Abstract
Background
The bone marrow (BM) of breast cancer patients harbors tumor-reactive memory T cells (TCs) with therapeutic potential. We recently described the immunologic effects of adoptive transfer of ex vivo restimulated tumor-reactive memory TCs from the BM of 12 metastasized breast cancer patients in a clinical phase-I study. In this trial, adoptive T cell transfer resulted in the occurrence of circulating tumor antigen-reactive type-1 TCs. We here describe the long-term clinical outcome and its correlation with tumor-specific cellular immune response in 16 metastasized breast cancer patients, including 12 included in the original study.
Methods
Sixteen metastatic breast cancer patients with preexisting tumor-reactive BM memory TCs were included into the study. The study protocol involved one transfusion of TCs which were reactivated in vitro with autologous dendritic cells pulsed with lysates of MCF-7 breast cancer cells as source of tumor antigens. The presence of tumor-reactive memory TCs was analyzed by IFN-γ ELISpot assays.
Results
Tumor-reactive memory TCs in the peripheral blood were induced de novo in 7/16 patients (44 %) after adoptive TC transfer. These patients were considered immunologic responders to the therapy. Positive adoptive immunotherapy (ADI) response was observed significantly more often in patients without bone metastases (p = 0.0051), in patients with high levels of tumor-reactive BM TCs prior to therapy (p = 0.036) and correlated significantly with the estimated numbers of transferred tumor-reactive TCs (p = 0.0021). After the treatment, we observed an overall median survival of 33.8 months in the total cohort with three patients alive at last follow-up and more than 7 years after ADI. Numbers of transferred tumor-reactive TCs correlated significantly with the overall survival of patients (p = 0.017). Patients with an immunologic response to ADI in the peripheral blood had a significantly longer median survival than nonresponders (median survival 58.6 vs. 13.6 months; p = 0.009).
Conclusion
In metastasized breast cancer patients, adoptive transfer of BM TCs can induce the presence of tumor antigen-reactive type-1 TCs in the peripheral blood. Patients with immunologic response after ADI show a significantly longer overall survival. Patients with bone metastases significantly less frequently respond to the treatment and, therefore, might not be optimal candidates for ADI. Although the present study does not yet prove the therapeutic effect of ADI, these findings shed light on the relation between immune response and cancer prognosis and suggest that transfer of reactivated BM TCs might bear therapeutic potential.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1414-x) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Bone marrow, T cell immunity, Adoptive T cell therapy
Introduction
Breast cancer as a systemic disease with a primarily loco-regional component is often recurrent due to an early dissemination of tumor cells. Although only 5 % of breast cancer patients show clinically detectable metastases at first diagnosis, up to 40 % of those patients have occult metastases at that time [1, 2]. A predominant organ for dissemination and maintenance of breast cancer tumor cells is the bone marrow (BM) [3]. Malignant tumors, however, can be recognized by the host’s immune system revealing an innate, specific recognition of breast cancer antigens. In this context, a distinct immunologic relevance of the BM in breast cancer was proposed after the detection of tumor antigen-reactive cytotoxic and type-1 memory T cell responses in this organ [4–10]. Upon restimulation, memory T cells can exert a particularly high proliferative potential [11–13], sustain the generation of secondary effector T cells [13, 14] and induce long-lasting tumor regressions in mouse models [15, 16]. While tumor-specific memory T cells are found in the BM of a majority of breast cancer patients, the detection of cellular immune responses in the peripheral blood, however, is rare [4, 5, 10]. Hypothetically, insufficient reactivation of BM T cells may contribute to their absence in the blood since it was shown that T cell reactivation in lymphoid organs precedes profound changes in expressed homing receptors, which is followed by their emigration into the blood and subsequent infiltration into inflamed tissues [17]. Therefore, specific reactivation of BM-resident T cells from breast cancer patients in vitro might result in their acquisition of protective capacity in vivo. Indeed, bone marrow-derived memory T cells can be reactivated to interferon (IFN)-γ producing, cytotoxic effector cells and reject autologous, xenotransplanted tumors in NOD/SCID mice after specific restimulation with autologous dendritic cells (DC) [4, 5].
