Abstract
Tuftsin (TF) is an immunomodulator tetrapeptide (Thr-Lys-Pro-Arg) that binds to the receptor neuropilin-1 (Nrp1) on the surface of cells. Many reports have described anti-tumor activity of tuftsin to relate with nonspecific activation of the host immune system. Lidamycin (LDM) that displays extremely potent cytotoxicity to cancer cells is composed of an apoprotein (LDP) and an enediyne chromophore (AE). In addition, Ec is an EGFR-targeting oligopeptide. In the present study, LDP was used as protein scaffold and the specific carrier for the highly potent AE. Genetically engineered fusion proteins LDP-TF and Ec-LDP-TF were prepared; then, the enediyne-energized fusion protein Ec-LDM-TF was generated by integration of AE into Ec-LDP-TF. The tuftsin-based fusion proteins LDP-TF and Ec-LDP-TF significantly enhanced the phagocytotic activity of macrophages as compared with LDP (P < 0.05). Ec-LDP-TF effectively bound to tumor cells and macrophages; furthermore, it markedly suppressed the growth of human epidermoid carcinoma A431 xenograft in athymic mice by 84.2 % (P < 0.05) with up-regulated expression of TNF-α and IFN-γ. Ec-LDM-TF further augmented the therapeutic efficacy, inhibiting the growth of A431 xenograft by 90.9 % (P < 0.05); notably, the Ec-LDM-TF caused marked down-regulation of CD47 in A431 cells. Moreover, the best therapeutic effect was recorded in the group of animals treated with the combination of Ec-LDP-TF with Ec-LDM-TF. The results suggest that tuftsin-based, enediyne-energized, and EGFR-targeting fusion proteins exert highly antitumor efficacy with CD47 modulation. Tuftsin-based fusion proteins are potentially useful for treatment of EGFR- and CD47-overexpressing cancers.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1604-1) contains supplementary material, which is available to authorized users.
Keywords: Tuftsin, Lidamycin, Fusion protein, EGFR, CD47
Introduction
Tuftsin is a natural immunomodulating tetrapeptide that is formed by enzymatic cleavage of the Fc portion of the immunoglobulin (IgG) molecule (residues 289–292) [1, 2]. This peptide binds specifically to the receptor neuropilin-1 (Nrp1) of macrophages/microglia, and the binding equilibrium dissociation constant was found to be 5.3 × 10−8 M [3]. Several studies have indicated that tuftsin stimulated phagocytosis in macrophages and enhanced immunomodulatory activity in the host [4, 5]. Many reports have also described the antitumor activity of tuftsin in animal models [6]. Tuftsin-bearing liposomized etoposide was found to be more effective than free etoposide in treatment of fibrosarcoma in mice [5]. Tuftsin-based scFv (anti-CA125 antibody) may provide protective immunity against ovarian cancer in vivo [7]. It is evident that the antitumor activity of tuftsin may be related to phagocytosis. Recently, CD47 has caused much attention for its role in the suppression of phagocytosis. Because the growth of tumor requires avoidance of being phagocytosed by the macrophages, the expression of CD47 in cell surface is a common mechanism by which cells protect themselves from phagocytosis [8]; hence, it is needed to understand the effect of tuftsin or its analogs on CD47 modulation. Some reports showed that tuftsin involves in Nrp1 and the canonical TGF-β signaling pathways [9]. However, there are no any reports yet on tuftsin in association with CD47 signaling pathway. Therefore, it is of interest to investigate the antitumor activity of tuftsin and tuftsin-derived agents relating to the possible involvement of CD47 modulation.
Lidamycin (LDM), an antitumor antibiotic displays extremely potent cytotoxicity to cultured cancer cells in vitro and high efficacy against various experimental tumors in vivo, contains an active enediyne (AE) responsible for the highly potent cytotoxicity and a non-covalently bound apoprotein LDP [10]. Notably, LDP and AE can be dissociated and reconstituted in vitro [11]. Our previous studies with tissue microarray have shown that LDP could bind to various human tumors with significant difference from the corresponding normal tissues [12]. The AE of LDM may induce major changes in the cell, including proliferation inhibition, apoptosis, and DNA double-strand breaks (DSB) [13].
The overexpression of epidermal growth factor receptor (EGFR) has been observed in many human tumors and the receptor evaluated as important target for the development of new targeted therapeutics [14, 15]. In this study, we generated tuftsin-based fusion proteins, LDP-TF and Ec-LDP-TF, and their corresponding AE integrated analogs, LDM-TF and Ec-LDM-TF, to enhance antitumor efficacy associated with immunomodulation. The genetically engineered LDP-TF fusion protein comprises tuftsin and LDP; in addition, Ec-LDP-TF is composed of an oligopeptide Ec and LDP-TF. As reported, the oligopeptide Ec can be used as EGFR-targeting molecule [16]. Our results suggest that Ec-LDP-TF and Ec-LDM-TF may be potential agents for anticancer therapy. Moreover, the induced reduction of CD47 expression in tumor cells might provide a basis for understanding the mechanism of action of tuftsin-based agents.
Materials and methods
Cell culture
Human epidermoid carcinoma A431 cells were routinely grown in DMEM (GIBCO) supplemented with 10 % fetal bovine serum (GIBCO). Macrophages J774A.1, breast cancer MCF-7 and lung carcinoma A549 cells were routinely grown in RPMI-1640 (GIBCO) supplemented with 10 % fetal bovine serum (GIBCO), penicillin (100 IU/ml) and streptomycin (100 μg/ml).
