Abstract
Translation of most eukaryotic mRNAs and many viral RNAs is enhanced by their poly(A) tails. Hepatitis C virus (HCV) contains a positive-stranded RNA genome which does not have a poly(A) tail but has a stretch of 98 nucleotides (X region) at the 3′-untranslated region (UTR), which assumes a highly conserved stem-loop structure. This X region binds a polypyrimidine tract-binding protein (PTB), which also binds to the internal ribosome entry site (IRES) in HCV 5′-UTR. These RNA-protein interactions may regulate its translation. We generated a set of HCV RNAs differing only in their 3′-UTRs and compared their translation efficiencies. HCV RNA containing the X region was translated three- to fivefold more than the corresponding RNAs without this region. Mutations that abolished PTB binding in the X region reduced, but did not completely abolish, enhancement in translation. The X region also enhanced translation from another unrelated IRES (from encephalomyocarditis virus RNA), but did not affect the 5′-end-dependent translation of globin mRNA in either monocistronic or bicistronic RNAs. It did not appear to affect RNA stability. The free X region added in trans, however, did not enhance translation, indicating that the translational enhancement by the X region occurs only in cis. These results demonstrate that the highly conserved 3′ end of HCV RNA provides a novel mechanism for enhancement of HCV translation and may offer a target for antiviral agents.
All eukaryotic cellular mRNAs and many viral RNAs have a cap structure at the 5′ end and a poly(A) tail at the 3′ end, both of which, individually or in concert, play essential roles in the regulation of translation. Each of these elements works by associating with a specific RNA-binding protein, and together they function synergistically to stimulate translation (see reference 29 for a review). In the yeast system, a translation initiation factor, eIF4G, which is a subunit of eIF4F, associates with the poly(A)-binding protein (Pab1p) (34). This interaction mediates the ability of the poly(A) tail to stimulate translation in vitro (35). These data support the model that the 5′ and 3′ ends of mRNA interact via eIF-4G and Pab1p. However, some viral RNAs lack either the cap structure, poly(A) tail, or both; the mechanism of translational regulation in these RNAs is still unclear.
Hepatitis C virus (HCV) is now recognized as the principal agent of parenterally transmitted non-A, non-B hepatitis. The viral genome has been cloned and sequenced (9) and shown to be a 9.5-kb single-stranded, positive-sense RNA encoding a large polyprotein with a size of about 3,008 to 3,037 amino acids (see reference 10 for a review). Although the overall sequence of HCV RNA shows significant diversity within the coding region among various isolates (see reference 8 for a review), the 5′-untranslated region (5′-UTR) and the 3′-end 98 nucleotides (nt) (termed X region) are highly conserved (7, 22, 33). Conceivably, both the 5′- and 3′-end sequences are important for viral RNA replication and/or translation. The 5′-UTR of HCV RNA has been shown to form extensive secondary structures (6, 37). In this region, the majority of HCV genotypes possess five AUG codons, which are not used for initiation of translation. Tsukiyama-Kohara et al. demonstrated that the HCV 5′-UTR could regulate translation initiation in an internal ribosome entry site (IRES)-dependent manner (37) as in the picornaviruses (19). Furthermore, the HCV 3′-UTR does not have a poly(A) tail but has a variable poly(U-C) stretch plus a conserved X region at the 3′ end (22, 33), which forms a three-stem-loop structure and binds a polypyrimidine tract-binding protein (PTB) (4, 17, 36). PTB binding requires strict primary sequence in the loops and stem structures (17). Interestingly, PTB has also been reported to bind to the IRES regions of several RNA viruses, including HCV, and regulate their translation (1, 2).
The experiments in this study are designed to examine whether the X region of HCV 3′-UTR plays a role in the IRES-dependent translation of HCV RNA. By using an in vitro translation system in rabbit reticulocyte lysate and transfection in mammalian cells, we showed that the HCV X region specifically enhanced the IRES-dependent translation from the 5′ end of HCV RNA. This effect does not depend on the primary sequence of the 5′ end of HCV RNA, since translation from another IRES of an unrelated virus (encephalomyocarditis virus [EMCV]) was also enhanced, although at a lower efficiency. Furthermore, we showed that this enhancement effect was not due to stabilization of RNA by the X region. This effect was seen only in cis, and PTB binding to the X region may be partially responsible, suggesting an interaction between the IRES sequence and the X region, possibly mediated by PTB and other proteins. In contrast, the X region did not stimulate the 5′-end-dependent translation from other RNAs.
Our results indicate that the X region of HCV RNA, probably together with its RNA-binding proteins, can stimulate the IRES-dependent translation from HCV RNA, thus providing a new mechanism for regulating HCV translation. The 5′- and 3′-end interaction in HCV RNA translation may provide a potential target for antiviral therapy.
MATERIALS AND METHODS
Plasmid constructions.
Plasmid pHCV-5CL, which contains a T7 promoter, the 5′-UTR, and the entire core protein-encoding region of HCV-1b strain (1 to 914 nt) and the luciferase gene fused in frame, was made from pT-gHCV (17) and pGL-basic vector (Promega). The cDNA fragment containing the T7 promoter and the 5′-UTR and core region of HCV were made by PCR with pT-gHCV as a template and appropriate primers containing the restriction enzyme sites. After digestion with the appropriate restriction enzymes, this PCR product was cloned into the pGL-basic vector. The cDNA representing the 3′-end 98 nt (X region) of the HCV RNA and an XbaI site at the 5′ end and a BamHI site at the 3′ end was made by PCR from a plasmid, HCV-X(+) (17), and subcloned into the pCR 2.1 vector (Invitrogen) by the TA cloning method (41). The resulting plasmid, pCR-X(+)-XBI, was confirmed by sequencing (30). To generate pHCV-5CL-X, pCR-X(+)-XBI was digested by XbaI and subcloned to pHCV-5CL. The site-directed mutants pHCV-5CL-Xa and pHCV-5CL-Xg were generated by subcloning the PCR fragments containing the corresponding mutations in the plasmid HCV-X(+), as previously described (17).
