Abstract
One alternative approach for the treatment of lung cancer might be the activation of the immune system using vaccination strategies. However, most of clinical vaccination trials for lung cancer did not reach their primary end points, suggesting that lung cancer is of low immunogenicity. To provide additional experimental information about this important issue, we investigated which type of immune cells contributes to the protection from lung cancer development. Therefore, A/J mice induced for lung adenomas/adenocarcinomas by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) were depleted of CD4+ or CD8+ T cells, CD11b+ macrophages, Gr-1+ neutrophils and asialo GM1+ natural killer (NK) cells. Subsequent analysis of tumour growth showed an increase in tumour number only in mice depleted of NK cells. Further asking by which mechanism NK cells suppressed tumour development, we neutralized several death ligands of the tumour necrosis factor (TNF) family known to be involved in NK cell-mediated cytotoxicity. However, neither depletion of TNF-α, TNF-related apoptosis-inducing ligand, TNF-like weak inducer of apoptosis or FasL alone nor in combination induced an augmentation of tumour burden. To show whether an alternative cell death pathway is involved, we next generated A/J mice deficient for perforin. After challenging with NNK, mice deficient for perforin showed an increase in tumour number and volume compared to wild-type A/J mice. In summary, our data suggest that NK cells and perforin-mediated cytolysis are critically involved in the protection from lung cancer giving promise for further immunotherapeutic strategies for this disease.
Keywords: Lung cancer, Natural killer cells, Perforin-mediated cytolysis, Tumour necrosis factors, Apoptosis, Immunogenicity
Introduction
Lung cancer is the leading cause of cancer death worldwide among men and the second leading cancer site in women. In fact, 1.6 million of people were diagnosed with lung cancer in 2008 accounting for 13 % of total cases. In the same year, 1.4 million patients died from lung cancer according to 18 % of total cancer deaths [1]. Despite improved surgery, radio- and chemotherapy, the 5-year survival remains low with approx. 16 % [2], suggesting that alternative treatment options are needed for this disease.
Such an alternative treatment strategy might encompass immunotherapy which has been successfully applied for other types of cancer such as prostate cancer [3] or melanoma [4]. A prerequisite for the administration of immunotherapy to certain types of cancer is their immunogenicity which describes the property of cancer cells to induce a detectable reaction of the immune system. Whether lung cancer is sufficiently accessible to the immune system is an unsolved issue yet; 80 % of lung cancer is associated with smoking [1]. Early studies demonstrated that carcinogens from tobacco smoke provoke an impaired function of the immune system. In this context, a decrease in NK cell function was found in both mice and humans [5, 6]. However, an impaired function of immune effector cells by tobacco smoke would not exclude an immunogenicity of lung tumours. Some preliminary answers to the question whether lung cancer is able to induce a sufficient reaction of the immune system were provided by vaccination studies. These studies made use of tumour antigens such as MAGE (melanoma-associated antigen)-A3 or Mucin 1 which are known to be overexpressed in non-small cell lung cancer (NSCLC) and associated with poor prognosis [7, 8]. Phase II clinical trials were conducted using these proteins for vaccination. Albeit trends for a positive clinical response to both vaccines were detectable, both studies did not reach their primary endpoint in terms of significantly prolonged survival [9, 10]. Combining the results of these clinical trials together with the demonstrated immunosuppression by tobacco smoke, these data indicate that lung cancer is not or only a weak inducer of an immune response. However, this conclusion might be amended by an encouraging clinical trial using the vaccine belagenpumaucel-L (LucanixTM) which is a mixture of allogeneic NSCLC cell lines stably transfected to secret an antisense nucleotide to TGF-β. This trial demonstrated a survival benefit of patients with NSCLC as well as an immune reaction to the vaccine [11].
The aim of the present work was to further shed light into the matter how the immune system is involved in the protection from lung cancer. To this end, we used a mouse model of chemically induced lung cancer. A/J mice treated with the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) developed adenomas/adenocarcinomas of the lung [12, 13] and are a well-known mouse model mainly used for chemoprevention studies. In this context, it was shown that certain substances such as isothiocyanates or methoxsalen which are believed to inhibit the mutagenic activation of NNK were able to decrease tumour growth in A/J mice [14, 15]. Also, FTI-276 an inhibitor of the enzyme farnesyltransferase which facilitates the activation of the proto-oncogene Ras demonstrated beneficial effects in the A/J mouse model [16]. The effect of FTI-276 was attributed to mutations of the K-ras gene shown for a high percentage of lung tumours in A/J mice [17, 18]. However, only a few data exist about how the immune system contributes to the carcinogenesis in A/J mice. Splenic and peripheral NK cell number seems to be lower in A/J mice compared with C57BL/6 mice [19], and their cytolytic activity was shown to be lower compared to strains of mice which are not susceptible for the development of lung cancer [20]. Moreover, administration of NNK was reported to further decrease the activity of splenic NK cells in A/J mice, an effect which was converted by the administration of non-steroidal anti-inflammatory drugs [5]. Since non-steroidal anti-inflammatory drugs were additionally shown to inhibit tumour growth in NNK-induced A/J mice [21], NK cells were attributed to play a role in the carcinogenesis of this tumour model. Whether other immune cells contribute to the development of tumours in A/J mice and which mechanisms might be utilized has been investigated in the study presented here.
