PE, CD8-FITC/PE and CD14-APC/CD19-FITC/PE antibodies for 30 min (BD PharMingen, San Diego, CA). The incubated bloods were hemolyzed with FACS lysing solution (PharMingen, San Diego, CA) and incubated at room temperature away from light for another 15 min. After this, the cells were washed and resuspended in phosphate-buffered saline, and fluorescence labeling was measured by FACSCalibur flow cytometry (BD Biosciences). Data were analyzed using CellQuest software (BD Biosciences).
Simulation of TLRs with ligands
PBMCs resuspended in RPMI 1640 medium (Gibco, Gaithersburg, MD) with 10 % human AB serum were dispensed into a 96-well plate (Greiner, Frickenhausen, Germany), with 1 × 105 cells per well. The cells were incubated either with or without TLR ligands. TLR ligands were added at the concentration recommended by the manufacturer, which were as follows: Pam3CSK4 (TLR1 ligand)—100 ng/ml, heat-killed L. monocytogenes (HKLM; TLR2 ligand)—108 cells/ml, FSL-1 (TLR6/2 ligand)—100 ng/ml (Invivogen, San Diego, CA). After 24 h stimulation, the cells were collected and detected for the mRNA expression of IL-1β, IL-6, IL-8 and TNFα by RT-PCR.
Coculture of PBMCs with SK-OV-3
Transwell culture experiments were performed in 24-well plates which had an inner well pore size of 0.4 μm (Greiner, Frickenhausen, Germany). SK-OV-3 were cultured in the outer wells of the plates, with 1 × 105 cells per well in 1.5 ml McCoy’s 5A medium (Gibco, Gaithersburg, MD) containing 10 % FBS (Gibco, Gaithersburg, MD). Isolated human PBMCs were added into the inner wells, at 5 × 105 cells per well and in 500 μl of the same medium. The density ratio of PBMCs to SK-OV-3 cells was 5:1. After 24 h of incubation, the PBMCs were collected and analyzed using RT-PCR and western blot.
Antibody blocking and cytokine assays
PBMCs and THP-1 cells were pretreated with either 10 ng/ml control IgG1 antibody, anti-TLR1, anti-TLR2 or anti-TLR6 monoclonal antibody in 24-well plates, at 5 × 105 cells per well. They were treated for 1 h at 37 °C, before being cocultured with SK-OV-3 cells for 24 h in a transwell culture system at 1 × 105 cells per well. The PBMCs and THP-1 cells were collected and analyzed using western blot or RT-PCR. IL-1β, IL-6, IL-8 and TNFα were tested using PCR primers for the detection of human proinflammatory cytokines (Table 1).
Western blot analysis
PBMCs or THP-1 cells were harvested, and protein concentrations were determined for the samples using a BCA protein assay kit (Beyotime, Shanghai, China). Equal amounts of protein per lane were separated by SDS-PAGE and transferred to PVDF membrane (Bio-Rad, Hercules, CA). Blotting membranes were blocked and subsequently incubated with antibodies against either MyD88, TRAF6, TANK, NF-κB p65, p-NF-κB p65 or GAPDH (Cell Signaling Technology, Danvers, MA). After being washed, the membranes were reacted with horseradish peroxidase (HRP)–conjugated goat anti-mouse or anti-rabbit IgG (Zhongshan Biological, Beijing, China) as the secondary antibody and then analyzed for protein expression using the enhanced chemiluminescence method (Millipore, Billerica, MA) on X-ray film.
Statistical analysis
Statistical analysis was performed using the independent sample t test or by one-way ANOVA on SPSS 16.0 statistics software (SPSS Inc., Chicago, IL). All analyses were two-sided with the level of statistical significance set to 0.05.
