Abstract
Cancer, the most devastating chronic disease affecting humankind, is treated primarily by surgery, chemotherapy, and radiation therapy. Surgery and radiotherapy are mainly used for debulking the primary tumor, while chemotherapy is the most efficient anti-metastatic treatment. To control better metastatic cancer, the host immune system should be stimulated. Yet, successful specific stimulation of the immune system against tumors was seldom achieved even in antigenic tumors. Our working hypothesis is that aggressive in situ tumor ablation can release tumor antigens and danger signals, which will enhance anti-tumor T cell responses resulting in the destruction of residual malignant cells in primary tumors and distant metastases. We developed two efficient in situ ablation treatments for solid cancer, which can be used to destroy the primary tumors and stimulate anti-tumor immune responses. The first treatment, electrochemical ablation, is applied through intratumoral electrodes, which deliver unipolar-pulsed electric currents. The second treatment, diffusing alpha-emitters radiation therapy (DaRT), is based on intratumoral 224Ra-loaded wire(s) that release by recoil its daughter atoms. These short-lived alpha-emitting atoms spread in the tumor and spray it with lethal alpha particles. It was confirmed that these treatments effectively destroy various malignant animal and human primary solid tumors. As a consequence of such tumor ablation, tumor-derived antigenic material was released and provoked systemic T cell-dependent anti-tumor immunological reactions. These reactions conferred protection against a secondary tumor challenge and destroyed remaining malignant cells in the primary tumor as well as in distant metastases. Such anti-tumor immune responses could be further amplified by the immune adjuvant, CpG. Electrochemical ablation or DaRT together with chemotherapy and immunostimulatory agents can serve as treatment protocols for solid metastatic tumors and can be applied instead of or in combination with surgery.
Keywords: Pulsed electric currents, Alpha radiation, Anti-tumor immunity, Electrochemical ablation, Tumor ablation, CITIM 2013
Introduction
Tumor immunology and anti-tumor immunity started with the discovery that microbial agents can trigger immune responses, which will cause tumor regression. Cancer immunotherapy research started with general immunostimulation (microbial products), before the role of danger signals was understood. The discovery of tumor-associated antigens (TAA) (1950) boosted the hope that efficient anti-tumor vaccines will be developed, yet, in spite of the knowledge about TAA the identity of many such molecules is still obscure.
Today, we have a more profound understanding of the function and intercellular interactions of immune cells, antigen recognition, the involvement of antigen-presenting cells (APC), and cross-presentation mechanisms to trigger helper and cytotoxic T lymphocytes, danger signals for proper activation of APC, and expression of costimulatory molecules. We also understand better the role of suppressor cells such as T regulatory and myeloid-derived suppressor cells (MDSC) in anti-tumor immunity.
Thus, it is required to properly expose TAA to the cell-mediated immune response and boost the response with strong adjuvants, which facilitate the recognition of TAA, and stimulate cytokine production by APC and T helper cells. It is also imperative that the tumor-specific antigens will be processed and presented on the tumor cells; otherwise, the CTL we induce will not attack these cells [1, 2].
The attempts to recruit the immune response to cure cancer were mostly disappointing although many approaches were taken and suggested [for review see 3]. Currently, vaccination against cancer after removal of the primary tumor is not a viable option even for an antigenic tumor such as melanoma [4, 5], although some recent clinical trials with dendritic cells have promising results [6, 7].
Insufficient anti-tumor reactivity could be due to structural and functional changes both in tumor and in stroma cells. These mechanisms include the absence of costimulatory molecules, down-regulation of tumor antigens or MHC molecules as well as an up-regulation of pro-apoptotic proteins on tumor cells. The secretion of immunosuppressive factors by tumor or host cells and the presence of immunosuppressive cells like CD4 + CD25 + FoxP3 + regulatory T cells (Treg) or myeloid-derived suppressor cells (MDSC) in the tumor microenvironment were also implicated [8, 9].
The lack of efficient tumor vaccines due to either unknown TAA or weak responses prompted attempts to find alternative to the conventional approach of developing injected tumor vaccines to activate anti-tumor immunity, which will fight primary and metastatic cancer.
It is argued that in situ tumor ablation (destruction) can make the tumor its own cellular vaccine. Tumor tissue ablation may release tumor antigens and DAMPS and create an inflammatory milieu, which will attract dendritic cells (DCs). Such DCs may undergo maturation after internalizing apoptotic and necrotic cellular debris and can mediate antigen-specific cellular immunity via presentation of processed antigens to T cells.
In situ tumor ablation
The primary goal of most ablation procedures is to eradicate all viable malignant cells within a designated target volume [10]. Tumor ablation of focal malignancies can be performed by chemical agents, radiotherapy, photodynamic therapy, cryoablation, high-temperature ablation (radiofrequency, microwave, laser, and ultrasound), and electric-based ablation.
Accumulating evidence indicates that the innate and adaptive immune systems can be enforced by in situ ablative cancer treatments. Various therapeutic and ablative modalities including chemotherapy [11], radiotherapy [12], cryoablation [13], radiofrequency ablation [14], thermal ablation [15], photodynamic therapy [16], and high intensity focused ultrasound [17], may release tumor-associated antigens in the context of danger-associated molecular patterns (DAMPs) that activate the immune system and manipulate the tumor microenvironment.
Our studies focused on the activation of anti-tumor immunity following ablation of solid tumors by two new intratumoral treatment modalities developed in our laboratories. An electrochemical treatment, which utilizes unipolar-pulsed electric fields/currents and a radioactive treatment, utilizes alpha particle emitting atoms.