Plasmid pGL-EMCV, which contains the IRES of EMCV and the luciferase gene under the T7 promoter, was made from pTF7.25EMC-1 (13) and pGL-basic vector by the strategy used for pHCV-5CL. To construct plasmid pGL-α-globin, which contains the 5′-UTR and the coding region of α-globin gene fused in frame to the luciferase (LUC) gene under the T7 promoter, the αglobin gene was amplified from phαG (32) by PCR and subcloned into pGL-basic vector. pGL-EMCV-X and pGL-αglobin-X were generated by insertion of the X region into pGL-EMCV and plasmid pGL-αglobin, respectively.
To construct the plasmid for bicistronic RNA, pCAT-5CL, the chloramphenicol acetyltransferase (CAT) gene in the pOPI3CAT vector (Stratagene) was digested by NotI and subcloned into the NotI site, which had been artificially introduced between the T7 promoter and the 5′-UTR of HCV in pGL-5CL. To generate pCAT-5CL-X, the X gene was inserted at the XbaI site of the pCAT-5CL; the resulting pCAT-5CL-X and pCAT-5CL plasmids transcribe bicistronic RNAs containing a CAT gene preceded by the vector sequence (6 nt) and a LUC gene preceded by the 5′-UTR and the core-encoding sequence of HCV genome.
Thermodynamic calculation of RNA secondary structure.
The optimal and suboptimal secondary structures for the wild-type and mutant X region of HCV RNA molecules were predicted by the method of Zuker (42) with the MulFold program in the Genetics Computer Group sequence analysis software package.
In vitro RNA transcription and translation.
Plasmids were linearized by digestion with various enzymes, including XbaI (for pHCV-5CL, pGL-EMCV, pGL-αglobin-X, and pCAT-5CL), HpaI (for pHCV-5CL, pGL-EMCV, and pCAT-5CL), BamHI (for pHCV-5CL-X, pCAT-5CL-X, and related mutants), or EcoRV (for pGL-EMCV-X and pGL-αglobin-X). RNA was transcribed by T7 RNA polymerase according to the protocol supplied by the manufacturer (Promega). After transcription, 1 U of RQ DNase I (Promega) was added to the reaction mixture to digest DNA templates, extracted with phenol-chloroform, and precipitated with ethanol–7.5 M ammonium acetate. The concentration of RNA was determined by spectrophotometry. To generate capped RNAs, the mMESSAGE kit (Ambion) was used for in vitro transcription reactions.
In vitro translation was carried out in micrococcal nuclease-treated rabbit reticulocyte lysates (Flexi; Promega). Translation reactions (25 μl) were programmed with 2 μg of RNA, 10 μl of lysates, 0.5 U of RNase inhibitor (RNasin; Promega), 2 mM dithiothreitol, 20 μM amino acid mixture minus methionine, and various concentrations of KCl in the presence of 1 μl of [35S]methionine (10 mCi/ml; NEN) and carried out at 30°C for 90 min. For RNAs containing an IRES sequence (e.g., HCV-5CL and [EMCV]) and for α-globin RNAs, translation was carried out in the presence of 120 and 70 mM KCl, respectively. At the end of the reactions, stop buffer (50 μg of RNase A per ml, 10 mM EDTA [pH 7.5]) was added to the reaction mixture. Aliquots of the translation products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5 to ∼10% polyacrylamide gels.
Primer extension.
Two micrograms of each of the various RNAs was incubated with rabbit reticulocyte lysates in the presence of 120 mM KCl under the conditions for in vitro translation. RNA was extracted from the lysates 30 and 90 min after the reaction by using TRIZOL (GIBCO BRL), and one-half of the total RNA was analyzed by primer extension with a 32P-end-labeled primer (5′-AACACTACTCGGCTAGCAGT-3′) complementary to the 5′-UTR of HCV RNA as previously described (17). HCV-5CL-X RNA was used to determine the linear range in which primer extension was performed.
Cell culture and transfection.
Huh7 cells (25), a human hepatoma cell line, were seeded onto 35-mm-diameter tissue culture dishes 48 h prior to transfection. Cells (80% confluent) were infected with recombinant vaccinia virus vTF7-3 (expressing T7 RNA polymerase) (14) in 100 μl of Dulbecco’s modified Eagle’s medium (DMEM) at a multiplicity of infection (MOI) of 10. After incubation at 37°C for 1 h, the virus inoculum was removed and replaced with a mixture containing 2.5 μg of linearized plasmid DNA and 15 μl of DOTAP (Boehringer Mannheim) in 75 μl of 20 mM HEPES buffer (pH 8.0), followed by the addition of 2 ml of DMEM supplemented with 10% fetal bovine serum. After a 24-h incubation at 37°C, the cells were washed with phosphate-buffered saline twice and harvested with 200 μl of the cell lysis buffer (Promega) and prepared for CAT (31) and LUC (12) assays.
LUC and CAT assays.
Cellular lysates of transfected Huh7 cells (from approximately 3 × 105 cells) were centrifuged at 10,000 × g for 5 min. Ten microliters of the supernatant was mixed with 100 μl of luciferase assay reagent (Promega), and the LUC activity was measured after 20 s by Luminometer (Berthold). For the LUC assay of the in vitro translation product, 5 μl of reaction mixture was used.
For the CAT assay, cellular lysates were incubated at 60°C for 10 min, and 100 μl of cell extract was mixed with 15 μl of 0.25 M Tris-HCl (pH 7.4), 3 μl of [14C]chloramphenicol (0.025 mCi/ml; Dupont NEN), and 5 μl of n-butyryl coenzyme A (5 μg/μl; Sigma). After incubation for 8 h at 37°C, the mixture was extracted with 300 μl of xylenes (Mallinckrodt Chemical Works). The phase of xylenes was back-extracted twice with 100 μl of 0.25 M Tris-HCl (pH 8.0), and 200 μl of upper xylenes was measured for the 14C count in a scintillation counter (Beckman Instruments model LS6000IC).