Results
Increased expression of TLR1, TLR2 and TLR6 in monocytes of OC patients
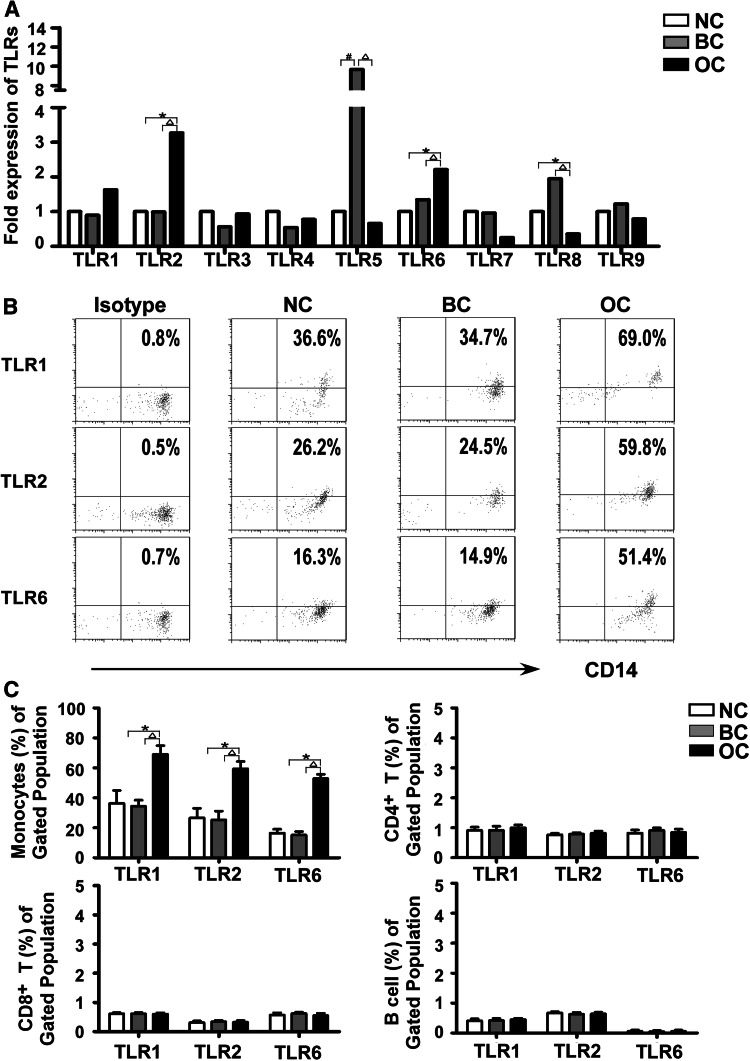
We utilized semiquantitative RT-PCR to study the expression of TLRs mRNA in PBMCs from OC patients, patients with benign diseases and healthy subjects. As shown in Fig. 1a, TLR1-9 all had varying degrees of expression in the PBMCs from the three groups, and TLR2 and TLR6 mRNA had higher expression levels in PBMCs from OC patients than in those from healthy subjects (3.27-fold change and 2.21-fold change, both P < 0.05). PBMCs from OC patients also showed increased levels of TLR2 and TLR6 mRNA relative to those from patients with benign diseases (3.24-fold change and 1.66-fold change, both P < 0.05). TLR5 was expressed more highly in benign disease group than in both OC and healthy subject groups (14.71-fold change and 9.66-fold change, both P < 0.05). TLR8 expression, however, was significantly lower in PBMCs from OC patients than in those from either the benign disease group or the healthy subject group (0.18-fold change and 0.36-fold change, both P < 0.05).
Fig. 1.
Increased expression of TLR1, TLR2 and TLR6 in monocytes of ovarian cancer patients. a Expression of TLR1-9 mRNA in PBMCs from ovarian cancer patients (OC; N = 24), benign disease patients (BC; N = 22) and healthy subjects (NC; N = 22). TLR2 and TLR6 mRNA expression levels were higher in PBMCs from ovarian cancer patients than in those from benign disease patients or healthy subjects. b The expression of TLR1, TLR2 and TLR6 in monocytes was analyzed by flow cytometry. The data shown were representative results of FACS analysis of monocytes from ovarian cancer patients, benign disease patients and healthy subjects. An isotype-matched antibody was served as a negative control. c The differential expression of TLR1, TLR2 and TLR6 in PBMCs cellular subsets was analyzed by flow cytometry. One-way ANOVA was used to assess the differences in expression of TLR1, TLR2 and TLR6 in cellular subsets of PBMCs from ovarian cancer patients, benign disease patients and healthy subjects. *P < 0.05 compared with healthy subjects; △ P < 0.05 compared with benign disease patients
Although the expression levels of TLR2 and TLR6 mRNA were expressed more highly in PBMCs from OC patients than in those from benign disease and healthy subject groups. TLR2 is generally considered that could form heterodimers with either TLR1 or TLR6. Specifically, the TLR2-TLR1 heterodimer can recognize triacylated lipopeptides from Gram-negative bacteria and mycoplasma, whereas diacylated lipopeptides from Gram-positive bacteria and mycoplasma are recognized by the TLR2–TLR6 heterodimer [23–25]. We conducted further analysis of the protein expression levels of TLR1, TLR2 and TLR6 in cellular subsets of PBMCs from OC patients, benign disease patients and healthy control subjects using flow cytometry (Fig. 1b). We found that TLR1, TLR2 and TLR6 were mainly expressed in monocytes. In addition to this, CD8+ T cells also expressed considerable levels of TLR1 and TLR6, but relatively low levels of TLR2. CD4+ T cells were characterized by intermediate expression levels of TLR2 and moderate levels of TLR1 and TLR6. The expression of TLR1, TLR2 and TLR6 on B cells was very low, but detectable (Fig. 1c). Whilst expression of TLR1, TLR2 and TLR6 in monocytes was higher in OC patients than in benign disease and healthy subject groups, there was no significant difference between benign disease and healthy subject groups (Fig. 1c). However, there was no significant difference in expression of TLR1, TLR2 and TLR6 on CD4+ T cells, CD8+ T cells and B cells among OC patients, benign disease and healthy controls (P > 0.05). Additional flow cytometry and real-time gene expression analysis revealed an up-regulation of TLR1, TLR2 and TLR6 expression in monocytes from OC patients.