Construction of expression vector pET-Ec-ldp-TF
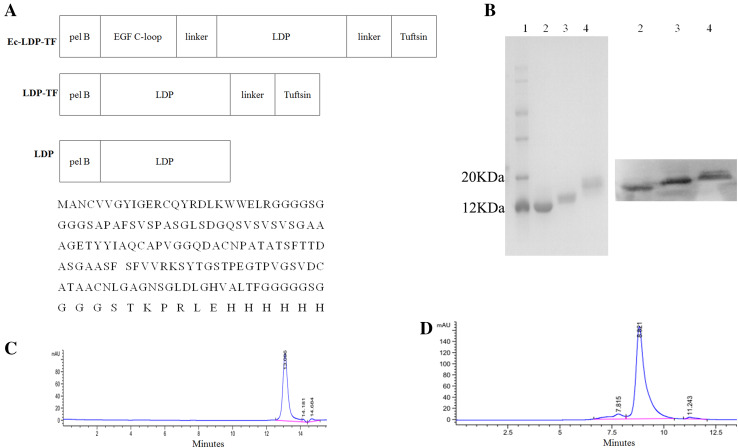
As shown in Fig. 1a, the full gene of the fusion protein Ec-LDP-TF (from 5′ to 3′) consisted of pelB signal peptide (22 amino acids; [16]), C-loop of EGF (the 22 amino acids of EGF COOH terminal; [17]), apoprotein LDP (110 amino acids; [17]) and tuftsin (4 amino acids; [2]). Two (GGGGS)2 linkers were inserted into the space separately between the coding sequences of EGF C-loop and LDP, and that of LDP and tuftsin, respectively. After PCR and DNA cloning process, the resultant 549-bp fragment was digested by NdeI/XhoI and was inserted into pET30a expression vector to generate plasmid pET-Ec-ldp-TF. Coding sequence for the fusion protein LDP-TF was created by the same way.
Fig. 1.
Purification and characterization of the Ec-LDP-TF or its enediyne-energized analogue Ec-LDM-TF. a Diagram of NdeI/XhoI gene fragments encoded for the fusion proteins Ec-LDP-TF, LDP-TF, and LDP (top). Bottom, the amino acid sequence of Ec-LDP-TF. b SDS-PAGE (left) and Western blot (right) analysis of fusion proteins. Lane 1 molecular weight marker; Lane 2 LDP protein; Lane 3 LDP-TF protein; Lane 4 Ec-LDP-TF protein. (c) and (d) The purity of LDP-TF and Ec-LDP-TF were analyzed by HPLC on S3000 column, respectively
Expression and purification of the fusion proteins
Expression plasmids pET-Ec-ldp-TF, pET-ldp-TF, and pET-ldp were transformed into Escherichia coli strain BL21 (DE3) star (Novagen). After overnight culture, bacteria were grown at 37 °C until the OD600 reaches 0.8–1.0. Gene expression was induced with the addition of IPTG at 0.1-mmol/L concentration at 37 °C for 12 h. The fusion proteins through a C-terminal 6 × His-tag were purified by affinity chromatography (HisTrap HP, GE Healthcare) according to the manufacturer’s instruction and the purity of fusion proteins was analyzed by high-performance liquid chromatography (HPLC) on a S3000 column (Tosoh).
Preparation of enediyne-energized fusion proteins
The fusion proteins (Ec-LDP-TF and LDP-TF) were packed with the enediyne AE as described [18, 19]. Then free AE was removed by using a Sephadex G-75 column (GE Healthcare) and the assembled energized fusion proteins (Ec-LDP-TF-AE and LDP-TF-AE) were confirmed by reverse-phase HPLC using a Vydac C4 300A column (Grace). Absorbance at 350 nm was measured.
Binding specificity with cancer cells
The binding specificity of the tuftsin-based fusion protein Ec-LDP-TF to A431 cells was evaluated with fluorescence microscope. This assay was performed as previously reported [19].
To quantitatively compare the binding affinity of each fusion protein to target cells, we used a fluorescence-activated cell sorting (FACS)-based saturation binding assay [19].The data were analyzed with the Prism 5.0 software (GraphPad Software).
In vivo fluorescence imaging
A431 cells were inoculated to the right armpit of nude mice. After 14 days, Dylight 680 antibody-labeled Ec-LDP-TF (20 mg/kg) was intravenously injected into the xenograft-bearing mice (n = 3). Then the mice were anesthetized by inhalation of isoflurane and the images were observed with the Xenogen Ivis 200 system and recorded by built-in camera (Caliper Life Sciences).
In vitro phagocytosis assay
For in vitro phagocytosis assay, macrophages were plated at 5 × 104 per well in a 24-well plate. Bovine serum albumin was labeled with FITC. Macrophages were incubated in serum-free medium for 2 h before adding 1 μM FITC-labeled BSA. The indicated Ec-LDP-TF, LDP-TF or LDP protein (10 μM) was added and incubated for 2 h at 37 °C. Macrophages were repeatedly washed and subsequently imaged with an inverted microscope.
To quantitatively compare the effect on the phagocytosis affinity of macrophages by various fusion proteins, FITC-labeled BSA fluorescence was then determined by flow cytometry.
Cytotoxicity assay by cell counting kit-8
Cells were plated in 96-well plates at 2 × 103 cells/well. After 24 h, the cells were then serum-starved for 8 h and further incubated with various concentrations of Ec-LDP-TF, LDP-TF, LDP, or PBS for 24 h at 37 °C, respectively. After adding 10 μL CCK-8 solution (Dojindo, Kumamoto, Japan) to 100 μl of culture media, optical density was measured at 450 nm.
RT-RCR analysis
Total mRNA was extracted from the cells by TRIzol reagent (Invitrogen) with an extra step of acid phenol extraction. RT-PCR was carried out using AMV Reverse transcriptase (promega) as described previously [20]. Oligonucleotide primers used were as follows: CD47 P1, 5′-CGG CGG GCG CGG AGA TGT-3′; CD47 P2, 5′-TCA CCT GGG ACG AAA AG AAT GG-3′; β-actin P1, 5′-CCC AGG CAC CAG GGC GTG ATG GT-3′; β-actin P2, 5′-GGA CTC CAT GCC CAG GAA GGA A-3′. β-actin mRNA was analyzed as internal control. A measure of 1 μg of total RNA was reverse-transcribed to synthesize cDNA at 37 °C for 2 h, and then the cDNA was subjected to PCR amplification with specific primers in 25 μl mixtures. PCR comprised 25 cycles with denaturing at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 40 s in each cycle using an MJ PCR system (Bio-Rad). The PCR products were then subjected to 2 % agarose gel electrophoresis.
Western blot analysis
After incubation with Ec-LDP-TF or Ec–LDM–TF for 24 h, the whole cell lysates were prepared and analyzed by Western blot. The polyvinylidene fluoride membrane samples containing cell proteins were probed with anti-CD47 antibody (Epitomics, Abcam Company, USA), then with anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology), and finally visualized with Immobiolon Western Chemiluminescent HRP Substrate (Millipore).