Electric-based cancer ablation by unipolar-pulsed low-electric fields and currents
Electric-based cancer ablation was developed for in situ ablation of solid tumors. The electrical parameters used for the treatment range from several volts per cm delivered for a long time period to very high electric fields (up to 300 kV/cm). The treatment can be delivered as a continuous treatment or pulses. These treatments are either based on electro-stimulation alone or in conjunction with chemotherapeutic drugs [for review see 18].
We have introduced a novel method to ablate solid tumors utilizing a unipolar train of pulsed low-electric fields/currents which can destroy tumors either alone or by facilitating the uptake of chemotherapeutic drugs into tumor cells [19, 20] making it an efficient electric ablation method when used in combination with chemotherapy.
The treatment was first introduced as low-electric field cancer treatment (LEFCT) and was delivered by intratumoral stainless steel electrodes, connected to an electric pulse generator. A unipolar-pulsed direct current was delivered for 12 min [21, 22] (field strength, 40 V/cm; repetition frequency, 500 Hz; and pulse width, 180 μs). The electric treatment was given also with intratumoral or intravenous administration of chemotherapy. In later experiments, we focused on the electrochemical effect of the electric current and designated the treatment, pulsed electric current tumor ablation (PECTA) (electric current of 5–150 mA, repetition frequency of 500–1000 Hz, pulse width of 200 μs, and total exposure duration of 15–120 min). Treatment by PECTA was executed by platinum-iridium needles connected to a stable current stimulator. In recent studies, we found that solid subcutaneous tumors in mice can be completely ablated by 100 Coulomb per cm3 tumor tissue [23].
Extensive studies were performed on ablation of solid metastatic tumors with pulsed low-electric fields alone (LEFCT) or with chemotherapeutic agents (LEFCT-EC). The treatment was applied against mouse metastatic tumors, such as breast carcinoma (DA3) [24, Hochman manuscript in preparation], colon carcinoma (CT-26) [25–27, Hochman et al., unpublished results], squamous cell carcinoma (SQ2) [25, 28], prostate cancer (TRAMP-C1) [19], and melanoma (B16F10) [22]. The treatment was applied to established subcutaneous solid tumors (5–15 mm in diameter) at the stage when most treated mice already had lung or abdominal metastases. Treatment was delivered without or with local or systemic injection of various chemotherapeutic drugs.
The treatment eradicated or inhibited the growth of the primary tumor lesion, reduced the progression of metastases, and prolonged the survival of the tumor-bearing animals. The effects of the electric fields with or without chemotherapy were superior to the effects of chemotherapy and of surgery with or without chemotherapy [for review, see also: 18, 29].
Stimulation of anti-tumor immune reactivity following electrochemical cancer ablation
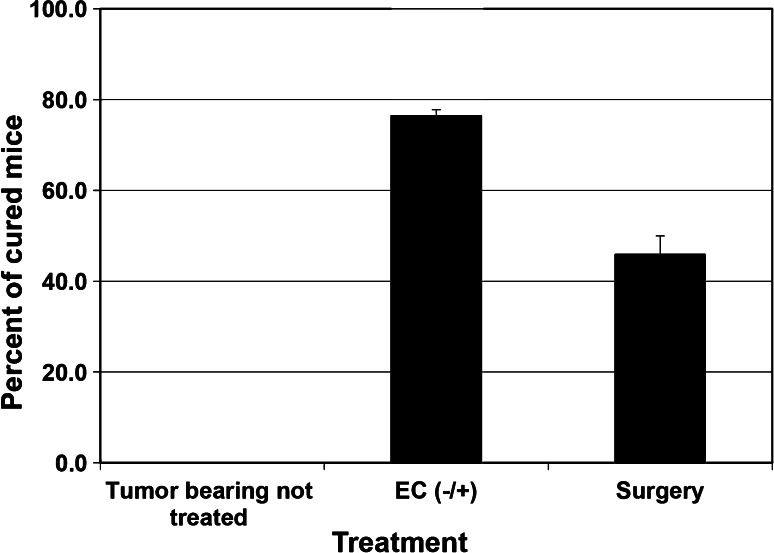
The ability of electrochemical ablation to stimulate the immune response of treated mice was first hinted by the results of an experiment in which we compared the long-term survival of breast carcinoma (DA3) tumor-bearing mice treated by either surgery or electrochemical treatment or non-treated mice (Fig. 1). We observed that all tumor-bearing non-treated mice died from metastases. Surgery cured 46 % of the animals because either they had no metastases at the time of treatment or local and systemic effects eliminated any residual metastases. Among the electrochemical-treated mice, 76 % lived after treatment. Thus, it can be postulated that the electrochemical treatment triggered local or systemic effects, probably anti-tumor immunity, which eliminated metastases in an additional 30 % of the animals.
Fig. 1.
Effect of electrochemical (EC) treatment on long-term survival of tumor-bearing mice. Mice-bearing s.c DA3 tumors of 6–9 mm in diameter were treated by either surgery or EC treatment (one anode and one cathode; EC±). Tumor-bearing non-treated mice served as control. Mice survival was followed for up to 5 months. EC treatment was statistically more effective than the surgery treatment. Each group, n = 12–22, p < 0.05
We further investigated the possibility that electrochemical ablation of four experimental tumors stimulated anti-tumor immune reactivity and compared it to animals in which the primary tumors were removed by surgery with and without chemotherapy.
