Abstract
Backgroud
Cancer stem cells (CSCs) are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Development of specific therapies targeted at CSCs holds hope for the improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease. This is particularly true in chronic myeloid leukemia (CML).
Methods
In this study, we isolated fetal liver kinase-1-positive (Flk1+) cells carrying the BCR/ABL fusion gene from the bone marrow of Philadelphia chromosome-positive (Ph+) patients with stem cells property. We examined their biological characteristics as well as immunological function and further detected the possible molecular mechanism involved in the leukemia genesis.
Results
We showed that CML patient-derived Flk1+CD31−CD34− MSCs had normal morphology, phenotype and karyotype but appeared impaired immunomodulatory function. The capacity of Flk1+CD31−CD34− MSCs from CML patients to inhibit T lymphocyte activation and proliferation was impaired in vitro. CML patient-derived MSCs have dampening immunomodulatory functions, suggesting that the dysregulation of hematopoiesis and immune response might originate from MSCs rather than HSCs. These Ph+ putative CML hemangioblast upregulated TGF-β1 and resultantly activated matrix metalloproteinase-9 (MMP-9) to enhance s-KitL and s-ICAM-1 secretion, which activated c-kit+ HSCs from the quiescent state to proliferative state. Further studies showed that phosphatidylinositol-3 kinase (PI3K)/Akt/nuclear factor (NF)-κB signaling pathway was involved in CML pathogenesis.
Conclusions
Flk1+CD31−CD34− MSCs that express BCR/ABL leukemia oncogene are CSCs of CML and they play a critical role in the progression of CML through PI3K/Akt/NF-κB/MMP-9/s-ICAM-1/s-KitL signaling pathway beyond HSCs.
Keywords: Chronic myeloid leukemia (CML), Cancer stem cells (CSCs), Hematopoietic stem cell (HSC), Immunological function, Matrix metalloproteinase-9 (MMP-9)
Introduction
Cancer stem cells (CSCs) are cancer cells (found within tumors or hematological cancers) that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are, therefore, tumorigenic (tumor-forming), perhaps in contrast to other nontumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for the improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease. This is particularly true in chronic myeloid leukemia (CML) [1–3]. The discovery of the Philadelphia chromosome followed by identification of its BCR/ABL fusion gene product and the resultant constitutively active P210 BCR/ABL tyrosine kinase prompted the unravelling of the molecular pathogenesis of CML. However, regardless of greatly reduced mortality rates with BCR/ABL targeted therapy, most patients harbor quiescent CML CSCs that may be a reservoir for disease progression to blast crisis. The existence of CSCs has several implications in terms of future cancer treatment and therapies. These include disease identification, selective drug targets, prevention of metastasis, and development of new intervention strategies.
Under steady state conditions, these CSCs are localized in a microenvironment known as the stem cell microenvironment, where they are maintained in an undifferentiated and quiescent state. These microenvironment are critical for regulating the self-renewal and cell fate decisions, yet molecular mechanisms underlying maintenance of quiescent stem cells in these specialized environments and why and how these cells are recruited to affect leukemia progression are not well known. Local secretion of proteases has been implicated in this tumor–stroma crosstalk. Matrix metalloproteinase-9 (MMP-9) is one of the proteases that has the preferential ability to degrade denatured collagens (gelatin) and collagen type IV, the 2 main components of basement membranes, and, therefore, plays a critical role in tumor progression and metastasis [4]. Previous studies have demonstrated localization of MMP-9 on the plasma membrane of various tumor cells [5–7], and recently, the role of MMP-9 in CML pathogenesis has became a focus of attention [8–11]. But the research is mainly focusing on the MMP-9 inducing molecules [12–14] or the effect of MMP-9 inhibitors [15]. However, it has become clear that the role of MMP-9 in CML is not limited to simple extracellular matrix (ECM) degradation [16]. The regulation of MMP-9 is found to be involved in multiple pathways induced by different kinds of cytokines in different cell types and illness [17]. Therefore, it is necessary to verify a specific MMP-9-induced pathway in a given cell type.
We have identified fetal liver kinase-1-positive (Flk1+) cells carrying the BCR/ABL fusion gene from the bone marrow of Philadelphia chromosome-positive (Ph+) patients with CML and found that these cells could differentiate into malignant blood cells and phenotypically defined endothelial cells at the single-cell level, suggesting these cells have the properties of CSCs [18, 19]. Membrane-bound cytokines, such as m-KitL and m-ICAM-1, not only convey survival signals, but support the adhesion of stem cells to the stroma. CML-specific BCR/ABL oncogene induced TGF-β1-activated PI3K/Akt/NF-κB/MMP9 signaling pathway resulting in the release of s-KitL and s-ICAM-1, enhanced recruitment, and mobilization of CSCs to the peripheral circulation.
Materials and methods
Regents
The following antibodies and reagents were used: antiphospho-Akt (Ser473; Calbiochem, San Diego, CA); anti-MMP-9 (Chemicon, Temecula, CA); anti-Akt1/2, mouse immunoglobulin G (IgG) agarose beads, anti-IKKα agarose beads, anti-IKKα, anti-IKKβ, protein A/G agarose beads (Santa Cruz Biotechnology, CA); Wortmannin, MG-132, SH-5 (Alexis Biochemicals, San Diego, CA); phosphoglycogen synthase kinase (GSK)-3α/β (Ser21/9) antibody, GSK-3 fusion protein, kinase buffer, adenosine 5′-triphosphate (ATP; Cell Signaling Technology, Beverly, MA); elution buffer (Pierce Biotechnology, Rockford, IL); nuclear extract kit, TransAMTM NF-κB transcription factor assay kit (Active Motif, Carlsbad, CA).
Patient samples
Twenty patients with newly diagnosed CML (12 male and 8 female, aged 17–63 years) were recruited in this study. All were Ph+ patients with CML in chronic phase as revealed by bone marrow histology and cytogenetic analysis. The immunophenotypes of thawed cells were quite variable. None was treated with hydroxyurea or interferon before. The control samples were from 20 healthy donors (12 male and 8 female, aged 21–60 years). Bone marrow samples were collected after obtaining informed consent according to procedures approved by the Ethics Committee at the 309th Hospital of Peoples Liberation Army.
Cell preparations and culture
Isolation and culture of bone marrow-derived CML CSCs were performed as described previously with some modifications [19–21]. Briefly, mononuclear cells were separated by a Ficoll-Paque gradient centrifugation (specific gravity 1.077 g/ml; Nycomed Pharma AS, Oslo, Norway) and the sorted cells were plated at concentration of 1 cell/well by limiting dilution in a total of 96× + 0 wells coated with fibronectin (Sigma, St. Louis, MO) and collagen (Sigma) for each patient. Culture medium was Dulbecco modified Eagle medium and Ham F12 medium (DF12) containing 40 % MCDB-201 medium complete with trace elements (MCDB) (Sigma), 2 % fetal calf serum (FCS; Gibco Life Technologies, Paisley, United Kingdom), 1× insulin transferrin selenium (Gibco Life Technologies), 10−9 M dexamethasone (Sigma), 10−4 M ascorbic acid 2-phosphate (Sigma), 20 ng/ml interleukin-6 (Sigma), 10 ng/ml epidermal growth factor (Sigma), 10 ng/ml platelet-derived growth factor BB (Sigma), 50 ng/ml fetal liver tyrosine kinase 3 (Flt-3) ligand (Sigma), 30 ng/ml bone morphogenetic protein-4 (Sigma), 100 U/ml penicillin and 100 ug/ml streptomycin (Gibco Life Technologies) at 37 °C and a 5 % CO2 humidified atmosphere. Culture media were changed every 4–6 days.