For this reason, we recently performed a clinical pilot trial in advanced metastasized breast cancer patients to evaluate the feasibility of an adoptive T cell transfer approach based on the aspiration, ex vivo reactivation and subsequent intravenous application of tumor-reactive CD4 and CD8 BM T cells and to investigate whether such approach increases the numbers of functional tumor antigen-reactive T cells in the peripheral blood [18]. Into this previous study, 12 patients with preexisting tumor-reactive memory T cells in the BM were included and subsequently treated by a single transfer of their own BM-derived T cells that were reactivated against breast cancer-associated antigens derived from the breast cancer cell line MCF-7. That adoptive immunotherapy (ADI) approach turned out feasible as well as tolerable and showed immunologic efficacy in terms of increased frequencies of activated type-1 tumor-reactive T cells in the peripheral blood of a distinct proportion of breast cancer patients [18].
In this long-term follow-up analysis of sixteen treated patients, twelve of whom were included in our previous report [18], we observed a median overall survival of 33 months. Post-therapeutically, tumor-reactive memory T cells in the peripheral blood were induced in 7 patients (44 %). These patients were considered immunologic responders to the therapy and showed a significantly prolonged overall survival compared to nonresponding patients. Although this does not yet prove the therapeutic effect of adoptive T cell transfer, these findings shed light on the relation between immune response and cancer prognosis and suggest that transfer of reactivated BM TCs might bear therapeutic potential.
Materials and methods
Patients
Blood and BM samples were taken from patients with histologically approved metastasized breast carcinomas as described earlier [18]. The study protocol was approved (# L285/2004) by the Ethical Committee of the University of Heidelberg (Heidelberg, Germany). Protocol-specified primary endpoints were safety and toxicity with efficacy as secondary endpoint. Heparinized BM and blood samples were processed as described [18]. Patients received no concomitant treatment except for bisphosphonates in the case of bone metastases.
Generation of dendritic cells
Generation of dendritic cells (DC) for diagnostic and therapeutic purposes was performed as described earlier [18]. B cells and T cells were removed by magnetic beads labeled with mAbs against CD19 and CD3 (Dynal, Oslo, Norway). DC were pulsed with cell lysates (200 μg protein/1 × 106 cells/ml) for 20 h.
T cell culture
Mononuclear cells were cultured as described [18]. T cells from therapeutic BM aspirates were exclusively used for adoptive transfer and sterility tests. T cells were purified using magnetic beads labeled with mAbs against CD19, CD15 and CD56 (Dynal, Oslo, Norway) and kept in cytokine-free RPMI-1640 with 10 % AB serum before stimulation [18].
Antigens
Lysates of the breast cancer cell line MCF-7 [19] as source of tumor antigens were generated as described [4]. Cell fragments were removed by 30-min centrifugation at 13,000 rpm. The supernatant was tested for protein content and stored at −80 °C. Lysates of the promonocytic tumor cell line U937 as source of control antigen were generated likewise.
ELISpot assays
Interferon-γ-producing T cells were determined by IFN-γ ELISpot test kits (Mabtech, Hamburg, Germany) and automatically quantified by ELISpot reader as described [4]. Antigen-pulsed DC were coincubated with autologous T cells (DC/T cell ratio 1:5) for 40 h in triplicate wells. The number of IFN-γ-spot-forming cells was analyzed using a microscope Axioplan 2 and KS ELISpot software (Carl Zeiss Vision, Hallbergmoos, Germany). Spots measured in the presence of DCs pulsed with negative control antigens were considered as nonspecific background. Individuals were designated as responders if the average number of spots in the presence of DCs loaded with TA was significantly higher (p ≤ 0.05; Student’s t-test) than that in negative control wells. Frequencies of tumor-reactive T cells were calculated only in case of significant tumor antigen reactivity as follows: (spots in test wells—spots in control wells)/T cell numbers per well.
T cell stimulation and adoptive cell transfer regimen
Purified T cells were coincubated with autologous MCF-7 lysate-pulsed DCs (DC/TC ratio between 1:5 and 1:13) at 1 × 106 TCs/ml medium (RPMI + 10 % AB serum) for 72 h. Half of the medium was substituted after 48 h. After 72 h, all cells in suspensions were washed, passed through a single cell filter, suspended in 100 ml 0.9 % NaCl solution and finally transfused intravenously over 15 min. For microbial testing, aliquots were collected before (Test 1) and after stimulation (Test 2). When microbial contamination was undetectable in Test 1 after 3 days of blood culture by the institution’s microbiology department, adoptive transfer was performed. Test 2 was performed as an additional safety measure and to adapt the antibiotic scheme if necessary. In none of those samples, however, microbiological contamination was detected. Antibiotic prophylaxis with i.v. administered ciprofloxacin (3 × 400 mg Ciprobay®; Bayer, Leverkusen, Germany) and fluconazole (2 × 400 mg Diflucan® i.v.; Pfizer, Karlsruhe, Germany) was given to all patients for one day. Patients received treatment at the intensive care unit under 24-h observation.