Increased proinflammatory cytokines in ovarian cancer patient PBMCs in response to TLR1, TLR2 and TLR6 ligands
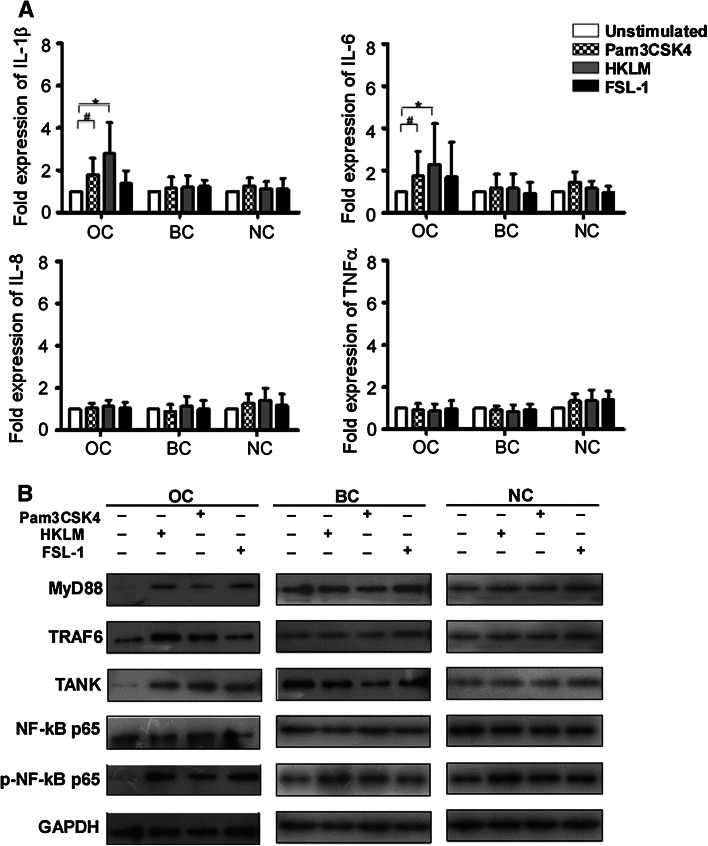
To evaluate the effects of up-regulated TLRs in OC patient PBMCs, we assessed the ability of PBMCs from OC patients, benign disease controls and healthy controls to produce related cytokines upon stimulation with TLR1, TLR2 and TLR6 ligands (Pam3CSK4, HKLM and FSL-1, respectively). As shown in Fig. 2a, compared with unstimulated PBMCs, Pam3CSK4-stimulated PBMCs from OC patients exhibited an observable increase in expression of inflammatory cytokines IL-1β and IL-6 (P < 0.05), but no significant difference in IL-8 and TNFα expression. Similarly, HKLM induced higher expression of IL-1β and IL-6 in PBMCs from OC patients than in unstimulated cells (P < 0.05). There was, however, no difference found in IL-8 and TNFα expression. In addition, no significant up-regulation of IL-1β, IL-6, IL-8 and TNFα expression was observed in OC patients PBMCs after FSL-1 stimulation. In particular, PBMCs from benign disease and healthy subject groups showed no significant up-regulation of IL-1β, IL-6, IL-8 or TNFα expression in response to Pam3CSK4, HKLM or FSL-1 stimulation.
Fig. 2.
Increased proinflammatory cytokines in ovarian cancer patient PBMCs in response to TLR1, TLR2 and TLR6 ligands. a Induction of IL-1β, IL-6, IL-8 and TNFα in PBMCs upon stimulation with Pam3CSK4 (TLR1 ligand), HKLM (TLR2 ligand) and FSL-1 (TLR6 ligand) in ovarian cancer patients (n = 13), benign disease patients (n = 13) and healthy subiects (n = 13). The fold expression of cytokines in PBMCs stimulated with TLRs ligands was determined relative to the expression of cytokines in PBMCs without TLRs ligands stimulation. *P < 0.05 compared with unstimulated group by one-way ANOVA. # P < 0.05 compared with unstimulated group by one-way ANOVA. b Activation of TLRs signaling pathways was assessed by western blot analysis. The expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 in ovarian cancer patient PBMCs increased after stimulated with Pam3CSK4, HKLM or FSL-1
We also found that treating PBMCs with Pam3CSK4, HKLM or FSL-1 increased expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 (Fig. 2b). These results indicated that the up-regulation of TLRs in OC patients may promote activation of NF-κB p65 signaling pathway and induce the production of proinflammatory cytokines.