Fluorescence-activated cell sorting (FACS)
For immunophenotype analysis, expanded clonal cells were stained with antibodies against Flk1, CD29, CD31, CD34, CD44, CD45, CD105, HLA-ABC, vWF (all from Becton–Dickinson Immunocytometry Systems, Mountain View, CA). For intracellular antigen detection, cells were first fixed in 2 % paraformaldehyde (Sigma) for 15 min at 4 °C and permeabilized with 0.1 % saponin (Sigma) for 1 h at room temperature. Cells were washed and labeled with fluorescein isothiocyanate (FITC) conjugated secondary goat antimouse, goat antirabbit, or sheep antigoat antibodies (Sigma) and then washed and analyzed using a FACS Calibur flow cytometer (Becton–Dickinson, San Jose, CA).
RT-PCR
RNA isolation and reverse transcription were performed as previously described [22]. Oligonucleotide primer sequences were as follows: β-actin (264 bp), forward: 5′-GAG ACC TTC AAC ACC CCA GCC-3′, reverse: 5′-AAT GTC AC G CAC GATT TCC C-3′; s-KitL (263 bp), forward: 5′-AGA GGT CTC AGA AGG GAC CG-3′, reverse: 5′-GGG CCA TAC AGG ACA CGA AG-3′; s-ICAM-1(167 bp), forward: 5′-TGC TTT TTC CAG GGG TGT GTT-3′, reverse: 5′-TAC TTC CTG CAC TAA TTT GGC A-3′; TGFβ1 (198 bp), forward: 5′-GGA AAC CCA CAA CGA AAT CTA TGA C-3′, reverse: 5′-TTG CTG AGG TAT CGC CAG GAA T-3′; TGFβ2 (213 bp), forward: 5′-CGC CAA GGA GGT TTA CAA AAT AGA C-3′, reverse: 5′-TCA ATC CGT TGT TCA GGC ACT CT-3′; TGFβ3 (233 bp), forward: 5′-CTG GCG GAG CAC AAC GAA CT-3′, reverse: 5′-AGG ATA TCT CCA TTG GGC TGA AAG-3′; MMP-9 (201 bp), forward: 5′-TCC CCA TCG CCA TCC CC-3′, reverse: 5′-CAC CAT GGC CTC GGC TGG-3′. For all the above genes, amplification was performed under the same cycling conditions (1 min at 94 °C, 50 s at 57 °C, 1 min at 72 °C), except the number of cycles that was specified for each gene (31 for s-KitL, s-ICAM-1, TGFβ1, TGFβ2 and TGFβ3, 32 for MMP-9).
Western blot and immunoprecipitation
CML CSCs were harvested at specific times after treatment with reagents as indicated in each experiment. Cells were mixed with loading buffer and subject to electrophoresis. After electrophoresis, proteins were transferred to polyvinyl difluoride membranes (Pall Filtron) using a semidry blotting apparatus (Pharmacia) and probed with mouse mAbs, followed by incubation with peroxidase-labeled secondary antibodies. Detection was performed by the use of a chemiluminescence system (Amersham) according to the manufacturer’s instructions. Then, membrane was striped with elution buffer and reprobed with antibodies against the nonphosphorylated protein as a measure of loading control. Controls for the immunoprecipitation used the same procedure, except agarose beads contained only mouse IgG.
Mitogen proliferative assays
In mitogen proliferative assays, triplicate wells containing responder 1 × 105 MNCs were cultured with 50 μg/ml PHA (Roche, USA) in a total volume of 0.1 ml medium at 37 °C in 5 % CO2, and Flk1+CD31−CD34− MSCs were added on day 0. Irradiated Flk1+CD31−CD34− MSCs (30 Gy) were cocultured with the MNCs at different ratios (MSCs to MNCs = 1:2, 1:10, 1:100). Control wells contained only MNCs. Cultures were pulsed with 1u Ci/well [3H]-TdR (Shanghai Nucleus Research Institute, China) on day 2 and harvested 18 h later with a Tomtec (Wallac Inc., Gaithersburg, MD) automated harvester. Thymidine uptake was quantified using a liquid scintillation and luminescence counter (Wallac TRILUX).
Mixed lymphocyte reaction (MLR) assays
Blood mononuclear cells (MNCs) were prepared from normal volunteers’ peripheral blood by Ficoll-Paque density gradient centrifugation and suspended in RPMI 1640 medium supplemented with 10 % (vol/vol) FCS, 2 mM l-glutamine, 0.1 mM nonessential amino acids (Life Technologies, Grand Island, NY), 1 mM sodium pyruvate, and 100 U/ml penicillin.
Effect of MSCs on T cell cycle
MSCs and MNCs were prepared as described before. T cells, stimulated with PHA (50 μg/ml, final concentration) stimulation for 3 days, were cultured alone or cocultured with MSCs (derived from normal and MDS patient) or 3T3 cell line and then harvested and quantified. One million T cells were fixed with 70 % cold ethanol at 4 °C for 30 min, washed with PBS twice, and stained with 50 μg/ml PI (Sigma, USA) at room temperature for 5 min. Data were analyzed with Mod-FIT software.
Effect of MSCs on T cell activation
MSCs and MNCs were prepared as described before, respectively. T cells were cultured alone or cocultured with prepared MSCs and stimulated with PHA (50 μg/ml final concentration). The expression of CD25 (BD, USA) and CD69 (BD, USA) was detected by flow cytometry at 24 h, and CD44 (BD, USA) was detected at 72 h.
Effect of MSCs on T cell apoptosis
MSCs and MNCs were prepared as described before. T cells were cultured alone or cocultured with MSCs with PHA (50 μg/ml final concentration) stimulation for 3 days, then harvested and quantified, stained with Annexin-V kit (BD, USA), and analyzed by flow cytometry (FACS Vantage).
FISH analysis
We cultured BCR/ABL+ CSCs from male CML patients (n = 12) and Y chromosome was detected using a probe (CEP Y Spectrum Red; Vysis, Downers Grove, IL) according to the manufacturer’s instructions. Normal cells showed two red abl signals and two green bcr signals. BCR/ABL+ CSCs showed a single red and a single green signal representing normal abl and bcr genes and the yellow signal representing fusion of abl and bcr genes.
RNA-i experiments
The si-RNA sequence targeting human MMP-9 (from mRNA sequence; Invitrogen online) corresponds to the coding region 377–403 relative to the first nucleotide of the start codon (target = 5′-AAC ATC ACC TAT TGG ATC CAA ACT AC-3′). Computer analysis using the software developed by Ambion Inc. confirmed this sequence to be a good target. si-RNAs were 21 nucleotides long with symmetric 2-nucleotide 3′ overhangs composed of 2′-deoxythymidine to enhance nuclease resistance. The si-RNAs were synthesized chemically and high-pressure liquid chromatography purified (Genset, Paris, France). Sense si-RNA sequence was 5′-CAU CAC CUA UUG GAU CCA AdT dT-3′. Antisense si-RNA was 5′-UUG GAU CCA AUA GGU GAU GdT dT-3′. For annealing of si-RNAs, mixture of complementary single-stranded RNAs (at equimolar concentration) was incubated in annealing buffer (20 mM Tris–HCl pH 7.5, 50 mM NaCl, and 10 mM MgCl2) for 2 min at 95 °C followed by a slow cooling to room temperature (at least 25 °C) and then proceeded to storage temperature of 4 °C. Before transfection, cells cultured at 50 % confluence in 6-well plates (10 cm2) were washed two times with OPTIMEM 1 (Invitrogen) without FCS and incubated in 1.5 ml of this medium without FCS for 1 h. Then, cells were transfected with MMP-9-RNA duplex formulated into Mirus TransIT-TKO transfection reagent (Mirus Corp, Interchim, France) according to the manufacturer’s instructions. Unless otherwise described, transfection used 20 nM RNA duplex in 0.5 ml of transfection medium OPTIMEM 1 without FCS per 5 × 105 cells for 6 h and then the medium volume was adjusted to 1.5 ml per well with RPMI 2 % FCS. Silencer™ negative control 1 si-RNA (Ambion Inc.) was used as negative control under similar conditions (20 nM).The efficiency of silencing is 80 % in our assay.