Statistical analysis.
Data from the repeated experiments were averaged and expressed as means ± standard deviations. The effects of the X region on translation were analyzed by Student’s t test for paired samples. P < 0.05 was taken as the level of statistical significance.
RESULTS
The X region of HCV RNA enhances its translation in monocistronic RNAs.
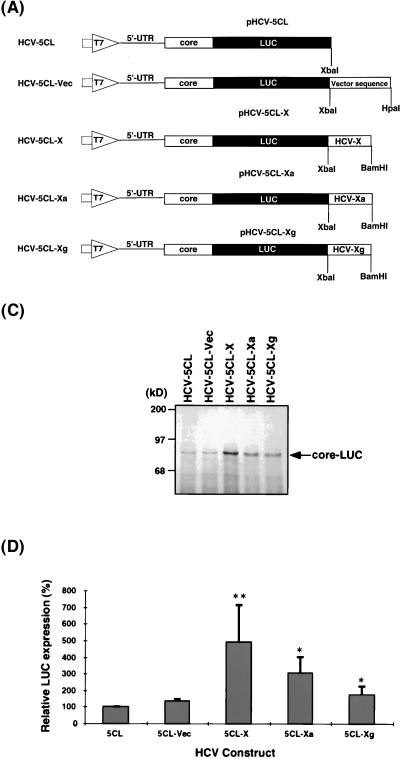
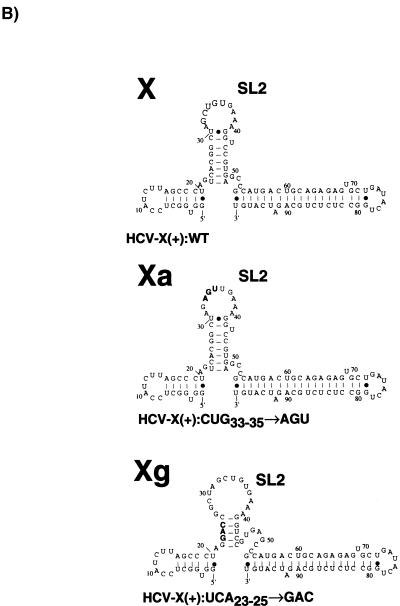
In order to investigate the biological function of the 3′-end 98-nt sequence (X region) on the translation of HCV RNA, we compared the translational efficiency of several synthetic RNAs which contain the authentic 5′-end sequence of HCV RNA, but different sequences at their 3′ ends. Because the 5′ end of the core protein-encoding sequence is part of the IRES structure of HCV RNA (27, 28) and because the 3′ end of the core protein-encoding region has a strong PTB-binding site (18), we included both the 5′-UTR and the entire core protein-encoding region (1 to 914 nt from the 5′ end of the viral RNA) as part of the 5′-end sequence in all of these constructs. These constructs represent the authentic 5′-end structure of HCV RNA at the translation initiation site. The core protein sequence was fused in frame with the LUC gene. Thus, the core-LUC fusion protein is the primary translation product of these constructs. HCV-5CL-X mimics the native HCV RNA in structure, consisting of the entire 5′-UTR at the 5′ end, the X region at the 3′ end, and the coding sequence for the core-LUC fusion protein in the middle (Fig. 1A). HCV-5CL-Vec has 160 nt of vector sequence at the 3′ end, whereas HCV-5CL-Xa and -Xg contain mutations in the X region which affect PTB binding (17). The secondary structure of the HCV-5CL-Xa RNA is predicted to be the same as that of the wild type, but the stem-loop 2 structure of the X region is predicted to be altered in the HCV-5CL-Xg RNA (Fig. 1B). Neither of these two mutant RNAs binds PTB (17). In vitro translation of these RNAs was carried out in rabbit reticulocyte lysates in the presence of 120 mM potassium chloride, which is the physiological salt concentration and allows HCV RNA translation in an IRES-dependent manner (3, 5). The translation products were examined by SDS-PAGE autoradiographs of the core-LUC fusion protein (Fig. 1C) and the LUC activity of the fusion protein (Fig. 1D). The results showed that the translation level of HCV-5CL-X was three- to fivefold higher than that of the control RNAs (HCV-5CL and HCV-5CL-Vec), while the mutations in the X region that affected PTB binding reduced the level of, but did not completely abolish, the translational enhancement. These results indicate that the 3′ end 98 nt of HCV RNA serves as a translational enhancer for its own RNA, and this enhancement may involve, at least partially, the sequence or structure of the PTB-binding site in this region.
FIG. 1.
Functional analysis of the HCV X region by in vitro translation. (A) Schematic diagrams of pHCV-5CL and its related plasmids used in this study. pHCV-5CL contains T7 promoter (large open arrow), the 5′-UTR (single line), and core-encoding region (open box) of the HCV 1b strain fused to LUC genes (closed box) in the pGL vector (Promega). pHCV-5CL-X, -Xa, and -Xg contain, in addition, the X region and its mutants, respectively, at the 3′ end. The plasmids were linearized with the appropriate restriction enzymes and transcribed with T7 RNA polymerase to generate transcripts. (B) Computer-predicted secondary structures of the X region and its mutants in HCV-5CL-X, -Xa, and -Xg RNAs (17). SL2, stem-loop 2. (C) In vitro translation products of various RNAs separated by SDS-PAGE on 7.5% polyacrylamide gels. In vitro translation was carried out in rabbit reticulocyte lysates at 120 mM KCl. An arrow indicates the core-LUC fusion protein. Computer imaging was generated by Adobe Photoshop, version 3.0. (D) Relative LUC activity of the translation products of various RNAs. The LUC activity of HCV-5CL RNA is artificially set at 100%. The columns and bars represent the means and standard deviations of two sets of triplicate studies. The asterisks indicate that the translational enhancement of these RNAs compared to the translational level of HCV-5CL RNA is significant. ∗, P < 0.05; ∗∗, P < 0.01.
