Abstract
Immunotherapeutic concepts in neurooncology have been developed for many decades but have mainly been hampered by poor definition of relevant antigens and selective measures to target the central nervous system. Independent of the recent remarkable successes in clinical immunooncology with checkpoint inhibitors and vaccines, immunotherapy of brain tumors in general and gliomas in particular has evolved with novel neurooncology-specific concepts over the past years providing new phase 1 approaches of individualized immunotherapy to first phase three clinical trials. These concepts are driven by a high medical need in the absence of approved targeted therapies and refute the classic dogma that the central nervous system is immune-privileged and hence inaccessible to potent antitumor immunity. Instead, measures have been taken to improve the odds for successful immunotherapies, including rational targeting of relevant antigens and integration of immunotherapies into standard of care primary radiochemotherapy to increase the efficacy of antitumor immunity in a meaningful time window. This review highlights concepts and challenges associated with epitope discovery and selection and trial design.
Keywords: Glioblastoma, Vaccine, IDH1, EGFRvIII, Checkpoint inhibition, CIMT 2015
Introduction
It is obvious that immunotherapy for brain tumors shares many concepts and opportunities with tumor immunotherapy in general but also provides unique challenges and chances for highly selective therapeutic approaches. Immunotherapy for brain tumors has been explored for many decades, starting from systemic or local administration of lymphokine-activated killer cells [1] to modern truly patient-specific and neoantigen-specific vaccine concepts [2]. Similar to immunotherapy of non-central nervous system (CNS) tumors, the field has long suffered from lack of appropriate predictive markers for efficacy, in part owing to improperly controlled clinical trials. Thus, even more than for other entities, CNS tumor immunotherapy has not yet proven efficient. However, there are also challenges specific to brain tumor immunity, which are mainly related to the fact that these tumors grow in a sanctuary site. But the dogma that the brain is absolutely immune privileged and as a consequence transferring relevant antitumor immunity to brain tumors is impossible is to be challenged based on several observations: First, the brain is constantly surveyed by antigen-specific T cells to keep viral agents such as John Cunningham virus (JCV) in check [3]. Second, there are autoimmune diseases such as multiple sclerosis, where spontaneous antigen-specific T cell responses to CNS antigens result in deleterious inflammation and tissue destruction [4], indicating that the CNS is permissive for both induction of peripheral antigen-specific immunity and transfer of adaptive immunity back from the periphery. It has recently been shown in an experimental autoimmune encephalomyelitis animal model that effector T cells enter the CNS from the blood via the leptomeninges, from which they may be released to cerebrospinal fluid (CSF) and re-attach to leptomeninges, whereas non-activated T cells are released into the CSF [5]. Third, to enable this communication, a lymphatic system is necessary, which has long been neglected but recently been shown to be present [6, 7]. And fourth, the concern of relevant CNS adverse events occurring when targeting brain tumors by local or systemic immunotherapy has not been supported in clinical trials [8]. Based on change in dogma and even before the recent renewed interest in immunooncology, immunotherapeutic concepts for brain tumors have evolved, which are quite exclusive and distinct from other tumor entities to meet the unique makeup and localization of a CNS tumor. Growing in the CNS, gliomas have a distinct microenvironment per se, in addition to shaping it toward an immunosuppressive milieu. Hallmarks and characteristics of the glioma microenvironment have extensively been described elsewhere [9]. Here we highlight the recent developments and current concepts in brain tumor immunotherapy, focusing on vaccines and checkpoint inhibitors for gliomas.