Enzyme-linked immunosorbent assays
This was carried out according to the manufacturer’s recommendations (Oncogene Research Products). Results were compared with those obtained with serially diluted solutions of commercially purified controls. Anti-human cytokine antibodies (R&D Systems, Minneapolis, MN) were added at 0.4 μg/ml in 0.05 M bicarbonate buffer (pH 9.3) to 96-well, U-bottom, polyvinyl microplates (Becton–Dickinson and Co., Oxnard, CA) and the cell number was 1 × 105/100 μl. After incubation overnight at 4 °C, the plates were washed and blocked with 1 % gelatin for 1 h. Samples (50 μl) or standard protein diluted in 0.5 % gelatin was added to the wells. After incubation for 1 h at 37 °C, the plates were washed again, and 50 ng/ml biotinylated antimouse antibody (R&D Systems) was added for 1 h at 37 °C. The plates were then washed and incubated with streptavidin-HRP for 1 h at 37 °C. After washing, 0.2 mM ABTS (Sigma Chemical Co.) was added to the wells, and after 10 min, the colorimetric reaction was measured at 405 nm with an ELISA reader VERSAmax (Molecular Devices, Sunnyvale, CA).
Preparation of nuclear extracts and the electrophoretic mobility shift assay (EMSA)
CML CSCs or cells pretreated as indicated were harvested, and then nuclear extracts were prepared. Oligonucleotides corresponding to the downstream NF-kB binding sequences 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ were synthesized, annealed and end-labeled with [g-32P] ATP using T4 polynucleotide kinase, and EMSA was performed as described previously [22].
Akt kinase assay
Akt kinase assay was performed as described previously with some modifications [22]. CML CSCs were harvested at specific times after treatment with reagents and lysed in lysis buffer. After equalizing the protein to 1.0 mg/200 μl in a 1.5-ml Eppendorf tube, 10 μl Akt antibody was added, and the samples were rotated overnight at 4 °C. Then, 20 μl protein A/G agarose bead slurry was added, and the samples were again rotated at room temperature for 2 h. The beads were washed twice with lysis buffer and kinase buffer, and the samples were suspended in 50 μl kinase buffer supplemented with 1 μl 10 mM ATP and 1 μg GSK-3 fusion protein and incubated at 30 °C for 30 min. The phosphorylation of GSK-3 fusion protein was analyzed by Western blot with phosphor-GSK-3α/β (Ser2119) antibodies.
NF-κB p65 activation assay
Cancer stem cells were harvested at specific times after treatment with reagents, and the nuclear extracts and the activation assay of p65 were performed according to the instructions of the manufacturer of the TransAM™/NF-κB kit. Briefly, 2 μg/well nuclear extract protein was added to a microtiter plate coated with a specific oligonucleotide of p65. The coated plate was then incubated for 1 h at room temperature with mild agitation, after which a primary antibody recognizing the p65 was added, and the plate was incubated for an additional 1 h. Anti-IgG horseradish peroxidase-conjugated secondary antibody was then added, and the plate was incubated for 1 h at room temperature. At the end of the hour, the developing and stop solution were added, and an optical density of 450 nm (OD450) was read on a Wallac Victor 1420 multilabel counter (Perkin Elmer Life Sciences, Shelton, CT). Three duplicates were performed for each sample.
Statistical analysis
Results are expressed as mean ± standard deviation. Data were analyzed using the unpaired two-tailed Student’s t-test and the log rank test. p values of p < 0.05 were considered significant.
Results
The biological characteristics of CML CSCs
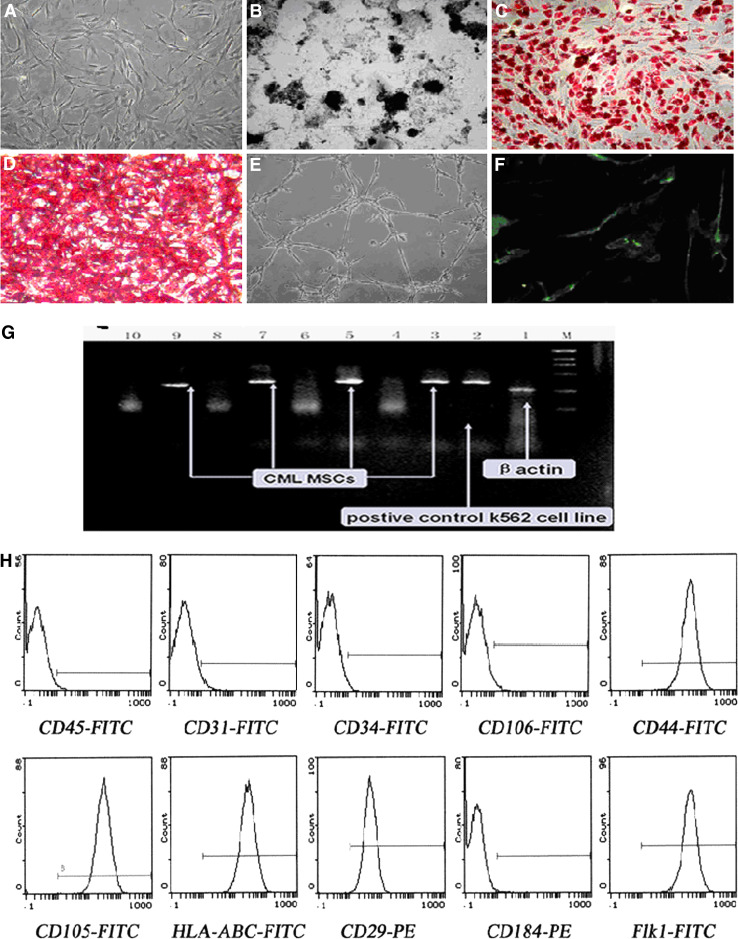
To establish the characteristics of CML CSCs, we first derived MSCs from the bone marrow of CML, and the differentiation experiments showed that the isolated cells were able to differentiate into bone, fat and cartilage cells, which indicated that the isolated cells had stem cells property (Fig. 1a–f). These stem cells persistently displayed fibroblast-like morphology, and CML-specific BCR/ABL oncogene was observed by FISH analysis (Fig. 1g). Isotype analysis indicated they were all persistently negative for CD34 and CD31 but positive for Flk1, CD29, CD44 and CD105 (Fig. 1h).
Fig. 1.