Microbial tests
Microbial tests were performed as described [18]. For 5 days, cell suspensions were subjected to BACTEC Plus/F-hemoculture flasks (Becton–Dickinson Biosciences, Heidelberg, Germany) supplemented with BACTEC-Fos-Supplement (BD Biosciences). After 3 and 5 days, microbial contamination was assessed by the institution’s microbiology department.
Toxicity assessment
Toxicity assessment was performed as described earlier [18]. In short, blood parameters were assessed before and 24 h after the treatment. Clinical examinations were performed over a period of 24 h at the intensive care unit (skin inspection, neurological observation, measurement of blood pressure, pulse, body temperature, ECG). All patients were asked to report on any adverse symptoms during the whole follow-up period.
Statistical evaluation
Statistical significance of differential findings between test and control groups was determined by two-sided, unpaired Student’s t-test (Fig. 1a, b) or two-tailed Mann–Whitney test (Fig. 2a, b). The significances of Kaplan–Meier curves (Fig. 3a, b) were calculated by log-rank (Mantel-Cox) test. Differences were regarded as significant (*) if p values were <0.05.
Fig. 1.
Detection of tumor-specific T cells (TCs) in metastasized breast cancer patients. a, b Representative results from IFN-γ EliSpot assays with BMTCs from two different individual patients demonstrate the presence (a) or absence (b) of tumor antigen-specific T cells tested for the recognition of MCF-7 breast cancer cell line lysates (MCF7-L, black bars) as compared to negative control antigens (gray bars) from U937 promonocytic tumor cell line lysates (U937-L). White bars: spot numbers in wells containing unstimulated TCs or DCs alone. Bars show mean ± SEM of triplicate wells. *Significant (p < 0.05) difference to control group by two-sided Student’s t-test; n.s. not significant. c Scheme of ADI treatment preparation; PB peripheral blood; BM bone marrow; DC dendritic cell
Fig. 2.
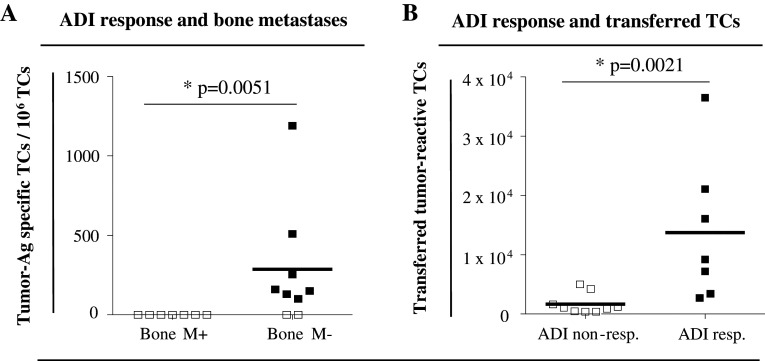
a Correlation of immune response with preexisting bone metastases. Post-therapeutic frequencies of PB memory TCs in bone-metastasized (Bone M+) and nonmetastasized (Bone M−) patients. b Estimated numbers of tumor-specific TCs transferred into patients (specific frequencies of tumor-reactive TCs in BM before treatment multiplied by numbers of transferred TCs). Left immunologic nonresponders (white squares), right immunologic responders (black squares). a, b *Significant (p < 0.05) difference between groups as analyzed by two-tailed Mann–Whitney test
Fig. 3.
Kaplan–Meier survival analysis relating to transferred memory TCs (TMCs) (a) and immunologic response after ADI (b). a Patients are grouped according to high (High TMC transfer) or low (Low TMC transfer) numbers of transferred tumor-reactive TCs. For distinction between high and low numbers, the cutoff was the median value of transferred TCs in the cohort (n = 16). b Patients are grouped according to immunologic response in the peripheral blood after adoptive TC transfer (ADI responder vs. ADI nonresponder). a, b *Significant (p < 0.05) difference between groups as analyzed by log-rank (Mantel-Cox) test
Results
Study design and therapeutic regimen
Into the present follow-up study, we included 16 metastatic breast cancer patients (mean age 53.4 years, range 43–73 years) with preexisting tumor-reactive memory TCs in their BM. All patients had a history of standard cytostatic treatment and/or hormone therapy (supplementary table) and were in a palliative treatment situation. Patients with secondary malignancies, autoimmune diseases, renal failure or pregnancy were excluded. In addition to our previous study (n = 12), 4 further patients have been treated meanwhile. One patient (#17) withdrew consent to participation after initial inclusion and was therefore excluded from data analysis.