ELISA
Blood samples were collected from the orbital blood capillary of mice 24 h after the second treatment. TNF-α and IFN-γ production following stimulation was measured using BioLegend’s ELISA Max™ Sets, according to the manufacturer’s instructions (BioLegend, San Diego, CA).
MTT assay
Cancer cells were detached by trypsinization and seeded at 4,000 cells/well in a 96-well plate (Costar) overnight. Then different concentrations of LDM, Ec-LDM-TF and LDM-TF were separately added and incubated for an additional 48 h. The effects on cell growth were examined by MTT assay, which was described previously [18].
Animal experiments
Antitumor experiment was carried out using A431 xenograft model. Female athymic mice (BALB/c, nu/nu) were purchased from the Institute for Experimental Animals, Chinese Academy of Medical Sciences and Peking Union Medical College. The study protocols were in accordance with the regulations of Good Laboratory Practice for Non-clinical Laboratory Studies of Drugs issued by the National Scientific and Technologic Committee of People’s Republic of China. The treatment and use of animals during the study were approved by the Animal Ethics Committee of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College (permission number:c1-2012-1220). Epidermoid carcinoma A431 cells were suspended in sterile saline (1 × 107 cells/mL) and 200 μL of the suspension were inoculated s.c. in the right armpit of nude mouse. After 3 weeks tumors in donor animals were aseptically dissected and mechanically minced. The tumor pieces tissue blocks (2 mm3 in size) were transplanted (subcutaneously) by a trocar needle into nude mice. When the tumor size reached approximately 100 mm3, mice were divided into groups (n = 6) and treated with different doses of fusion proteins or energized fusion proteins in a 200 μL volume of sterile saline by injected i.v. into the tail vein, in total of two injections with a weekly interval. One group of mice was administered i.v. with saline as control. Tumor volumes were measured as described previously [18, 19].
Statistical analysis
Statistical differences between experimental groups were determined by the unpaired t test using Prism software (GraphPad, La Jolla, CA). Bar graphs indicate means; error bars represent SD.
Results
Effect of tuftsin on epidermoid carcinoma A431 cells
As shown in Supplementary Fig. 1A, no obvious cytotoxicity was observed in A431, A549 or MCF-7 cells even at a concentration of 1,000 μmol/L of tuftsin. Additionally, tuftsin did not display obvious anti-tumor effects on A431 xenografts in vivo (Supplementary Fig. 1B). By treatment with high-dose tuftsin resulted in transient weight loss in treated animals (data not shown).
Construction and preparation of fusion proteins
Tuftsin-based fusion proteins were generated by genetic engineering. For LDP-TF, the C-terminal of LDP was tethered to the N-terminal of the tetrapeptide tuftsin with a linker peptide. In the case of Ec-LDP-TF, the apoprotein LDP was separately at the C-terminal and N-terminal tethered to tuftsin and the oligopeptide Ec that consists of a C-loop of EGF (Fig. 1a). As shown in Fig. 1b, the purity of fusion proteins was analyzed by SDS-PAGE and Western blot. The molecular mass of LDP-TF and Ec-LDP-TF are approximately 12.9 and 15.1 kD, respectively, as determined by MALDI. The purity of LDP-TF isolated by molecular sieve approached 98 % as assessed by HPLC (Fig. 1c); and Ec-LDP-TF approached 91 % (Fig. 1d).
Binding affinity of fusion proteins
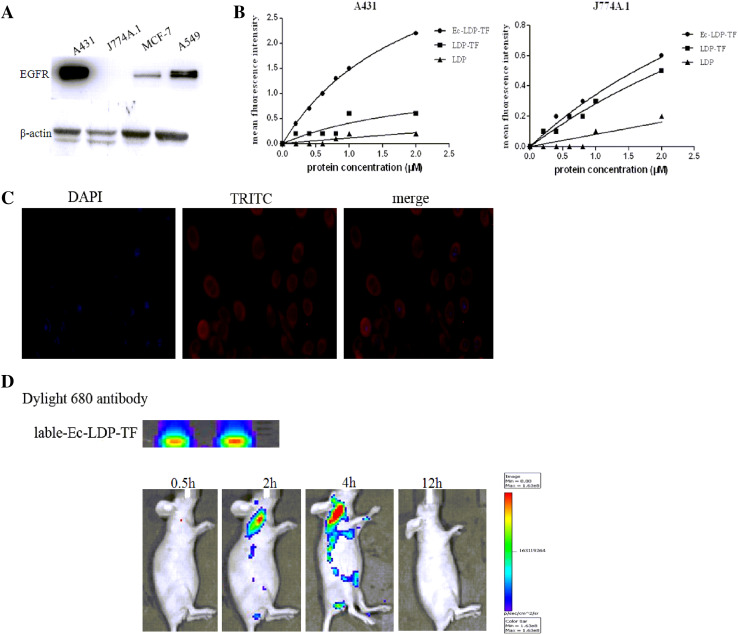
FaCS analysis revealed that Ec-LDP-TF obviously bound to the EGFR-overexpressed A431 cells; by contrast, LDP barely bound to A431 cells (Fig. 2b, Supplementary Table 1). As to the tuftsin receptor-integrated mouse macrophages, Ec-LDP-TF and LDP-TF both bound to J774A.1 cells efficiently; however, LDP showed no binding to J774A.1 cells (Fig. 2b, Supplementary Table 1). The binding specificity of Ec-LDP-TF with the cancer cells was also characterized by immunofluorescence. The results showed that Ec-LDP-TF protein bound specifically to the membrane of EGFR-overexpressed A431 cells (Fig. 2c). An optical in vivo imaging system was used to evaluate the tissue distribution and tumor targeting capability of Ec-LDP-TF. The images of Dylight 680 antibody-labeled Ec-LDP-TF showed fast tumor localization and accumulation in the A431 xenografts (Fig. 2d), which yielded images with good contrast as early as 2 h after injection.
Fig. 2.