Activation of TLRs signaling pathways and up-regulation of proinflammatory cytokines in PBMCs induced by ovarian cancer cells
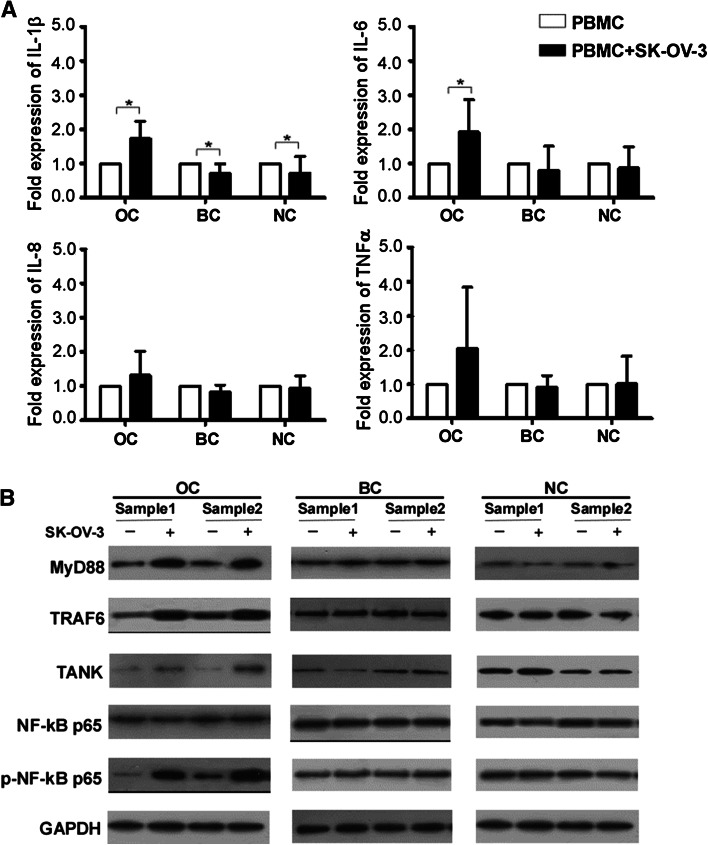
To determine whether OC cells could activate TLRs signaling pathways in PBMCs, resulting in the production of proinflammatory cytokines, PBMCs were cocultured with SK-OV-3 cells in a transwell culture system. As shown in Fig. 3a, PBMCs derived from OC patients exhibited an increase in IL-1β and IL-6 mRNA levels after being cocultured with SK-OV-3 for 24 h (1.74-fold change and 1.92-fold change, both P < 0.05). In contrast, PBMCs from benign disease and healthy subject control groups exhibited lower levels of IL-1β mRNA after being cocultured with SK-OV-3 (0.71-fold change and 0.72-fold change, both P < 0.05). There was no significant difference in either IL-8 or TNFα mRNA expression in PBMCs.
Fig. 3.
Activation of TLRs signaling pathways and up-regulation of proinflammatory cytokines in PBMCs induced by ovarian cancer cells. a PBMCs from ovarian cancer patients (n = 8), benign disease patients (n = 7) or healthy subjects (n = 8) were cultured with or without SK-OV-3 cells. IL-1β, IL-6, IL-8 and TNFα production was measured by RT-PCR. *P < 0.05 by independent sample t test. b The expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 was evaluated by western blot analysis
Following this, the expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 in TLRs signaling pathways was evaluated using western blot (Fig. 3b). The analysis revealed that levels of MyD88, TRAF6, TANK and p-NF-κB p65 significantly increased in PBMCs from OC patients after coculturing with SK-OV-3, though no significant difference was observed in expression of TLRs signaling pathway proteins in PBMCs from benign disease or healthy controls. These results suggested that there were some molecules in the growth environment of OC cells that could induce the activation of TLRs signaling pathways and promote the expression of proinflammation cytokines.