Checkpoint inhibitors in neurooncology
The remarkable success of checkpoint inhibitors in oncology has certainly extended into the neurooncology arena, although the number of clinical trials is limited. First clinical experiences, however, generally do not recapitulate the tremendous response rates seen in melanoma, lung cancer or mismatch-repair-deficient colon cancer, perhaps with the exception of brain metastases [10]. Nevertheless, randomized phase II trials are pursued in patients with recurrent glioblastomas [11], and a phase III trial testing the efficacy of nivolumab in addition to standard radiochemotherapy in patients with newly diagnosed glioblastoma is currently accruing patients (CheckMate 498, NCT0261758). Conceptually, the main argument against the effectiveness of checkpoint inhibitor monotherapy in brain tumors is as follows: Despite the challenge of dogma, the brain is still a comparatively immune privileged organ. Hence, peripheral immune responses to brain tumor antigens are scarce, and even if they occur, the immune privilege of the CNS prevents effective peripheral antitumor immunity to be transferred into the CNS. There are, however, several lines of evidence which refute this view: Spontaneous immune responses to tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) are observed in patients with intrinsic brain tumors [2, 12], which suggests that antigens are presented in the brain tumor microenvironment and induce an effective antigen-specific peripheral T cell response. In addition, preclinical studies using adoptive T cell transfer or monotherapy with checkpoint blockers in syngeneic mouse models using transplantable, chemically induced gliomas demonstrate that unleashing an endogenous or transferring a peripheral T cell response effectively eradicates these tumors [13]. Finally, in patients with systemic cancers, metastases to the brain may be effectively controlled by checkpoint inhibitors, even if response rates do not compare well with those observed with extra-CNS tumor manifestations [14]. The reason why particularly gliomas display a reduced sensitivity—if any—to checkpoint inhibition alone is most likely a comparatively low mutational load, because it has been shown that checkpoint inhibition unleashes mutation-specific T cell responses [15]. Gliomas—on average—contain 40–80 non-synonymous mutations, which is an order of magnitude lower than in melanoma or small-cell lung cancer, which mostly respond well to checkpoint inhibitors alone [16]. The therapeutic efficacy in preclinical animal models can thus be attributed to the comparatively high mutational load in chemically induced experimental murine gliomas, such as the GL261 model.
Collectively, these observations argue against further trials with mono-checkpoint inhibition in gliomas, perhaps with the exception of recurrent gliomas with mismatch repair deficiency and subsequent hypermutation as a consequence of prolonged alkylating chemotherapy [17, 18]. Instead, we would strongly advocate combination trials of checkpoint inhibitors with antigen-specific vaccines as the endogenous T cell response in this disease may lack the sufficient intensity and breadth. In any case, future clinical trials using checkpoint inhibitors ought to be performed in carefully selected patient populations and backed by a meaningful translational program for hypothesis testing. It will thus be interesting to see the outcomes of studies combining with vaccines (AVERT, NCT02529072). The type of checkpoint inhibitor to be chosen for glioma therapy will also depend on the compartment of target cells, because the blood–brain barrier (BBB) does not allow antibodies to cross easily [19]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) antibodies target receptors present on circulating T cells. But PD-1 ligand (PD-L1) inhibitors may not necessarily require brain penetrance either, as PD-L1 is expressed not only in the tumor microenvironment of gliomas [20–22] but also elevated in circulating antigen presenting cells (APCs) in glioma patients [23], which may indicate biological activity even if the therapeutic antibody does not reach sufficient intratumoral levels. This suggests that anti-PD-L1 antibodies such as atezolizumab may represent an additional attractive strategy in neurooncology. Here, intratumoral or peripheral PD-L1 expression may serve as a biomarker as is suggested for other indications [24, 25]. Another concept to cross the BBB could be the development of small molecule inhibitors instead of antibodies to block checkpoint signal transduction.