Binding affinity of Ec-LDP-TF protein in vitro and the optical imaging in vivo. a Expression of EGFR on different carcinoma cell lines analyzed by Western blotting. b Binding affinities of three fusion proteins with A431 and J774A.1 cells. Following FACS, the mean fluorescence intensity was plotted versus fusion protein concentration. c Immunorescence assay on binding affinity of Ec-LDP-TF using A431 cells. Red fluorescence located around the cells staining with anti-His-tag antibody (TRITC –conjugated antibody) as well as 4′,6-diamidino-2-phenylindole (DAPI). d Optical imaging in living nude mice bearing A431 xenografts treated with Dylight 680 antibody-labeled Ec-LDP-TF. The images collected at different times after i.v. injection of Dylight 680 antibody-labeled Ec-LDP-TF. Red circles the tumor areas
Tuftsin-based fusion proteins promoting phagocytosis of the macrophages and inhibiting tumor cell proliferation
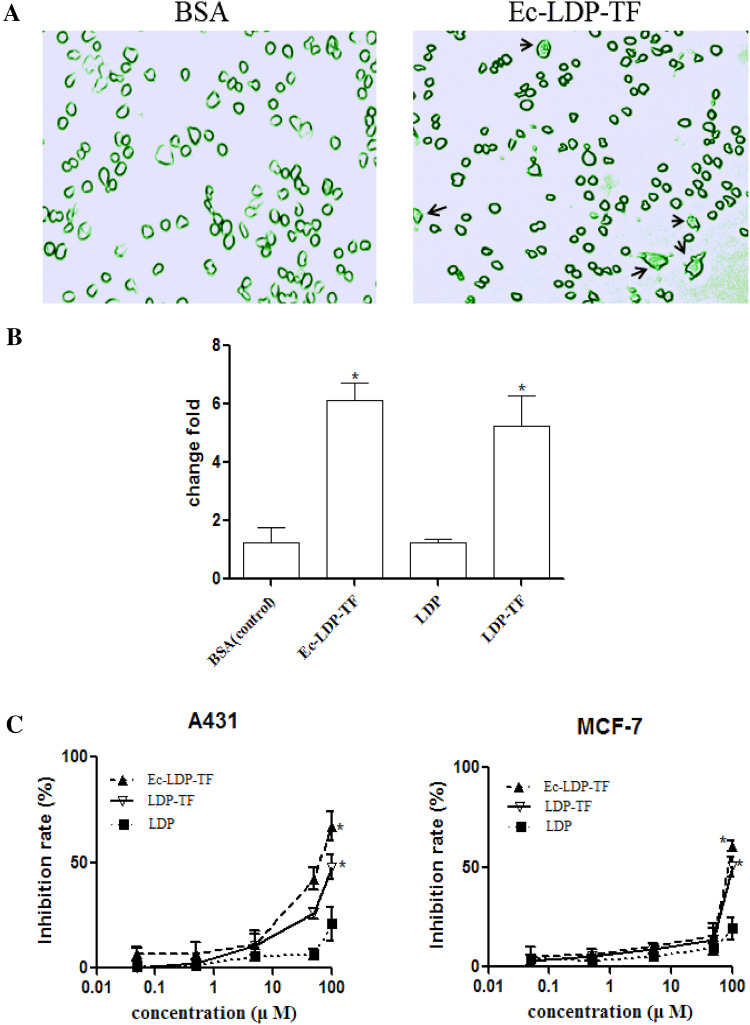
The fluorescence microscopy images in Fig. 3a shows that phagocytosis of BSA by mouse macrophages was promoted following Ec-LDP-TF treatment. FACS analysis suggested that mouse macrophages treated with tuftsin-based fusion proteins obviously enabled phagocytosis as compared with those treated with LDP and the control (Fig. 3b). As shown, the fusion proteins inhibited the growth of A431and MCF-7 cells in a dose-dependent manner. Ec-LDP-TF and LDP-TF displayed more marked cell growth inhibition than LDP did as compared at 100 μM concentration (Fig. 3c).
Fig. 3.
Cytotoxicity of tuftsin-fusion proteins against tumor cells. a Representative images of mouse macrophages phagocytosing FITC-labled BSA following treatment with fusion proteins. Arrows point to phagocytosed BSA. b Bar graph shows the mean fluorescence intensity of FITC-labeled BSA after being primed by BSA, Ec-LDP-TF, LDP-TF, or LDP for the 2-h time point. Fold change is expressed as a ratio of mean fluorescence intensity level of the different fusion proteins relative to the mean fluorescence intensity level of the BSA protein. *P < 0.05. c The growth inhibition ratio of A431 and MCF-7 cells were indicated by the absorbance of experimental groups compared with that of control group. Data represent mean ± SD of three repeats. Compared LDP treated group, *P < 0.05
Tuftsin-based fusion proteins displaying therapeutic effect against A431 xenograft in nude mice
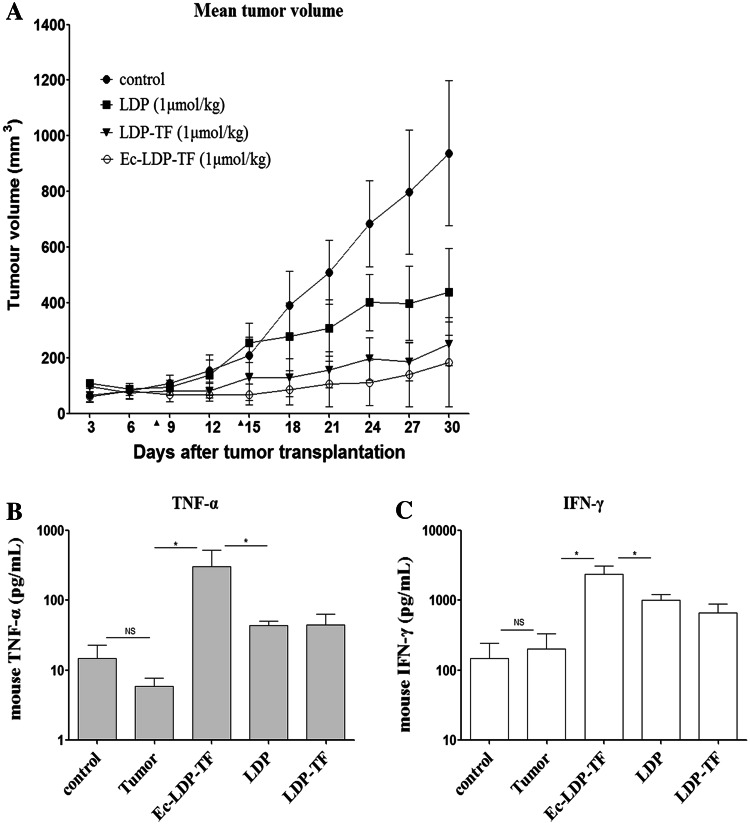
In comparison, Ec-LDP-TF was more effective than LDP-TF or LDP in the treatment of epidermoid carcinoma A431 xenograft. The treatment started when the tumors attained the size of approximately 100 mm3. Mice were treated with the proteins by intravenous route, once a week, a total of two injections. At doses of 1 μmol/kg, LDP-TF (12.9 mg/kg) and Ec-LDP-TF (15.1 mg/kg) were effective in suppressing tumor growth (P < 0.01) (Fig. 4a). LDP-TF delayed tumor growth and the efficacy was superior to that of LDP (P < 0.05). Ec-LDP-TF showed even better therapeutic effect (P < 0.01). Notably, treatment with Ec-LDP-TF markedly reduced tumor growth down to 69.8 ± 30.2 mm3 from the original 100 mm3 with in eight days after the initiation of therapy. As evaluated on day 30 (Supplementary Table 2), Ec-LDP-TF treatment showed an inhibition rate of 84.2 %. The therapeutic effects on the tumors were further confirmed by histopathological examination. Tumor cells were found to arrange in loose pattern and disappearance of mitosis (Supplementary Fig. 2).
