Abstract
Background/aims
This study aimed to investigate and compare the efficacy and safety of first-line and second-line selective laser trabeculoplasty (SLT) in Japanese patients with normal-tension glaucoma (NTG).
Methods
100 patients with NTG were enrolled in this study. Patients were treated with SLT as a first-line or second-line treatment for NTG. Main outcome measures were intraocular pressure (IOP) reduction rate, outflow pressure improvement rate (ΔOP), success rate at 1 year and complications. Success was defined as ΔOP≥20% (criterion A) or an IOP reduction ≥20% (criterion B) without additional IOP-lowering eye-drops, repeat SLT or additional glaucoma surgeries. The incidence of transient IOP spike (>5 mm Hg from the pretreatment IOP), conjunctival hyperaemia, inflammation in the anterior chamber and visual impairment due to SLT were assessed.
Results
A total of 99 patients (99 eyes) were initially enrolled in this study, including 74 eyes assigned to the first-line SLT group and 25 eyes to the second-line SLT group. The mean IOP of 16.3±2.1 mm Hg before SLT decreased by 17.1%±9.5% to 13.4±1.9 mm Hg at 12 months after SLT in the first-line group (p<0.001), and the mean IOP of 15.4±1.5 mm Hg before SLT decreased by 12.7%±9.7% to 13.2±2.0 mm Hg at 12 months after SLT (p=0.005) in the second-line group. Both groups showed significant reductions in IOP. Higher pre-SLT IOP and thinner central corneal thickness were associated with greater IOP reduction. The success rate at 1 year was higher in the first-line compared with the second-line group, with lower pretreatment IOP and the use of IOP-lowering medication before SLT being associated with treatment failure. Most post-treatment complications were minor and transient.
Conclusions
SLT may be an effective and safe treatment option for NTG, as either a first-line or second-line treatment.
Trial registration number
The study was registered in the UMIN-CTR (UMIN Test ID: UMIN R000044059).
Keywords: Glaucoma, Intraocular pressure, Treatment Lasers
WHAT IS ALREADY KNOWN ON THIS TOPIC
Selective laser trabeculoplasty (SLT) was effective and safe as a primary treatment for primary open-angle glaucoma and ocular hypertension, and achieved drop-free disease control in approximately 75% of eyes at 3 years, with lower overall costs and a reduced risk of surgical intervention. Furthermore, SLT is associated with fewer adverse events, such as blepharitis and conjunctival hyperaemia, than intraocular pressure (IOP)-lowering eye-drops therapy, and is considered an intervention that does not affect adherence and can maintain the patient’s quality of life.
WHAT THIS STUDY ADDS
Both first-line and second-line SLT may be effective and safe treatments for patients with normal-tension glaucoma (NTG), leading to a substantial decrease in IOP over a period of 1 year, with no serious adverse events.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study will help to elucidate the efficacy of SLT in eyes with NTG.
Introduction
Selective laser trabeculoplasty (SLT) is a well-established treatment with a good safety profile and good repeatability. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial1–5 recently demonstrated that SLT was effective and safe as a primary treatment for primary open-angle glaucoma (POAG) and ocular hypertension (OHT), and achieved drop-free disease control in approximately 75% of eyes at 3 years, with lower overall costs and a reduced risk of surgical intervention. Furthermore, SLT is associated with fewer adverse events, such as blepharitis and conjunctival hyperaemia, than intraocular pressure (IOP)-lowering eye-drops therapy,6 and is considered an intervention that does not affect adherence and can maintain the patients’ quality of life (QOL).1–5 In Western countries, glaucoma treatment guidelines have been revised to support or encourage the use of SLT as a first-line treatment.
Normal tension glaucoma (NTG) is a progressive optic neuropathy despite an IOP of 21 mm Hg or less7 and has been shown to account for the majority of POAG in the population older than 40 years in Asian countries; the Tajimi study from Japan reported NTG in 92% of patients with POAG8 and the Namil study from Korea reported 77%.9 However, information on the outcomes of SLT in patients with NTG is currently lacking. Lee et al previously demonstrated significant reductions in IOP and medication use after SLT during 2 years of follow-up in patients with medically treated NTG.10 Nevertheless, data on the efficacy of first-line SLT for newly diagnosed NTG are scarce. We previously studied the effect of first-line SLT in 42 eyes from 42 Japanese patients with NTG at a single centre, comprising 37 treatment-naïve patients and 5 patients who had discontinued IOP-lowering drops therapy before SLT.11 We found that the mean preoperative IOP of 15.8 mm Hg was significantly reduced by 15.8% to 13.2 mm Hg at 1 year and by 12.7% to 13.5 mm Hg at 3 years after first-line SLT,11 suggesting that SLT may be an effective treatment for NTG.
In this multicentre prospective study, we investigated and compared the clinical efficacy and safety of first-line and second-line SLT in Japanese patients with NTG.
Materials and methods
Study design
This multicentre cohort interventional study was conducted at 26 medical institutions in Japan.
Study participants
Patients diagnosed with NTG between January 2020 and June 2021 at the participating medical institutions and judged to require first-line or second-line SLT were eligible for inclusion in the study. Patients who fully understood the purpose of the study and who met the following criteria were administered SLT: (1) age ≥20 years; (2) at least one of the last three IOP values ≥14 mm Hg; (3) mean deviation (MD) of the visual field ≥−15 dB and (4) central corneal thickness (CCT) 450–600 µm. All participants provided written informed consent before participation.
The inclusion criteria for first-line SLT were newly diagnosed patients with NTG or patients who had previously used one-component IOP-lowering eye-drops but discontinued them after the onset of allergic symptoms.