The development of anti-tumor immunity was assessed by two parameters: (1) resistance to a rechallenge with a tumorigenic dose of the same tumor cell and (2) manifestation of augmented ability of immune cells from treated mice to protect naïve animals against a tumorigenic dose of tumors cells in a passive transfer assay [30]. The schedule of treatments is outlined in Table 1.
Table 1.
Timetable for ablation procedures and rechallenge or Winn assays
| Time | Day 0 | Day 14 | Day 21 | |
|---|---|---|---|---|
| Rechallenge assay | Ablation of 50–120 mm3 tumors by electrochemical or DaRT treatment | Excision of residual local tumors (only for DaRT-treated tumors) | Inoculation of 5 × 105 tumor cells/treated or control mouse | Follow-up for 5 months |
| Winn assay | Ablation of 50–120 mm3 tumors by electrochemical or DaRT treatment | Excision of residual local tumors (only for DaRT-treated tumors) | Inoculation of 5 × 105 tumor cells + 5 × 107 splenocytes from treated or control mice/normal mouse | Follow-up for 5 months |
We found that in all four tumors studied, the electrochemical treatment prolonged the survival of tumor-bearing mice, augmented the immune response in the treated mice against a tumor rechallenge, and reduced the development of metastases.
Electric treatment of mice bearing an immunogenic and highly metastatic clone of B16-F10.9 melanoma cured twice as many animals compared to surgery and chemotherapy [22]. Histological examination of treated tumors revealed massive necrosis after a transient step of apoptotic cell death and massive infiltration of T cells and macrophages [21]. Mice cured by electrochemical treatment showed enhanced resistance to a second tumor inoculation [22]. We also examined the anti-tumor activity of splenocytes from treated animals. Mice-bearing B16-F10.9 tumors were treated by electrochemical ablation and killed 3 weeks later. Their splenocytes were harvested and mixed 100:1 with tumor cells and inoculated to naïve mice. Tumor volumes measured 14 days after s.c. cell inoculation revealed an average volume of 286 ± 125 mm3 in the electrochemical-treated group compared with 847 ± 164 mm3 in the group inoculated with a mixture of tumor cells and normal splenocytes (n = 6, p < 0.023).
Splenocytes from animals treated by the electric ablation also expressed increased levels of IL-2 (2.36-fold), IL-4 (1.5-fold) and IFN-γ (1.47-fold) mRNA compared to splenocytes from non-treated tumor-bearing mice [22].
Animals with metastatic mouse mammary adenocarcinoma (DA3), which their primary tumors were ablated by the electrochemical treatment, also developed resistance to a tumor rechallenge as compared to the first-time inoculated normal mice or mice cured by surgery. The long-term anti-tumor immunological memory lasted 5 months after treatment. Tumors treated by electrochemical ablation and the immunostimulator CpG were more resistant to a tumor challenge than animals cured by electrochemical ablation only (Hochman unpublished results). Next, we checked whether immune cells from the treated mice could transfer anti-tumor resistance when mixed with tumor cells and injected to normal mice. The results indicate that splenocytes from mice cured by electrochemical ablation of the primary tumor retarded tumor growth in naïve mice (42 ± mm3) better than splenocytes from surgery-treated mice (90 ± 17 mm3) or normal mice (135 ± 14 mm3), (n = 10 per group, p < 0.03).
Furthermore, in the group, which was inoculated with electrochemical-treated splenocytes + DA3 tumor cells, 29 % of the animals did not develop a tumor at all, while only 5 % of the animals which recieved splenocytes from surgery treated mice were tumor free [24, Hochman et al. unpublished results]. We also demonstrated that the immune response, developed against the tumor cells, depends on the polarity of the electrode inserted into the tumor, where the anode was more efficient than the cathode (unpublished results).
Prostate cell line (TRAMP C-1) derived tumors that were ablated by pulsed electric field treatment became necrotic with progressive infiltration of inflammatory cells into the tumor tissue. While 40 % of the tumor-bearing mice were cured by the electrochemical treatment, the percent of cured animals dropped to zero following treatment with the immunosuppressive agent cyclosporine A. The immunosuppressed mice died from either recurrent tumors or large abdominal metastases. The results indicate a strong involvement of anti-tumoral immune responses in the eradication of residual disease following electrochemical ablation of prostate-derived tumors [19].
Extensive studies on tumor ablation by the electrochemical treatment and the stimulation of anti-tumor immunity were performed with the immunogenic CT-26 colon carcinoma metastatic tumor. Cure of the primary tumor as well as that of metastases was monitored and compared with the curative effects of surgery alone or combined with chemotherapy [25, 26].
Electrochemical ablation of 15 mm in diameter tumors achieved a cure rate of 15 % and the combination with BCNU increased the cure rate to 93 % of the animals. All the animals, which were not cured by electrochemical ablation died from recurrent tumors at the primary site with no detectable metastases in the lungs and liver. Surgery eliminated the primary tumor as efficient as the electric fields, yet many of the surgery-treated animals died from metastases without recurrence at the primary tumor site. The immunosuppressive drug, cyclosporine A, injected into mice treated by a combination of electric currents and chemotherapy decreased the cure rate from 83 to 0 %. Moreover, 66 % of the cyclosporine A-treated animals developed large metastases, including atypical abdominal metastases, which were the major cause of death [27]. These results suggest the involvement of anti-tumor immune mechanisms in the cure of animals treated by the electrochemical ablation.