Fig. 4.
In vivo efficacy of tuftsin-based fusion proteins. a Antitumor effects of Ec-LDP-TF alone on epidermoid carcinoma A431 xenograft. Triangles, the day of injection (day 8 and 14). Production of cytokine in blood serum treated with fusion proteins. Blood samples were taken 24 h after the injection of second dose of drugs. Results are presented in histograms IFN-γ (b) and TNF-α (c) per milliliter ± SD. Asterisks (*) indicate significant difference (P < 0.05) between untreated tumor group and drugs treated groups. Control is normal animal
As reported, TNF-α and IFN-γ were used as a parameter to study the antitumor effect of the tuftsin [4, 21]. In the present study, the level of TNF-α decreased in A431-bearing animals compared with untreated healthy ones (Fig. 4b). Elevated expression level of TNF-α was recorded in the animals treated with tuftsin-based fusion proteins. Compared with those treated with PBS, the expression levels of TNF-α in tumor-bearing mice treated with Ec-LDP-TF, LDP-TF and LDP increased by 250-fold, 7-fold and 5-fold, respectively (Fig. 4b). As shown, the levels of IFN-γ increased more significantly in mice treated with Ec-LDP-TF than those treated with LDP (P < 0.05) (Fig. 4c). These results indicated that tuftsin-based fusion proteins could influence the cytokine levels that might modulate the immune responses and be potentially useful for cancer immunotherapy.
Enediyne-energized, tuftsin-based fusion proteins displaying highly potent cytotoxicity to cancer cells
The enediyne-energized fusion proteins Ec-LDM-TF and LDM-TF were prepared by integrating AE molecule of LDM into Ec-LDP-TF and LDP-TF, respectively. Using CCK-8 assay, the fusion proteins showed moderate antitumor effects in vitro with IC50 values varied from levels of 10−2–10−4 M. The tuftsin-based fusion proteins LDP-TF and Ec-LDP-TF displayed moderate cell growth inhibition (Supplementary Fig. 4). For A431 and MCF-7 cells, the IC50 values of LDP-TF were 1.72 × 10−4 and 2.3 × 10−4 M; and those of Ec-LDP-TF were 1.0 × 10−4 and 2.2 × 10−4 M, respectively. Notably, as determined by MTT assay, the enediyne-energized fusion proteins LDM-TF and Ec-LDM-TF displayed highly potent cytotoxicity to cancer cells. For A431 and MCF-7 cells, the IC50 values of LDM-TF were 6.93 × 10−11 and 2.6 × 10−10 M; and the IC50 values of Ec-LDM-TF were 2.94 × 10−12 and 9.14 × 10−11 M, respectively (P < 0.01). Evidently, the EGFR-overexpressed A431 cells were more sensitive to Ec-LDM-TF than the low-expressing MCF-7 cells (Supplementary Fig. 3).
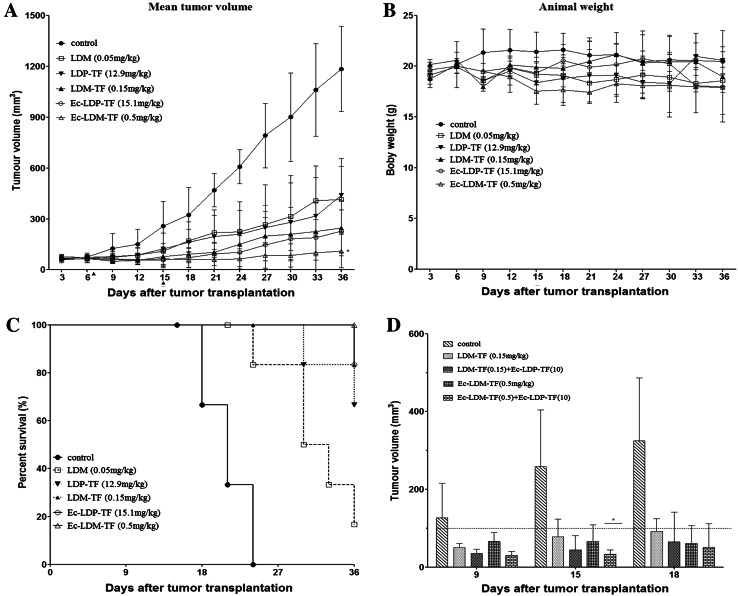
Enediyne-energized analogs exerting higher antitumor efficacy in vivo.
Therapeutic experiments were performed with epidermoid carcinoma A431 xenograft in nude mice. As shown in Fig. 5a, the enediyne-energized fusion proteins LDM-TF and Ec-LDM-TF exerted higher antitumor efficacy as compared with respective non-enediyne analogs LDP-TF and Ec-LDP-TF. In comparison, LDM-TF and Ec-LDM-TF as well as LDP-TF and Ec-LDP-TF suppressed A431 tumor growth by 79.2, 90.9, 61.2, and 80.8 %, respectively. Free LDM at tolerated dosage of 0.05 mg/kg resulted in a 65.0 % inhibition. Figure 5b shows that body weight loss in the animals treated with different doses did not exceed 10 % of the pre-treatment body weight. Thus, the dosages of Ec-LDM-TF and LDM-TF were tolerated. Ec-LDM-TF significantly enhanced the probability of survival of the mice compared with the control group (Fig. 5c). In comparison, the best therapeutic effect was recorded in the group of animals treated with Ec-LDM-TF (P < 0.05; compared with control). Notably, in therapeutic experiments with A431 xenograft in nude mice, the combination of Ec-LDP-TF (10 mg/kg) with Ec-LDM-TF (0.5 mg/kg), exerted high antitumor efficacy (P < 0.05; compared with Ec-LDM-TF treatment) (Fig 5d).