The involvement of TLR1, TLR2 and TLR6 signaling pathways in the production of proinflammatory cytokines in ovarian cancer patients PBMCs
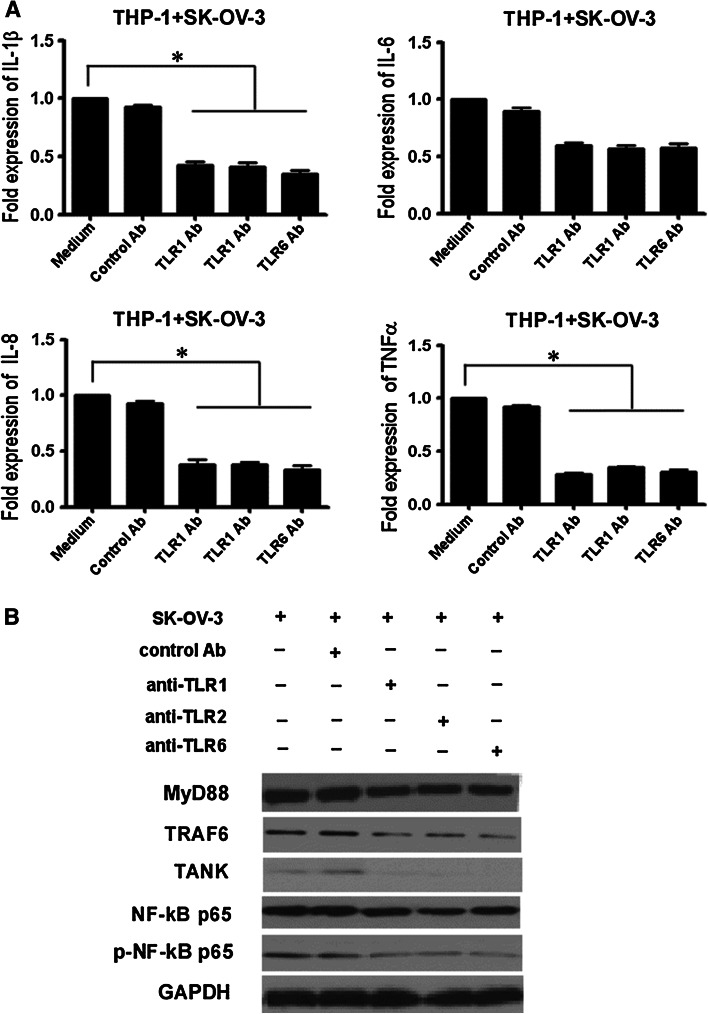
The results above suggested that TLRs signaling pathways may play an important role in the production of proinflammatory cytokines in OC patients PBMCs. Because TLR1, TLR2 and TLR6 were overexpressed in monocytes from OC patients, we explored whether these TLR were involved in proinflammatory cytokine production. THP-1, a monocyte cell line of the mononuclear cell lineage, can be induced to differentiate into mature mononuclear macrophages and express TLR1-9 [26]. We used THP-1 cells as model system to examine proinflammatory cytokines production after incubation with TLR1, TLR2 and TLR6 antibodies.
In this experiment, THP-1 cells were cultured with SK-OV-3 cells in a transwell culture system. THP-1 cells exhibited an increased expression of proinflammatory cytokines and TLRs signaling proteins. Pretreatment of THP-1 cells with TLR1, TLR2 or TLR6 antibodies (anti-hTLR1-IgG, anti-hTLR2-IgG and anti-hTLR6-IgG, respectively) led to a significant reduction of IL-1β, IL-8 and TNFα production, but did not significantly inhibit IL-6 production. Pretreatment with the control antibody, however, did not affect IL-1β, IL-8, IL-6, nor TNFα expression (Fig. 4a). These blocking antibodies also blocked the activation and expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65. Pretreatment of THP-1 with control antibody did not affect the expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 (Fig. 4b). These results revealed that these blocking antibodies could block the potential ligands interaction with these TLRs. It also suggested that TLR1, TLR2 and TLR6 signaling pathways were involved with proinflammatory cytokine production in THP-1 cells.
Fig. 4.
The involvement of TLR1, TLR2 and TLR6 signaling pathways in the production of proinflammatory cytokines in THP-1. a Anti-TLR1, anti-TLR2 and anti-TLR6 antibodies inhibited IL-1β, IL-8 and TNFα production in THP-1 cocultured with SK-OV-3. Pretreatment of THP-1 with or without 10 μg/ml antibody (control IgG1 Ab, anti-hTLR1-IgG, anti-hTLR2-IgG or anti-hTLR6-IgG) for 1 h at 37 °C and then cocultured with SK-OV-3 for 24 h in a transwell culture system. The expression of cytokines in THP-1 was measured by RT-PCR. b The blocking effect of anti-TLR1, anti-TLR2 and anti-TLR6 antibodies on MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 activation in THP-1 was analyzed by western blot
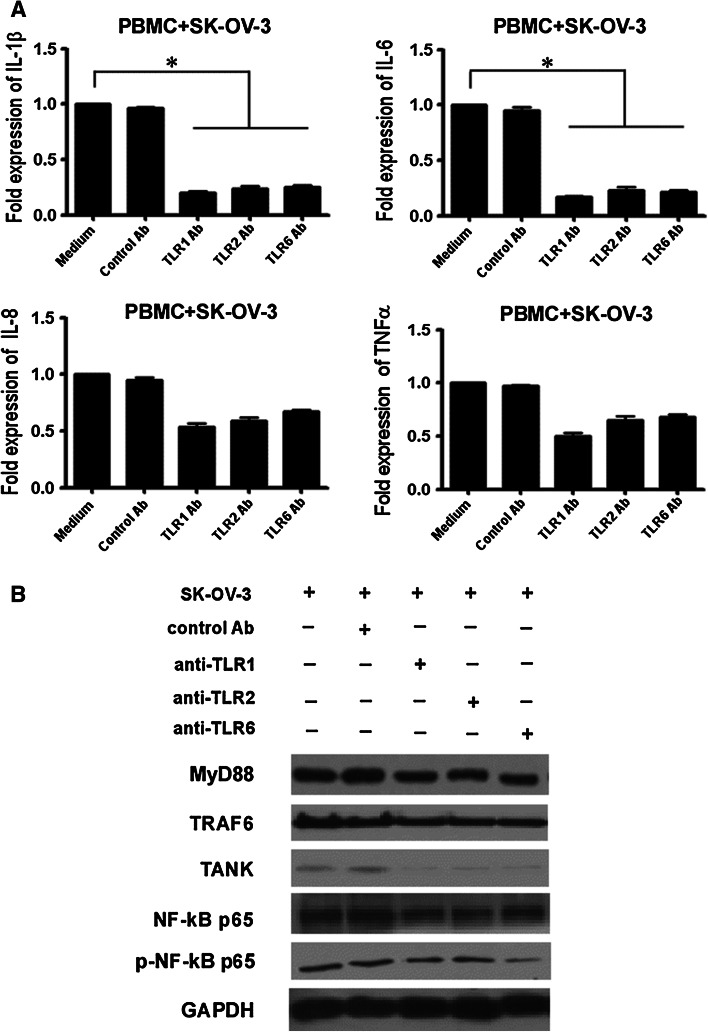
Following this, we performed the same assay with PBMCs from OC patients and found that pretreatment of PBMCs with anti-TLR1, anti-TLR2 or anti-TLR6 antibodies led to a significant decrease in IL-1β and IL-6 production when they were stimulated by SK-OV-3. There was, however, no significant decrease in IL-8 and TNFα production in PBMCs pretreated with blocking antibodies. Pretreatment of PBMCs with control antibody did not affect the IL-1β, IL-8, IL-6 or TNFα production (Fig. 5a). All three blocking antibodies exhibited inhibitory activity on TLRs signaling proteins MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 in PBMCs. The pretreatment of PBMCs