Biological characteristics of the CML CSCs. a The morphology of CSCs from CML (magnification ×100). b Osteogenic differentiation (von-Kossa staining, magnification ×100); c adipogenic differentiation (Oil Red-O staining, magnification ×100); d chondrogenic differentiation (Lycopene-O staining, magnification ×100); e endothelial differentiation (magnification ×100); f epidermis differentiation (vWF indirect immunofluorescence staining, magnification ×100); g BCR/ABL fusion gene was detected by RT-PCR (line 4, 6, 8, 10 correspond to nonspecial amplification). h Isotype analysis showed they were all persistently negative for CD34 and CD31 but positive for Flk1, CD29, CD44 and CD105
Immunomodulatory decrease in CML CSCs on T cell proliferation
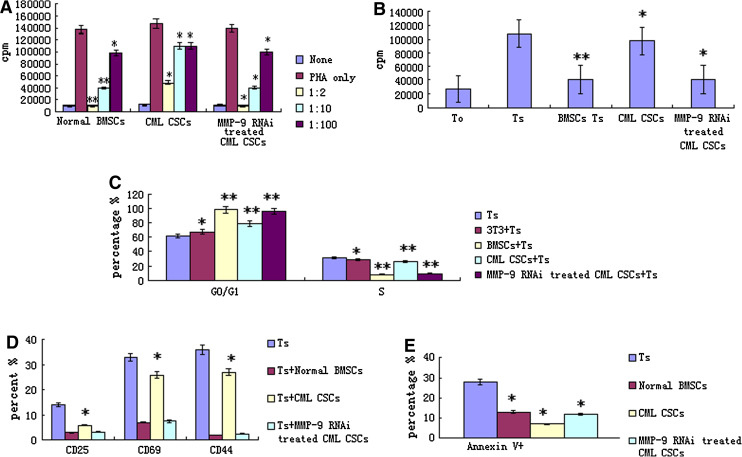
To analyze immunomodulatory effects on T cell proliferation, irradiated MSCs were added to mitogen-stimulated T cell proliferation reactions and mixed lymphocyte reactions (MLR). A previous study showed that MSCs from healthy volunteers could obviously inhibit the proliferation of T cells not only stimulated with mitogen but also with MLR [17]. Additionally, this inhibitory effect occurred in a dose-dependent manner. In mitogen-stimulated T cell proliferation assays, the proliferation of T cells at 1:2 ratio (MSCs to MNCs) was significantly inhibited to about 1 % with normal MSCs, but proliferation at the same ratio was inhibited only to about 37 % with CML-derived MSCs (compared with coculture system of normal MSCs, p < 0.05). Similarly, inhibitory rates were impaired at 1:10 ratio (MSCs to MNCs) in CML-derived MSCs (compared with coculture system of normal MSCs, p < 0.05). Also, the inhibitory effect was dose dependent in CML-derived MSCs (Fig. 2a). In MLR, a similar impaired inhibitory effect with CML-derived MSCs was observed (Fig. 2b).
Fig. 2.
The effects of CML CSCs on T lymphocyte. a The effects of CML CSCs on T lymphocyte proliferation in mitogen proliferative assays. There are three groups, including nonstimulated T cells (none), PHA-stimulated T cells (Ts) and PHA-stimulated T cells cocultured with MSC at different ratios (MSC to T cell = 1:2, 1:10, :100). Data are shown as mean ± SD of three independent experiments (*p < 0.05, **p < 0.005 vs. Ts). b The effects of CML CSCs on T lymphocyte proliferation in MLR. CML CSCs at 1:10 ratios (irradiated MSCs to T cells); there are four groups, including nonstimulated responder T cells (T0), irradiated stimulator cells plus responder T cells; normal MSC plus MLR (BMSC Ts), CML-derived MSC plus MLR (CML Ts). Data are shown as mean ± SD of three independent experiments (*p ≥ 0.05, **p = 0.001 vs. Ts). c Effects of CML CSCs on T cell cycle. CML CSCs or 3T3 at 1:10 ratios (MSCs to T cells); the data are expressed as mean ± SD. Of triplicates of five separate experiments with similar results. Cell cycles of PHA-stimulated T cells were analyzed in T cells alone (Ts), cocultured with MSCs (MSC + Ts) group and MSCs derived from CML patient group (CML MSC + Ts). 3T3 cell line was used as control (3T3 + Ts). Data are shown as mean ± SD of five independent experiments (*p ≥ 0.05, **p < 0.05 vs. Ts). d Effects of CML CSCs on T lymphocyte activation. CML CSCs at 1:10 ratios (MSCs to T cells); the data are expressed as mean ± SD of triplicates of five separate experiments with similar results. Activators of T cells were analyzed including CD25, CD69 and CD44. The activation of T cells was analyzed in T cells alone (Ts), normal MSC cocultured with activated T cells (BMSC + Ts), and CML-derived MSC cocultured with activated T cells (MDS MSC + Ts). Data are shown as mean ± SD of five independent experiments (*p ≥ 0.05, **p < 0.05 vs. Ts). e Effect of CML CSCs on T cell apoptosis. CML CSCs at 1:10 ratios (MSCs to T cells); the data are expressed as mean ± SD of triplicates of five separate experiments with similar results. The test was conducted by Annexin V and PI double staining and analyzed by flow cytometry. Apoptosis of T cells was analyzed in T cells alone (Ts), normal MSC cocultured with activated T cells (MSC + Ts), and CML patient-derived MSC cocultured with activated T cells (CMLMSC + Ts). Annexin V + means the cells were PI negative and Annexin V positive. Data are shown as mean ± SD of five independent experiments (*p < 0.05 vs. Ts)
Immunomodulatory attenuation of CML CSCs on T cell cycle
A previous study showed that MSCs could silence T cells in G0/G1 phase, which might be one of the possible mechanisms of MSC’s inhibitory effect on T cells [17]. When the inhibitory effect of CML-derived MSC on T cell proliferation was impaired, the related inhibitory effect on cell cycle was analyzed. In a PHA-stimulating system without MSC coculture, there were 67.3 ± 3.7 and 28.4 ± 2.9 % T cells in G0/G1 phase and S phase, respectively. When normal MSCs were present in coculture, the percentages of T cells in G0/G1 phase and S phase were 94.0 ± 1.9 and 3.1 ± 1.9 %, respectively (compared with PHA-stimulated T cells, p < 0.05). MSCs from healthy volunteers could have most of their T cells in G0/G1 phase with fewer cells entering S phase. However, T cells in G0/G1 phase and S phase remained 74.5 ± 1.2 and 22.1 ± 2.4 % in the coculture system of CML-derived MSCs (compared with coculture system of normal MSCs, p < 0.05). This result was confirmed by five independent tests (Fig. 2c). These results suggested that the inhibitory effect of CML-derived MSCs on cell cycle arrest was also impaired.
Impaired effects of CML CSCs on T cell activation
MSCs from CML patients could significantly inhibit activation of T cells. The percentage of CD25, CD69 and CD44 in PHA-induced T lymphocyte was 12.3 ± 3.5, 34.5 ± 5.9 and 29.4 ± 7.0 %, respectively. But they were 3.1 ± 2.3, 6.4 ± 3.2 and 2.1 ± 1.7 % when cocultured with normal MSCs, and when cocultured with CML CSCs, they were 5.4 ± 2.3, 31.5 ± 6.8 and 24.5 ± 3.6 %, respectively. All data presented here were confirmed by repeated tests (Fig. 2d). These results also indicated that MSCs from CML patients were impaired in their immunomodulatory function.
Dampening effect of CML CSCs on T cell apoptosis
In apoptosis tests, we have observed that MSCs from healthy volunteers could significantly dampen the effect of activation-induced apoptosis of T cells. Following stimulation with PHA for 3 days, the rate of apoptosis of T cells was 23.37 ± 2.71 %. When PHA-stimulated T cells were cocultured with MSCs obtained from healthy volunteers, the percentage of apoptotic T cells decreased to 14.1 ± 0.65 % (compared with PHA-stimulated T cells, p < 0.05). In the same condition, the apoptosis percentage of T cells cocultured with MDS-derived MSCs further decreased to 8.36 ± 1.31 % (compared with coculture system of normal MSCs, p < 0.05). We repeated the experiment five times to confirm this result (Fig. 2e). These results suggested the dampening effect of CML-derived MSCs on activation-induced T apoptosis seemed to be enhanced.