The inclusion criterion for second-line SLT was patients with NTG who chose to receive SLT because the IOP-lowering effect of first-line one-component eye-drops (prostaglandin analogues or β-blockers) was insufficient (IOP reduction rate, <15% from baseline).
The exclusion criteria were as follows: (1) IOP-lowering eye-drops already in use IOP-lowering with at least two components (fixed combination considered as two components); (2) oral steroids and/or steroid eye-drops used within 1 month before SLT, or sub-Tenon’s triamcinolone acetonide injection performed within 6 months before SLT; (3) a history of laser therapy; (4) a history of refractive surgery; (5) a history of intraocular surgery (except if >3 months since cataract surgery); (6) difficulty in measuring IOP with a Goldmann tonometer and (7) the presence of potentially advanced retinal disease with no confirmed cure.
Laser procedure
All patients underwent SLT using a Q-switched Nd:YAG laser (Tango Ophthalmic Laser; Ellex Medical, Adelaide, Australia). The SLT was delivered to 360° of the trabecular meshwork using a gonioscope. Non-overlapping shots were used with the minimum laser energy at which bubble formation was visible.
All eyes were instilled with apraclonidine hydrochloride 1% (IOPIDINE UD Ophthalmic Solution 1%; Novartis Pharma K.K., Tokyo, Japan) 1 hour before and immediately after SLT. The use of steroids and non-steroidal anti-inflammatory eye-drops was prohibited after SLT.
Follow-up examinations
Patients underwent the following examinations at baseline, before SLT: slit-lamp examination, visual acuity test (decimal visual acuity), IOP measurement with a Goldmann applanation tonometer (GAT) (measured twice each time), gonioscopy, CCT, endothelial cell density (ECD) measurement with specular microscopy, automated visual field assessment using the Humphrey Field Analyzer and Swedish interactive threshold algorithm standard, 30-2 or 24-2 programme (Carl Zeiss Meditec, Dublin, California, USA).
Post-treatment examinations were conducted 1 week and 1, 3, 6, 9 and 12 months after SLT. At each visit, the patients were examined using slit-lamp microscopy, and IOP was measured using GAT. Automated visual field assessment, CCT measurements and ECD measurement with specular microscopy were performed at 6 and 12 months after SLT, and gonioscopy was performed at 12 months. IOP, corrected IOP and corneal hysteresis were measured using an ocular response analyser before and 12 months after SLT.
In the first-line treatment group, all IOP-lowering eye-drops were discontinued for >1 month before SLT. Concomitant eye-drops in the second-line treatment were limited to prostaglandin analogues or β-blockers.
Outcome measures
The primary outcome measures were the IOP reduction rate (IOP at enrolment—IOP after SLT)/(IOP at enrolment)×100) and the proportion of patients with an outflow pressure improvement rate (ΔOP) ≥20% after SLT, where ΔOP=(IOP pre-SLT–IOP post-SLT)/(IOP pre SLT–10)×100 with an episcleral venous pressure (EVP) of 10 mm Hg. A study examining the changes in aqueous humour dynamics before and after SLT reported that only outflow facilities increased significantly after SLT, with no changes in aqueous humour flow rate, uveoscleral outflow or EVP. To better evaluate the IOP-lowering effect of SLT in patients with NTG, the ΔOP was included as an endpoint to assess the outflow facility.12 This report describes the baseline EVP as 9.89±1.09 mm Hg in the control group, based on which the EVP was set at 10 mm Hg in this study.
The secondary outcome measures included IOP, CCT, ECD, number of SLT irradiation spots, SLT irradiation energy and success rate at 12 months after SLT.
Criteria for success
Success was defined as ΔOP ≥20% (criterion A) or an IOP reduction ≥20% (criterion B) without additional IOP-lowering eye-drops, repeat SLT or additional glaucoma surgeries.
Safety
The incidences of transient IOP spike (>5 mm Hg from pretreatment IOP), conjunctival hyperaemia, inflammation in the anterior chamber and visual impairment due to SLT were assessed.
Statistical analyses
A power calculation revealed that a sample size of 80 would allow detection of the relationships between the two groups (allocation ratio 2:1) and the aforementioned factors using a two-group Student’s t-test at a significance level of 5% with 80% power for a large-size effect of 0.6.
All statistical analyses were conducted by using SPSS V.22.0 (IBM). IOP values before and after SLT were compared using Wilcoxon’s signed-rank test. Between-group comparisons were carried out using Student’s t-test, the Mann-Whitney U test and χ2 test. The cumulative surgical success rate was determined using Kaplan-Meier survival analysis.
Univariate and multivariate Cox proportional hazards regression models were used to determine the associations between pretreatment factors and IOP reduction or success at 1 year after SLT. Univariate and multivariate multiple linear regression analyses were used to determine the associations between factors and IOP reduction at 12 months. Values of p<0.05 were considered statistically significant.
Results
Study population and baseline characteristics
A total of 100 patients (100 eyes) were initially enrolled in this study, including 74 eyes assigned to the first-line SLT group and 26 eyes to the second-line SLT group. One patient in the second-line group who used steroid eye-drops during the study due to the development of epidemic keratoconjunctivitis with subepithelial opacity of the cornea was excluded, because of failure to meet the inclusion criteria. Therefore, data for 99 eyes from 99 Japanese patients with NTG were included in the analysis: 74 eyes (74 patients) in the first-line SLT group and 25 eyes (25 patients) in the second-line SLT group.
During the 12 months following SLT, three patients started or added IOP-lowering eye-drops (two patients in the first-line group and one in the second-line group), one patient in the first-line group underwent cataract surgery, and one patient in the first-line group underwent goniotomy with a Kahook Dual Blade combined with phacoemulsification. We used data for these patients up to the time immediately before each event.
The baseline characteristics of the participants are shown in table 1. There were significant differences in mean age, pretreatment IOP, and visual field MD between the first-line and second-line SLT groups (table 1).