Vaccines in neurooncology—self-antigens vs. neoepitopes
Vaccines targeting self-antigens
As in general oncology, vaccines in neurooncology have mainly focused on embryonal self-antigens in the past years. For instance, gliomas frequently share these antigens with melanomas, such as melanoma-associated antigen (MAGE)-A1/3, tyrosinase-related protein 2 (TRP-2) or glycoprotein 100 (gp100). In fact, there are few glioma-specific self-antigens reported to date. These self-antigens are expressed in the majority of gliomas albeit at highly variable levels [12]. In addition, the degree of presentation of these antigens in the tumor tissue remains unclear, a problem currently targeted by mass-spectrometry-based human leukocyte antigen (HLA) ligandome analyses aiming at enriching the pool of tumor-associated self-antigens [12]. Recent developments include targeting of antigens, which are believed to be specifically expressed in brain tumor-initiating cells, such as SRY-box (SOX) 2/11 or CD133. Vaccines targeting glioma-associated self-antigens are generally viewed as safe, but the induction of an efficacious antitumor immunity may be hampered by the fact that many of these self-antigens are expressed in the thymus, resulting in central T cell tolerance and the development of antigen-specific suppressive T-regulatory cells [26]. In the absence of defined universal self-antigens, current clinical trials aim at combining multiple epitopes to multi-peptide vaccines. Examples currently in phase I/II testing include IMA950 (NCT01920191), ICT-107 (NCT01280552) and SL-701 (NCT02078648). These vaccines are typically restricted to HLA-A2+ patients and may involve intradermal injection combined with immune enhancers (IMA950, SL-701) or prior loading on autologous dendritic cells (DC; ICT-107). Semi-personalized approaches are undertaken using warehouse concepts, where peptide vaccines are selected based on the individual patient’s tumor antigen profile (IMA950). Based on encouraging results from a randomized phase II clinical trial in HLA-A2+ patients with newly diagnosed glioblastoma, a phase III clinical trial has been initiated for the multi-peptide vaccine ICT-107 (EORTC1587, NCT02546102).
Also in neurooncology, concepts are being developed to enhance the induction of antigen-specific anti-tumor immunity by using RNA-based vaccines [27, 28], coupling of peptides to heat-shock proteins [29] and enhancing the efficacy of DC-based vaccines by priming with recall antigens [30].
TSAs such as mutated or splice variant neoantigens are usually private antigens. There are, however, some exceptions to this rule in neurooncology. These exceptions have been consequently and rapidly taken from bench to bedside:
EGFRvIII as a tumor antigen
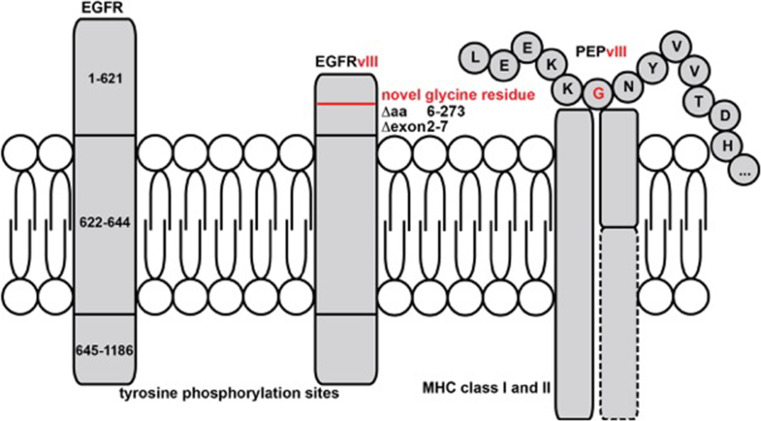
The variant III of the epidermal growth factor receptor (EGFRvIII) is a tumor-specific antigen generated by alternative splicing of exons 2–7—representing the ligand-binding domain—with subsequent generation of a neoepitope by fusion of exon 1 with exon 8. Functionally, EGFRvIII is constitutively active in the absence of epidermal growth factor (EGF), leading to enhanced proliferation and inhibition of apoptosis. EGFRvIII is detected in approx. 