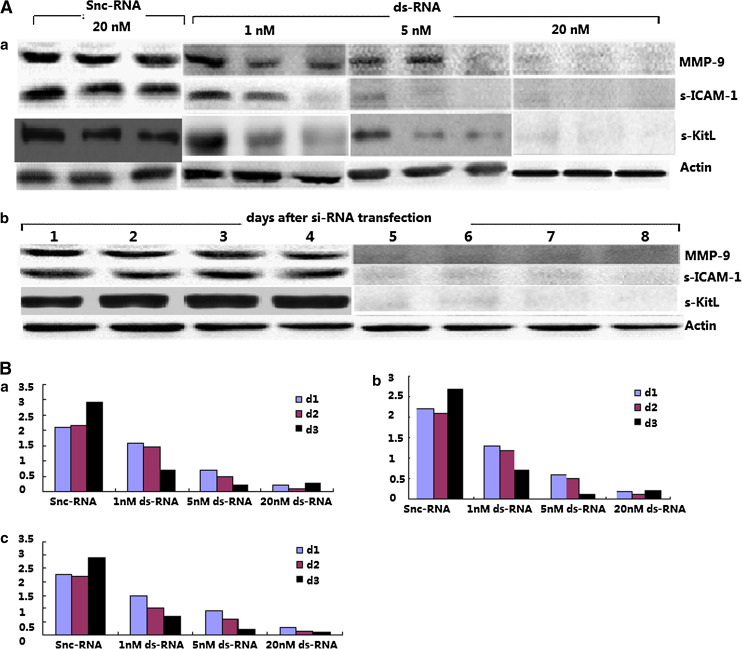
Efficient extinction of MMP-9 expression in CML CSCs by RNAi strategy and the concomitantly upregulation of s-ICAM-1 and s-KitL
We used an RNAi method to target MMP-9 in the CML MSC, and the constructs we designed encoded an RNA that targets the MMP-9 mRNA. The target sequence had no homology with other members of the MMP family. The ds-RNA and Silencer negative control si-RNA (snc) were each tested for their ability to suppress MMP-9 specifically. We first assessed whether RNAi was dose- and time-dependent. A MMP-9-dependent ds-RNA-mediated inhibition was observed in a dose- and time-dependent manner (Fig. 3a). The time-course assay performed with 20 nM ds-RNA-transfected CML MSC showed that the induced MMP-9 silencing could be maintained for at least 3 days (Fig. 3b). Besides, serum ICAM-1 was concomitantly changing with MMP-9. The Western blotting results were confirmed by enzyme-linked immunoadsorbent assay. CML snc-RNA-transfected cells cultured up to 3 days spontaneously released high amount of MMP-9 into the culture conditioned medium, whereas ds-RNA-transfected cells showed a marked time- and dose-dependent inhibition in MMP-9 protein levels. Importantly, levels of s-ICAM-1 and s-KitL were also affected with ds-RNA transfection (Fig. 3c).
Fig. 3.
Efficient inhibition of MMP-9 in CML CSCs using RNAi. a The ds-RNA and Silencer negative control si-RNA (snc) were each tested for their ability to suppress MMP-9 specifically. A MMP-9-dependent ds-RNA-mediated inhibition was observed in a dose- and time-dependent manner by WB. b ELISA results confirmed that MMP-9-dependent ds-RNA-mediated inhibition in a dose- and time-dependent manner (a MMP-9, b s-ICAM-1, c s-KitL)
Abl kinase upregulated TGF-β1 in CML
To investigate the mechanism involved in MMP-9 synthesis, we first assayed the expression of TGF-β in CSCs from CML and healthy donors from mRNA and protein level, respectively. As TGF-β has 3 isotype, we examined the expression of them. The results showed that TGF-β1 expression was apparently higher than TGF-β2 and TGF-β3 as shown by ELISA, PCR and Western blot (Fig. 4a, b). Thus, further research was aimed to TGF-β1. The effect of various doses of TGFβ1 on MMP-9 production by CML CSCs was assessed to correlate the MMP-9 levels with the induction of signaling components leading to the synthesis of the enzyme (Fig. 4c). Results from ELISA (Fig. 4d) showed TGF-β1 upregulated MMP-9 production both in CSCs from CML and healthy donors. Those results demonstrated that BCR/ABL oncogene and TGF-β1 were involved in the regulation of MMP-9 production. We then examined the relationship of CML-specific BCR/ABL oncogene with TGF-β1, MMP-9, s-KitL and s-ICAM-1. Intimab, inhibitor of BCR/ABL oncogene, blocked the induction of TGF-β1 and concomitantly MMP-9, s-KitL and s-ICAM-1 in a dose-dependent manner (Fig. 4e).
Fig. 4.
BCR/ABL tyrosine kinase-induced TGF-β1 upregulates MMP-9 in CML CSCs. a The supernatants from CML CSCs and healthy donor MSCs were collected from ELISA to examine the expression of three isotypes of TGF-β, and the results were representative of at least three independent experiments. *p < 0.05. b RT-PCR results of three isotypes of TGF-β. The results were representative of at least three independent experiments. c CML CSCs were treated with TGF-β1 and then cell lysates were analyzed by Western blot with GAPDH as the loading control. d Supernatants were also collected and MMP-9 was analyzed by ELISA. Columns represent cytokine concentration mean ± SD of three different experiments, *p < 0.05. e CML CSCs were treated with Intimab for 2 h and then cell lysates were analyzed by Western blot
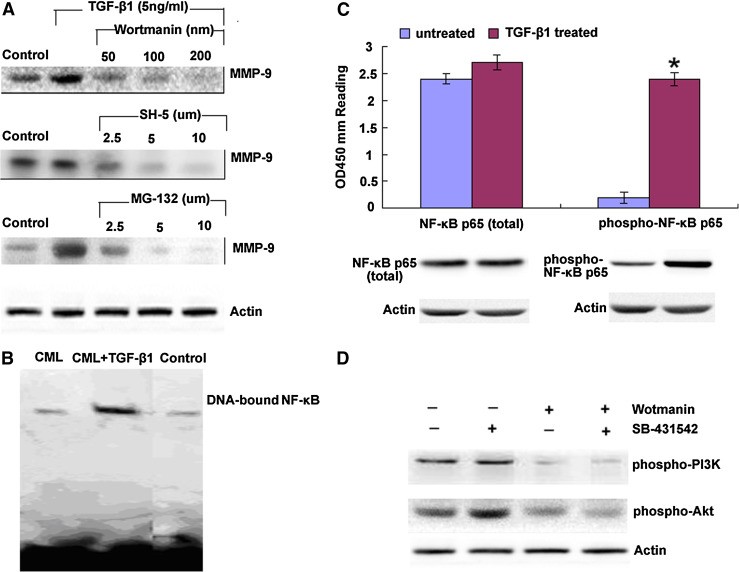
TGF-β1 upregulates MMP-9 production via PI3K/Akt/NF-κB but independent of Smad signalling pathway
To determine the involvement of PI3K signalling pathway in CML CSCs, we initially examined the effect of PI3K activity on the induction of MMP-9 by TGF-β1. The addition of Wortmannin, inhibitor of PI3K and SH-5, inhibitor of Akt and MG-132, inhibitor of NF-κB, suppressed the MMP-9 synthesis induced by TGF-β1, respectively (Fig. 5a). We then analyzed nuclear extraction by EMSA for DNA binding activity of NF-κB. As shown by Fig. 5b, lane 1, 2 and 3 represented the results of nuclear extraction of CML CSCs. CML CSCs with 5 ng/ml TGF-β1 preculturing for 2 h and the results indicated that NF-κB was involved in TGF-β1-induced MMP-9 production. We at the same time analyzed total cytoplasmic NF-κBp65 and phosphorylated NF-κBp65 in the presence or absence of TGF-β1 (5 ng/ml) treatment using Western blot and ELISA (Fig. 5c, d), and the results showed that TGF-β1 upregulated phosphorylated NF-κBp65 while had little effects on total NF-κBp65 production. As Smad was the main regulating pathway of TGF-β1, we then investigated whether Smad regulated PI3K/Akt in CML CSCs. As shown in Fig. 4e, Smad inhibitor did not blocked the phosphorylated PI3K and Akt production, indicating that PI3K regulated MMP-9 synthesis independent of Smad signaling pathway.