Stimulation of anti-tumor immunity following electrochemical ablation was further substantiated by the finding that animals cured by the treatment were significantly more resistant to a challenge of a tumorigenic dose of CT-26 cells than mice cured by surgery. It is important to note that animals cured by a combination of electric currents and chemotherapy were more resistant to challenge than mice cured by surgery and chemotherapy, which indicates that the chemotherapy did not abolish the anti-tumor immune response [27].
Mice with CT-26 tumors cured by the electrochemical treatment were not only more resistant to a tumor challenge in the skin, but also to the development of tumor foci in the lungs. Mice previously bearing CT-26 tumor, treated by either electrochemical treatment or surgery, were injected i.v with CT-26-mCherry tumor cells. Lung images taken by CRI™ Maestro showed that the mCherry signal (expressed as CRI fluorescence units) in the lungs of electric-ablated mice was lower than that in surgery-treated mice [surgery = 1.63 ± 0.66; electrochemical = 0.07 ± 0.03 (n = 15, p < 0.03)].
In order to confirm the role of immune cells in the anti-tumor effects after electrochemical ablation, we examined the anti-tumor activity of splenocytes from treated and non-treated animals bearing CT-26 tumors. The spleens of treated animals contained a high proportion of lymphocytes, which could protect normal mice from a tumorigenic dose of CT-26 cells. Passive transfer of splenocytes from animals cured by electrochemical ablation protected 11 out of 12 mice from a tumor dose, which killed all mice injected with splenocytes from normal mice. Splenocytes from mice cured by surgery protected only 5 out of 12 mice from the same tumor cell dose. Fractionation of the splenocytes into T lymphocyte subpopulations indicated that only CD8 and CD4 lymphocytes carried the protective capacity [27].
The experiments performed with four histotypes of metastatic tumors indicate that ablation of tumors by pulsed low-electric currents in the presence or absence of chemotherapeutic agents increased tumor cell destruction. Massive cell death during treatment accompanied by realization of large amounts of tumor-associated antigens (TAA) attracts inflammatory cells that directly (via activation of phagocytes and NK cells) and indirectly (via APC-T helper mechanism) may induce effective macrophage and cytotoxic T lymphocyte-mediated immune responses and generate population of anti-tumor memory cells capable of recognizing and eliminating tumor cells at primary as well as metastatic tumor sites.
Direct evidence for the involvement of immune defense mechanisms in eradication of metastatic lesions was obtained for antigenic tumors. Cured mice showed increased resistance to a tumor challenge, resistance, which was abolished by treatment with an immunosuppressive drug. Further experiments revealed that inoculation of tumor cells simultaneously with splenocytes from tumor-cured mice attenuated tumor growth and prolonged the survival of inoculated animals, as compared with those which received tumor cells with splenocytes from normal mice. In the colon carcinoma model (CT-26), it was established that the protective immune cells belong to the T helper and T cytotoxic lineage.
The curative effect of the combination of electric fields and chemotherapy was better than electric fields alone although the chemotherapeutic drugs reduced the anti-tumor immune response. It might be postulated that the cytotoxic drug acts to directly destroy the tumor cells and those that are not destroyed become more vulnerable to attack by he immune cells.
Alpha radiation-mediated cancer ablation and stimulation of anti-tumor immune reactivity
Ablation of solid tumors by intratumoral alpha radiation
Along with surgery and chemotherapy, radiation therapy is one of the most important methods of cancer treatment, and approximately 50–70 % of cancer patients will receive radiation therapy. Radiation therapy involves photons (e.g., X-rays) or particles (e.g., protons, neutrons, alpha particles, heavy ions, and electrons). The most prevalent radiation treatment is the use of gamma or X-ray radiation (external beam radiation therapy—EBRT). It is useful for the treatment of local and regional disease sites, or where surgical excision of the tumor is not feasible due to the size and site of tumor, or patients` medical condition. The effectiveness EBRT is limited due to hypoxia in the tumor.
In order to maximize the dose administered to the target while minimizing the dose to other regions, intratumoral implantation of radioactive sources (internal radiotherapy) was developed. The placement of sealed radioactive sources into or immediately adjacent to the tumor is referred to as brachytherapy. The isotope used in brachytherapy can be embedded in surface applicator placed directly on the tumor, inserted into various tubular organs (intraluminal), or directly through the tumor (interstitial) [31].
Photons and electrons are characterized by a low linear energy transfer (low LET) and considerable range in the tissue (millimeters for beta particles, centimeters for photons). About two-thirds of the biological damage by low-LET radiations (sparsely ionizing radiations) is due to indirect action [32].
Heavier particles (neutrons, protons, alpha particles, and heavy ions) are defined as high-LET radiation and deposit more energy along the path they take through tissue than do X-rays or gamma rays and cause more damage to the cells they hit [33, 34]. High-LET radiation interacts directly with the critical target in the cell. The atoms of the target itself may be ionized or excited through Coulomb interactions, leading to the chain of physical and chemical events that eventually produce the biological damage. High-LET radiations, such as alpha particles, have a high relative biological effectiveness (RBE), and only a few hits are required to ensure cell lethality [35–37].
The signature of high-LET radiations, such as alpha radiation, is the formation of complex DNA damage that comprises of closely spaced DNA lesions forming a cluster of DNA damage [double strand breaks (DSBs) and non-DSB oxidative clustered DNA lesions (OCDL)] [38, 39].