Patients were tested for preexisting tumor-reactive memory TCs in their BM by short-term IFN-γ ELISpot assays before inclusion into the study. In Fig. 1a, b, representative IFN-γ ELISpot data are shown. We used autologous DCs pulsed with breast cancer tumor antigens derived from the allogeneic breast cancer cell line MCF7 for restimulation of ex vivo isolated BM TCs during 40 h ELISpot assays [4, 9]. DCs pulsed with lysates of the promonocytic leukemia cell line U937 were employed as negative controls. Figure 1a depicts a representative patient with antigen-specific TCs who was suitable for inclusion into the study. Figure 1b shows a representative patient, who was not suitable for study inclusion, as no specific antigen response was detectable in the ELISpot analysis. Figure 1c depicts the therapeutic regimen of the present study as described in the “Materials and methods” section. Patients were released into ambulatory surveillance 24 h after T cell transfer. None of the patients showed any relevant clinical or laboratory signs of treatment-related toxicity.
Immunologic effects and overall survival
All 16 included metastatic breast cancer patients showed tumor-reactive BM memory TCs at the beginning of the study (pre-ADI; supplementary figure), while in none of these patients, tumor-reactive TCs were detected in the peripheral blood at that time. After adoptive TC transfer (ADI), however, 7 out of 16 patients (44 %) showed tumor-reactive TCs in the peripheral blood according to IFN-γ ELISpot analysis (supplementary figure). Patients with tumor-reactive TCs in the peripheral blood after ADI were regarded as immunologic responders. One month after the therapy, 67 % of tested patients (8/12) showed tumor-specific TCs in the BM, without a significant correlation with ADI response in the peripheral blood. Three months after therapy in all tested patients (8/8), tumor-specific TCs were detectable in the BM (supplementary figure).
We analyzed clinical and experimental parameters (supplementary table) for potential correlation with an immunologic response after ADI. In this context, neither the patients’ age nor the disease status before treatment had a considerable impact on the occurrence or frequencies of circulating tumor-reactive TC. In accordance with our previous results [18], we found reduced immune responsiveness in patients with overt bone metastases. In none of the 7 patients with bone metastases, circulating tumor-reactive memory TCs were detected after ADI, whereas 7 out of 9 patients (77.8 %) without bone metastases were considered immunologic responders (p = 0.0051; Fig. 2a, supplementary table). Correlating the frequencies of tumor-reactive TCs in the BM at the beginning of the study (pre-ADI) with immunologic responses to ADI, we observed significantly higher levels of tumor-reactive BM TCs in patients with subsequently positive ADI response compared to nonresponding patients (p = 0.036; data not shown). Finally, we correlated the estimated numbers of transferred tumor-specific TCs with the immunologic response after ADI. As depicted in Fig. 2b, patients showing an immunologic response after ADI had received a significantly higher number of tumor-reactive TCs compared to nonresponders, which is also in accordance with our previous study (p = 0.0021).
Analyzing the overall survival of the 16 advanced metastatic breast cancer patients treated, we observed a median survival of 33.8 months within the total cohort. Three out of 16 (18.8 %) patients were still alive at the time of latest follow-up, currently showing a mean survival of 96.2 months after adoptive TC transfer. Those 3 patients all presented with stable disease at the beginning of the study after a history of multiple cytostatic and/or endocrine therapies. Nevertheless, we could not determine a correlation between disease state at the beginning of the study and overall survival within the total cohort (n.s.; data not shown).
Subsequently, we correlated the numbers of tumor-reactive TCs in the BM at the beginning of the study (supplementary figure; pre-ADI) with the overall survival of patients after adoptive TC transfer. However, we did not detect a significant impact of this parameter (n.s.; data not shown). Considering next the numbers of transferred specific memory TCs, we observed a significantly prolonged survival (median survival 47.4 months) after adoptive TC transfer in patients with high numbers of TCs transferred during ADI (p = 0.017; Fig. 3a) compared to patients receiving low numbers of tumor-reactive memory TCs (median survival 14.6 months).