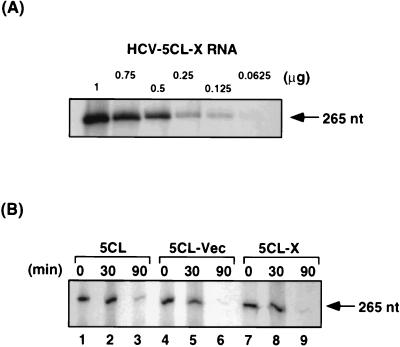
To investigate whether the translational enhancement by the X region was due to possible stabilization of mRNAs, we monitored the stability of these RNAs in rabbit reticulocyte lysates during in vitro translation, by primer extension study with a primer complementary to the 5′-UTR sequence. Primer extension was performed under the condition in which the primer-extended products reflected the amounts of RNA (HCV-5CL-X RNA) in a linear relationship within the range of RNA amounts used in this study (Fig. 2A). The results showed that the amounts of all three RNAs decreased at approximately the same rate (Fig. 2B), indicating that these three RNAs had comparable stabilities and that the translation enhancement effect by the X region was not due to RNA stabilization by this region.
FIG. 2.
Primer extension study of the HCV RNA constructs. (A) Calibration of the primer extension reactions. Decreasing amounts of HCV-5CL-X RNA were used in the primer extension reactions with a 5′-UTR primer, yielding a 265-nt product (arrow). (B) RNA stability of HCV-5CL (lanes 1 to 3), 5CL-Vec (lanes 4 to 6), and 5CL-X (lanes 7 to 9) RNA in rabbit reticulocyte lysates. Two micrograms of each RNA was used in in vitro translation in rabbit reticulocyte lysates. Reactions were stopped at 0 min (lanes 1, 4, and 7), 30 min (lanes 2, 5, and 8), and 90 min (lanes 3, 6, and 9). RNAs were extracted, and half of the amounts from each time points were used in primer extension experiments as in panel A.
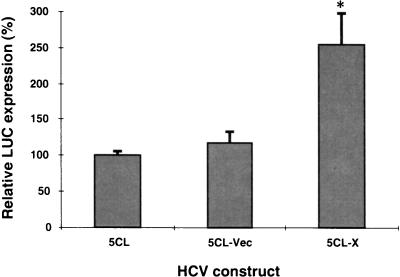
To examine whether this translation enhancement effect of the X region was also seen in intact cells, the plasmid DNAs (pHCV-5CL and pHCV-5CL-X) which had been linearized by the appropriate restriction enzymes were transfected into Huh7 cells infected with the recombinant vaccinia virus expressing T7 RNA polymerase (vTF7-3) (14). The translation efficiency of the transcribed RNA containing the X region (HCV-5CL-X) was found to be two- to threefold higher than those of HCV-5CL and 5CL-Vec (Fig. 3). Thus, the X region can enhance translation in both rabbit reticulocyte lysates and intact cells.
FIG. 3.
Effects of the X region on HCV translation in vivo. Linearized plasmids were transfected into Huh7 cells infected with a recombinant vaccinia virus expressing T7 RNA polymerase. Relative LUC activities in the lysates were determined 24 h after transfection. The columns and bars represent the means and standard deviations of three independent transfections. ∗, P < 0.05 compared with HCV-5CL.
The X region of HCV RNA enhances translation of another IRES-containing RNA but not non-IRES-dependent translation.
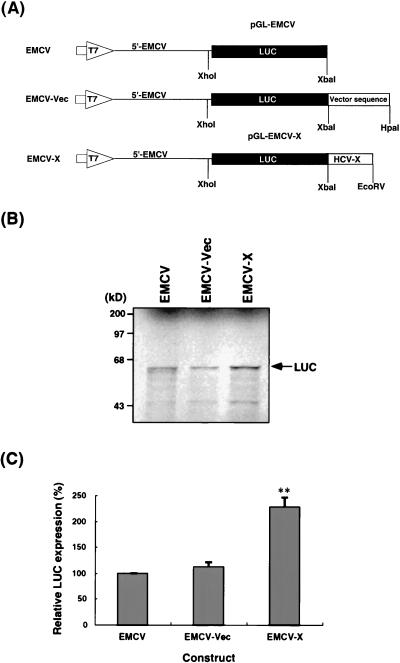
To analyze the mechanism of translational enhancement by the X region, we next asked whether the IRES derived from unrelated RNAs such as EMCV RNA (20) can be stimulated by the HCV X sequence (Fig. 4A). The EMCV-X construct is a chimeric RNA which consists of the entire 5′-UTR of EMCV RNA, LUC coding sequence, and the X region of HCV. An in vitro translation study under the same condition (120 mM KCl) described above showed that the presence of the X region at the 3′ end also enhanced the translation of RNAs by approximately twofold, compared with that of the corresponding RNAs containing vector sequences at the 3′ end or no 3′-UTR at all (Fig. 4B and C). These results indicate that the translational enhancement by the HCV X region is not restricted to the HCV 5′-UTR sequence.
FIG. 4.
Effects of the X region on translation from an EMCV IRES. (A) Schematic diagrams of the plasmids used. pGL-EMCV contains the T7 promoter (large open arrow), the 5′-UTR of EMCV (single line), and LUC genes (closed box) in the pGL vector. pGL-EMCV-X contains, in addition, the X region of HCV at the 3′ end. The plasmids were linearized with the appropriate restriction enzymes and transcribed with T7 RNA polymerase to generate transcripts. (B) In vitro translation products of the various RNAs were separated by SDS-PAGE on 7.5% polyacrylamide gels. In vitro translation was performed in rabbit reticulocyte lysates at 120 mM KCl. An arrow indicates the LUC protein. Computer imaging was generated by Adobe Photoshop, version 3.0. (C) Relative levels of LUC expression of the various RNAs. The activity of the EMCV transcripts is set at 100%. The columns and bars represent the means and standard deviations of two sets of triplicate studies. ∗∗, P < 0.01 compared with EMCV RNA.