with control antibody did not affect the expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 (Fig. 5b). These results revealed that these blocking antibodies could really block the potential ligands interaction with these TLRs. They also suggested that the up-regulated TLR1, TLR2 and TLR6 in OC patients PBMCs was related to recognition of SK-OV-3 secreted factors in the tumor cell culture environment. As such, activation of TLR1, TLR2 and TLR6 signaling pathways was correlated with proinflammatory cytokine production in OC patient PBMCs.
Fig. 5.
The involvement of TLR1, TLR2 and TLR6 signaling pathways in the production of proinflammatory cytokines in ovarian cancer patient PBMCs. a Anti-TLR1, anti-TLR2 and anti-TLR6 antibodies inhibit IL-1β and IL-6 in PBMCs cocultured with SK-OV-3 cells. Pretreatment of PBMCs with or without 10 μg/ml antibody (control IgG1 Ab, anti-hTLR1-IgG, anti-hTLR2-IgG or anti-hTLR6-IgG) for 1 h at 37 °C, and then cocultured with SK-OV-3 for 24 h in a transwell culture system. The expression of cytokines in PBMCs was measured by RT-PCR. b The blocking effect of anti-TLR1, anti-TLR2 and anti-TLR6 antibodies on MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 activation in PBMCs was analyzed by western blot
Discussion
Infection, chronic irritation and inflammation are among the main causes for the initiation of different types of cancer [27]. TLRs, as potential activators of NF-κB, are considered to be the gateway to inflammation and tumorigenesis [25, 28].
This study provided the first comprehensive characterization of TLRs expression in PBMCs and their subsets from OC patients, benign disease patients and healthy subjects. Our study demonstrated that TLR1, TLR2 and TLR6 expression was significantly increased in subsets of PBMCs from OC patients, when compared with those from benign disease and healthy subject control groups. TLR1, TLR2 and TLR6 were expressed most highly in monocytes from OC patients, and to approximately, the same degree in subsets of PBMCs from benign disease and healthy subject groups. However, recent evidence has shown that activation of TLR5 by its ligand flagellin elicits potent antitumor activity and thus inhibits growth and cell proliferation of colon tumor and breast cancer in vivo [29, 30]. This antitumor role of TLR5 may be the answer to why it was expressed so highly in the PBMCs of benign disease patients. It thus became very important to explore further the functions of TLR1, TLR2 and TLR6 in OC patient PBMCs.
Subsequently, we studied the functions of TLR1, TLR2 and TLR6, which were overexpressed in PBMCs from OC patients. We observed differential responses to TLR ligands by PBMCs, which could be attributed to this overexpression. Our results showed increased production of several proinflammatory cytokines, including IL-1β and IL-6, in OC patient PBMCs in response to TLR1, TLR2 and TLR6 ligands. Meanwhile, we also found that the ligands (Pam3CSK4, HKLM and FSL-1) elevated the expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 in PBMCs. Our results suggested that overexpressed TLRs were functional and had the potential to influence tumor progression through production of proinflammatory cytokines and activation of NF-κB p65.