Materials and methods
Reagents
NNK was purchased from Toronto Research Chemicals, Canada. The stock solution was prepared by solving 100 mg NNK in 200 μl DMSO. The stock solution was further diluted to a final volume of 10 ml with saline. From this solution, 200 μl (2 mg NNK) were injected to mice. For depletion of immune cells, the following antibodies were used: rat anti-CD4, clone GK 1.5 [22]; rat anti-CD8, clone 53-6.7 [23, 24]; rat anti-CD11b, clone 5C6 [25]; and rat anti-Gr-1, clone RB6-8C5 [26], all produced in the laboratory of Hideo Yagita. Rabbit anti-asialo GM1 purchased from Wako Pure Chemical Industries, Osaka, Japan, was used for the depletion of NK cells [23]. Cytokines of the TNF family were depleted by the following antibodies: rat anti-TRAIL, clone N2B2 [27]; rat anti-TWEAK, clone MTW-1 [28]; and hamster anti-FasL, clone MFL4 [29], all produced in the laboratory of Hideo Yagita. In addition, rat anti-TNF, clone V1q [30] which was prepared in the laboratory of Werner Falk was used for neutralization of TNF-α. Control groups were treated with rat IgG or rabbit IgG (both from Sigma, St. Louis, MO) or hamster IgG (Jackson ImmunoResearch, Suffolk, UK).
Handling of mice
All animal experiments were approved by the Kantonale Tierversuchskommission (Kanton Bern, Switzerland). Five-week-old female A/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in isolated ventilated cages. Treatment was applied as described below. All applications of antibodies and NNK were performed as i.p. injections. After completion of the treatment, mice were killed by an overdose of pentobarbital, and lungs were harvested. The tumour number was counted, and the size of tumours was measured using a digital calliper. The latter experiments were all executed with two investigators in which the person who measured tumour number and volume was blinded.
Generation of perforin-deficient A/J mice
Perforin-deficient (PKO) mice on a C57BL/6 background [31] were kindly provided by Hans Hengartner (Institute of Experimental Immunology, University of Zurich, Switzerland). These mice were backcrossed for ten generations with A/J mice. Mice with mutated perforin allele were identified by PCR analysis as described before [32]. In brief, genomic DNA was isolated from ear biopsies using the DNeasy Blood and Tissue kit from QIAGEN (Valencia, CA, USA). For the PCR, two different primer pairs were used. 5′-TTT TTG AGA CCC TGT AGA CCC A-3′, 5′-GCA TCG CCT TCT ATC GCC TTC T-3′) which gives no product for the wild-type (wt) allele and which produces a band of 665 bp for the mutated perforin allele. 5′-CCG GTC CTG AAC TCC TGG CCA A-3′, 5′-CCC CTG CAC ACA TTA CTG GAA G-3′ yields a 300-bp fragment for wt and a 1,300-bp fragment for the mutated allele. Only the second primer pair was able to distinguish between mice harbouring a heterozygous or homozygous mutation of the perforin allele. Perforin-deficient A/J mice mice used in this study will be archived and distributed as live mice or frozen material via the European Mouse Mutant Archive (EMMA), which is part of the INFRAFRONTIER Research Infrastructure (www.infrafrontier.eu) under the strain name A.B6-Prf1tm1Sdz/Biat (EMMA ID EM:07765).
Analysis of Foxp3-positive regulatory T cells (Tregs or CD4+Foxp3+ cells) and myeloid-derived suppressor (CD11+Gr-1+) cells
Splenocytes from wt A/J mice and A/J mice deficient for perforin were freshly isolated as described before [33]. Cells were then incubated with Fc receptor-blocking mAb (clone 2.4G2, BD Biosciences) followed by incubation with fluorochrome-labelled mAbs. For Tregs, after staining for CD4, cells were fixed and permeabilized followed by incubation with the anti-Foxp3 Ab. Staining was measured on an LSRII flow cytometer (BD Biosciences) and analysed using FlowJo software (Tree Star, Ashland, OR).The following Abs were used: anti-CD4 (clone RM4-5, Caltag), anti-Foxp3 (clone 150D, BioLegend), anti-CD11 (clone M1/70, BioLegend) and anti-Gr-1 (clone RB6-8C5, Caltag).
Cytotoxicity assay
NK cell-sensitive Yac-1 cells (ATCC, Manassas, VA) were used as target cells. Yac-1 cells were labelled with 5-(and-6)-carboxyfluorescein diacetate succinimydyl ester (CFSE) using the 7-AAD/CFSE Cell-Mediated Cytotoxicity Assay Kit from Abcam (Cambridge, UK). Labelled Yac-1 cells were then added to a 96-well plate with 1 × 105 cells per well. As effector cells, freshly isolated splenocytes from wt A/J mice, AJ.PKO+/− or AJ.PKO−/− mice were added at an effector/target ratio of 20:1. The plate was centrifuged for 4 min at 30×g to allow cell contact followed by incubation for 4 h at 37 °C. Immediately before analysis, samples were incubated with 7-amino-actinomycin D (7-AAD), and death of target cells was subsequently determined by flow cytometry analysing the number of CFSE+/7-AAD+ events. The final experiments were realized in a manner that the conducting person was blinded for the groups of mice from which splenocytes were isolated.
Statistical analysis
Differences in tumour number and volume were evaluated for significance using Student’s t test or Mann–Whitney U test. A P < 0.05 was considered statistically significant.
Results
Depletion of NK cells promotes tumour development in A/J mice
First, we examined whether certain types of immune cells contribute to the development of lung cancer in A/J mice. Therefore, mice were depleted of CD4+ or CD8+ T cells, CD11b+ macrophages, Gr-1+ neutrophils or asialo GM1+ NK cells using antibodies which were shown before to effectively reduce the number of targeted cells [22–26]. At 6 weeks of age, depletion was initiated with 100 μg of antibody for CD4 [22], CD8 [23, 24], CD11b [25] and Gr-1 [26] and 300 μg for asialo GM1 [23], each in a volume of 200 μl. Treatment was performed twice a week for 20 weeks. Two days after the first application of depleting antibodies, a single dose of 2 mg NNK was injected to mice. As a result, neither the depletion of CD4-, CD8- or CD11b-positive cells nor depletion of neutrophils yielded enhanced tumour growth compared to saline-treated mice or to mice which received the respective control IgG (Fig. 1a, b). Only the treatment with anti-asialo GM1, an antibody which has been widely used for the depletion of NK cells, evoked a significant effect on tumour growth. While there was no difference between saline-treated mice and mice treated with rabbit IgG, NK cell-depleted mice showed a significantly increased tumour number compared to either of these control groups (n = 5; P = 0.047 saline vs anti-asialo GM1 and P = 0.039 rabbit IgG vs anti-asialo GM1, Fig. 1c). No difference was found between the groups when analysing the tumour volume (Fig. 1d). However, the missing difference in tumour volume might be a result of the chosen time point for killing of the animals and would have been different most likely to a later time point.