20–30 % of glioblastoma samples, in general concurrently with the wild-type variant even on a single-cell level. A vaccine using the peptide sequence from the neoepitope (EGFRvIII Pep) conjugated to the adjuvant keyhole limpet hemocyanin (KLH) administered with granulocyte–macrophage colony-stimulating factor (GM-CSF) induces robust anti-EGFRvIII antibody responses and possibly CD8+ T cell responses in patients with EGFRvIII-positive tumors due to neoantigen presentation on tumoral major histocompatibility complex (MHC) class I [31] (Fig. 1). Rigorous phase I–II trials tested the efficacy of EGFRvIII Pep-KLH (rindopepimut®) in glioblastoma patients. Of note, the vast majority of patients in the phase I/II program with recurrent disease after the vaccine had lost expression of EGFRvIII in the tumor tissue, indicating immune escape [32]. The explanation is that in an EGFRvIII-expressing tumor, this variant–albeit oncogenic—only affects a distinct subclone of the tumor [33]. The phase III randomized ACT-IV registration trial was initiated, testing the efficacy of rindopepimut® compared to placebo in addition to combined radiochemotherapy with temozolomide in patients with newly diagnosed EGFRvIII-positive glioblastoma (NCT01480479); however, the interim analysis has shown that there was no statistical significance in the primary endpoint overall survival (OS) between the rindopepimut® group (20.4 months) and the control group (21.1 months; http://www.nature.com/nrneurol/journal/v12/n4/full/nrneurol.2016.38.html). Biologically, further analyses will show whether immune escape via antigen loss or other immune modulatory mechanisms by the tumor cells is the reason for the lack of therapeutic efficacy.
Fig. 1.
EGF receptor (EGFR) is a transmembrane protein with an extracellular N-terminus and intracellular tyrosine phosphorylation sites for signaling. Fusion of exons 1 and 8 results in the constitutively active EGFRvIII, which has a truncated extracellular domain containing a novel glycin residue. This makes EGFRvIII an extracellular antigen, which is accessible for direct antibody binding. The fusion epitope PEPvIII is additionally loaded onto MHC class I and II molecules after proteasomal cleavage within the mutated tumor cell, making it accessible for EGFRvIII-specific T cell responses. PEPvIII is the fusion epitope used for vaccination. Novel glycine residue is shown in red
IDH1R132H as a tumor antigen
The clinical experience with the EGFRvIII vaccine illustrates the challenges associated with tumor heterogeneity, which often results in antigen heterogeneity and therefore antigen loss after vaccination. Conceptually, targeting a true driver mutation, which occurs at the top of the hierarchy, will circumvent heterogeneity-driven immune escape. In the past years, several attempts have been made to dissect the phylogenetic tree of gliomagenesis by dissecting the mutational profile in different areas of a human tumor [34]. These analyses demonstrate that a mutation in the gene for isocitrate dehydrogenase type 1 (IDH1), which occurs in 70–80 % of diffuse and anaplastic gliomas and which affects the catalytic site of the protein, in the vast majority (>90 %), resulting in an amino acid exchange (Arg to His) at position 132 of the protein (IDH1R132H), is the earliest mutation in these tumors, rendering all tumor cells positive for IDH1R132H even during malignant progression [35, 36]. While the mutation is a unique characteristic of these gliomas, it is also observed in other tumor types such as acute myeloid leukemia and chondrosarcoma albeit at a much lower frequency. Mechanistically, IDH1R132H results in a neomorphic enzyme function, leading to the production of the oncometabolite 2-hydroxyglutarate in excess amounts [37], which results in genetic instability via epigenetic modifications and hence tumorigenicity [38].