Table 1.
Baseline characteristics of participants
| All | First line | Second line | P value | |
| Eyes (n) | 99 | 74 | 25 | |
| Age (mean±SD) (years) | 60.8±12.7 | 58.6±11.5 | 67.4±14.0 | 0.002* |
| Eye laterality, (right/left) | 43/56 | 30/44 | 13/12 | 0.318† |
| Sex (F/M) | 59/40 | 45/29 | 14/11 | 0.672† |
| Pretreatment IOP (mean±SD) (mm Hg) | 16.1±2.0 | 16.3±2.1 | 15.4±1.5 | 0.015* |
| Visual field, mean deviation (mean±SD) (dB) | −4.1±3.9 | −3.4±3.6 | −6.1±3.9 | 0.01‡ |
| Refractive error (spherical D) | −3.66±3.76 | −3.79±3.94 | −3.25±3.13 | 0.538‡ |
| Decimal visual acuity | 1.20±0.30 | 1.22±0.31 | 1.15±0.26 | 0.334‡ |
| CCT (mean±SD) (mm) | 532.9±29.9 | 533.6±31.5 | 530.7±25.0 | 0.720‡ |
| ECD (mean±SD) (/mm2) | 2668.4±304.2 | 2703.4±282.1 | 2564.8±347.8 | 0.048‡ |
*Student’s t-test.
†Pearson’s χ2 test.
‡Mann-Whitney U test.
CCT, central corneal thickness; ECD, endothelial cell density; F, female; IOP, intraocular pressure; M, male.
Treatment outcomes
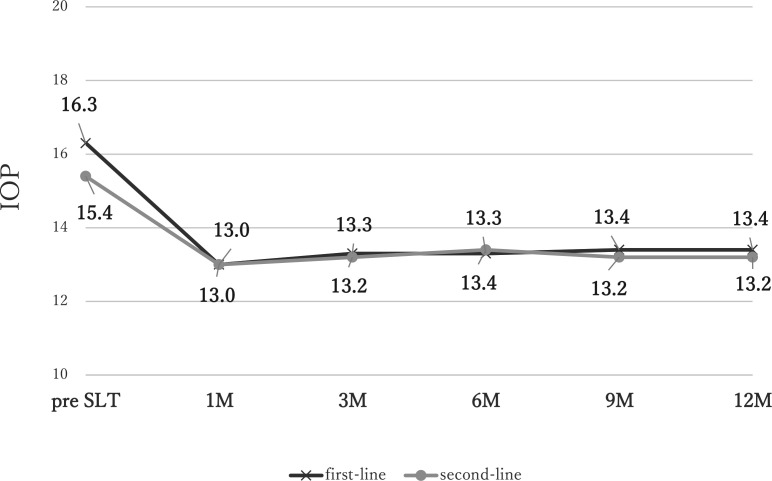
The results for the 99 patients (99 eyes) who completed the 12 months of follow-up are shown in table 2. Overall, the mean pre-SLT IOP of 16.1±2.0 mm Hg decreased by 16.0%±9.7% to 13.3±1.9 mm Hg at 12 months (p<0.001) (table 2). Scatter plot shows that approximately half of patients achieved a reduction in IOP of ≥20% at 3 months (online supplemental figure 1). The mean IOP of 16.3±2.1 mm Hg before SLT decreased by 17.1%±9.5% to 13.4±1.9 mm Hg at 12 months in the first-line group (p<0.001), and the mean IOP of 15.4±1.5 mm Hg before SLT decreased by 12.7%±9.7% to 13.2±2.0 mm Hg at 12 months (p=0.005) in the second-line group (figure 1). There were no significant differences in IOP between the groups during the 12 months after SLT. Although the IOP reduction rate was significantly greater in the first-line SLT group compared with the second-line SLT group at 6 months (p=0.0369) (table 2).
Table 2.
Intraocular pressure values before and after selective laser trabeculoplasty
| All | First line | Second line | P value | |
| Eyes (n) | 99 | 74 | 25 | |
| Pretreatment IOP (mean±SD) (mm Hg) | 16.1±2.0 | 16.3±2.1 | 15.4±1.5 | 0.015* |
| 1 month | ||||
| IOP (mean±SD) (mm Hg) | 12.9±2.1 | 13.0±2.2 | 13.0±1.8 | 0.236* |
| IOP reduction rate (mean±SD) (%) | 19.8±9.5 | 20.2±10.0 | 18.8±8.0 | 0.494* |
| 3 months | ||||
| IOP (mean±SD) (mm Hg) | 13.3±2.2 | 13.3±2.3 | 13.2±2.3 | 0.822* |
| IOP reduction rate (mean±SD) (%) | 17.3±10.5 | 18.2±10.8 | 14.5±9.2 | 0.100* |
| 6 months | ||||
| IOP (mean±SD) (mm Hg) | 13.3±2.0 | 13.3±2.0 | 13.4±2.1 | 0.903* |
| IOP reduction rate (mean±SD) (%) | 16.8±9.2 | 18.0±8.7 | 13.1±9.8 | 0.037* |
| 9 months | ||||
| IOP (mean±SD) (mm Hg) | 13.4±2.0 | 13.4±2.0 | 13.2±2.0 | 0.634* |
| IOP reduction rate (mean±SD) (%) | 16.2±9.6 | 17.1±9.6 | 13.5±9.4 | 0.115* |
| 12 months | ||||
| IOP (mean±SD) (mm Hg) | 13.3±1.9 | 13.4±1.9 | 13.2±1.9 | 0.722* |
| IOP reduction rate (mean±SD) (%) | 16.0±9.7 | 17.1±9.5 | 12.7±9.7 | 0.063* |
*Mann-Whitney U test.