Its characteristics should have posed the alpha irradiation as a natural candidate for the treatment of cancerous tissues, but the short range of alpha particles in tissue (<0.1 mm) has so far limited their medical applicability. The main alpha particle-based treatment modality is alpha radio-immunotherapy [40], and clinical experiment with alpha particles was done in order to treat breast and prostate cancer patients with skeletal metastases [41].
We developed a new approach that potentially enables the application of alpha particles against solid tumors, i.e., diffusing alpha-emitters radiation therapy (DaRT). DaRT is the only modality currently available, which provides an efficient and secure method for prolonged treatment of the entire volume of solid tumors by alpha particles.
The basic idea of this method is to insert into the tumor radioactive sources (wires), which continually release short-lived alpha-emitting atoms from their surface. The parent alpha-emitting atoms (224Ra) are embedded closely below the surface of the source, at a depth that prevents their release into the tumor, but allows considerable release of their daughter atoms by recoil. These daughter atoms spread within the tumor forming a region where a lethal radioactive dose is delivered through their alpha decays several millimeters away from the wire, causing intensive tumor tissue damage. As 224Ra is itself the result of the alpha decay of 228Th (1.91-y half-life), the production of 224Ra-bearing sources is based on the use of a 228Th generator as described [42]. The source is a thin wire carrying a small activity of 224Ra, and it is inserted into the tumor. Once inside the tumor and over a period determined by 224Ra half-life (3.66 d), the source releases, by recoil, 220Rn (55.6-s half-life), 216Po (0.15 s half-life) and 212Pb (10.64-h half-life) atoms, while the remaining atoms of 224Ra stay below its surface. 212Pb beta decays to 212Bi (60.6-min half-life), which either alpha decays to 208Tl (3.05-min half-life) or beta decays to 212Po (0.3-μs half-life), which then alpha decays to stable 208Pb [42].
In vivo experiments tested the ablative performance of DaRT against murine- and human-derived solid tumors, from various histological origins. The responses to the interstitial radiation varied between tumors. DaRT achieved substantial tumor growth retardation, extended survival, reduced lung metastases, and even complete cure of animals bearing murine squamous cell carcinoma (SCC) [42–46], pancreatic [47], colon [48], prostate [49], and breast (Confino et al., manuscript in preparation) mouse-derived tumors, and human-derived tumors [50].
The DaRT modality thus combines the advantages of local intra-tumoral irradiation with the high efficacy of alpha particles against cancer cells.
The extent of radioactive atoms spread in the tumor may be affected by several characteristics such as the tumor tissue compactness, disposal of the radioactive atoms (presence or absence of blood vessels) in the tumor and the cell’s sensitivity to radiation. Dosimetric measurements of the intra-tumoral spread of radioactivity in different tumor models revealed biologically significant doses (asymptotically exceeding 10 Gy) of 212Pb over a region a few millimeters in size. The average region diameter was largest in SCC tumors [42], smallest in pancreatic tumors [47] and intermediate for lung [46], breast, and colon tumors [48]. A close correlation was found between the tumor growth inhibition, the area of necrotic damage inside the tumor, and the region covered by alpha radiation [43]. This indicates that the volume of necrotic tissue directly depends on the distance radioactive atoms traveled from the DaRT wires. The tissue and cell characteristics, which affect the efficacy of this treatment, are not entirely clear.
In situ tumor ablation by alpha radiation and activation of anti-tumor immunity
Development of anti-tumor immunity after the ablation of primary tumors was studied in two tumor models of mouse metastatic tumors, the DA3 breast carcinoma and the CT-26 colon carcinoma. CT-26 is highly antigenic, while DA3 is a relatively weak one. The induction of anti-tumor immunity was assessed by several parameters: (1) the development of specific anti-tumor immune resistance in treated mice, (2) inhibition or destruction of metastases, (3) presence of immune cells with anti-tumor activity in the treated mice, and (4) the feasibility to enforce the anti-tumor immunity by a combined treatment of DaRT and immune adjuvants.