Fig. 5.
In vivo efficacy of tuftsin-based fusion proteins and corresponding enediyne-energized fusion proteins. a Mean tumor volumes and b Mean body weights of mice in each group are shown. Triangles the days were injected by day 7 and day 15. Compared with LDM group, *P < 0.05. c The Kaplan–Meyer survival curve (end-points defined as tumor load of 400 mm3) demonstrated that compared with other groups, probability of survival (i.e., probability of maintaining a tumor volume <400 mm3) of mice treated with Ec-LDM-TF (0.5 mg/kg) was significantly improved (P < 0.05). d, Antitumor effects of Ec-LDP-TF (10 mg/kg) combined with Ec-LDM-TF (0.5 mg/kg) on the growth of epidermoid carcinoma A431 xenograft in nude mice. Triangles the day of injection (day 8 and 14). *P < 0.05
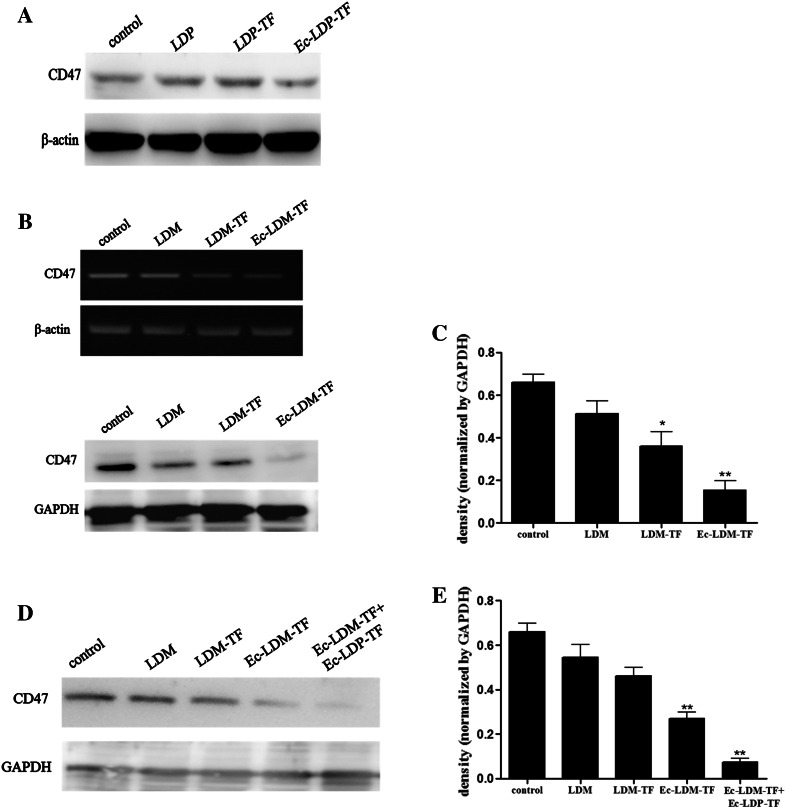
Enediyne-energized tuftsin-based fusion proteins down-regulating CD47 expression in A431 cells
CD47, known as a “don’t eat me” signal for phagocytic cells, is expressed on the surface of all human solid tumor cells. In comparison with adjacent normal (non-tumor) tissue, CD47 is overexpressed on cancer cells [21]. Previous studies imply that CD47 overexpression might lead tumor cells more resistant to phagocytosis. Therefore, it is important to determine whether tuftsin-based proteins could modulate CD47 expression on tumor cells, rendering the cell more susceptible to phagocytosis. In the present study, the expression levels of CD47 on different carcinoma cell lines were analyzed by Western blot. Among the tested cell lines, epidermoid carcinoma A431 cells showed the highest level of CD47 expression (Supplementary Fig. 5a). Notably, the level of CD47 protein was markedly reduced in A431 cells treated with Ec-LDM-TF (P < 0.01, Fig. 6b, c). Moreover, the levels of CD47 protein were significantly reduced in A431 cells, which were treated with the combination of Ec-LDP-TF with Ec-LDM-TF (Fig. 6d, e). It is evident that Ec-LDM-TF can down-regulate the level of CD47 expression.
Fig. 6.
CD47 modulation by tuftsin-based fusion proteins and corresponding enediyne-energized fusion proteins. a A431 cells treated with tested samples (100 μg), including LDP, LDP-TF, and Ec-LDP-TF, respectively. b A431 cells treated with 1 nM LDM, LDM-TF, and Ec-LDM-TF by RT-PCR (upper panel) and Western blot (lower panel). c Mean ± SEM density of Western blot. The experiments repeated at least three times. *P < 0.05, **P < 0.01. d, 1 nM LDM, LDM-TF, Ec-LDM-TF, and Ec-LDM-TF (1 nM) + Ec-LDP-TF (5 μM), respectively. e Mean ± SEM. *P < 0.05, **P < 0.01
Discussion
Because of its extremely potent cytotoxicity to cancer cells, LDM has been considered an effector molecule (“warhead” agent) for the construction of antibody-based, molecule-targeted drugs [17]. In previous studies, a series of antibody-based fusion proteins with enhanced antitumor efficacy have been prepared and evaluated, such as EGFR/HER2-specific fusion protein Ec-LDP-Hr-AE [19], anti-gelatinase diabody format dFv-LDP-AE [18], anti-EGFR fusion protein Fv-LDP-AE [22], and the endostatin-based fusion protein Es-LDP-AE [23]. It is of interest to construct novel fusion proteins using LDP as scaffold and comprising active “effector” agents. As well known, tuftsin, a tetrapeptide, promotes phagocytic activity of those cells of monocytic origin, such as macrophages and microglia, and also exerts other stimulatory effects, including enhanced cell migration/chemotaxis and antigen presentation. Tuftsin can affect T-cell function as well [24, 25]. However, the degradation of this tetrapeptide in serum, especially caused by leucine aminopeptidase, greatly limits its clinical use [26]. Therefore, it is crucial to develop target-oriented, highly active and metabolically stable analogs of tuftsin.