IOP, intraocular pressure.
Figure 1.
Changes in intraocular pressure (IOP) before and after selective laser trabeculoplasty (SLT). The mean IOP of 16.3±2.1 mm Hg before SLT decreased by 17.1%±9.5% to 13.4±1.9 mm Hg at 12 months in the first-line group (p<0.001), and the mean IOP of 15.4±1.5 mm Hg before SLT decreased by 12.7%±9.7% to 13.2±2.0 mm Hg at 12 months (p=0.005) in the second-line group.
bmjophth-2023-001563supp002.pdf (112.8KB, pdf)
bmjophth-2023-001563supp003.pdf (244.1KB, pdf)
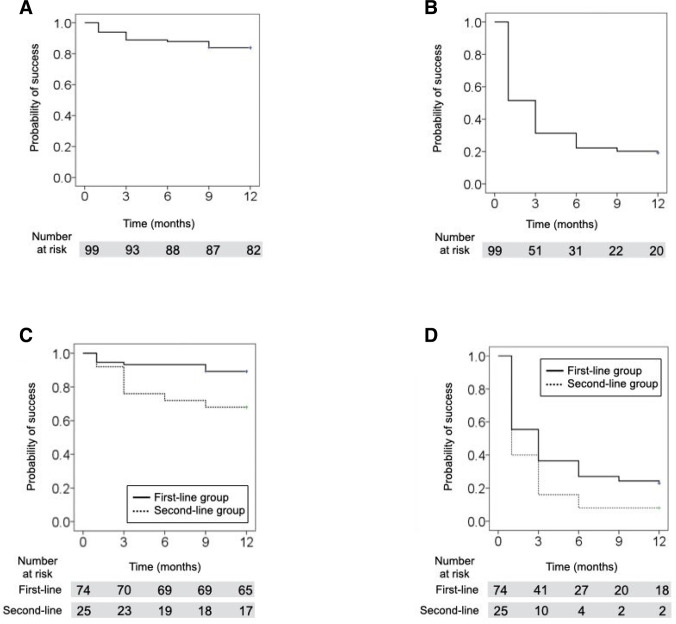
The success rate for criterion A was 83.8% (figure 2A) and that for criterion B was 19.2% (figure 2B) at 12 months. Comparing the two groups at 12 months, the success rate for criterion A was 89.2% in the first-line group and 68.0% in the second-line group (figure 2C), and the success rate for criterion B was 23.0% in the first-line group and 8.0% in the second-line group (figure 2D). The success rate was greater in the first-line group compared with the second-line group for both criteria (p=0.011, 0.046, respectively) (figure 2C,D).
Figure 2.
Success rates for criteria A, B according to Kaplan-Meier survival analyses. The success rate for criterion A was 83.8% (A) and that for criterion B was 19.2% (B) at 12 months. Comparing the two groups at 12 months, the success rate for criterion A was 89.2% in the first-line group and 68.0% in the second-line group (C), and the success rate for criterion B was 23.0% in the first-line group and 8.0% in the second-line group (D). The success rate was greater in the first-line group compared with the second-line group for both criteria (p=0.011, 0.046, respectively) (C, D).
Univariate analysis of criterion A showed that success was related to medication use before SLT (p=0.019). Multivariate regression analysis for criterion A confirmed that pretreatment IOP (OR 1.431; 95% CI 1.036 to 1.976; p=0.030) and medication use (OR 0.152; 95% CI 0.046 to 0.503; p=0.002) were associated with the success of SLT at 12 months post-treatment, when medication use, pretreatment IOP, visual field MD and CCT were included in the Cox proportional hazards model. No factors were related to treatment success in univariate or multivariate analysis of criterion B (table 3).
Table 3.
Cox proportional hazard model for risk factors for success of selective laser trabeculoplasty (criterion A or B)
| Univariate model | OR | 95% CI | P value | |
| Lower limit | Upper limit | |||
| Medications | ||||
| Criterion A | 0.310 | 0.116 | 0.827 | 0.019 |
| Criterion B | 0.689 | 0.423 | 1.123 | 0.135 |
| Pretreatment IOP | ||||
| Criterion A | 1.261 | 0.978 | 1.625 | 0.078 |
| Criterion B | 0.906 | 0.808 | 1.015 | 0.088 |
| Visual field MD | ||||
| Criterion A | 0.994 | 0.876 | 1.129 | 0.993 |
| Criterion B | 0.975 | 0.923 | 1.030 | 0365 |
| CCT | ||||
| Criterion A | 1.017 | 1.000 | 1.034 | 0.053 |
| Criterion B | 1.003 | 0.996 | 1.010 | 0.407 |
| Multivariate model | Adjusted OR | 95% CI | P value | |
| Lower limit | Upper limit | |||
| Medications | ||||
| Criterion A | 0.152 | 0.046 | 0.503 | 0.002 |
| Criterion B | 0.777 | 0.459 | 1.314 | 0.347 |
| Pretreatment IOP | ||||
| Criterion A | 1.431 | 1.036 | 1.976 | 0.030 |
| Criterion B | 0.904 | 0.797 | 1.024 | 0.113 |
| Visual field MD | ||||
| Criterion A | 1.035 | 0.898 | 1.193 | 0.635 |
| Criterion B | 0.994 | 0.938 | 1.053 | 0.834 |
| CCT | ||||
| Criterion A | 1.017 | 0.998 | 1.036 | 0.087 |
| Criterion B | 1.005 | 0.997 | 1.012 | 0.226 |
CCT, central corneal thickness; IOP, intraocular pressure; MD, mean deviation.