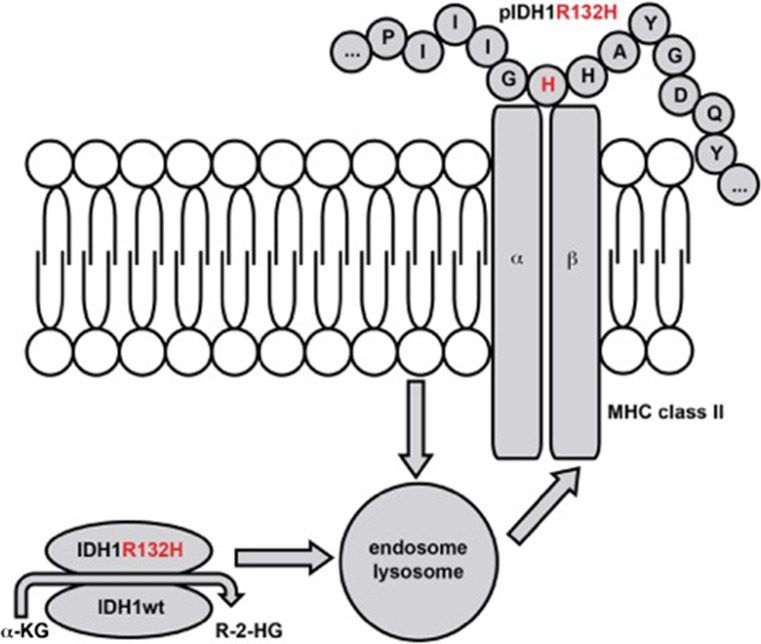
From an immunological point of view, IDH1R132H represents an attractive tumor antigen specifically expressed in tumor but not normal cells, which is readily detectable in routine diagnostic immunohistochemistry using a mutation-specific antibody [39]. A subset of patients with IDH1R132H-mutated gliomas spontaneously harbors mutation-specific CD4+ T helper cells and antibodies, indicating that IDH1R132H is specifically presented to and recognized by the immune system in a mutation-specific manner [2]. Vaccination of MHC-humanized but also wild-type C57BL/6 mice with the IDH1 peptide vaccine comprised of the neoepitope sequence and the adjuvant Montanide-ISA51® results in a mutation-specific CD4-dependent immune response [2, 40], effective in controlling IDH1R132H-expressing tumors in a preventive and a therapeutic manner without causing toxicity [2]. Since IDH1R132H is—in contrast to EGFRvIII—an intracellular antigen, the biological and therapeutic relevance of the antibody response is probably limited. However, antibodies may be able to bind the MHC class II-bound peptide, which holds true for the diagnostic antibody (Fig. 2) [41]. Whether this could lead to an antitumor immunity is unclear. Clinically, the production of an IDH1R132H-specific antibody can be used for monitoring purposes as an easy-to-evaluate biomarker for response to vaccination. A newly developed companion diagnostic, which allows for prediction of antigen presentation in paraffin-embedded tumor tissue using proximity ligation assay [41], may aid patient selection for vaccine trials. Conceptually, the IDH1R132H vaccine differs from the EGFRvIII vaccine as it represents as a CD4 epitope, which requires endosomal/lysosomal processing and—most likely –presentation by professional APCs instead of tumor cells (Fig. 2).
Fig. 2.
IDH1 is a cytosolic protein with enzymatic activity. A point mutation leads t a single amino acid exchange (R132H), resulting in a neomorphic enzymatic function, the production of R-2-HG. IDH1(R132H) is processed in the endosomes and lysosomes within tumor cells and APCs that have taken up the protein from necrotic and apoptotic tumor cells, from where the epitope pIDH1R132H is loaded onto MHC class II. This makes it accessible for IDH1(R132H-specific CD4+ T cells. Amino acid exchange to histidine is shown in red
A multicenter phase I trial is underway (NCT02454634), enrolling a planned population of 39 patients with newly diagnosed grade 3 or grade 4 astrocytomas at eight German sites. Patients receive a total of eight vaccines comprised of a 20-mer peptide emulsified in Montanide-ISA51®. Importantly, the vaccine is integrated into the primary therapy. The translational program associated with the trial and ongoing preclinical studies using MHC-humanized mice will address important questions for further development, chiefly with respect to the mechanism of action of CD4+ IDH1R132H-specific T cells. Possible scenarios include the induction of senescence by Th1 cytokines produced by mutation-specific T cells [42], the induction of cytotoxic CD4+ mutation-specific T helper cells or the induction of a bystander CD8 response against (a) different antigen (s) [43]. We have acquired data, indicating that efficient killing requires activation of CD8+ T cells (unpublished data), which has been shown by others as well [43]. Therefore, antigen spreading, which occurs via cross-presentation of an unrelated antigen on MHC class I by the same APC presenting the IDH1R132H epitope on MHC class II, is most likely required for vaccine efficacy. As it is likely that also in gliomas the majority of neoepitopes are private and MHC class II-restricted, as suggested in preclinical models [41, 43], it will be important to delineate the mechanism of action in the current phase I trial.