Fig. 1.
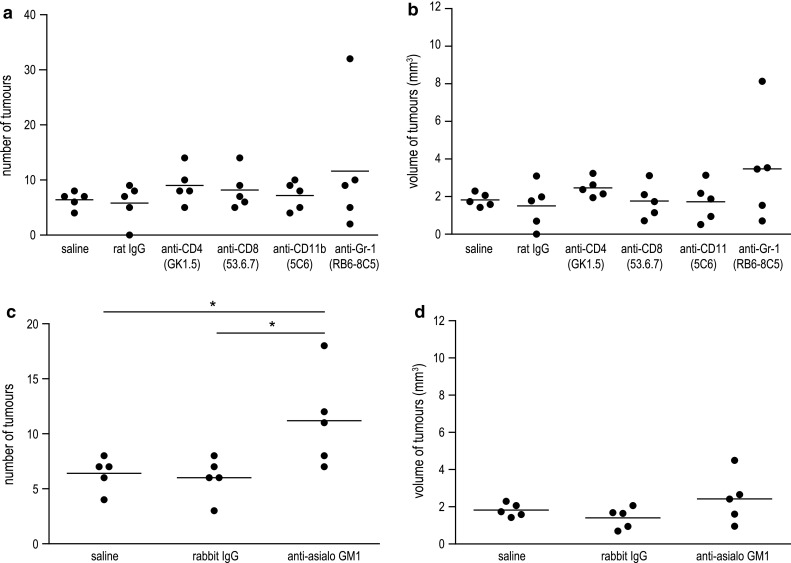
Effect of immune cell depletion on lung tumour development in A/J mice induced with NNK. Depletion of CD4+ or CD8+ T cells, macrophages, neutrophiles and NK cells was initiated in 6-week-old A/J mice using i.p. injections with 100 μg of anti-CD4, anti-CD8, anti-CD11b or anti-Gr-1 mAb or 300 μg of anti-asialo GM1 ab, respectively. Two days after first application of depleting antibodies, mice were induced for lung cancer with 2 mg of NNK. Treatment with antibodies or control IgG was performed twice a week for 20 weeks. The tumour burden was assessed in a blinded manner. a Tumour number and b tumour volume for mice depleted of CD4+ or CD8+ T cells, macrophages or neutrophils demonstrated no significant differences compared to saline- or control rat IgG-treated mice. c Depletion of NK cells with anti-asialo GM1 Ab showed an increased tumour number compared to saline-treated mice or mice treated with control rabbit IgG (n = 5; P = 0.047 saline vs anti-asialo GM1 and P = 0.039 rabbit IgG vs anti-asialo GM1, Student’s t test). d Differences in tumour volume of NK cell-depleted mice were not significant compared to control groups. *P < 0.05
Death-inducing cytokines of the TNF family are not involved in NK cell-mediated tumour suppression in A/J mice
Providing a contribution of NK cells to the suppression of lung cancer in A/J mice, we next asked which mechanisms NK cells used to suppress tumour growth in these mice. Death-inducing cytokines of the TNF family, especially TNF-related apoptosis-inducing ligand (TRAIL) and FasL (CD95L), were demonstrated to be involved in NK cell-mediated cytotoxicity [34]. To explore whether and which of these cytokines might be responsible for the effects of NK cell-mediated tumour suppression, A/J mice were depleted of TRAIL, FasL, TNF-α or TWEAK using specific neutralizing antibodies [27–30]. At 6 weeks of age, mice received a single dose of 2 mg NNK followed by application of 100 μg of neutralizing antibody. Control groups were treated with saline, or rat or hamster IgG. Treatment with antibodies was carried out twice weekly for 21 weeks. The subsequent analysis of tumour burden in the lung revealed no differences between groups neutralized for these cytokines and control groups (Fig. 2a–d). To exclude that this result was caused by redundancy of the neutralized cytokines, we next performed an experiment where TRAIL, FasL and TWEAK were neutralized in combination. The control group received the respective amount of hamster and rat IgG. Similarly to the separate neutralization, the combined neutralization of TRAIL, FasL and TWEAK had no effect on tumour growth, neither on tumour number nor on tumour volume (Fig. 2e, f). Collectively, these data suggest that cytokines of the TNF family do not considerably contribute to the suppression of lung cancer in A/J mice.
Fig. 2.