Fig. 5.
TGF-β1 upregulates MMP-9 production via PI3K/Akt/NF-κB, but independent of Smad signalling pathway. a CML CSCs were treated with a Wortmannin, b SH-5 and c MG-132 for 1 h prior to the addition of TGF-β1. Culture supernatants were harvested after 48 h and analyzed for MMP-9 by Western blot. Me2SO was also added to one of the cultures as a control. b Cell nuclear extracts were prepared and the DNA binding ability of NF-κBp65 was examined by EMSA. c Nuclear and cytoplasmic protein were collected, respectively, to assay the total and phosphorylated NF-κBp65 by Western blot and ELISA (PathScan phospho-NF-κBp65 Sandwich ELISA kit) in the presence or absence of TGF-β1 treatment. OD450 reading was shown in the top figure, and the corresponding Western blot using phospho-NF-κBp65 (Ser536) rabbit mAb (right panel) or NF-κBp65 antibody (left panel) was shown in the bottom figure. Columns represent cytokine concentration mean ± SD of three different experiments, *p < 0.05. d Cell extracts from CML CSCs treated with Wortmannin or SB-431542 were examined for phosphorylated PI3K and Akt by Western blot
PI3K and Akt regulate NF-κB in CML CSCs via IκB degradation
Previous assays indicated that Akt regulated NF-κB expression through its activation with IKKα. IKKα was closely related with the phosphorylation of IκB, which was ubiquitinated and degraded by the proteasome complex. NF-κB was then activated and translocated to the nucleus [23]. To determine whether Akt regulated NF-κB in CML in this way, we first examined the expression of the 3 isotype of IKK, IKKα, IKKβ and IKKγ on MSCs from both CML and healthy donor. As evidenced by Fig. 6A, IKKα level was obviously higher in CML CSCs. Next, CML CSCs were treated with TGF-β1 for 2 h and then cell lysates were analyzed by Western blot with anti-IκB. The results showed that TGF-β1 suppressed IκB in a dose-dependent manner. Thirdly, we treated CML CSCs with Wortmannin, SH-5 and MG-132 for 1 h prior to the addition of TGF-β1 for 2 h and harvested cell lysates for immunoprecipitation to verify the relationship of phospho-Akt and phospho-IKKα, and the results indicated that the phosphorylation of Akt and IKKα was blocked by Wortmannin and SH-5 but not MG-132 (Fig. 6b). Finally, we examined the degradation of IκB by Western blot (Fig. 6d) and the activation of NF-κB p65 by EMSA (Fig. 6e) after the treatment of TGF-β1 in the absence or presence of the PI3K/Akt/NF-κB-specific inhibitors, respectively. The results indicated that elevated levels of TGF-β1 led to IκB degradation and activation of NF-κB p65. The fact that this process could be blocked by the inhibitors demonstrated that PI3K and Akt in the pathway led to the downstream activation of NF-κB in CML and subsequently upregulated MMP-9 synthesis.
Fig. 6.
PI3K/Akt/NF-κB signaling pathway regulates TGF-β1 in BCR/ABL+ CSCs. a Cell extracts (60 μg protein) from BCR/ABL+ CSCs and healthy donors were analyzed by Western blot with anti-IKKα, anti-IKKβ and anti-IKKγ, and GAPDH was used as the loading control. b BCR/ABL+ MSCs were treated with Wortmannin, SH-5 and MG-132 for 1 h prior to the addition of TGF-β1 for 2 h, cell lysates were prepared and immunoprecipitated with anti-IKKα, and then analyzed by Western blot with antiphospho-Akt or antiphospho-IKKα. c CML CSCs were treated with TGF-β1 for 2 h, and then cell lysates were analyzed by Western blot with anti-IκB and GAPDH as the loading control. d CML CSCs were treated with Wortmannin, MG-132 and SH-5 for 1 h prior to the addition of TGF-β1 for 2 h, and then cell lysates were prepared and analyzed by Western blot with anti-IκB or anti-GAPDH. e Nuclear extracts were also harvested to examine the NF-κB binding ability by EMSA
Discussion
Chronic myeloid leukemia is a clonal hematopoietic stem cell disorder characterized by the t(9;22) chromosome translocation and resultant production of the constitutively activated BCR/ABL tyrosine kinase [24]. Although interferon-α, Intimab (a BCR/ABL tyrosine kinase inhibitor) and stem cell transplantations are the standard therapeutic options, transplant-related morbidity from graft-versus-host disease and mortality rates of 10–20 % have greatly reduced the allogeneic hematopoietic cell transplantation in clinics [25], while interferon-α is only effective in some patients to some degree and chemotherapeutic intervention does not result in prolonged overall survival [26, 27], and the reason is possibly due to some unknown biology of the CML CSCs [28].
Our laboratory have identified the Flk-1+CD34−CD31− MSCs as the CML-initiating cells and proved the rearrangement of the BCR/ABL gene might happen at the level of this MSCs instead of HSCs [21]. Based on this concept, we first examined the biological characteristics of CML CSCs. FISH analysis of the Flk-1+CD34−CD31− MSCs derived from CML bone marrow showed that they were BCR/ABL+, which indicated that this oncogene had already mutated at the CSCs level. More importantly, the ability of CML-derived MSCs (or CML CSCs) to inhibit T lymphocytes was weaker than that of the normal MSCs. Besides, a lot of blood disease such as ALL, CLL and MDS are found to express high level of s-ICAM-1, and clinical researches indicated the level of it correlated directly with disease stage, prognosis and survival period [29–31]. All these inspired us to further examine the s-KitL and s-ICAM-1, and both of them were higher in MSCs from CML than those from normal donors. So, we believe that enhanced expression of s-ICAM-1 may facilitate tumor cells immune evasion by blocking the ICAM/LFA connection and prevent them from being recognized by T lymphocyte and NK cells. Increased s-KitL may also promote c-kit+ HSCs recruitment and mobilization in CML, resulting in extramedullary leukemia cells infiltration. Besides, it further confirms our belief that purified true leukemia stem cells could provide a target for immune-based therapies and biological response modifiers [32].