The cytokine profile of the tumor microenvironment is largely a result of factors produced by tumor cells themselves, nearby cells and infiltration of white blood cells, all of which can have a profound effect upon tumor progression [11, 31]. Known to mediate acute immune responses, IL-1β is one of the main pleiotropic proinflammatory cytokines produced by antigen-presenting cells and is connected to innate and adaptive immune responses [32]. Excessive IL-1β production has been implicated in the development of chronic inflammatory diseases and malignancies [33, 34]. IL-1β can promote myeloid-derived suppressor cells recruiting to the tumor position to inhibit the antitumor response [35, 36]. Furthermore, IL-1β can involve in the process of angiogenesis and facilitate tumor cell growth [37–39]. Generally speaking, IL-6 is secreted by OC cells, tumor-associated macrophages and peritoneal mesothelial cells that exist in ascites [40]. The role that IL-6 plays in the autocrine growth of OC cells has been demonstrated. Cell signaling pathway related-IL-6 in OC cells can regulate tumor cell proliferation, invasion and metastasis, which results in shorter progression-free survival [41].
In order to further explore the causes of the high TLR1, TLR2 and TLR6 expression in OC patients PBMCs, we first determined whether or not OC cells could activate TLRs signaling pathways to produce proinflammatory cytokines in PBMCs when cultured with SK-OV-3 in a transwell coculture system. We found that PBMCs taken from OC patients exhibited increased IL-1β and IL-6 mRNA levels after being cocultured with SK-OV-3, as compared to PBMCs cultured in the absence of SK-OV-3. Furthermore, expression of MyD88, TRAF6, TANK, NF-κB p65 and p-NF-κB p65 also increased significantly. Our results suggested that the presence of natural TLR ligands (e.g., apoptotic bodies and cellular debris from necrotic cells) could exert a role in activating TLRs signaling pathways. Furthermore, the results also pointed out the importance of natural TLR ligands in tumor microenvironment, as they could induce proinflammatory cytokine secretion through activation of TLRs signaling pathways.
Following this, we used the transwell coculture system to explore which TLRs were involved in proinflammatory cytokine secretion. Antibody blocking analysis revealed that TLR1, TLR2 and TLR6 recognized natural TLR ligands and played a crucial role in activating TLRs signaling pathways which promote proinflammatory cytokine secretion. The pretreatment of THP-1 and PBMCs with antibodies against TLR1, TLR2 and TLR6 led to significant inhibition of IL-1β, IL-6, IL-8 and TNFα production. It has been well demonstrated that recruiting leukocytes could enhance the formation ability of the inflammatory environment and cytokines released from leukocytes can dramatically alter the neoplastic development [42]. The promotion of these cytokines through TLR1, TLR2 and TLR6 activation may therefore contribute to the recruitment of inflammatory cells, as well as increased tumor growth and neoplastic progression. In addition, antibodies against TLR1, TLR2 and TLR6 blocked the natural ligands and further prevented activation of TLRs signaling pathways in OC cells coculture system. Our results indicated that the overexpression of TLR1, TLR2 and TLR6 in OC patients PBMCs may be crucial for disease maintenance and progression, although it is still unknown what the relevant natural ligands are.
Our study demonstrated a significant increase of TLR1, TLR2 and TLR6 in the PBMCs of OC patients, but not in those of benign disease and healthy subjects. Those TLR levels were mostly elevated in monocytes. After stimulation with TLR2/TLR6 ligands, an increase in IL-1β and IL-6 was detected in OC patients PBMCs. The factors presented in tumor microenvironment also appeared to develop their functions through activating TLRs signaling proteins (MyD88, TRAF6, TANK and p-NF-κB p65 pathway), resulting in increased expression of IL-1β, IL-6 and IL-8. That is to say, the TLR1, TLR2 and TLR6 expressed in OC patients PBMCs appeared to participate in the recognition of the factors and the activation of TLR1/TLR2/TLR6 brought about increased expression of TLRs signaling pathway proteins and increased secretion of IL-1β, IL-6 and IL-8. These results supported the idea that up-regulated TLR1, TLR2 and TLR6 in OC patient PBMCs was significant, as it had the potential to influence the production of proinflammatory cytokines, which were necessary for the maintenance and progression of OC.
Acknowledgments
We are grateful to the technical support from National Key Clinical Department of Laboratory Medicine of Jiangsu Province Hospital. This work was supported by National Natural Science Foundation of China (81272324, 81371894), Key Laboratory for Medicine of Jiangsu Province of China (No. XK201114), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Abbreviations
- BC
Benign disease control
- EOC
Epithelial ovarian cancer
- FBS
Fetal bovine serum
- HRP
Horseradish peroxidase
- IL
Interleukin
- MyD88
Myeloid differentiation factor 88
- NC
Healthy normal controls
- NF-κB
Nuclear factor-kappa B
- OC
Ovarian cancer
- PAMPs
Pathogen-associated molecular patterns
- PBMCs
Peripheral blood mononuclear cells
- p-NF-κB
Phospho-Nuclear factor-kappa B
- RT-PCR
Real-time PCR
- TANK
TRAF family member-associated NF-κB activator
- TLRs
Toll-like receptors
- TNFα
Tumor necrosis factorα
- TRAF6
Tumor necrosis factor receptor-associated factor 6
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Xiaojie Zhang, Juan Xu and Xing Ke have contributed equally to this work.