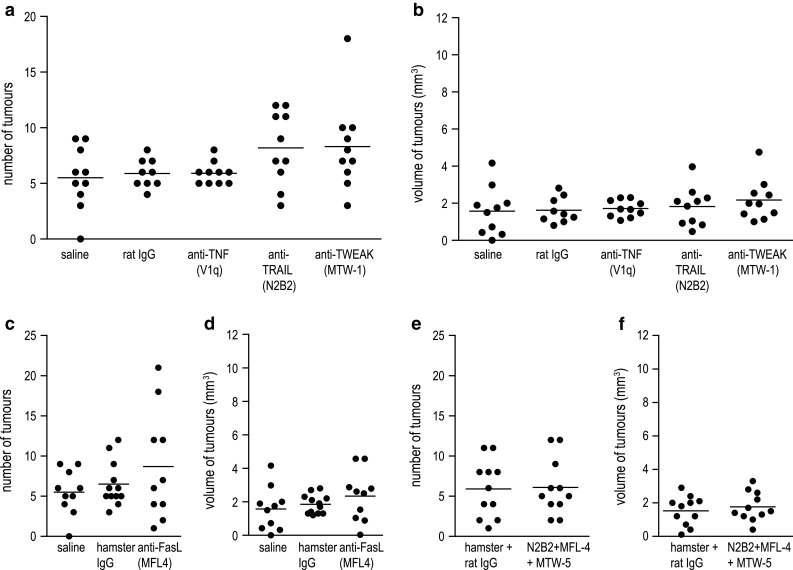
Neutralization of death-inducing TNF family ligands has no influence on tumour development in NNK-treated A/J mice. Six-week-old female A/J mice were exposed to a single dose of 2 mg NNK and subsequently treated with antibodies neutralizing TNF-α, TRAIL, TWEAK or FasL. Treatment with antibodies or control IgG was performed twice a week for 21 weeks. Tumour number (a) and (c) and tumour volume (b) and (d) of animals depleted of single TNF family ligands revealed no differences in tumour growth compared to saline- or IgG-treated groups. Tumour number (e) and tumour volume (f) of mice depleted of TRAIL, FasL and TWEAK in combination were statistically not different from those of the control IgG-treated group
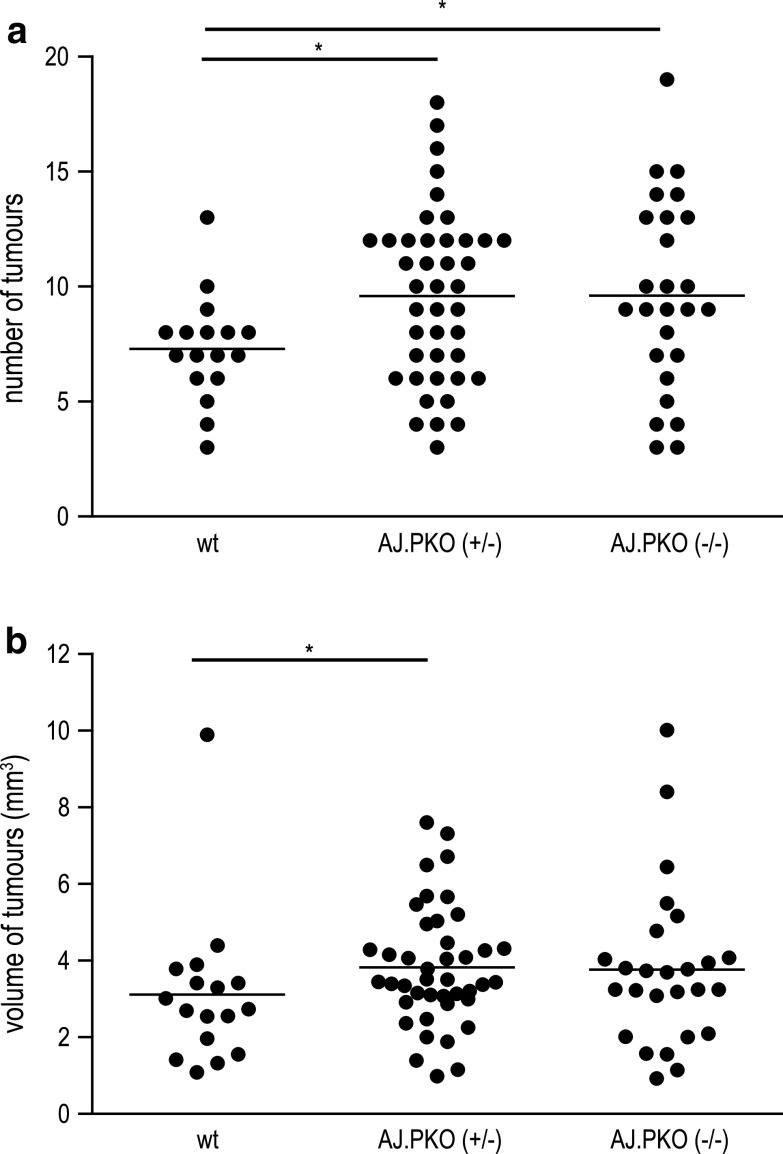
Tumour suppression in A/J mice depends on perforin-mediated cytotoxicity
Another mechanism for cell death induced by NK cells is represented by the perforin/granzyme pathway. While the function of certain members of the granzyme family for immunosurveillance of cancer is uncertain yet, it has been shown that NK cell activity against a number of tumours highly depends on an intact perforin molecule [35]. To answer the question whether perforin-mediated cytolysis is involved in NK cell-mediated tumour suppression in A/J mice, A/J mice deficient for perforin (referred as AJ.PKO) were generated. These mice were induced for lung cancer with 2 mg NNK at 6 weeks of age. Twenty-two weeks later, mice were killed and the tumour burden of A/J mice heterozygously (+/−) or homozygously (−/−) deficient for perforin were compared with wild-type (wt) A/J mice. We performed eight independent experiments with varying numbers of animals. From these eight experiments, one was excluded due to an exceptional high tumour number and volume in all three experimental groups. Analysis of the remaining animals revealed that A/J mice deficient for perforin had a higher susceptibility for NNK-induced lung cancer. Both AJ.PKO+/− and AJ.PKO−/− showed a significantly higher number of lung tumours compared with wt A/J mice (n = 17 for wt, n = 42 for AJ.PKO+/−, n = 26 for AJ.PKO−/−; P = 0.031 for wt vs AJ.PKO+/−, P = 0.036 for wt vs AJ.PKO−/−; Fig. 3a). The assessment of tumour volume demonstrated a significant difference for AJ.PKO+/− compared to wt (P = 0.034; Fig. 3b) but not for AJ.PKO−/− compared to wt (P = 0.16; Fig. 3b).
Fig. 3.