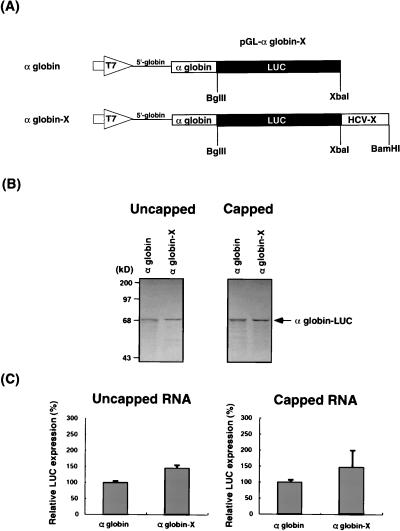
We next asked whether the HCV X region can also enhance the translation of RNAs without IRES. We constructed RNAs containing the natural 5′-UTR of α-globin mRNA, which does not contain IRES and is translated by a 5′-end-dependent mechanism, and the entire globin-coding sequence fused to LUC reporter gene followed by a different 3′-UTR (Fig. 5A). The α-globin-X RNA construct contains the X region of HCV at the 3′-end, whereas the α-globin construct does not have a 3′-UTR. Both uncapped and capped RNAs were translated at 70 mM KCl in rabbit reticulocyte lysates, which allowed more efficient translation of RNAs without an IRES (3, 5) (described below). Figure 5B and C showed that these RNAs, both capped and uncapped, were translated to approximately the same extent. The presence of the HCV X region slightly increased translation efficiency over that of the RNA without a 3′-UTR, but this difference was not statistically significant. These results combined indicate that the X region of HCV can enhance only the IRES-dependent translation but not the 5′-end-dependent translation, including the cap-dependent translation.
FIG. 5.
Effects of the X region on α-globin translation from the 5′-UTR of the α-globin gene. (A) Schematic diagrams of the plasmids used. pGL-αglobin-X contains T7 promoter (large open arrow), the 5′-UTR (single line) and coding region (open box) of α-globin gene fused to LUC genes (closed box), and the X region of HCV in the pGL vector. The plasmids were linearized with the appropriate restriction enzymes and transcribed with T7 RNA polymerase to generate uncapped and capped RNAs. (B) In vitro translation products of uncapped (left) and capped (right) RNAs separated by SDS-PAGE on 7.5% polyacrylamide gels. Translation was performed in rabbit reticulocyte lysates at 70 mM KCl. An arrow indicates the α-globin–LUC fusion protein. Computer imaging was generated by Adobe Photoshop, version 3.0. (C) Relative LUC expression of uncapped (left) and capped (right) RNAs. α-Globin RNA is set at 100%. The columns and bars represent the means and standard deviations of two sets of triplicate studies.
Specific enhancement of the IRES-dependent translation by the X region in bicistronic RNAs.
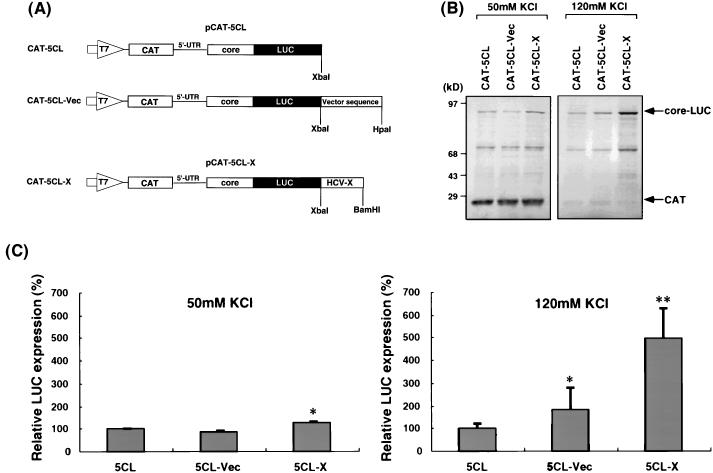
The results presented above showed that the HCV X region can enhance the IRES-dependent translation, but not the 5′-end-dependent translation. To rule out the trivial explanation that these two types of RNA have completely different RNA structures, we combined these two types of translation in bicistronic RNA constructs. These bicistronic RNAs contain a minimum vector sequence (8 nt) preceding the CAT reporter, followed by the HCV 5′-UTR sequence preceding the HCV core-LUC fusion gene (Fig. 6A). The CAT gene is translated in the 5′-end-dependent manner, while the core-LUC fused gene is translated in the IRES-dependent manner. Figure 6B and C showed that when translation was carried out under the physiological salt concentration (120 mM KCl), the translation of core-LUC from CAT-5CL-X, which was translated from an IRES, was three- to fivefold higher than that from RNAs without the X region (CAT-5CL and CAT-5CL-Vec), similar to the translational enhancement observed with the monocistronic RNAs (Fig. 1). In this experiment, there was a marginally significant enhancement of translation by the presence of the vector sequence at the 3′ end of RNA (CAT-5CL-Vec). Determination of the significance of this observation was not further pursued. Under the same conditions, the CAT translation was barely detectable; nevertheless, it can be seen that the presence of the 3′ X sequence in CAT-5CL-X RNA did not enhance the CAT translation at all.
FIG. 6.
Effects of the X region on translation from bicistronic RNAs. (A) Schematic diagrams of plasmids used. pCAT-5CL contains the T7 promoter (large open arrow), the CAT gene (hatched box), the 5′-UTR (single line), and the core protein-encoding region (open box) of HCV fused to a LUC gene (closed box) in the pGL vector. pCAT-5CL-X contains, in addition, the X region at the 3′ end. The plasmids were linearized with the appropriate restriction enzymes and transcribed with T7 RNA polymerase to generate transcripts. (B) In vitro translation products of RNAs with 50 mM KCl (left) or 120 mM KCl (right) after separation by SDS-PAGE on 10% polyacrylamide gels. The core-LUC fusion protein (upper arrow) and CAT (lower arrow) are indicated. (C) Relative LUC expression of RNAs with 50 mM KCl (left) or 120 mM KCl (right). The columns and bars represent the means and standard deviations of two sets of triplicate studies. ∗, P < 0.05; ∗∗, P < 0.01 (compared with CAT-5CL RNA).