Finally, we correlated the induction of tumor-reactive memory TCs in the peripheral blood after ADI with the overall survival. Strikingly, patients with immunologic response after adoptive TC transfer presented with a significantly higher overall survival (median survival 58.6 months) compared to patients without the induction of tumor-reactive TCs in the peripheral blood after ADI (median survival 13.6 months; p = 0.009; Fig. 3b).
Discussion
In the present study, we observed a median overall survival of 33.8 months in the total cohort of patients with significantly longer overall survival in patients with high numbers of transferred tumor-reactive TCs and subsequent therapeutic response in the form of induction of tumor-reactive TCs in the peripheral blood. Overall survival did not correlate with the frequency of TA-specific TCs in the BM at the beginning of the study. This supports the hypothesis of a therapeutic effect of an adoptive TC transfer. Regarding conventional therapies prior or subsequent to our T cell treatment, we did not observe significant differences in this cohort of 16 patients in respect to overall survival.
Given its objectivity and the benefit derived by patients, overall survival (OS) has been historically considered the most important therapeutic objective in advanced breast cancer [20]. As the present trial was designed as a single-arm study, a conclusion regarding the absolute benefit in overall survival cannot be drawn within the total cohort. Generally, however, a recent meta-analysis of 76 phase III trials, including a total of 29,442 patients, has shown a median overall survival of 20.5 months in metastatic breast cancer patients [21]. In our present study, we observed a median overall survival of 13.6 months in patients not responding to the therapeutic regimen and of 58.6 months in responding patients. This is remarkable considering the fact that all of our study patients were multiply pretreated by chemo- and/or endocrine therapies. Although a comparison with the above meta-analysis might not be valid methodologically, our data hint, however, at a relation between immune response and cancer prognosis and suggest that preferably patients with immunologic response to adoptive TC transfer might benefit from this treatment option.
As tumor antigen-reactive TC responses were not inducible in all of the treated patients, the question arises whether or not tumor-immune escape mechanisms might be responsible for poorer prognosis. In none of the patients with bone metastasis, tumor-reactive TCs were detectable in the blood after adoptive TC transfer. An osseous metastatic process enhances the release of activated growth factors from the bone during the bone resorption process and the production of growth factors and cytokines by BM stromal cells [22]. A central cytokine that is released in active form from the bone in osseous metastatic breast cancer is TGF-β. Breast carcinoma cells produce parathyroid hormone-related peptide (PTHrP), which induces osteoclastic bone resorption and releases TGF-β from the bone matrix [23]. TGF-β as an immunosuppressive cytokine inhibits DC maturation, antigen presentation, and the production of activation-induced mediators of inflammation [9, 24]. Besides indirect influences on TC induction as mediated by DC, TGF-β can trigger the generation of immunosuppressive regulatory TCs (Treg) in situ [25] that might inhibit the priming or reactivation of tumor-specific TCs [9].
A critical aspect of our therapeutic regimen was the overall low number of tumor-reactive T cells obtained by BM aspiration. This has to be of special interest as numbers of transferred tumor-reactive TCs correlated with both immune response and overall survival. Besides the major technical restrictions of BM aspiration, low TC numbers retrieved can most likely be attributed to the late-stage situation of the patients after multiple palliative cytostatic treatments. One amendment for future trials might therefore be a series of TC transfers in the very same patient, which appears possible as the presence of TA-reactive BM TCs seems to be unaffected by adoptive TC transfer. Furthermore, insufficient numbers of tumor-reactive T cells were particularly obtained from patients with bone metastases [18] and may be caused by increased frequencies of Treg in the BM of these patients, as Treg function as effective inhibitors of activation and subsequent clonal expansion of TCs [26, 27]. This might be due to the influence of TGF-β in osseous metastatic lesions as discussed above. Altogether, these data suggest a dominant activation of Treg in some patients and call for a redesign of future adoptive TC transfer studies with sufficient depletion of regulatory TCs prior to or during therapy. Here, one promising approach might be a therapeutic combination with immunomodulating agents, such as metronomic cyclophosphamide, which transiently reduces Treg in advanced breast cancer patients [28, 29].
Other current studies focus on adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) [30–32]. In patients receiving TIL therapy, objective responses have correlated well with the persistence of these cells and increased migration to the sites of disease. Therefore, in further studies with adoptive transfer of tumor-reactive BM-derived TCs, it would be of interest to analyze metastatic breast cancer tissue for actual TC infiltration and monitor trafficking of transferred TCs by radiologic means as, for example, by positron emission tomography.