Univariate analysis showed that pre-SLT IOP, IOP reduction at 3 months and CCT were associated with IOP reduction by SLT at 12 months (p=0.005, p<0.001, p=0.029, respectively). Multivariate regression analysis confirmed that pretreatment IOP (beta 0.269; 95% CI 0.365 to 2.403; p=0.008), IOP reduction at 3 months (beta 0.353; 95% CI 0.162 to 0.538; p<0.001) and CCT (beta −0.259; 95% CI −0.144 to –0.023; p=0.008) were associated with the IOP reduction by SLT at 12 months post-treatment, when medication use, pretreatment IOP, visual field MD, total energy of SLT and CCT were included in the analysis (online supplemental table 1).
bmjophth-2023-001563supp004.pdf (23.8KB, pdf)
Complications
Adverse events associated with SLT either at 60 min or between 1 and 12 months after the procedure are shown in online supplemental table 2. No eyes had a postlaser IOP spike (>5 mm Hg from pretreatment IOP). Transient symptoms were reported in 43.4% of patients. Macular oedema (ME) due to branch retinal vein occlusion (BRVO) occurred after SLT in one case. The patient had no ME on 6 January 2020 and underwent left second-line SLT on 10 March 2020. On 15 April 2020, ME appeared and a close examination revealed macular BRVO. Meanwhile, the prostaglandin analogues continued. Without additional treatment such as steroids, ME was confirmed to have resolved on 21 May 2020. Visual acuity was 1.5. Since the date of onset of macular BRVO is unknown, the causal relationship between SLT and ME is unknown. ECD was 2668.4±304.2/mm2 pre-SLT and 2674.4±314.2 /mm2 at 12 months after SLT, with no significant change between the pre-SLT and post-SLT values (p=0.628). There were no severe adverse events, such as hyphema or prolonged iritis, associated with SLT either during or after the procedure (online supplemental table 2).
bmjophth-2023-001563supp005.pdf (13KB, pdf)
Discussion
Both first-line and second-line SLT significantly reduced IOP in Japanese patients with NTG during 12 months of follow-up. Post-SLT IOP values and IOP reduction were comparable between the two groups. A higher IOP and thinner CCT prior to SLT were identified as factors associated with a greater SLT-induced reduction in IOP in NTG patients. The success rate at 12 months was higher in the first-line group than the second-line group, with lower pretreatment IOP and use of IOP-lowering eye-drops before SLT identified as factors related to treatment failure. Post-treatment complications were mostly minor and transient.
Our findings were consistent with previous studies that showed a substantial reduction in IOP after first-line SLT for NTG. In a study of Japanese patients with NTG including 37 treatment-naïve patients and 5 patients who had discontinued IOP-lowering medications before SLT, we found that the pretreatment mean IOP of 15.8 mm Hg was significantly reduced by 15.8% to 13.2 mm Hg at 1 year after first-line SLT, and by 12.7% to 13.5 mm Hg at 3 years, while 25% of the subjects started IOP-lowering eye-drops after first-line SLT and 15.0% underwent SLT retreatment.11 Lee et al evaluated a single session of SLT in medicated patients with NTG after a 1-month washout of IOP-lowering eye-drops.13 They reported that the mean IOP was 12.2 mm Hg and mean number of eye-drops was 1.1 at 1 year after a single session of SLT, which resulted in an additional 15% reduction in IOP while using 27% less medication. The success rate was 22%, when success was defined as an IOP reduction ≥20% from pre-SLT without any additional IOP-lowering eye-drops at 1 year.13 In their 2-year study, they found a reduction in IOP of 22.0% from pre-SLT IOP and a medication decrease of 41.1% after initial SLT and a success rate of 11.1% at 2 years, using the same success criteria as at 1 year.10 In the LiGHT trial, the SLT group showed better adherence and consequently better QOL. In terms of cost, SLT was more cost-effective than IOP-lowering eye-drops.1 SLT is expected to improve symptoms related to ocular surface diseases and improve adherence to the remaining medications.
According to the Guidelines on Design and Reporting of Glaucoma Surgical Trials issued by the World Glaucoma Association,14 the success rate for NTG, defined by a 20% reduction in IOP, is lower than that for POAG because the baseline IOP falls within the normal range. Notably, patients treated with three to four types of IOP-lowering eye-drops with a preoperative IOP of 18 mm Hg and postoperative IOP of 15 mm Hg, may be categorised as treatment failures. In the current study, we, therefore, also determined the ΔOP to evaluate the treatment outcomes for patients with NTG, with response defined as ΔOP≥20% for SLT. For example, if a patient’s IOP decreased from 13 mm Hg before SLT to 11 mm Hg after SLT, it would be beneficial for glaucoma treatment. However, the percentage reduction in IOP was only 15.3%, and this IOP-lowering effect was underestimated with respect to its clinical significance. Therefore, we considered the usual success criteria undesirable. A study examining outflow facilities before and after SLT12 reported that only outflow facilities increased significantly after SLT with no changes in aqueous humour flow rate (Q), uveoscleral outflow (U) or EVP. To assess the outflow facility, we included the ΔOP as an endpoint to better evaluate the IOP-lowering effect of the SLT. Since EVP=10 mm Hg was assumed for the calculation of ΔOP, a low IOP close to EVP still has the problem of underestimation of the effect of SLT. Therefore, patients were not included in the study unless their IOP was 14 mm Hg or higher. In addition, in practice, a large IOP-reducing effect cannot be expected in cases with extremely low preoperative IOP.
First-line or second-line SLT for NTG was deemed beneficial in this study, with an average reduction in IOP of 16.0% at 12 months. The scatter plot (online supplemental files figure 1) demonstrates that numerous patients achieved a reduction in IOP of ≥20%. Although the success rate based on criterion B was low (19.2%), the success rate according to criterion A, which assessed ΔOP, was 83.8%, suggesting that ΔOP is a valuable metric for evaluating treatment outcomes in patients with NTG.