Personalized concepts
IDH1R132H and EGFRvIII are rare examples of recurrent neoepitopes. Most of the neoepitopes also in neurooncology are private antigens [16]. This constitutes the need for personalized vaccine concepts targeting these private neoantigens after identification by whole-exome sequencing (WES). Based on a computational pipeline predicting HLA binding of mutated epitopes [44], a phase I study in patients with newly diagnosed O-6-methylguanine-DNA methyltransferase (MGMT)-unmethylated glioblastoma is underway to test the safety and immunogenicity of a personalized peptide vaccine (NeoVax) encompassing neoepitopes relevant for the individual patient (NCT02287428). The vaccine is given after completion of radiotherapy. An even more complex setting of neoepitope discovery is applied in the European Glioma Actively Personalized Vaccine Consortium (GAPVAC), which currently conducts a multicenter phase I clinical trial in patients with newly diagnosed glioblastoma (GAPVAC-101, NCT02149225). Here, the selection and production of the personalized peptide vaccine are based not only on WES but also on HLA-ligandome analyses providing additional information of the actual presentation of the relevant epitopes on HLA molecules in the tumor tissue [12]. This effort not only increases the complexity of epitope discovery but also tissue requirement, which is certainly a bottleneck in neurooncology. The turnaround time for vaccine production in both trials is within limits to allow for integration of the vaccine into primary therapy. It is evident that these personalized concepts represent a regulatory challenge, which has to be met if vaccines ought to incorporate private antigens.
Conclusion
Immunotherapy in neurooncology has evolved considerably in the past years with novel unique concepts targeting true tumor antigens (EGFRvIII, IDH1R132H) and patient-specific vaccine approaches targeting private mutated antigens (GAPVAC, NeoVax). Lessons from the shared neoepitopes taught us the importance of selecting evolutionary and functionally relevant antigens, albeit immunotherapy may at the first glance be independent of targeting proteins associated with survival and proliferation when compared to targeted agents. However, for long-term effects this is equally important in immunotherapy. The regulatory challenges associated with patient-specific approaches will have to be met along with challenges associated with the cost-intensive and time-consuming process of patient-specific neoepitope discovery. It will be of utmost importance to obtain proof of concept in well-controlled trials enrolling selected patients with newly diagnosed tumors to increase the chance of retrieving meaningful signals of efficacy. This is possible since standard of care therapy is restricted to radiation therapy and alkylating chemotherapy, both of which may even be beneficial for inducing an effective immune response and increasing the influx of effector T cells into the tumor tissue, respectively. It will be equally important to attach to these trials a comprehensive immune monitoring program including analysis of tissue at recurrence, which has been an obstacle in previous trials but will be necessary to answer pivotal questions with respect to the nature and magnitude of intratumoral antigen-specific immune responses, escape variants and antigen spreading. Finally, active vaccines ought to be combined with checkpoint inhibitors early on to block T cell tolerance and exhaustion as well as with therapies targeting the immunosuppressive tumor microenvironment. With these strategies already being realized in early clinical trials, there are exciting times ahead in brain tumor immunotherapy.