Influence of perforin deficiency on lung tumour development. Six-week-old female AJ.PKO mice (heterozygous or homozygous) were exposed to 2 mg NNK. Twenty-two weeks later, lung tissue was analysed. a AJ.PKO+/− and AJ.PKO−/− had a significantly higher number of lung tumours compared with wt A/J mice (n = 17 for wt, n = 42 for AJ.PKO+/−, n = 26 for AJ.PKO−/−; P = 0.031 for wt vs AJ.PKO+/−, P = 0.036 for wt vs AJ.PKO−/−). b The tumour volume was significantly higher in AJ.PKO+/− compared to wt (P = 0.034) but not in AJ.PKO−/− compared to wt (P = 0.16); Mann–Whitney U test. *P < 0.05
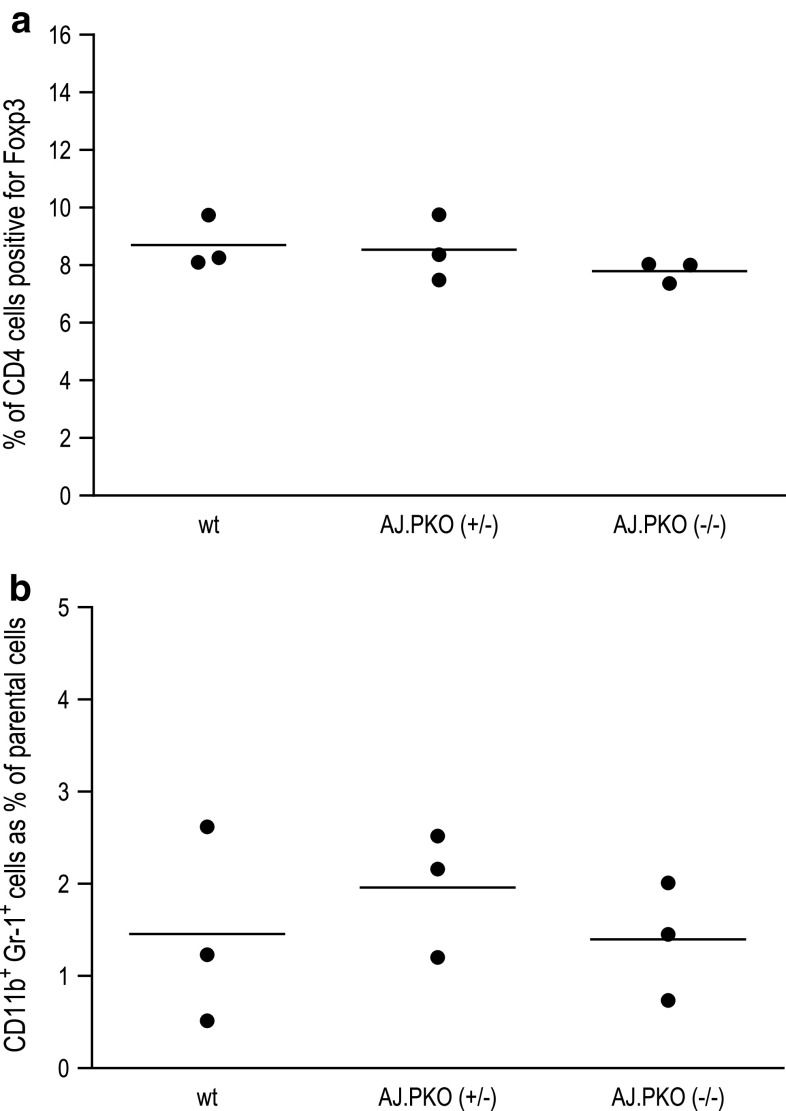
To rule out that immunosuppression other than decreased NK cell activity might play a role in AJ.PKO mice, we measured the level of Tregs (CD4+Foxp3+ cells) and the level of myeloid-derived suppressor (CD11+Gr-1+) cells. Analysis of these cell types in the splenocyte population revealed no difference between wt A/J mice and AJ.PKO mice (Fig. 4a, b), indicating that decreased immunosurveillance in perforin-deficient mice is due to an impaired function of NK cells. To provide more direct evidence for this suggestion, we subsequently measured in vitro cytotoxic splenic NK cell activity of perforin-deficient mice. As shown in Fig. 5, the cytotoxic activity of both AJ.PKO−/+- and AJ.PKO−/−-derived splenic NK cells was significantly lower than the NK cell activity of wt A/J mice. These findings propose that NK cells and the perforin-mediated cytolytic pathway indeed contribute to the prevention of chemically induced lung cancer.
Fig. 4.
Analysis of Tregs (CD4+Foxp3+ cells) and myeloid-derived suppressor (CD11+Gr-1+) cells in perforin-deficient A/J mice. Splenocytes from wt A/J mice, AJ.PKO−/+ and AJ.PKO−/− mice were stained with fluorochrome-labelled mAbs and analysed by flow cytometry
Fig. 5.