Comparing first-line and second-line SLT, although the rate of IOP reduction was higher in the first-line compared with the second-line group at 6 months, the post-treatment IOP values and reduction rates were comparable between the two groups at other time points. However, the success rate at 12 months was higher in the first-line group than in the second-line group (23.0% vs 8.0%, respectively). These results were in line with previous reports. Woo et al 15 retrospectively evaluated the additional effect of SLT in patients with POAG, OHT, exfoliation glaucoma or pigmentary glaucoma, classified into four groups according to the number of pre-SLT IOP-lowering medications (0–3) and followed up for 5 years. They showed that although the number of pre-SLT eye-drops did not affect the IOP-lowering effect of SLT, a higher proportion of patients receiving more medications required additional interventions such as trabeculectomy, SLT or additional medications. The increased need for additional interventions or medications in patients with more pre-SLT medications may be the result of the limited response to SLT due to the reduced natural capacity of the patient’s trabecular meshwork and physiological outflow caused by prior treatment with topical aqueous suppressants.16 The current study also revealed that use of IOP-lowering eye-drops before SLT was one of the factors related to treatment failure at 12 months post-SLT. Patients who received eye-drops before SLT (ie, the second-line group) had lower pre-SLT IOP values, which may have reduced the success rate. Treatment-naïve patients with NTG were more likely to respond favourably to SLT than medically treated patients, in accord with previous results in patients with POAG or OHT.1–5
The IOP-lowering effect of 17.1% for first-line SLT in the current study is unlikely to be sufficient to achieve drop-free IOP control in patients with newly diagnosed NTG, given an individual IOP target of a 30% reduction from baseline IOP indicated by the Collaborative Normal Tension Glaucoma study.17 Nevertheless, Kashiwagi et al 18 assessed the long-term effect of latanoprost monotherapy in Japanese patients with glaucoma, including 65% with NTG, and demonstrated that it reduced IOP by 15.5%, which was equivalent to the IOP-lowering effect of first-line SLT in this study. In addition, El Mallah et al found that adjunctive SLT decreased mean IOP by 14.7% and also reduced intervisit variations in IOP in patients with NTG.19 SLT may, thus, help to prevent glaucoma progression by reducing IOP fluctuations,20–23 as well as improving treatment adherence and patient QOL, by decreasing the number of IOP-lowering eye-drops in patients with NTG.
In this study, higher IOP and thinner CCT before SLT were identified as factors associated with a greater reduction in IOP at 1 year. In addition, a lower IOP and use of IOP-lowering eye-drops before SLT were factors related to failure at 1 year. Previous studies have suggested that a higher pre-SLT IOP may be a predictor of a successful outcome in patients with POAG.16 24 25 The LiGHT trial demonstrated that first-line SLT was more likely to be effective in female patients, patients with higher pretreatment IOP, and those with mild POAG or OHT. A high energy of SLT irradiation and low IOP at 2 months after SLT were also shown to sustain a long-term IOP-reduction rate ≥20%.2 Regarding NTG, Lee et al 13 studied 60 eyes in medicated patients with NTG after a 1-month washout of medication, and showed that a higher pre-SLT IOP and a greater IOP reduction at 1 week post-SLT were predictors of a successful outcome. As stated in the Guidelines on Design and Reporting of Glaucoma Surgical Trials published, the success rate for NTG, defined as an IOP reduction ≥20%, is lower than the success rate for POAG. This difference is attributed to baseline IOP values being within the normal range. Therefore, a higher pre-SLT IOP was also associated with a greater IOP reduction and a higher success rate in this study.14 The factors associated with success of SLT may differ between NTG and POAG.
Complications after SLT include transient IOP spike, anterior chamber haemorrhage, iritis, ME and corneal oedema.26–32 There were no cases of a transient IOP increase ≥5 mm Hg in the current study. After SLT, 43.4% of patients in this study reported ocular discomfort, headache, blurred vision, photophobia and nausea, all of which were transient symptoms. One patient had ME due to macular BRVO, but this resolved within 3 months without treatment, and we failed to identify any causal relationship between SLT and BRVO. Because transient corneal endothelial cell damage has been reported following SLT,28 33 we also examined the ECD after SLT and found no significant decrease in this parameter between pre-SLT and 1-year post-SLT.
This study had several limitations. First, we were unable to determine the long-term IOP-lowering effects of first-line and second-line SLT because the observation period was only 1 year, and further interventions may be required to maintain long-term IOP control. We aim to analyse the long-term SLT outcomes of the participants in this study. Second, there is no control group, and the clinical backgrounds of the two groups are different.
This prospective study was designed to evaluate the efficacy and safety of SLT in patients who were to undergo SLT as either first-line or second-line treatment. In the real world of glaucoma, the second line of patients undergo SLT for a longer period of time and with more advanced stage than the first-line SLT groups. In Japan, IOP-lowering eye-drops remain the first-line treatment in most glaucoma cases. For patients with poorly controlled glaucoma, the target IOP is set even lower, and additional treatment is administered, which often leads to adverse events caused by the IOP-lowering eye-drops (eg, allergy, superficial punctate keratopathy, bradycardia and chronic obstructive pulmonary disease). This indicates that the patients eligible for second-line SLT included those with varied clinical backgrounds. Further IOP reduction with SLT in such patients would be clinically ‘beneficial’ and is highly expected to ‘delay’ the surgical decision. Therefore, patients in the second-line SLT group were included in this study.
In conclusion, both first-line and second-line SLT may be effective and safe treatments for patients with NTG, leading to a substantial decrease in IOP over a period of 1 year, with no serious adverse events. Further investigations are warranted to identify the long-term efficacy of first-line and second-line SLT in patients with NTG.
bmjophth-2023-001563supp001.pdf (50.6KB, pdf)
Acknowledgments
We thank Susan Furness, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. And we also thank Yoshinari Takahashi, from inary (https://www.inary.co) for analysing the data of this study.