Data from functional tests proved that CML CSCs had abnormal immunomodulatory function, although they showed normal karyotype. An inhibitory effect on T cell proliferation is an important characteristic of CML CSCs in immunomodulatory action. A previous study [21], in accordance with another report, suggested that the inhibitory effect on T cell proliferation might be through cell cycle arrest. MSCs from healthy volunteers could obviously block T cells in G0/G1 phase. In this study, inhibitory effects of CML CSCs on T cell proliferation were obviously impaired. Moreover, no significant cell cycle arrest was observed in PHA-stimulated T cells cocultured with CML CSCs. In addition, an inhibitory effect on T cell activation is another key point of immunomodulatory function for MSCs, although there are still disputes [24, 28]. CD25, CD69 and CD44 are candidates for T cell activation in different phases. In our study, MSCs from healthy volunteers showed significant inhibitory effects on expression of T cell activation markers, but CML CSCs showed very limited inhibitory effects. These results suggested that CML CSCs have immunological abnormalities and their application in immunomodulation might be limited.
Normally, the invasion and metastasis by malignant tumor cells consists of three major steps: the receptor-mediated adhesion of tumor cells to the ECM, the degradation of the ECM by the proteinase secreted by the tumor cells, and the transfer and proliferation of tumor cells [32]. Pathological conditions will change the tumor cell fate leading to invasion and metastasis [33]. Local secretion of proteases has been implicated in this tumor–stroma crosstalk.
MMP-9 is important for maintaining normal hematopoietic process, and abnormally higher MMP-9 will degrade extra-cellular matrix and change the cytokines and molecular that have bearing with HSCs adhesion and motivation. In recent years, MMP-9 is found to influence cells recruitment and differentiation [33–35]. In the present investigation, we found that CML CSCs expressed high level of MMP-9. We also found that the s-KitL and s-ICAM-1 were MMP-9 dependent. To further investigate the mechanism behind MMP-9 production in CSCs, we examined the expression of the 3 isotypes of TGFβ and found TGF-β1 was abnormally higher, which upregulated MMP-9 in a dose-dependent manner. Thereby, in the next research, we focused on the relationship between BCR/ABL oncogene and TGF-β1. We found that Intimab, inhibitor of BCR/ABL oncogene, blocked the induction of TGF-β1 in a dose-dependent manner. Further evidence shows that Intimab also blocked s-KitL and s-ICAM-1 in a dose-dependent manner. An important downstream signaling molecule of TGF-β1 is Smad. There is also Smad-independent pathway, such as Ras [36], Rho proteins [37], extracellular signaling kinase [38] and p38 [39]. Recently, phosphatidylinositol 3-kinase (PI3K) is found to be involved in TGF-β1 signaling positively or negatively at least in some cell types as both stimulation and inhibition effects have been described [40–42]. To investigate whether TGF-β1 was Smad dependent or independent in CML CSCs, we used Smad and PI3K inhibitor, respectively, as indicated, and the results showed that it depended on PI3K instead of Smad. Actually, TGF-β and PI3K are involved in many cell lines [43, 44].
It was reported that PI3K was activated by tyrosine kinase [45, 46], and our results further verified that in CML, it was activated by tyrosine kinase-induced TGF-β1. Following its recruitment to these receptors in the plasma membrane, PI3K is activated and phosphorylated to generate the second messenger PIP3, whose levels are tightly regulated by the action of phosphatases. PIP3 does not activate Akt directly but instead appears to recruit Akt to the plasma membrane and to alter its conformation to allow subsequent phosphorylation by the phosphoinositide-dependent kinase [47]. When the capability for PI3K to produce PIP3 was blocked in BCR/ABL+ CSCs by Wortmannin, MMP-9 production was inhibited, which indicated that the PI3K pathway had a central role in the regulation of CML MMP-9 synthesis.
Furthermore, when SH-5 blocked Akt phosphorylation specifically, MMP-9 production was also inhibited. These data showed that PIP3 mediated MMP-9 production in BCR/ABL+ CSCs, primarily through Akt and not other downstream components of PI3K containing a PH domain. MG-132 inhibition of MMP-9 production indicated that NFκB was also involved in this pathway which coordinated with the reports of other cell lines [48].
Three members of the IKK family have been isolated, and these are now referred to as IKK1/IKKα, IKK2/IKKβ and IKK3/IKKγ. Expression and the ratio of IKKα to IKKβ vary among cell types. NF-κB, in the cells with a high proportion of IKKα to IKKβ, is sensitive to Akt activity [49]. Our data showed that IKKα was predominant in human BCR/ABL+ CSCs, which was apparently different from that in healthy donors where the three subunits existed at similar level. Based on this data, we hypothesized that Akt might bind to IKKα constitutively and their phosphorylation might occur following TGF-β1 stimulation. Immnoprecipitation confirmed our idea which showed that the phosphorylation of Akt was regulated by PI3K and Akt subsequently phosphorylated IKKα which regulated the activation of NF-κB.
Finally, IκB proteins are degraded rapidly by the proteasome complex once ubiquitinated, thereby freeing NF-κB, which then enters the nucleus, binds to DNA and activates transcription [50]. It had been shown that Akt regulated NF-κB activation directly through the activation of IKKα, and our findings from two aspects, EMSA and Western blot, demonstrated that the PI3K/Akt pathway also regulated IκB degradation and activation of NF-κB in BCR/ABL+ CSCs stimulated by TGF-β1.
Generally speaking, we demonstrated here a decisive checkpoint for MMP-9 to rapidly release the stem cell-active cytokines s-KitL and s-ICAM-1, thereby directing tumor cell immune evasion and mobilization to extramedullary infiltration. An intriguing aspect of this study is the illustration of stem cell–stromal cell interactions being amplified by membrane-anchored cytokines which are mediated by CML-specific BCR/ABL oncogene-induced signalling pathway. Modulation of local cytokines relating to HSCs fates provides promising hope for clinical targeting therapy.
Acknowledgments
Supported by grants from the National Natural Science Foundation of China (No. 81100366).