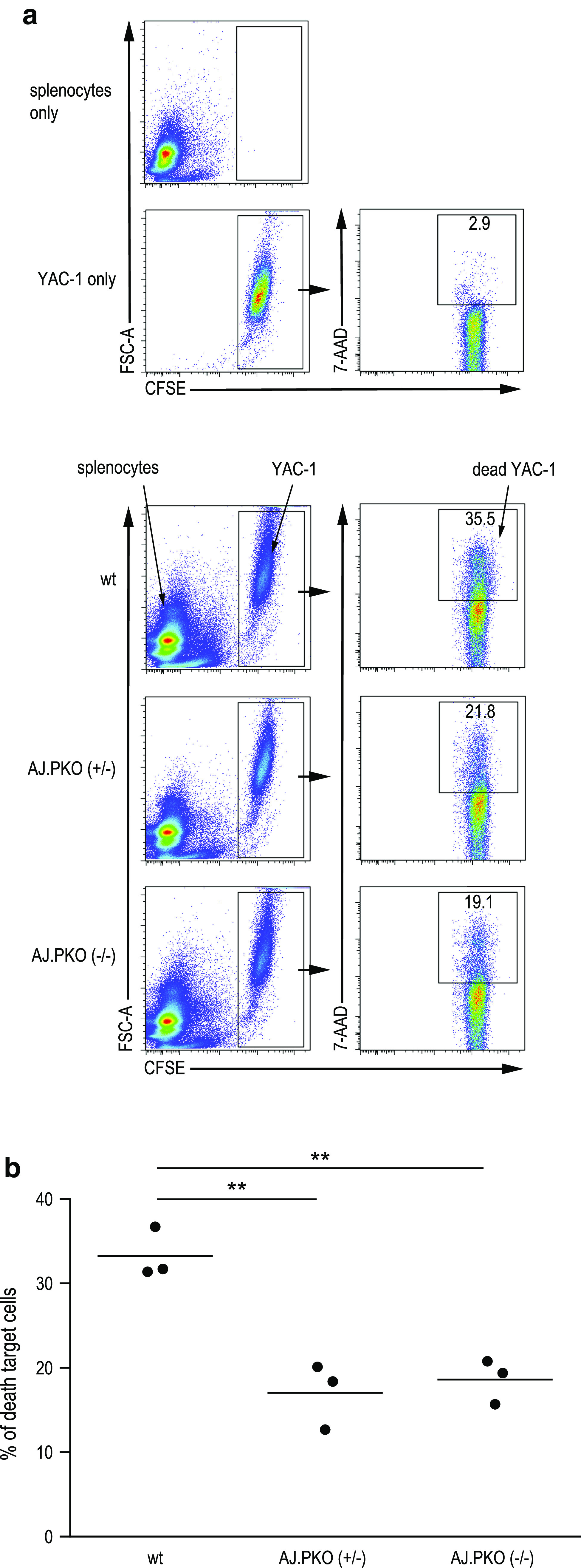
Cytolytic NK cell activity in perforin-deficient A/J mice. NK cell-sensitive Yac-1 cells were used as target cells. Yac-1 cells were labelled with 5-(and-6)-carboxyfluorescein diacetate succinimydyl ester (CFSE). As effector cells, freshly isolated splenocytes from wt A/J mice, AJ.PKO+/− or AJ.PKO−/− were added at an effector/target ratio of 20:1 followed by an incubation for 4 h at 37 °C. Samples were then stained with 7-amino-actinomycin D (7-AAD), and death of target cells was subsequently determined by flow cytometry analysing the number of CFSE+7/AAD+ events. a Representative dot plots for target and effector cells alone (upper panel) and when incubated together (lower panel). b Comparison of cytotoxic NK cell activity of the different groups of mice with n = 3 per group. P = 0.0046 wt A/J versus AJ.PKO+/− and P = 0.0031 wt A/J versus AJ.PKO−/−, Student’s t test. **P < 0.01
Discussion
The immunogenicity of lung cancer remains an unsolved question until now. As referred above, most of the clinical trials for lung cancer using vaccination strategies failed to achieve their primary end points. This problem might have two reasons: One could be that the antigens used for vaccination were not ideal and the other could be that lung cancer is in general not able to induce a sufficient reaction of the immune system. Our study was delineated to elucidate the second issue whether lung cancer has the ability to provoke an immune reaction or not.
In a first set of experiments, A/J mice which were induced for lung cancer with NNK were depleted of different types of immune cells. However, depletion of CD4+ T cells, CD8+ T cells, CD11b+ macrophages or Gr-1+ neutrophils did not significantly affect the tumour burden in A/J mice. These results were not expected because the deficiency of Foxp3-positive regulatory T cells was shown to reduce tumour growth in A/J mice [36]. Tregs are a subset of CD4-positive T cells. Since we depleted in our experiments all CD4-positive cells, one might ask whether a proposed beneficial effect of losing these Tregs had been negotiated by the loss of effector CD4+ T cells. Therefore, we do not exclude from our data that in wild-type A/J mice CD4-positive cells other than Tregs contribute to the suppression of lung cancer.
Our results further demonstrated that depletion of NK cells resulted in an increased tumour number, suggesting a pivotal role of NK cells for the control of tumour growth in this lung cancer model. NK cells execute their cytotoxic effects either by direct target cell lysis or by secretion of cytokines such as interferon-γ, but without the need of antigen-specific recognition as required for cytotoxic T lymphocytes. In this context, NK cell activity was shown to be important for the development or rejection of MHC class-I-deficient lymphomas [37], and depletion of NK cells was also found to promote tumour growth of fibrosarcomas [38]. Furthermore, there is evidence that lung cancer development depends on the function of NK cells. In a recent publication, Kreisel and colleagues demonstrated that depletion of NK cells promoted urethane-induced lung tumour growth in a mouse strain which is normally not susceptible to lung cancer [20]. Moreover, NK cells were shown to have lower activity in A/J mice [19], which are prone to develop lung cancer induced by certain chemicals such as the nitrosamine NNK. NNK itself was demonstrated to further reduce NK cell activity in A/J mice [5], an effect which has been observed in human smokers as well [6]. Despite the assumption of lower NK cell activity in NNK-induced A/J mice, our data suggest that the remaining NK cell activity was sufficient to reduce lung tumour formation. However, once the lung tumours are established, another situation might arise comprising a direct inhibition of NK cell activity by lung cancer cells. Thus, it has been shown that lung tumour cells from malignant pleural effusions inhibit NK cell activity [39], and it was further demonstrated that NK cells isolated from lung tumours exhibit decreased cytotoxic activity [40].