Footnotes
Collaborators: The FSS Study group: Koji Nitta, Kae Sugihara, Akiko Narita, Tomoko Naito, Takako Miki, Maki Katai, Shiro Mizoue, Keiji Yoshikawa, Masaki Tanito, Kazuhisa Sugiyama, Yasushi Ikuno, Takuji Matsuda, Hiroaki Ozaki, Kazuyuki Hirooka, Kaori Komatsu, Yoshiaki Saito, Itaru Kimura, Tairo Kimura, Takeshi Sagara, Katsuyoshi Suzuki, Aika Tsutsui, Akiko Ishida, Toru Nakazawa, Satoru Tsuda, Toyoaki Tsumura, Naoki Tojo, Naoto Tokuda, Tadashi Nakano, Tomoyuki Watanabe, Kenji Nakamoto, Naka Shiratori, Mami Nanno, Naoya Nezu, Yoshitaka Tasaka, Shigeru Mori, Shigeki Yamabayashi, Kimihito Konno and Miyuki Domoto.
Contributors: Conception and design: KN, MT, KSugihara and KSugiyama. Data collection: KN, KSugiyama, AN, TN, TM, MK, SM, KY, MT and FSS Study group. Analysis and interpretation: KSugihara. Acts as Guarantor of Work: KN
Funding: Supported by the Japan Glaucoma Society Research Project Support Programme (2020-001).
Disclaimer: The sponsor or funding organisation had no role in the design or conduct of this research.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The FSS Study group, Koji Nitta, Kae Sugihara, Akiko Narita, Tomoko Naito, Takako Miki, Maki Katai, Shiro Mizoue, Keiji Yoshikawa, Masaki Tanito, Kazuhisa Sugiyama, Yasushi Ikuno, Takuji Matsuda, Hiroaki Ozaki, Kazuyuki Hirooka, Kaori Komatsu, Yoshiaki Saito, Itaru Kimura, Tairo Kimura, Takeshi Sagara, Katsuyoshi Suzuki, Aika Tsutsui, Akiko Ishida, Toru Nakazawa, Satoru Tsuda, Toyoaki Tsumura, Naoki Tojo, Naoto Tokuda, Tadashi Nakano, Tomoyuki Watanabe, Kenji Nakamoto, Naka Shiratori, Mami Nanno, Naoya Nezu, Yoshitaka Tasaka, Shigeru Mori, Shigeki Yamabayashi, Kimihito Konno, and Miyuki Domoto
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All protocols approved by the ethics committee of each institution or the Medical Research Ethics Committee of the Shimane University Faculty of Medicine (for medical institutions without an ethics committee) (IRB ID:2019-047). This study was conducted in accordance with the ethical principles of the Declaration of Helsinki; the research protocol; the standards stipulated in Article 14, Paragraph 3 and Article 80-2 of the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices; and related regulatory laws, including the Ministerial Ordinance on Standards for Conducting Clinical Trials of Pharmaceuticals (GCP). All participants provided written informed consent.
References
- 1. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 2019;393:1505–16. 10.1016/S0140-6736(18)32213-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garg A, Vickerstaff V, Nathwani N, et al. Primary selective laser trabeculoplasty for open-angle glaucoma and ocular hypertension: clinical outcomes, predictors of success, and safety from the laser in glaucoma and ocular hypertension trial. Ophthalmology 2019;126:1238–48. 10.1016/j.ophtha.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 3. Garg A, Vickerstaff V, Nathwani N, et al. Efficacy of repeat selective laser trabeculoplasty in medication-naïve open-angle glaucoma and ocular hypertension during the LiGHT trial. Ophthalmology 2020;127:467–76. 10.1016/j.ophtha.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 4. Wright DM, Konstantakopoulou E, Montesano G, et al. Visual field outcomes from the multicenter, randomized controlled laser in glaucoma and ocular hypertension trial (LiGHT). Ophthalmology 2020;127:1313–21. 10.1016/j.ophtha.2020.03.029 [DOI] [PubMed] [Google Scholar]
- 5. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (LiGHT) trial: six-year results of primary selective laser trabeculoplasty versus eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology 2023;130:139–51. 10.1016/j.ophtha.2022.09.009 [DOI] [PubMed] [Google Scholar]
- 6. Ang GS, Fenwick EK, Constantinou M, et al. Selective laser trabeculoplasty versus topical medication as initial glaucoma treatment: the glaucoma initial treatment study randomised clinical trial. Br J Ophthalmol 2020;104:813–21. 10.1136/bjophthalmol-2018-313396 [DOI] [PubMed] [Google Scholar]
- 7. King D, Drance SM, Douglas G, et al. Comprison of visual field defects in normal-tension glaucoma and high-tension glaucoma. Am J Ophthalmol 1986;101:204–7. 10.1016/0002-9394(86)90596-9 [DOI] [PubMed] [Google Scholar]
- 8. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi study. Ophthalmology 2004;111:1641–8. 10.1016/j.ophtha.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 9. Kim C, Seong GJ, Lee N, et al. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 2011;118:1024–30. 10.1016/j.ophtha.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 10. Lee JWY, Shum JJW, Chan JCH, et al. Two-year clinical results after selective laser trabeculoplasty for normal tension glaucoma. Medicine 2015;94:e984. 10.1097/MD.0000000000000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nitta K, Sugiyama K, Mawatari Y, et al. Results of selective laser trabeculoplasty (SLT) as initial treatment for normal tension glaucoma. J Japanese Ophthalmol Soc 2013;117:335–43. [PubMed] [Google Scholar]