Conclusions
From the present clinical pilot trial, we conclude that adoptive transfer of BM TCs can induce the presence of tumor antigen-reactive type-1 TCs in the peripheral blood of late-stage metastasized breast cancer patients. An immunologic response appears to depend significantly on numbers of transferred specific memory TCs and on the absence of BM metastases. Patients with high numbers of transferred memory TCs and subsequent immunologic response to ADI show a prolonged overall survival. Although this does not prove the therapeutic effect of ADI, these findings shed light on the relation between the induced immune response and cancer prognosis and suggest a clinical benefit for at least patients with immunologic response to ADI. Clearly, these preliminary data have to be analyzed further by upcoming controlled, randomized studies in larger cohorts of patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (SFB 938).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- ADI
Adoptive immunotherapy
- BM/BMTC
Bone marrow/bone marrow T cell
- CD
Cluster of differentiation
- DC
Dendritic cell
- ELISpot
Enzyme-linked immunosorbent spot
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- IFN
Interferon
- IL
Interleukin
- PBMC
Peripheral blood mononuclear cell
- TA
Tumor antigen
- TC
T cell
Footnotes
Philipp Beckhove and Florian Schuetz contributed equally.
References
- 1.Clare SE, Sener SF, Wilkens W, Goldschmidt R, Merkel D, Winchester DJ. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol. 1997;4:447–451. doi: 10.1007/BF02303667. [DOI] [PubMed] [Google Scholar]
- 2.Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmüller G, Schlimok G. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 3.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 4.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 5.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 2005;105:2132–2134. doi: 10.1182/blood-2004-01-0366. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz-Winnenthal FH, Volk C, Z’graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Büchler MW, Beckhove P. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 8.Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- 9.Domschke C, Schuetz F, Ge Y, Seibel T, Falk C, Brors B, Vlodavsky I, Sommerfeldt N, Sinn HP, Kühnle MC, Schneeweiss A, Scharf A, Sohn C, Schirrmacher V, Moldenhauer G, Momburg F, Beckhove P. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res. 2009;69:8420–8428. doi: 10.1158/0008-5472.CAN-09-1627. [DOI] [PubMed] [Google Scholar]
- 10.Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/S0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8 + and CD4 + effector memory T cells. J Immunol. 2005;175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8 + T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8 + T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dailey M. Expression of T lymphocyte adhesion molecules: regulation during antigen-induced T cell activation and differentiation. Crit Rev Immunol. 1998;18:153–184. doi: 10.1615/CritRevImmunol.v18.i3.10. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz F, Ehlert K, Ge Y, Schneeweiss A, Rom J, Inzkirweli N, Sohn C, Schirrmacher V, Beckhove P. Treatment of advanced metastasized breast cancer with bone marrow-derived tumour-reactive memory T cells: a pilot clinical study. Cancer Immunol Immunother. 2009;58:887–900. doi: 10.1007/s00262-008-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 20.Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006;33(suppl 2):S2–S5. doi: 10.1053/j.seminoncol.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–1962. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 22.Roodman GD. Role of stromal-derived cytokines and growth factors in bone metastasis. Cancer. 2003;97(suppl 3):733–738. doi: 10.1002/cncr.11148. [DOI] [PubMed] [Google Scholar]
- 23.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases [review] Crit Rev Eukaryot Gene Expr. 2000;10:159–178. doi: 10.1615/CritRevEukarGeneExpr.v10.i2.50. [DOI] [PubMed] [Google Scholar]
- 24.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4 + CD25 − naive T cells to CD4 + CD25 + regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nummer D, Suri-Payer E, Schmitz-Winnenthal H, Bonertz A, Galindo L, Antolovich D, Koch M, Büchler M, Weitz J, Schirrmacher V, Beckhove P. Role of tumor endothelium in CD4 + CD25 + regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst. 2007;99:1188–1199. doi: 10.1093/jnci/djm064. [DOI] [PubMed] [Google Scholar]
- 27.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25 + regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, Beckhove P, Schuetz F. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353–362. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radvanyi LG, Bernatchez C, Zhang M, Fox P, Miller P, Chacon J, Wu RC, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson V, McMannis JD, Shpall EJ, Prieto VG, Papadopoulos NE, Kim KB, Homsi J, Bedikian AY, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P (2012) Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 31.Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met O, Hölmich LR, Andersen RS, Hadrup SR, Andersen MH, Straten PT, Svane IM. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.