To evaluate more precisely the effects of the X gene in these bicistronic RNAs, we also performed translation under the low-salt condition (50 mM KCl). This condition allowed the efficient translation of the CAT gene, which represents the 5′-end-dependent translation, from the bicistronic RNA, similar to the monocistronic RNA (Fig. 4). The results showed that the translation level of the CAT gene from CAT-5CL-X RNA was not significantly different from those from the other two RNAs (Fig. 6B and C), indicating that the X region did not enhance the 5′-end-dependent translation. The core-LUC was translated very inefficiently under this condition; nevertheless, there was still a marginal but statistically significant enhancement of translation by the X region. Thus, the results obtained with the bicistronic RNAs are consistent with those from the monocistronic RNAs and suggest that IRES-dependent, but not 5′-end-dependent, translation can be enhanced by the X region. These results further suggest that the enhancement of the IRES-dependent translation by the X region was not because of the stabilization of the RNA by the X region, since the 5′-end-dependent translation from the same RNA was not enhanced by the presence of the X region.
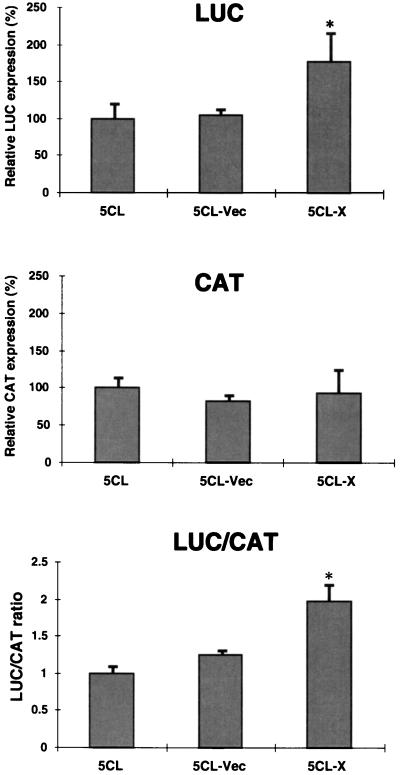
To determine whether the translation-enhancement effects of the X region were also observed in intact cells, these bicistronic RNAs were transfected into Huh7 cells infected with the recombinant vaccinia virus expressing T7 RNA polymerase. The results show that the LUC/CAT ratio from CAT-5CL-X RNA was significantly higher than that from the other RNAs by approximately twofold, confirming the results of in vitro translation, although the level of enhancement was lower in intact cells (Fig. 7). These results indicate that the X region of HCV 3′-UTR can specifically enhance the IRES-dependent translation in vitro and in vivo.
FIG. 7.
Effects of the X region on translation in vivo from bicistronic RNA constructs. Linearized DNAs were transfected into Huh7 cells infected with a recombinant vaccinia virus expressing T7 RNA polymerase. Luciferase and CAT activities were determined at 24 h posttransfection. The relative LUC and CAT activities of the various RNAs and their LUC/CAT ratios are shown. The columns and bars represent the means and standard deviations of three independent transfections. ∗, P < 0.05 compared with CAT-5CL RNA.
The X region added in trans does not have effects on translation.
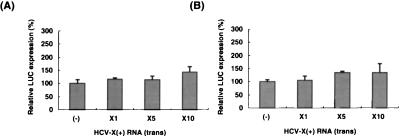
To investigate the possible mechanism of the translational regulation by the HCV X region, we asked whether the X region has any effects on translation when added in trans. Various amounts of X region RNA transcript were added to in vitro translation reaction mixtures containing CAT-5CL RNA, which did not have a 3′-UTR, and the LUC activity was determined. Figure 8A showed that the free X region added in trans did not significantly enhance the translation of RNA without the 3′-UTR, suggesting that the HCV X region can enhance the IRES-dependent translation only as a cis-acting element. We then studied whether the X region added in trans could inhibit the translational enhancement effect of the X region in the CAT-5CL-X. If the enhancement effect of the X region is mediated by a trans-acting factor, then the free X region added in trans is expected to deplete this factor and inhibit CAT-5CL-X translation. The results showed that the X region added in trans did not inhibit the LUC activity of CAT-5CL-X RNA (Fig. 8B). These results combined indicate that the translation enhancement effect of the HCV X region works only in cis and may involve not only translation factors but an RNA element as well.
FIG. 8.
The trans effects of the X region on translation. A 1- to 10-fold excess of HCV X(+) RNA (17) was added to rabbit reticulocyte lysate containing CAT-5CL RNA (A) or CAT-5CL-X RNA (B). In vitro translation was carried out at 120 mM KCl. Translation without free HCV-X(+) RNA [(−)] is set at 100%. The columns and bars represent the means and standard deviations of three independent translation reactions.
DISCUSSION
In this study, we have demonstrated that the X region in HCV 3′-UTR enhanced HCV RNA translation in rabbit reticulocyte lysates and in intact mammalian cells (Fig. 1). This enhancement was not due to RNA stabilization by the X region (Fig. 2). Mutations in the X region which affected PTB binding reduced, but did not completely abolish, the translational enhancement, suggesting that the PTB–3′-UTR interaction may be at least partially involved in the regulation of HCV translation. Furthermore, this region also enhanced translation from another IRES derived from EMCV RNA, but did not affect the 5′-end-dependent translation of α-globin mRNA (Fig. 4 and 5). Bicistronic RNAs which contain an IRES between two reporter genes confirmed that the X region enhanced only IRES-dependent translation (Fig. 6). Thus, the X region in HCV 3′-UTR very likely regulates HCV RNA translation in natural infection.