The mechanisms how NK cells induce direct target cell lysis might vary; both the engagement of cytokines of the TNF family as well as the perforin-mediated granule exocytosis pathways are known [34]. Many studies were published showing a significant contribution of TNF family cytokines to the protection from different types of cancer. While the presence of TRAIL and FasL was demonstrated to have anti-cancer attributes [41–43], the genetic ablation or antibody-mediated depletion of TNF-α inhibited the development of skin cancer [44, 45]. Similar to TNF-α, TWEAK seems to act in a pro-cancerogenic manner [46]. However, in our experimental setup, neither the neutralization of TRAIL, FasL, TWEAK or TNF-α alone nor in combination had an influence on NNK-induced lung tumour development. This was somewhat surprising especially for TRAIL since our previous laboratory works provided promising results when treating lung cancer cells with this cytokine [47, 48]. As an alternative pathway, we found in vivo and in vitro evidence that the perforin-mediated pathway contributes to NK cell-mediated tumour suppression in A/J mice, as perforin-deficient A/J mice exhibited increased tumour development and reduced cytolytic NK cell activity. Moreover, the number of other immunosuppressive cells such as Tregs was not different in perforin-deficient mice compared to their wild-type counterpart what might speak against a significant contribution. However, the effect of PKO on tumour growth was less pronounced than the effect of NK cell depletion. Therefore, the involvement of other factors such as IFN-γ or lymphotoxin-α which were shown before to be involved in NK cell-mediated cytotoxicity cannot be excluded [49, 50].
Collectively, our data suggest a significant contribution of NK cells to the suppression of lung cancer. This finding might have important consequences since NK cell activity can be stimulated using the glycolipid α-galactosylceramide (α-GalCer), which activates invariant NKT cells and dendritic cells to produce IFN-γ and IL-12, respectively. Subsequent activation of NK cells by IFN-γ and IL-12 is again accompanied by profound production of IFN-γ [51]. The effects of α-GalCer on NKT and NK cells were further explored in clinical trials. While systemic administration of α-GalCer induced a prolonged depletion of NKT cells from peripheral blood [52], the ex vivo activation of respective NK cells using α-GalCer is another promising approach. A phase I/II study including patients with advanced or recurrent NSCLC has been published recently with encouraging results. A prolonged survival was observed in patients who showed increased IFN-γ production in peripheral blood mononuclear cells stimulated with α-GalCer [53]. However, further studies are needed especially in an adjuvant setting of radically resected T1 and T2 NSCLC in order to see whether stimulation of NK cells lowers the risk of local or metastatic recurrence.
Acknowledgments
We thank Beatrice Zumkehr for technical assistance. We are also thankful to Hans Hengartner for providing a breeding pair of PKO mice on a C57BL/6 background. This work was supported by the Bernische Krebsliga and by the Stiftung für Klinisch-Experimentelle Krebsforschung Bern, both grants to Steffen Frese.
Conflict of interest
None.
Abbreviations
- NSCLC
Non-small cell lung cancer
- NNK
Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NK
Natural killer
- TNF
Tumor necrosis factor
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TWEAK
TNF-like weak inducer of apoptosis
- PKO
Perforin-deficiency
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Midthun DE, Jett JR (2008) Lung tumors. In: Albert RK, Spiro SG, Jett JR (eds) Clinical respiratory medicine, 3rd edn. Mosby Elsevier, Philadelphia, p 605–632
- 3.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rioux N, Castonguay A. Recovery from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced immunosuppression in A/J mice by treatment with nonsteroidal anti-inflammatory drugs. J Natl Cancer Inst. 1997;89(12):874–880. doi: 10.1093/jnci/89.12.874. [DOI] [PubMed] [Google Scholar]
- 6.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42(4):241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gure AO, Chua R, Williamson B, Gonen M, Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ, Altorki NK. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 8.Guddo F, Giatromanolaki A, Koukourakis MI, Reina C, Vignola AM, Chlouverakis G, Hilkens J, Gatter KC, Harris AL, Bonsignore G. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J Clin Pathol. 1998;51(9):667–671. doi: 10.1136/jcp.51.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer. 2009;10(5):371–374. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 10.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, Cormier Y, Ellis P, Price A, Sawhney R, Davis M, Mansi J, Smith C, Vergidis D, Ellis P, MacNeil M, Palmer M. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 11.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, Maples PB, Liu L, Chamberlin T, Shawler DL, Fakhrai H. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, Morse MA, Amin S, Stoner GD, Jordan KG, Choi CI, Chung FL. Rapid single-dose model for lung tumor induction in A/J mice by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the effect of diet. Carcinogenesis. 1989;10(10):1901–1904. doi: 10.1093/carcin/10.10.1901. [DOI] [PubMed] [Google Scholar]
- 13.Belinsky SA, Stefanski SA, Anderson MW. The A/J mouse lung as a model for developing new chemointervention strategies. Cancer Res. 1993;53(2):410–416. [PubMed] [Google Scholar]
- 14.Hecht SS. Chemoprevention by isothiocyanates. J Cell Biochem Suppl. 1995;22:195–209. doi: 10.1002/jcb.240590825. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi H, Saoo K, Yokohira M, Ikeda M, Maeta H, Miyazaki M, Yamazaki H, Kamataki T, Imaida K. Pretreatment with 8-methoxypsoralen, a potent human CYP2A6 inhibitor, strongly inhibits lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Cancer Res. 2003;63(22):7581–7583. [PubMed] [Google Scholar]
- 16.Lantry LE, Zhang Z, Yao R, Crist KA, Wang Y, Ohkanda J, Hamilton AD, Sebti SM, Lubet RA, You M. Effect of farnesyltransferase inhibitor FTI-276 on established lung adenomas from A/J mice induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2000;21(1):113–116. doi: 10.1093/carcin/21.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, Anderson MW. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989;49(19):5305–5311. [PubMed] [Google Scholar]