During the past decade, biodegradable polymers or oligopeptides that recognize particular cell-surface receptors have been shown to increase drug specificity with lowering systemic drug toxicity [19]. Thus, for construction of tuftsin-based fusion protein, we used a ligand-based oligopeptide (Ec) for the receptor EGFR binding, an apoprotein LDP as protein scaffold and specific carrier for assembled with the AE, and the tetrapeptide tuftsin for immunostimulating effects. This genetically engineered EGFR-targeting fusion protein Ec-LDP-TF not only efficiently bound to carcinoma A431 cells, but also mouse macrophage J774A.1 cells (Fig. 2). Evidently, Ec-LDP-TF showed higher antitumor efficacy than that of LDP-TF in epidermoid carcinoma A431 model; the inhibition rates by LDP-TF and Ec-LDP-TF at equimolar/kg dosage level on A431 tumor growth were 76.3 and 84.2 %, respectively (Supplementary Table 2). By checking several factors that may activate macrophages, TNF-α and IFN-γ expression levels have shown remarkable up-regulation (Fig. 4). As reported, TNF-α is able to induce apoptotic cell death, inflammation and to inhibit tumorigenesis [27]; and IFN-γ is a cytokine being critical for innate and adaptive immunity against viral and intracellular bacterial infections and for tumor control [28]. In particular IFN-γ is an important activator of macrophages. In addition, Supplementary Table 2 showed that weight loss resulting from the Ec-LDP-TF treatment at the termination of the experiment at different doses did not exceed 10 % of the pre-treatment weight. Thus, we suggested the anticancer potential of Ec-LDP-TF against human epidermoid carcinoma xenograft on the basis that Ec-LDP-TF displays cytotoxic property and immunomodulatory property. Ec-LDP-TF more effectively bound to tumor cells compared with LDP-TF; furthermore, it markedly suppressed the growth of human epidermoid carcinoma A431 xenograft with up-regulated expression of TNF-α and IFN-γ. Thus, we propose that the Ec oligopeptide of Ec-LDP-TF might be relevant to the higher efficacy that Ec-LDP-TF suppresses tumor growth. Our results confirm that Ec-LDP-TF is a promising therapeutic agent for various forms of cancers. Moreover, the enediyne-energized LDM-TF and Ec-LDM-TF could further improve the antitumor efficiency as compared with the corresponding non-energized fusion proteins and the dosage used was about 20-fold less. Treatment with combination therapy resulted in the better therapeutic effect compared with Ec-LDM-TF (Fig. 5d). However, treatment with combination therapy resulted in transient body weight loss in treated animals. Thus, the dosages of combination therapy were might be not well tolerated.
It is interesting to determine whether the expression level of CD47 in cancer cells is affected by the tuftsin-based fusion proteins. Notably, as the results shown, the survival rate of CD47-overexpressed A431 cells was lower than that of CD47-low expressed MCF-7 cells when treated with tuftsin-based proteins. The results of Western blot revealed that the CD47 in A431 cells was markedly down-regulated by enediyne-energized fusion protein Ec-LDM-TF (Fig. 6b and Supplementary Fig. 5b). Coincidently, the therapeutic experiment in A431 xenograft model showed that Ec-LDM-TF exerted high efficacy against tumor growth. The results of Western blot revealed that the CD47 in A431 cells was markedly down-regulated by the fusion proteins in combination therapy (Fig. 6d). Coincidently, treatment with combination therapy resulted in the better therapeutic effect compared with Ec-LDM-TF (Fig. 5d). These results suggest that the antitumor efficacy of tuftsin-based fusion proteins against the epidermoid carcinoma A431 might relate to, at least in part, the down-regulation of CD47. As reported, a series of human solid tumors require CD47 expression to avoid phagocytosis by innate immune surveillance and elimination [21]. Thus, we propose that CD47 might be relevant in the mechanism of action that tuftsin-based fusion proteins suppress tumor progression.
In summary, we have generated a novel engineered fusion protein Ec-LDP-TF that comprises oligopeptide Ec, apoprotein LDP, and the tetrapeptide tuftsin; in which, Ec is an EGFR-targeting peptide. LDP serves as protein scaffold and specific carrier for the highly potent cytotoxic enediyne AE. By molecular reconstitution, AE can be integrated into Ec-LDP-TF to prepare the enediyne-energized fusion protein Ec-LDM-TF. Notably, the tuftsin-based fusion protein Ec-LDP-TF markedly inhibited the growth of human epidermoid cancer A431xenograft; furthermore, the enediyne-energized fusion protein Ec-LDM-TF showed even higher therapeutic efficacy. The mechanism of action may relate to, at least in part, the down-regulation of CD47 in cancer cells. Study results indicate that tuftsin-based fusion proteins are potentially useful for treatment of EGFR- and CD47-overexpressing cancers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors acknowledge the National High Technology Research and Development Program (No. 2012AA02A301) and “Significant New Drug Development” Major Science and Technology Development Projects of China (No. 2013ZX09102064).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- AE
Active enediyne from lidamycin
- BSA
Bovine serum albumin
- CSCs
Cancer stem cells
- DSB
DNA double-strand breaks
- Ec
The C-loop of epidermal growth factor (22 amino acids of EGF COOH terminal)
- Ec-LDM-TF
The enediyne-energized fusion protein composed of Ec, LDP, TF and AE
- Ec-LDP-TF
The fusion protein composed of Ec, LDP and TF
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- FITC
Fluorescein isothiocyanate
- HER2
Human epidermal growth factor receptor-2
- HPLC
High-performance liquid chromatography
- HRP
Horseradish peroxidase
- IFN
Interferon
- IgG
Immunoglobulin
- LDM
Lidamycin (composed of LDP and AE)
- LDM-TF
The enediyne-energized fusion protein composed of LDP, TF and AE