Because of the absence of a poly(A) tail in HCV RNA, the mechanism of regulation of HCV RNA translation is predicted to be different from those of most eukaryotic mRNAs and other viral RNAs. The X region in HCV 3′-UTR binds PTB, which recognizes the primary sequences and stem structures in this region (17, 36). PTB has also been reported to interact with the IRESs of many RNA viruses, including HCV and EMCV (1, 2), and it was thought to be a translation factor (1, 2, 15, 16, 38–40), although this claim has been disputed (21, 27). The PTB–3′-UTR complex may play a role similar to that of the Pab1p-poly(A) tail complex in the translation of eukaryotic mRNAs. Our data from in vitro translation in rabbit reticulocyte lysates indeed showed that the X region enhanced translation nearly fivefold, when compared with control RNAs without the X region, at the physiological salt concentration (23) (Fig. 1 and 6C). When translation was carried out at a nonphysiological salt concentration (50 to 70 mM KCl), in which translation was predominantly from the 5′ end of RNA, the enhancement effect of the X region on translation from the HCV 5′-UTR was only marginal (Fig. 6B). Thus, the effects of translational enhancement by the X region are most significant when translation is strictly IRES dependent.
When RNAs were expressed in mammalian cells, the X region enhanced HCV translation two- to threefold (Fig. 3), but not the three- to fivefold stimulation as seen in translation in reticulocyte lysates, suggesting either that the in vitro and in vivo conditions are different or that the HCV RNA may undergo 5′-end-dependent translation as well as IRES-dependent translation in vivo. Regardless, our data clearly showed that the X region enhanced HCV RNA translation both in vitro and in vivo. This enhancement may be mediated, at least partially, by PTB interacting with the X region. However, mutations in the PTB-binding site did not completely abolish the translational enhancement by the X region. This may have been due to the binding of a residual amount of PTB (17). Alternatively, other translation factors or primary sequence or secondary structure of X region RNA may also be involved in this enhancement.
In vitro translation studies with another IRES-containing RNA from an unrelated virus (EMCV) and non-IRES RNAs (α-globin) showed that the X region in HCV 3′-UTR enhances the IRES-dependent translation only (Fig. 4 and 5). These results were confirmed by the experiments with bicistronic RNAs (Fig. 6), which also indicated that this enhancement was not because of the stabilization of RNA by the X sequence. Direct measurement of RNA stability in rabbit reticulocyte lysates also indicated that the X region does not protect the 5′ end of HCV RNA from degradation (Fig. 2). Thus, the most likely mechanism of the translational enhancement is that the X region interacts with the IRES-specific translational machinery. Recently, Pestova et al. reported that the mechanism of initiation of HCV translation is different from that of other IRES-containing RNAs, e.g., EMCV RNA, but is similar to that of prokaryotic RNAs (26). This may explain why the level of enhancement by the X region on EMCV translation (two- to threefold) is lower than that on HCV translation (fivefold). The fact that PTB binds to the IRES of both HCV and EMCV RNA (1, 16) suggests that PTB may be involved in the translational enhancement by the X region in both cases. The X region in HCV 3′-UTR may interact with these IRESs through PTB. Our recent data showed that the binding of PTB to the X region is at least 100 times stronger than that to the 5′-UTR of HCV (18). Thus, the binding of PTB to the X region may be the most significant factor involved in the translational enhancement. However, the role of PTB in this 3′-UTR-enhanced translation has not yet been directly tested. Also, it is likely that other protein factors and/or RNA structures are involved in this enhancement as well. It should be noted that most of the RNAs used in this study included the entire core protein-coding region. Recently, we found that the inclusion of this stretch of sequences in the RNAs is crucial for demonstrating the translational enhancement effects of the X region (18). Since the core protein-coding region also binds PTB (18), this observation further suggests the importance of the PTB-X region interactions in translational enhancement. Also, this observation suggests that the translational enhancement observed in this study likely reflects the regulation of translation in the virus-infected cells, because natural HCV infection has to utilize full-length viral RNA, including the core protein-coding region, for translation.
This study suggests that the functions of the X region may be similar to that of poly(A) in translation. However, poly(A) added in trans can inhibit translation from capped polyadenylated mRNAs and stimulate translation from capped RNA without a poly(A) tail (24). In contrast, the X region does not affect the translation of HCV RNA with or without the X region when it is added in trans (Fig. 8), suggesting that the mechanism of translational enhancement by the X region is different from that of the poly(A) tail. The function of poly(A) is thought to be mediated through Pab1p (11, 34). In yeast, a poly(A) tail enhances the mRNA translation by interacting with eIF4G through Pab1p (34), suggesting that mRNA can be circularized by the interaction between Pab1p and eIF4G. Recently Craig et al. also reported that interaction of poly(A)-binding protein with PAIP, which is an eIF4G homolog, enhances translation in human cells (11). This PAIP binds eIF4A to enable the 3′ end of mRNA to communicate with the 5′ end. Whether the function of the X region is mediated through PTB by a mechanism similar to that of poly(A)-Pab1p requires more stringent tests. Nevertheless, the failure of the X region to act in trans may be due to the possibility that PTB is in excess in rabbit reticulocyte lysates, so that PTB could not be completely depleted by the exogenous X RNA, whereas the poly(A)-binding protein is in limited supply. In any case, the enhancement of translation of HCV RNA by its 3′ end may provide a novel target for anti-HCV agents.
ACKNOWLEDGMENTS
We thank Daphne Shimoda for assistance in preparing the manuscript.