- 18.Matzinger SA, Crist KA, Stoner GD, Anderson MW, Pereira MA, Steele VE, Kelloff GJ, Lubet RA, You M. K-ras mutations in lung tumors from A/J and A/J x TSG-p53 F1 mice treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and phenethyl isothiocyanate. Carcinogenesis. 1995;16(10):2487–2492. doi: 10.1093/carcin/16.10.2487. [DOI] [PubMed] [Google Scholar]
- 19.Whyte AL, Miller SC. Strain differences in natural killer cell-mediated immunity among mice: a possible mechanism for the low natural killer cell activity of A/J mice. Immunobiology. 1998;199(1):23–38. doi: 10.1016/S0171-2985(98)80061-2. [DOI] [PubMed] [Google Scholar]
- 20.Kreisel D, Gelman AE, Higashikubo R, Lin X, Vikis HG, White JM, Toth KA, Deshpande C, Carreno BM, You M, Taffner SM, Yokoyama WM, Bui JD, Schreiber RD, Krupnick AS. Strain-specific variation in murine natural killer gene complex contributes to differences in immunosurveillance for urethane-induced lung cancer. Cancer Res. 2012;72(17):4311–4317. doi: 10.1158/0008-5472.CAN-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepin P, Bouchard L, Nicole P, Castonguay A. Effects of sulindac and oltipraz on the tumorigenicity of 4-(methylnitrosamino)1-(3-pyridyl)-1-butanone in A/J mouse lung. Carcinogenesis. 1992;13(3):341–348. doi: 10.1093/carcin/13.3.341. [DOI] [PubMed] [Google Scholar]
- 22.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 23.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, Johnstone RW, Smyth MJ. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci USA. 2008;105(42):16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199(4):437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong F, Hansen RD, Yan J, Allendorf DJ, Baran JT, Ostroff GR, Ross GD. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63(24):9023–9031. [PubMed] [Google Scholar]
- 27.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163(4):1906–1913. [PubMed] [Google Scholar]
- 28.Nakayama M, Harada N, Okumura K, Yagita H. Characterization of murine TWEAK and its receptor (Fn14) by monoclonal antibodies. Biochem Biophys Res Commun. 2003;306(4):819–825. doi: 10.1016/S0006-291X(03)01051-9. [DOI] [PubMed] [Google Scholar]
- 29.Kayagaki N, Yamaguchi N, Nagao F, Matsuo S, Maeda H, Okumura K, Yagita H. Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci USA. 1997;94(8):3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145(11):3762–3766. [PubMed] [Google Scholar]
- 31.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 32.Kagi D, Odermatt B, Seiler P, Zinkernagel RM, Mak TW, Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med. 1997;186(7):989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frese-Schaper M, Zbaeren J, Gugger M, Monestier M, Frese S. Reversal of established lupus nephritis and prolonged survival of New Zealand black × New Zealand white mice treated with the topoisomerase I inhibitor irinotecan. J Immunol. 2010;184(4):2175–2182. doi: 10.4049/jimmunol.0903153. [DOI] [PubMed] [Google Scholar]
- 34.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 35.Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. 2010;235(1):35–54. doi: 10.1111/j.0105-2896.2010.00896.x. [DOI] [PubMed] [Google Scholar]
- 36.Granville CA, Memmott RM, Balogh A, Mariotti J, Kawabata S, Han W, Lopiccolo J, Foley J, Liewehr DJ, Steinberg SM, Fowler DH, Hollander MC, Dennis PA. A central role for Foxp3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One. 2009;4(3):e5061. doi: 10.1371/journal.pone.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 38.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13(4):459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 39.Uchida A, Colot M, Micksche M. Suppression of natural killer cell activity by adherent effusion cells of cancer patients. Suppression of motility, binding capacity and lethal hit of NK cells. Br J Cancer. 1984;49(1):17–23. doi: 10.1038/bjc.1984.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 41.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168(3):1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 42.Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195(2):161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5(7):828–831. doi: 10.1038/10462. [DOI] [PubMed] [Google Scholar]
- 45.Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2(5):445–451. [PubMed] [Google Scholar]
- 46.Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom HN, Winkles JA. Soluble tumor necrosis factor-like weak inducer of apoptosis overexpression in HEK293 cells promotes tumor growth and angiogenesis in athymic nude mice. Cancer Res. 2004;64(24):8968–8972. doi: 10.1158/0008-5472.CAN-04-1879. [DOI] [PubMed] [Google Scholar]
- 47.Frese S, Frese-Schaper M, Andres AC, Miescher D, Zumkehr B, Schmid RA. Cardiac glycosides initiate Apo2L/TRAIL-induced apoptosis in non-small cell lung cancer cells by up-regulation of death receptors 4 and 5. Cancer Res. 2006;66(11):5867–5874. doi: 10.1158/0008-5472.CAN-05-3544. [DOI] [PubMed] [Google Scholar]
- 48.Frese-Schaper M, Schardt JA, Sakai T, Carboni GL, Schmid RA, Frese S. Inhibition of tissue transglutaminase sensitizes TRAIL-resistant lung cancer cells through upregulation of death receptor 5. FEBS Lett. 2010;584(13):2867–2871. doi: 10.1016/j.febslet.2010.04.072. [DOI] [PubMed] [Google Scholar]
- 49.Smyth MJ, Johnstone RW, Cretney E, Haynes NM, Sedgwick JD, Korner H, Poulton LD, Baxter AG. Multiple deficiencies underlie NK cell inactivity in lymphotoxin-alpha gene-targeted mice. J Immunol. 1999;163(3):1350–1353. [PubMed] [Google Scholar]
- 50.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97(1):192–197. doi: 10.1182/blood.V97.1.192. [DOI] [PubMed] [Google Scholar]
- 51.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99(4):1259–1266. doi: 10.1182/blood.V99.4.1259. [DOI] [PubMed] [Google Scholar]
- 52.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- 53.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, Hanaoka H, Shimizu N, Suzuki M, Yoshino I, Taniguchi M, Fujisawa T, Nakayama T. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182(4):2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]