Contributor Information
Fang Wang, Phone: +86 2586862814, Email: wangfang@njmu.edu.cn.
Shiyang Pan, Phone: +86 25 68135886, Email: sypan@njmu.edu.cn.
References
- 1.Zhou M, McFarland-Mancini MM, Funk HM, Husseinzadeh N, Mounajjed T, Drew AF. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol Immunother. 2009;58:1375–1385. doi: 10.1007/s00262-008-0650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachy PA, Karhadkar SS, Berman DM. Mending and malignancy. Nature. 2004;431:402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Coussens LM. Cancer—an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Setia S, Nehru B, Sanyal SN. Activation of NF-kappaB: bridging the gap between inflammation and cancer in colitis-mediated colon carcinogenesis. Biomed Pharmacother. 2014;68:119–128. doi: 10.1016/j.biopha.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Li S, Yan Q, Chen X, Yang Y, Liu X, Wan X. Interferon-beta Induced microRNA-129-5p down-regulates HPV-18 E6 and E7 viral gene expression by targeting SP1 in cervical cancer cells. PLoS One. 2013;8:e81366. doi: 10.1371/journal.pone.0081366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneda A, Matsusaka K, Aburatani H, Fukayama M. Epstein–Barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 2012;72:3445–3450. doi: 10.1158/0008-5472.CAN-11-3919. [DOI] [PubMed] [Google Scholar]
- 10.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 11.Costa NL, Valadares MC, Souza PPC, Mendonca EF, Oliveira JC, Silva TA, Batista AC. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49:216–223. doi: 10.1016/j.oraloncology.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 13.Stender JD, Glass CK. Epigenomic control of the innate immune response. Curr Opin Pharmacol. 2013;13:582–587. doi: 10.1016/j.coph.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116–133. doi: 10.3109/08830185.2013.774391. [DOI] [PubMed] [Google Scholar]
- 15.Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67–74. doi: 10.1111/j.1749-6632.2012.06786.x. [DOI] [PubMed] [Google Scholar]
- 16.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250:216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Marusawa H, Jenkins BJ. Inflammation and gastrointestinal cancer: an overview. Cancer Lett. 2013;345:153–156. doi: 10.1016/j.canlet.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Wang L, Chen S. Dual character of Toll-like receptor signaling: pro-tumorigenic effects and anti-tumor functions. Biochim Biophys Acta. 2013;1835:144–154. doi: 10.1016/j.bbcan.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang B, Zhao J, Shen SQ, Li HX, He KL, Shen GX, Mayer L, UnkelesS J, Li D, Yuan Y, Zhang GM, Xiong H, Feng ZH. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–4352. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- 21.He WG, Liu QY, Wang L, Chen W, Li N, Cao XT. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–2859. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Nakao Y, Funami K, Kikkawa S, Taniguchi M, Nishiguchi M, Fukumori Y, Seya T, Matsumoto M. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005;174:1566–1573. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira-Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu Y, Ding Y, Zou L, Tan Z, Liu T, Fu X, Xu W. Divergent roles of amino acid residues inside and outside the BB loop affect human Toll-like receptor (TLR)2/2, TLR2/1 and TLR2/6 responsiveness. PLoS One. 2013;8:e61508. doi: 10.1371/journal.pone.0061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto A, Morello S, Sorrentino R. Lung cancer and Toll-like receptors. Cancer Immunol Immunother. 2011;60:1211–1220. doi: 10.1007/s00262-011-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bode C, Diedrich B, Muenster S, Hentschel V, Weisheit C, Rommelsheim K, Hoeft A, Meyer R, Boehm O, Knuefermann P, Baumgarten G. Antibiotics regulate the immune response in both presence and absence of lipopolysaccharide through modulation of Toll-like receptors, cytokine production and phagocytosis in vitro. Int Immunopharmacol. 2014;18:27–34. doi: 10.1016/j.intimp.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 29.Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008;135:518–528. doi: 10.1053/j.gastro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011;71:2466–2475. doi: 10.1158/0008-5472.CAN-10-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeannin P, Duluc D, Delneste Y. IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: regulation by IFN-gamma. Immunotherapy. 2011;3:23–26. doi: 10.2217/imt.11.30. [DOI] [PubMed] [Google Scholar]
- 32.Chow MT, Moller A, Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012;22:23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello CA. A clinical perspective of IL-1 beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 35.Elkabets M, Ribeiro VSG, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, Vosshenrich CAJ. IL-1 beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell crosstalk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voronov E, Carmi Y, Apte RN. Role of IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol. 2007;601:265–270. doi: 10.1007/978-0-387-72005-0_28. [DOI] [PubMed] [Google Scholar]
- 39.Song XP, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, Shendler Y, Bjorkdahl O, Segal S, Dinarello CA, Apte RN. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:6448–6456. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- 40.Lane D, Matte I, Rancourt C, Piche A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo CW, Chen MW, Hsiao M, Wang SA, Chen CA, Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML, Wei LH. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71:424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 42.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–154. doi: 10.1016/S1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]