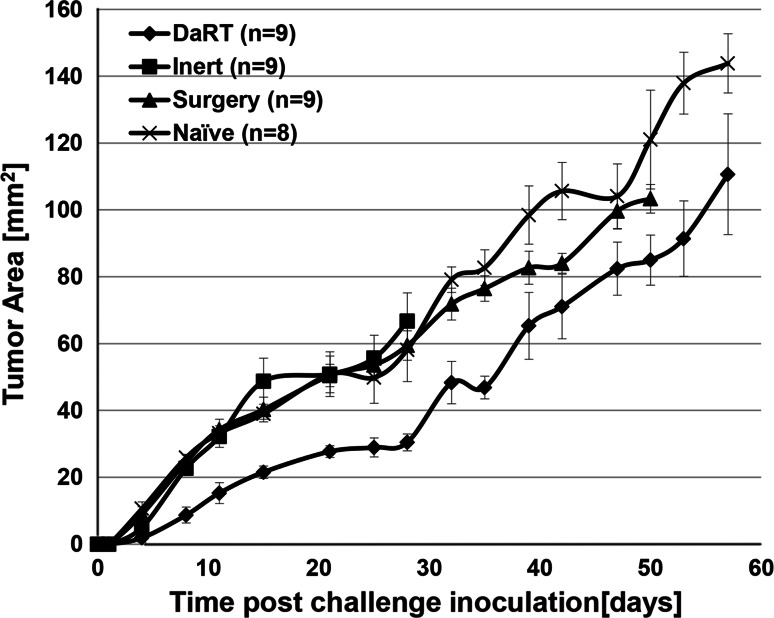
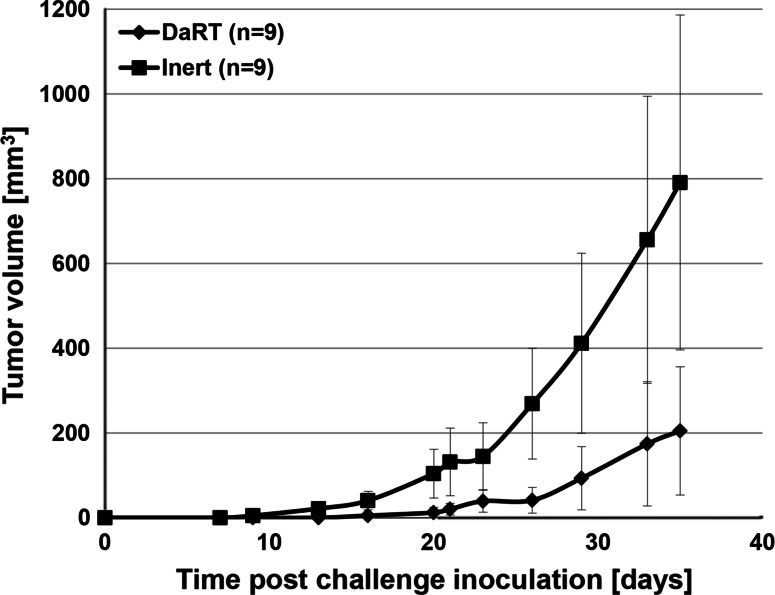
In order to test the induction of anti-tumor immunity, primary subcutaneous DA3 and CT-26 tumors (volume of ~50 mm3) were treated by the DaRT ablative system (Ra-224 loaded wire). If the primary tumors were not eliminated, they were surgically removed 2 weeks after treatment and 2 weeks later the mice were reinoculated with tumor cells (rechallenge assay). Both DA3- and CT-26-bearing mice developed resistance to a rechallenge of tumor cells after the treatment of the primary tumor with a Ra-224 loaded wire. In both DA3 (Fig. 2) and CT-26 (Fig. 3) models, the challenge tumors were the smallest in the DaRT-treated group or have not developed at all. As control served animals, which their primary tumors were treated by non-radioactive wires (inert).
Fig. 2.
Effect of DaRT treatment on the development of a DA3 tumor cell rechallenge. DA3 primary tumors were treated by DaRT followed by surgery, Inert wire + surgery or surgery alone. Two weeks after treatment, the mice were rechallenged with a tumorigenic dose of tumor cells and tumor development was monitored. p value (two-way ANOVA without replication, DaRT vs. controls) < 0.05
Fig. 3.
Effect of DaRT treatment on the development of a CT-26 tumor cell rechallenge. CT-26 primary tumors were treated by DaRT followed by surgery or Inert wires and surgery. Two weeks after treatment, the mice were rechallenged with a tumorigenic dose of tumor cells and tumor development was monitored. p value (two-way ANOVA without replication, DaRT vs. controls) < 0.05
No tumor grew in 67 % of CT-26-bearing animals treated by DaRT, compared with only 33 % tumor-free mice in the inert-treated group (Table 2). For the less immunogenic tumor, DA3, the DaRT treatment conferred protection in 15 % of the animals, while none of the mice in the control groups rejected the tumor cell challenge (Table 2). It is evident that the effect of in situ ablation is more pronounced in the more immunogenic tumor, CT-26.
Table 2.
Percentage of mice that did not develop a tumor after a rechallenge
| DA3 tumorsa | CT-26 tumorsb | |||||
|---|---|---|---|---|---|---|
| Days after rechallenge | 4 | 9 | 16 | 7 | 21 | 35 |
| DaRT | 77 % | 27 % | 15 % | 100 % | 88 % | 67 % |
| Inert | 62 % | 4 % | 0 % | 78 % | 56 | 34 % |
| Surgery | 65 % | 0 % | 0 % | 100 % | 57 | 43 % |
| Naïve | 69 % | 8 % | 4 % | 0 % | 0 % | 0 % |
DA3 or CT-26 primary tumors were treated by DaRT followed by surgery, inert + surgery or surgery alone. Three weeks after wire insertion, the mice were rechallenged with a tumorigenic dose of tumor cells and tumor development was monitored
aDA3 rechallenge was injected s.c. to 24–26 animals/group for each treatment
bCT-26 rechallenge was injected s.c. to 7–9 animals/group for each treatment
Next, we examined whether the DaRT ablation and the resulting anti-tumor immunity can reduce the lung metastatic load in mice with DA3 tumors. DA3-bearing mice, treated by a single Ra-224 loaded wire, were scanned by a CT imaging tool for lung metastases. Lung metastases were observed in 71 % of the DaRT-treated mice 49-days posttreatment, compared to 100 % in the inert-treated mice.
Since DA3 tumors are weakly immunogenic, DaRT treatment was given with the immunostimulant CpG. Tumors treated by DaRT and CpG had an average volume of 35 ± 8 mm3, DaRT alone 206 ± 64 mm3 and inert wires + CpG treated 174 ± 90 mm3. Thus, CpG augmented the effect of DaRT and improved the local control of tumor growth and prolonged survival.