This work was partially supported by research grant AI 40038 from the National Institutes of Health. T.I. is a Research Associate and M.M.C.L. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Ali N, Siddiqui A. Interaction of polypyrimidine tract-binding protein with the 5′ noncoding region of the hepatitis C virus RNA genome and its functional requirement in internal initiation of translation. J Virol. 1995;69:6367–6375. doi: 10.1128/jvi.69.10.6367-6375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsham G J, Sonenberg N. RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol Rev. 1996;60:499–511. doi: 10.1128/mr.60.3.499-511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann J E, Lodish H E. Translation of capped and uncapped vesicular stomatitis virus and reovirus mRNAs. J Biol Chem. 1979;254:459–468. [PubMed] [Google Scholar]
- 4.Blight K J, Rice C M. Secondary structure determination of the conserved 98-base sequence at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1997;71:7345–7352. doi: 10.1128/jvi.71.10.7345-7352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman A M, Bailly J-L, Girard M, Kean K M. Picornavirus internal ribosome entry segments: comparison of translation efficiency and the requirements for optimal internal initiation of translation in vitro. Nucleic Acids Res. 1995;23:3656–3663. doi: 10.1093/nar/23.18.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown E A, Zhang H, Ping L-H, Lemon S M. Secondary structure of the 5′ nontranslated regions of the hepatitis C virus and pestivirus genomic RNAs. Nucleic Acids Res. 1992;20:5041–5045. doi: 10.1093/nar/20.19.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukh J, Purcell R H, Miller R M. Sequence analysis of the core gene of 14 hepatitis C virus genotypes. Proc Natl Acad Sci USA. 1994;91:8239–8243. doi: 10.1073/pnas.91.17.8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukh J, Miller R H, Purcell R H. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin Liver Dis. 1995;15:41–63. doi: 10.1055/s-2007-1007262. [DOI] [PubMed] [Google Scholar]
- 9.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 10.Clarke B. Molecular virology of hepatitis C virus. J Gen Virol. 1997;78:2397–2410. doi: 10.1099/0022-1317-78-10-2397. [DOI] [PubMed] [Google Scholar]
- 11.Craig A W B, Haghighat A, Yu A T K, Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- 12.de Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elroy-Stein O, Fuerst T R, Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci USA. 1989;86:6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuerst J A, Niles E G, Studier F W, Moss B. Eucaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–81226. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellen C U T, Pestova T V, Litterst M, Wimmer E. The cellular polypeptide p57 (pyrimidine tract-binding protein) binds to multiple sites in the poliovirus 5′ nontranslated region. J Virol. 1994;68:941–950. doi: 10.1128/jvi.68.2.941-950.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellen C U T, Witherell G W, Schmid M, Shin S H, Pestova T V, Gil A, Wimmer E. A cytoplasmic 57 kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc Natl Acad Sci USA. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Lai M M C. Determination of the secondary structure of and cellular protein binding to the 3′-untranslated region of the hepatitis C virus RNA genome. J Virol. 1997;71:8698–8706. doi: 10.1128/jvi.71.11.8698-8706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, T., and M. M. C. Lai. Unpublished observation.
- 19.Jackson R J, Howell M T, Kaminski A. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem Sci. 1990;15:477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- 20.Jang S K, Davies M V, Kaufman R J, Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989;63:1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaminski A, Hunt S L, Patton J G, Jackson R J. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- 22.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews C K, van Holde K E. Biochemistry. Redwood City, Calif: Benjamin-Cummings Publishing Co.; 1990. pp. 298–332. [Google Scholar]
- 24.Munroe D, Jacobson A. mRNA poly(A) tail, a 3′ enhancer of translational initiation. Mol Cell Biol. 1990;10:3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakabayashi H, Taketa K, Miyano T, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated function in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 26.Pestova T V, Shatsky I N, Fletcher S P, Jackson R J, Hellen C U T. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C virus and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds J E, Kaminski A, Kettinen H J, Grace K, Clarke B E, Carroll A R, Rowlands D J, Jackson R J. Unique features of internal initiation of hepatitis C virus RNA translation. EMBO J. 1995;14:6010–6020. doi: 10.1002/j.1460-2075.1995.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rijnbrand R, Bredenbeek P, van der Straaten T, Whetter L, Inchauspe G, Lemon S, Spaan W. Almost the entire 5′ nontranslated region of hepatitis C virus is required for cap-independent translation. FEBS Lett. 1995;365:115–119. doi: 10.1016/0014-5793(95)00458-l. [DOI] [PubMed] [Google Scholar]
- 29.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seed B, Sheen J-Y. A simple phase-extraction assay for chloramphenicol acetyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 32.Tahara S M, Dietlin T A, Bergmann C C, Nelson G W, Kyuwa S, Anthony R P, Stohlman S A. Coronavirus translational regulation: leader affects mRNA efficiency. Virology. 1994;202:621–630. doi: 10.1006/viro.1994.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T, Kato N, Cho M-J, Sugiyama K, Shimotohno K. Structure of the 3′ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarun S Z, Sachs A B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 35.Tarun S Z, Welis S E, Deardorff J A, Sachs A B. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc Natl Acad Sci USA. 1997;94:9046–9051. doi: 10.1073/pnas.94.17.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchihara K, Tanaka T, Hijikata M, Kuge S, Toyoda H, Nomoto A, Yamamoto N, Shimotohno K. Specific interaction of polypyrimidine tract-binding protein with the extreme 3′-terminal structure of the hepatitis C virus genome, the 3′X. J Virol. 1997;71:6720–6726. doi: 10.1128/jvi.71.9.6720-6726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witherell G W, Gil A, Wimmer E. Interaction of polypyrimidine tract binding protein with the encephalomyocarditis virus mRNA internal ribosomal entry site. Biochemistry. 1993;32:8268–8275. doi: 10.1021/bi00083a030. [DOI] [PubMed] [Google Scholar]
- 39.Witherell G W, Schultz-Witherell C S, Wimmer E. cis-Acting elements of the encephalomyocarditis virus internal ribosomal entry site. Virology. 1995;214:660–663. doi: 10.1006/viro.1995.0081. [DOI] [PubMed] [Google Scholar]
- 40.Witherell G W, Wimmer E. Encephalomyocarditis virus internal ribosomal entry site RNA-protein interactions. J Virol. 1994;68:3183–3192. doi: 10.1128/jvi.68.5.3183-3192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou M Y, Clark S E, Gomez-Sanchez C E. Universal cloning method by TA strategy. BioTechniques. 1995;19:34–35. [PubMed] [Google Scholar]
- 42.Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamic and auxiliary information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]