References
- 1.Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5:2862–2866. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen HG, Copland M, Allan EK, Jiang X, Eaves A, Eaves C. Intermittent exposure of primitive quiescent chronic myeloid leukemia cells to granulocyte-colony stimulating factor in vitro promotes their elimination by imatinib mesylate. Clin Cancer Res. 2006;12:626–633. doi: 10.1158/1078-0432.CCR-05-0429. [DOI] [PubMed] [Google Scholar]
- 4.Ries C, Pitsch T, Mentele R, Zahler S, Egea V, Nagase H, Sede K. Identification of a novel 82 kDa proMMP-9 species associated with the surface of leukaemic cells: (auto-)catalytic activation and resistance to inhibition by TIMP-1. Biochem J. 2007;405(3):547–558. doi: 10.1042/BJ20070191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 6.Fridman R, Toth M, Chvyrkova I, Meroueh S, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22:153–166. doi: 10.1023/A:1023091214123. [DOI] [PubMed] [Google Scholar]
- 7.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 8.Paupert J, Mansat-De Mas V, Demur C. Cell-surface MMP-9 regulates the invasive capacity of leukemia blast cells with monocytic features. Cell Cycle. 2008;7(8):1047–1053. doi: 10.4161/cc.7.8.5645. [DOI] [PubMed] [Google Scholar]
- 9.Redondo-Muñoz J, José Terol M, García-Marco JA (2008) Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration Blood 111(1):383–386 [Epub 2007 Sept 21] [DOI] [PubMed]
- 10.Redondo-Muñoz J, Escobar-Díaz E, Samaniego R. MMP-9 in B-cell chronic lymphocytic leukemia is up-regulated by alpha4beta1 integrin or CXCR4 engagement via distinct signaling pathways, localizes to podosomes, and is involved in cell invasion and migration. Blood. 2006;108(9):3143–3151. doi: 10.1182/blood-2006-03-007294. [DOI] [PubMed] [Google Scholar]
- 11.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12(6):1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 12.Janowska-Wieczorek A, Majka M, Marquez-Curtis L, Wertheim JA, Turner AR, Ratajczak MZ. Bcr-abl-positive cells secrete angiogenic factors including matrix metalloproteinases and stimulate angiogenesis in vivo in Matrigel implants. Leukemia. 2002;16(6):1160–1166. doi: 10.1038/sj.leu.2402486. [DOI] [PubMed] [Google Scholar]
- 13.Kaneta Y, Kagami Y, Tsunoda T, Ohno R, Nakamura Y, Katagiri T. Genome-wide analysis of gene-expression profiles in chronic myeloid leukemia cells using a cDNA microarray. Int J Oncol. 2003;23(3):681–691. [PubMed] [Google Scholar]
- 14.Bruchova H, Borovanova T, Klamova H, Brdicka R. Gene expression profiling in chronic myeloid leukemia patients treated with hydroxyurea. Leuk Lymphoma. 2002;43(6):1289–1295. doi: 10.1080/10428190290026358. [DOI] [PubMed] [Google Scholar]
- 15.Ries C, Loher F, Zang C, Ismair MG, Petrides PE. Matrix metalloproteinase production by bone marrow mononuclear cells from normal individuals and patients with acute and chronic myeloid leukemia or myelodysplastic syndromes. Clin Cancer Res. 1999;5(5):1115–1124. [PubMed] [Google Scholar]
- 16.Kim JG, Sohn SK, Kim DH, Baek JH, Lee NY, Suh JS, Chae SC, Lee KS, Lee KB. Clinical implications of angiogenic factors in patients with acute or chronic leukemia: hepatocyte growth factor levels have prognostic impact, especially in patients with acute myeloid leukemia. Leuk Lymphoma. 2005;46(6):885–891. doi: 10.1080/10428190500054491. [DOI] [PubMed] [Google Scholar]
- 17.Yoon S-O, Shin S, Lee H-J. Isoginkgetin inhibits tumor cell invasion by regulating phosphatidylinosito 3 kinase/Akt dependent matrix metalloproteinase-9 expression. Mol Cancer Ther. 2006;5(11):344–349. doi: 10.1158/1535-7163.MCT-06-0321. [DOI] [PubMed] [Google Scholar]
- 18.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Fang B, Zheng C, Lianming L, Mingxia S, Shaoguang Y, Zhao RCH. Identification of human chronic myelogenous leukemia progenitor cells with hemangioblastic characteristics. Blood. 2005;105(7):2733–2740. doi: 10.1182/blood-2004-07-2514. [DOI] [PubMed] [Google Scholar]
- 20.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.V98.9.2615. [DOI] [PubMed] [Google Scholar]
- 21.Guo H, Fang B, Zhao RC. Hemangioblastic characteristics of fetal bone marrow-derived Flk1(+)CD31(−)CD34(−) cells. Exp Hematol. 2003;31:650–663. doi: 10.1016/S0301-472X(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKK/NF-κB pathway. J Leukoc Biol. 2005;78(1):259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- 23.Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, Guzman JR, Korgaonkar CK, Donner DB. Cell type-specific expression of the IκB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-κ B activation. J Biol Chem. 2004;279:1615–1620. doi: 10.1074/jbc.M306976200. [DOI] [PubMed] [Google Scholar]
- 24.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Lefde H. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karanes C, Nelson GO, Chitphakdithai P, Agura E, Ballen KK, Bolan CD, Wede N. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):8–15. doi: 10.1016/j.bbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Martin MG, Dipersio JF, Uy GL. Management of the advanced phases of chronic myelogenous leukemia in the era of tyrosine kinase inhibitors. Leuk Lymphoma. 2008;29:1–10. doi: 10.1080/10428190802517765. [DOI] [PubMed] [Google Scholar]
- 27.Martinelli G, Soverini S, Iacobucci I, Baccarani M. Intermittent targeting as a tool to minimize toxicity of tyrosine kinase inhibitor therapy. Nat Clin Pract Oncol. 2009;6(2):68–69. doi: 10.1038/ncponc1276. [DOI] [PubMed] [Google Scholar]
- 28.Catriona H, Jamieson Y. Chronic myeloid leukemia stem cell. Hematology Am Soc Hematol Educ Program. 2008;34:436–442. doi: 10.1182/asheducation-2008.1.436. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier SD, Hong DS, Hu Y, Sede J. Lack of the adhesion molecules P-selectin and intercellular adhesion molecule-1 accelerate the development of BCR/ABL-induced chronic myeloid leukemia-like myeloproliferative disease in mice. Blood. 2004;104:2163–2171. doi: 10.1182/blood-2003-09-3033. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Henao GA, Quiroga R, Sureda A, Petes D. L-selectin expression is low on CD34+ cells from patients with chronic myeloid leukemia and interferon-a up-regulates this expression. Haematologica. 2000;85:139–146. [PubMed] [Google Scholar]
- 31.Wertheim JA, Forsythe K, Druker BJ, Swede LH. BCR-ABL-induced adhesion defects are tyrosine kinase-independent. Blood. 2002;99(11):4122–4130. doi: 10.1182/blood.V99.11.4122. [DOI] [PubMed] [Google Scholar]
- 32.Fiorel E, Fuscol C, Romero P. Matrix metalloproteinase 9 (MMP-/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–5223. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 33.Darai E, Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 34.Molica S, Vitelli G, Levato D, Giannarelli D, Vacca A, Cuneo A. Increased serum levels of matrix metalloproteinase-9 predict clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Eur J Haematol. 2003;10:373–378. doi: 10.1034/j.1600-0609.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 35.Kamiguti AS, Lee ES, Till KJ, Harris RJ, Glenn MA, Lin K, Fedes KH. The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. Br J Haematol. 2004;125:128–140. doi: 10.1111/j.1365-2141.2004.04877.x. [DOI] [PubMed] [Google Scholar]
- 36.Yue J, Mulder KM. Transforming growth factor-h signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/S0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 37.Mucsi I, Skorecki KL, Goldberg HJ. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-h1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 38.Munshi HG, Wu YI, Mukhopadyay S. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2 and p38 MAPK-modulated tissue inhibitor of metalloproteinase expression controls transforming growth factor-h1-induced pericellular collagenolysis. J Biol Chem. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 39.Kim ES, Kim MS, Moon A. TGF-h-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- 40.Horowitz JC, Lee DY, Waghray M. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-h1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor b-medicated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 42.Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–7023. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- 43.Rahimi RA, Andrianifahanana M, Wilkes MC. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta. Cancer Res. 2009;69(1):84–93. doi: 10.1158/0008-5472.CAN-08-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assinder SJ, Dong Q, Mangs H. Pharmacological targeting of the integrated AKT, PTEN and TGF-{beta} pathways in prostate cancer. Mol Pharmacol. 2009;75(3):429–436. doi: 10.1124/mol.108.053066. [DOI] [PubMed] [Google Scholar]
- 45.Rexer BN, Engelman JA, Arteaga CL. Overcoming resistance to tyrosine kinase inhibitors: Lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle. 2009;8(1):18–22. doi: 10.4161/cc.8.1.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci. 2008;121(Pt 24):4124–4133. doi: 10.1242/jcs.032763. [DOI] [PubMed] [Google Scholar]
- 47.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/S0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKK/NF-κB pathway. J Leukoc Biol. 2005;78:259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- 49.Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, Guzman JR, Korgaonkar CK, Donner DB. Cell type-specific expression of the IκB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-κB activation. J Biol Chem. 2004;279:1615–1620. doi: 10.1074/jbc.M306976200. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]