Taken together, these results indicate that alpha radiation-based therapy inhibits both breast and colon primary tumor growth, reduced the lung metastatic load, and prolonged animal survival. The destruction of the tumor stimulated anti-tumor immunity, which could have been enforced when combined with an immune stimulating agent.
Summary
The results presented here highlight the effect of in situ electrochemical and alpha radiation-based ablation treatments on the immune response against tumor cells.
It was demonstrated that in situ ablation could augment the immune system of treated mice against metastases.
The treatments prolong life expectancy of treated mice in comparison with surgical removal of the tumors.
In situ ablation can augment the development of memory immunity response against the tumor cells more than control surgery-treated mice.
The enforced anti-tumor immunity, triggered by the ablation treatments, could be further augmented by combining it with an immune adjuvant.
Acknowledgments
This work was supported in part by The Roberts-Guthman Chair in Immunopharmacology, The German Israeli Fund, The Israel Cancer Association, and Althera Medical LTD.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Third International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2013), held in Krakow, Poland, 22nd–25th April 2013. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein O, Schmidt C, Knights A, Davis ID, Chen W, Cebon J. Melanoma vaccines: developments over the past 10 years. Expert Rev Vaccines. 2011;10:853–873. doi: 10.1586/erv.11.74. [DOI] [PubMed] [Google Scholar]
- 5.Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy–revisited. Nat Rev Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 6.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Myc LA, Gamian A, Myc A. Cancer vaccines. Any future? Arch Immunol Ther Exp (Warsz) 2011;59:249–259. doi: 10.1007/s00005-011-0129-y. [DOI] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meniawy TM, Nowak AK, Lake RA. Effect of chemotherapy on the tumor microenvironment and anti-tumor immunity. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 1–28. [Google Scholar]
- 12.McBride WH, Schaue D. In situ tumor ablation with radiation therapy: Its effect on the tumor microenvironment and anti-tumor immunity. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 109–119. [Google Scholar]
- 13.Sabel MS. The interrelationship between cryoablation, the immune response and the tumor microenvironment: stimulatory and suppressive effects. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 77–107. [Google Scholar]
- 14.Nierkens S, den Brok MH, Ruers TJ, Adema GJ. Radiofrequency ablation in cancer therapy: tuning into in situ tumor vaccines. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 39–59. [Google Scholar]
- 15.Calderwood SK. Hyperthermia, the tumor microenvironment and immunity. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 29–37. [Google Scholar]
- 16.Korbelik M. Tumor-localized insult delivered by photodynamic therapy and the breakdown of tumor immunotolerance. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 121–132. [Google Scholar]
- 17.Wu F. High intensity focused ultrasound (HIFU) ablation. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. pp. 61–75. [Google Scholar]
- 18.Keisari Y, Korenstein R. Anti-tumoral effects of pulsed low electric field enhanced chemotherapy: lessons from experimental malignant tumors. In: Spugnini EP, Baldi A, editors. Electroporation in laboratory and clinical investigations. Hauppauge, NY: Nova Science Publishers, Inc.; 2011. pp. 178–204. [Google Scholar]
- 19.Plotnikov A, Niego B, Ophir R, Korenstein R, Keisari Y. Effective treatment of mouse metastatic prostate cancer by low electric field enhanced chemotherapy. Prostate. 2006;66:1620–1630. doi: 10.1002/pros.20435. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Dov N, Rozman Grinberg I, Korenstein R. Electroendocytosis is driven by the binding of electrochemically produced protons to the cell’s surface. PLoS ONE. 2012;7(11):e50299. doi: 10.1371/journal.pone.0050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Entin I (2002) Low electric field cancer therapy as a treatment modality of melanoma and breast carcinoma in mice. Ph.D. Dissertation, Tel Aviv University, Israel
- 22.Entin I, Plotnikov A, Korenstein R, Keisari Y. Tumor growth retardation, cure, and induction of anti tumor immunity in B16 melanoma bearing mice by low electric field enhanced chemotherapy. Clin Cancer Res. 2003;9:3190–3197. [PubMed] [Google Scholar]
- 23.Hochman I, Confino H, Efrati M, Korenstein R, Keisari Y. Induction of anti-tumor immune responses by ablation of the primary tumor with pulsed electric currents. Tumor Biol. 2012;33(Suppl 1):S101. [Google Scholar]
- 24.Entin I, Ophir R, Korenstein R, Keisari Y. Cure of breast carcinoma bearing mice and induction of tumor immunity by low electric fields and chemotherapy. Int Immunol. 2010;22(Supl. 1):iii157. [Google Scholar]
- 25.Fishman D (2002) Low electric field enhanced cancer chemotherapy (LEFCT-EC) as a treatment modality of murine colon cancer and squamous cell carcinoma. Dissertation, Tel Aviv University, Israel
- 26.Plotnikov A, Fishman D, Tichler T, Korenstein R, Keisari Y. Low electric field enhanced chemotherapy can cure mice with CT-26 colon carcinoma and induce anti tumor immunity. Clin Exp Immunol. 2004;138:410–416. doi: 10.1111/j.1365-2249.2004.02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plotnikov A, Tichler T, Korenstein R, Keisari Y. Involvement of the immune response in the cure of metastatic murine CT-26 colon carcinoma by low electric field enhanced chemotherapy. Int J Cancer. 2005;117:816–824. doi: 10.1002/ijc.21261. [DOI] [PubMed] [Google Scholar]