- LDP
Lidamycin apoprotein (110 amino acids)
- LDP-TF
The fusion protein composed of LDP and TF
- Nrp1
Receptor neuropilin-1
- TF
Tuftsin (4 amino acids)
- TGF-β
Transforming growth factor beta
- TGI
Tumor growth inhibition
- TNF
Tumor necrosis factor
References
- 1.Fridkin M, Najjar VA. Tuftsin: its chemistry, biology, and clinical potential. Crit Rev Biochem Mol Biol. 1989;24:1–40. doi: 10.3109/10409238909082550. [DOI] [PubMed] [Google Scholar]
- 2.Nishioka K. Anti-tumour effect of the physiological tetrapeptide, tuftsin. Br J Cancer. 1979;39:342–345. doi: 10.1038/bjc.1979.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S, Amiji M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules. 2012;13:1074–1085. doi: 10.1021/bm2017993. [DOI] [PubMed] [Google Scholar]
- 4.Nissen JC, Selwood DL, Tsirka SE. Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway. J Neurochem. 2013;127:394–402. doi: 10.1111/jnc.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A, Khan AA, Dwivedi V, Ahmad MG, Hakeem S, Owais M. Tuftsin augments antitumor efficacy of liposomized etoposide against fibrosarcoma in Swiss albino mice. Mol Med. 2007;13:266–276. doi: 10.2119/2007-00018.Khan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishioka K, Babcock GF, Phillips JH, Banks RA, Amoscato AA. In vivo and in vitro antitumor activities of tuftsin. Ann N Y Acad Sci. 1983;419:234–241. doi: 10.1111/j.1749-6632.1983.tb37109.x. [DOI] [PubMed] [Google Scholar]
- 7.Yuan W, Xia G, Zhao C, Sui C, Ma J. Anti-idiotypic single chain mimicking CA125 linked with tuftsin provides protective immunity against ovarian cancer in mice. Mol Med Rep. 2012;5:388–394. doi: 10.3892/mmr.2011.643. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prud’Homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao RG, Zhen YS. Relationship between the molecular composition of C1027, a new macromolecular antibiotic with enediyne chromophore, and its antitumor activity. Yao Xue Xue Bao. 1995;30:336–342. [PubMed] [Google Scholar]
- 11.Tanaka T, Fukuda-Ishisaka S, Hirama M, Otani T. Solution structures of C-1027 apoprotein and its complex with the aromatized chromophore. J Mol Biol. 2001;309:267–283. doi: 10.1006/jmbi.2001.4621. [DOI] [PubMed] [Google Scholar]
- 12.Cai L, Chen H, Miao Q, Wu S, Shang Y, Zhen Y. Binding capability of the enediyne-associated apoprotein to human tumors and constitution of a ligand oligopeptide-integrated protein. J Biotechnol. 2009;144:142–150. doi: 10.1016/j.jbiotec.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy DR, Gawron LS, Ju J, Liu W, Shen B, Beerman TA. Single chemical modifications of the C-1027 enediyne core, a radiomimetic antitumor drug, affect both drug potency and the role of ataxia-telangiectasia mutated in cellular responses to DNA double-strand breaks. Cancer Res. 2007;67:773–781. doi: 10.1158/0008-5472.CAN-06-2893. [DOI] [PubMed] [Google Scholar]
- 14.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, Dobashi K, Greene MI. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-X. [DOI] [PubMed] [Google Scholar]
- 16.Rathore D, Nayak SK, Batra JK. Expression of ribonucleolytic toxin restrictocin in Escherichia coli: purification and characterization. FEBS Lett. 1996;392:259–262. doi: 10.1016/0014-5793(96)00825-3. [DOI] [PubMed] [Google Scholar]
- 17.Miao QF, Liu XY, Shang BY, Ouyang ZG, Zhen YS. An enediyne-energized single-domain antibody-containing fusion protein shows potent antitumor activity. Anticancer Drugs. 2007;18:127–137. doi: 10.1097/CAD.0b013e3280112779. [DOI] [PubMed] [Google Scholar]
- 18.Zhong G, Zhang S, Li Y, Liu X, Gao R, Miao Q, Zhen Y. A tandem scFv-based fusion protein and its enediyne-energized analogue show intensified therapeutic efficacy against lung carcinoma xenograft in athymic mice. Cancer Lett. 2010;295:124–133. doi: 10.1016/j.canlet.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Guo XF, Zhu XF, Shang Y, Zhang SH, Zhen YS. A bispecific enediyne-energized fusion protein containing ligand-based and antibody-based oligopeptides against epidermal growth factor receptor and human epidermal growth factor receptor 2 shows potent antitumor activity. Clin Cancer Res. 2010;16:2085–2094. doi: 10.1158/1078-0432.CCR-09-2699. [DOI] [PubMed] [Google Scholar]
- 20.Roh YJ, Park YG, Kang S, Kim SY, Moon JI. Effects of AFP-172 on COX-2-induced angiogenic activities on human umbilical vein endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2012;250:1765–1775. doi: 10.1007/s00417-012-2125-2. [DOI] [PubMed] [Google Scholar]
- 21.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng W, Shang Y, Miao Q, Li Y, Zhen Y. Antitumor efficacy of the scFv-based fusion protein and its enediyne-energized analogue directed against epidermal growth factor receptor. Anticancer Drugs. 2012;23:406–416. doi: 10.1097/CAD.0b013e32834f9801. [DOI] [PubMed] [Google Scholar]
- 23.Jiang WG, Lu XA, Shang BY, Fu Y, Zhang SH, Zhou D, Li L, Li Y, Luo Y, Zhen YS. Genetically engineered endostatin-lidamycin fusion proteins effectively inhibit tumor growth and metastasis. BMC Cancer. 2013;13:479. doi: 10.1186/1471-2407-13-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Guo J, Han S, Yao L, Chen A, Yang Q, Bo H, Xu P, Yin J, Zhang Z. Enhanced immune response induced by a potential influenza A vaccine based on branched M2e polypeptides linked to tuftsin. Vaccine. 2012;30:6527–6533. doi: 10.1016/j.vaccine.2012.08.054. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Nissen JC, Chen EI, Tsirka SE. Tuftsin promotes an anti-inflammatory switch and attenuates symptoms in experimental autoimmune encephalomyelitis. PLoS ONE. 2012;7:e34933. doi: 10.1371/journal.pone.0034933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siemion IZ, Kluczyk A. Tuftsin: on the 30-year anniversary of Victor Najjar’s discovery. Peptides. 1999;20:645–674. doi: 10.1016/S0196-9781(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 27.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.