Abbreviations
- APC
Antigen-presenting cell
- BBB
Blood–brain barrier
- CAR
Chimeric antigen receptor
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- DC
Dendritic cell
- EGF
Epidermal growth factor
- EGFRvIII
Epidermal growth factor receptor variant III
- GAPVAC
Glioma Actively Personalized Vaccine Consortium
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- gp100
Glycoprotein 100
- HLA
Human leukocyte antigen
- IDH1
Isocitrate dehydrogenase type 1
- JCV
John Cunningham virus
- KLH
Keyhole limpet hemocyanin
- MAGE
Melanoma-associated antigen
- MGMT
O-6-methylguanine-DNA methyltransferase
- MHC
Major histocompatibility complex
- OS
Overall survival
- PD-1
Programmed cell death 1
- PD-L1
PD ligand 1
- SOX
SRY-box
- TAA
Tumor-associated antigen
- TRP-2
Tyrosinase-related protein 2
- TSA
Tumor-specific antigen
- WES
Whole-exome sequencing
Compliance with ethical standard
Conflicts of interest
Michael Platten, Wolfgang Wick and Theresa Bunse are inventors on a patent application entitled “Means and methods for treating or diagnosing IDH1 R132H mutant-positive cancers” (WO 2013/102641 A1, PCT/EP2013/050048). Michael Platten, Lukas Bunse and Theresa Bunse are inventors on a patent application entitled “Method for the Detection of Antigen Presentation” (WO 2016/066524, PCT/EP2015/074506).
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Thirteenth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 11th–13th May, 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Merchant RE, Grant AJ, Merchant LH, Young HF. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988;62:665–671. doi: 10.1002/1097-0142(19880815)62:4<665::AID-CNCR2820620403>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512:324–327. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 3.Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman L. Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 5.Schlager C, Korner H, Krueger M, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 6.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16:1441–1458. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platten M, Ochs K, Lemke D, Opitz C, Wick W. Microenvironmental clues for glioma immunotherapy. Curr Neurol Neurosci Rep. 2014;14:440. doi: 10.1007/s11910-014-0440-1. [DOI] [PubMed] [Google Scholar]
- 10.Ajithkumar T, Parkinson C, Fife K, Corrie P, Jefferies S. Evolving treatment options for melanoma brain metastases. Lancet Oncol. 2015;16:e486–e497. doi: 10.1016/S1470-2045(15)00141-2. [DOI] [PubMed] [Google Scholar]
- 11.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutoit V, Herold-Mende C, Hilf N, et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135:1042–1054. doi: 10.1093/brain/aws042. [DOI] [PubMed] [Google Scholar]
- 13.Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic. Immunocompetent Model Cancer Immunol Res. 2016;4:124–135. doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- 14.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma [NIBIT-M1]: an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879–886. doi: 10.1016/S1470-2045(12)70324-8. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129:597–607. doi: 10.1007/s00401-015-1403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milojkovic Kerklaan B, van Tellingen O, Huitema AD, Beijnen JH, Boogerd W, Schellens JH, Brandsma D. Strategies to target drugs to gliomas and CNS metastases of solid tumors. J Neurol. 2016;263:428–440. doi: 10.1007/s00415-015-7919-9. [DOI] [PubMed] [Google Scholar]
- 20.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–1075. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubinski D, Wölfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, Wiendl H, Grauer OM. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol. 2016;18:807–818. doi: 10.1093/neuonc/nov280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powles T, Eder JP, Fine GD, et al. MPDL3280A [anti-PD-L1] treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 26.Platten M, Offringa R. Cancer immunotherapy: exploiting neoepitopes. Cell Res. 2015;25:887–888. doi: 10.1038/cr.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahin U, Kariko K, Tureci O. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 28.Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–1330. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16:274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster J, Lai RK, Recht LD, et al. A phase II, multicenter trial of rindopepimut [CDX-110] in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17:854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer. 2015;15:302–310. doi: 10.1038/nrc3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47:458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. New Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 37.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–741. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 39.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 40.Pellegatta S, Valletta L, Corbetta C, et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun. 2015;3:4. doi: 10.1186/s40478-014-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunse L, Schumacher T, Sahm F, et al. Proximity ligation assay evaluates IDH1R132H presentation in gliomas. J Clin Investig. 2015;125:593–606. doi: 10.1172/JCI77780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braumuller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 43.Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasagi M, Shukla SA, Fritsch EF, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]