- 28.Ohad S (2005) Involvement of the immune response in the cure of squamous cell carcinoma (SQ2) and colon cancer (CT-26) tumors, treated with Low electric field cancer therapy in the presence of chemotherapy. Dissertation, Tel Aviv University, Israel
- 29.Keisari Y, Korenstein R. In-situ ablation of solid tumors by electric forces and its effect on the tumor microenvironment and anti-tumor immunity. In: Keisari Y, editor. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Berlin: Springer; 2013. pp. 133–153. [Google Scholar]
- 30.Winn HJ. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunological activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1961;86:228–239. [PubMed] [Google Scholar]
- 31.Williamson JF. Brachytherapy technology and physics practice since 1950: a half-century of progress. Phys Med Biol. 2006;51:R303–R325. doi: 10.1088/0031-9155/51/13/R18. [DOI] [PubMed] [Google Scholar]
- 32.Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist. Lippincott Williams and Wilkins, Philadelphia, PA, USA
- 33.Behr TM, Behe M, Stabin MG, Wehrmann E, Apostolidis C, Molinet R, Strutz F, Fayyazi A, Wieland E, Gratz S, Koch L, Goldenberg DM, Becker W. High-linear energy transfer (LET) a versus low-LET β emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab’ fragments in a human colonic cancer model. Cancer Res. 1999;59:2635–2643. [PubMed] [Google Scholar]
- 34.Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res. 2011;711:150–157. doi: 10.1016/j.mrfmmm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Søyland C, Hassfjell SP. Survival of human lung epithelial cells following in vitro α-particle irradiation with absolute determination of the number of alpha-particle traversals of individual cells. Int J Radiat Biol. 2000;76:1315–1322. doi: 10.1080/09553000050151583. [DOI] [PubMed] [Google Scholar]
- 36.Suntharalingam N, Podgorsak EB, Hendry JH (2005) Basic radiobiology. In: Podgorsak EB (ed) Radiation oncology physics: a handbook for teachers and students. IAEA publication, Vienna (ISBN 92-0-107304-6), pp 485–504
- 37.Roeske JC, Stinchcomb TG. The average number of alpha-particle hits to the cell nucleus required to eradicate a tumour cell population. Phys Med Biol. 2006;51:N179–N186. doi: 10.1088/0031-9155/51/9/N02. [DOI] [PubMed] [Google Scholar]
- 38.Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res (Tokyo) 2008;49:203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- 39.Asaithamby A, Hu B, Chen DJ. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. PNAS. 2011;108:8293–8298. doi: 10.1073/pnas.1016045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang CY, Pourgholami MH, Allen BJ. Optimizing radioimmunoconjugate delivery in the treatment of solid tumor. Cancer Treat Rev. 2012;38:854–860. doi: 10.1016/j.ctrv.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, Salberg G, Bruland ØS. First clinical experience with α-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11:4451–4459. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 42.Arazi L, Cooks T, Schmidt M, Keisari Y, Kelson I. Treatment of solid tumours by interstitial release of recoiling short-lived alpha emitters. Phys Med Biol. 2007;52:5025–5042. doi: 10.1088/0031-9155/52/16/021. [DOI] [PubMed] [Google Scholar]
- 43.Cooks T, Arazi L, Schmidt M, Marshak G, Kelson I, Keisari Y. Growth retardation and destruction of experimental Squamous cell carcinoma by interstitial radioactive wires releasing diffusing alpha-emitting atoms. Int J Cancer. 2008;122:1657–1664. doi: 10.1002/ijc.23268. [DOI] [PubMed] [Google Scholar]
- 44.Cooks T, Arazi L, Efrati M, Schmidt M, Marshak G, Kelson I, Keisari Y. Interstitial wires releasing diffusing alpha emitters combined with chemotherapy improved local tumor control and survival in squamous cell carcinoma-bearing mice. Cancer. 2009;15:1791–1801. doi: 10.1002/cncr.24191. [DOI] [PubMed] [Google Scholar]
- 45.Raab S (2010) In vivo and in vitro killing of squamous cell carcinoma tumors, by diffusing radioactive atoms emitting alpha particles. M.Sc. Dissertation, Tel Aviv University, Israel
- 46.Cooks T, Schmidt M, Bittan H, Lazarov E, Arazi L, Kelson I, Keisari Y. Local control of lung derived tumors by diffusing alpha-emitting atoms released from intratumoral wires loaded with radium-224. Int J Radiat Oncol Biol Phys. 2009;74:966–973. doi: 10.1016/j.ijrobp.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 47.Horev-Drori G, Cooks T, Bittan H, Lazarov E, Schmidt M, Arazi L, Efrati M, Kelson I, Keisari Y. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224radium-loaded wires releasing alpha-emitting atoms. Transl Res. 2012;159:32–41. doi: 10.1016/j.trsl.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Reitkopf S (2009) Radiotherapy treatment of malignant colon cancer by intratumoral alpha emitting sources combined with chemotherapy. M.Sc. Dissertation, Tel Aviv University, Israel
- 49.Tal M (2008) Treatment of prostate and glioblastoma tumors by interstitial 224Ra wires releasing short-lived alpha emitting atoms. M.Sc. Dissertation, Tel Aviv University, Israel
- 50.Cooks T, Tal M, Raab S, Efrati M, Reitkopf S, Lazarov E, Etzyoni R, Schmidt M, Arazi L, Kelson I, Keisari Y. Intratumoral Ra-224-loaded wires spread alpha emitting atoms inside solid human tumors in athymic mice and can achieve local tumor control. Anticancer Res. 2012;32:5315–5321. [PubMed] [Google Scholar]