- 12. Gulati V, Fan S, Gardner BJ, et al. Mechanism of action of selective laser trabeculoplasty and predictors of response. Invest Ophthalmol Vis Sci 2017;58:1462. 10.1167/iovs.16-20710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JW, Ho WL, Chan JC, et al. Efficacy of selective laser trabeculoplasty for normal tension glaucoma: 1 year results. BMC Ophthalmol 2015;15:1. 10.1186/1471-2415-15-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaarawy TM, Sherwood MB, Grehn F. Guidelines on design and reporting of glaucoma surgical trials WGA consensus, consensus on definitions of success I. Intraocular pressure documentation 4. In: Confounding influences. The Hague, Amsterdam, The Netherlands: Kugler Publications, 2009: 17. [Google Scholar]
- 15. Woo DM, Healey PR, Graham SL, et al. Intraocular pressure-lowering medications and long-term outcomes of selective laser trabeculoplasty. Clin Exp Ophthalmol 2015;43:320–7. 10.1111/ceo.12452 [DOI] [PubMed] [Google Scholar]
- 16. Hirabayashi M, Ponnusamy V, An J. Predictive factors for outcomes of selective laser trabeculoplasty. Sci Rep 2020;10:9428. 10.1038/s41598-020-66473-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulzer M, Airaksinen PJ, Alward WLM, et al. Intraocular pressure reduction in normal-tension glaucoma patients. Ophthalmology 1992;99:1468–70. 10.1016/S0161-6420(92)31782-8 [DOI] [PubMed] [Google Scholar]
- 18. Kashiwagi K, Tsumura T, Tsukahara S. Long-term effects of latanoprost monotherapy on intraocular pressure in Japanese glaucoma patients. J Glaucoma 2008;17:662–6. 10.1097/IJG.0b013e318166656d [DOI] [PubMed] [Google Scholar]
- 19. El Mallah MK, Walsh MM, Stinnett SS, et al. Selective laser trabeculoplasty reduces mean IOP and IOP variation in normal tension glaucoma patients. Clin Ophthalmol 2010;4:889–93. 10.2147/opth.s11787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tojo N, Oka M, Miyakoshi A, et al. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma 2014;23:e138–43. 10.1097/IJG.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 21. Lee JWY, Fu L, Chan JCH, et al. Twenty-four-hour intraocular pressure related changes following adjuvant selective laser trabeculoplasty for normal tension glaucoma. Medicine (Baltimore) 2014;93:e238. 10.1097/MD.0000000000000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu D, Chen D, Tan Q, et al. Outcome of selective laser trabeculoplasty in young patients with primary open-angle glaucoma and ocular hypertension. J Ophthalmol 2020;2020:5742832. 10.1155/2020/5742832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pillunat KR, Kocket GA, Herber R, et al. Efficacy of selective laser trabeculoplasty on lowering intraocular pressure fluctuations and nocturnal peak intraocular pressure in treated primary open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2023;261:1979–85. 10.1007/s00417-022-05897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khawaja AP, Campbell JH, Kirby N, et al. Real-world outcomes of selective laser trabeculoplasty in the United Kingdom. Ophthalmology 2020;127:748–57. 10.1016/j.ophtha.2019.11.017 [DOI] [PubMed] [Google Scholar]
- 25. Ayala M, Chen E. Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin Ophthalmol 2011;5:573–6. 10.2147/OPTH.S19873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Regina M, Bunya VY, Orlin SE, et al. Corneal edema and haze after selective laser trabeculoplasty. J Glaucoma 2011;20:327–9. 10.1097/IJG.0b013e3181e6668d [DOI] [PubMed] [Google Scholar]
- 27. Moubayed SP, Hamid M, Choremis J, et al. An unusual finding of corneal edema complicating selective laser trabeculoplasty. Can J Ophthalmol 2009;44:337–8. 10.3129/i09-025 [DOI] [PubMed] [Google Scholar]
- 28. Ong K, Ong L, Ong LB. Corneal endothelial abnormalities after selective laser trabeculoplasty. J Glaucoma 2015;24:286–90. 10.1097/IJG.0b013e3182946381 [DOI] [PubMed] [Google Scholar]
- 29. Rhee DJ, Krad O, Pasquale LR. Hyphema following selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging 2009;40:493–4. 10.3928/15428877-20090901-09 [DOI] [PubMed] [Google Scholar]
- 30. Shihadeh WA, Ritch R, Liebmann JM. Hyphema occurring during selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging 2006;37:432–3. 10.3928/15428877-20060901-14 [DOI] [PubMed] [Google Scholar]
- 31. Kim DY, Singh A. Severe Iritis and choroidal effusion following selective laser trabeculoplasty. Ophthalmic Surg Lasers Imaging 2008;39:409–11. 10.3928/15428877-20080901-10 [DOI] [PubMed] [Google Scholar]
- 32. Wechsler DZ, Wechsler IB. Cystoid macular oedema after selective laser trabeculoplasty. Eye (Lond) 2010;24:1113. 10.1038/eye.2009.249 [DOI] [PubMed] [Google Scholar]
- 33. Lee JWY, Chan JCH, Chang RT, et al. Corneal changes after a single session of selective laser trabeculoplasty for open-angle glaucoma. Eye (Lond) 2014;28:47–52. 10.1038/eye.2013.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2023-001563supp002.pdf (112.8KB, pdf)
bmjophth-2023-001563supp003.pdf (244.1KB, pdf)
bmjophth-2023-001563supp004.pdf (23.8KB, pdf)
bmjophth-2023-001563supp005.pdf (13KB, pdf)
bmjophth-2023-001563supp001.pdf (50.6KB, pdf)
Data Availability Statement
Data are available on reasonable request.