Abstract
Cancer vaccines such as MVA-5T4 (TroVax®) must induce an efficacious immune response to deliver therapeutic benefit. The identification of biomarkers that impact on the clinical and/or immunological efficacy of cancer vaccines is required in order to select patients who are most likely to benefit from this treatment modality. Here, we sought to identify a predictor of treatment benefit for renal cancer patients treated with MVA-5T4. Statistical modeling was undertaken using data from a phase III trial in which patients requiring first-line treatment for metastatic renal cell carcinoma were randomized 1:1 to receive MVA-5T4 or placebo alongside sunitinib, IL-2 or IFN-α. Numerous pre-treatment factors associated with inflammatory anemia (e.g., CRP, hemoglobin, hematocrit, IL-6, ferritin, platelets) demonstrated a significant relationship with tumor burden and patient survival. From these prognostic factors, the pre-treatment mean corpuscular hemoglobin concentration (MCHC) was found to be the best predictor of treatment benefit (P < 0.01) for MVA-5T4 treated patients and also correlated positively with tumor shrinkage (P < 0.001). Furthermore, MCHC levels showed a significant positive association with 5T4 antibody response (P = 0.01). The latter result was confirmed using an independent data set comprising phase II trials of MVA-5T4 in patients with colorectal, renal and prostate cancers. Retrospective analyses demonstrated that RCC patients who had very large tumor burdens and low MCHC levels received little or no benefit from treatment with MVA-5T4; however, patients with smaller tumor burdens and normal MCHC levels received substantial benefit from treatment with MVA-5T4.
Keywords: Biomarker, Therapeutic vaccine, Renal cell carcinoma, 5T4, Antibody, Inflammatory anemia
Introduction
The field of cancer immunotherapy was given a boost recently by the approvals of sipuleucel-T for prostate cancer and ipilimumab for metastatic melanoma. Despite such advances, therapeutic cancer vaccines have yet to fulfill their promised potential to become standard treatment options for patients with cancer. The ability to identify patients who are most likely to benefit from immunotherapy approaches is needed [1, 2]. Here, we describe a potential predictive marker of treatment benefit for renal cancer patients treated with MVA-5T4.
Renal cell carcinoma (RCC) is the most common primary malignancy affecting the kidney. Approximately 20–30 % of RCC patients present with metastatic disease, which has a poor prognosis. Conventional cytotoxic chemotherapeutic agents and hormonal therapies have little impact on survival, and response rates are usually <10 %. Recognition of the immunogenic nature of RCC has resulted in the development of immunotherapy approaches, with high-dose interleukin-2 treatment being the best established and associated with durable disease control. More recently, patients with RCC have been given wider treatment options following the FDA approvals of the targeted therapies sunitinib, sorafenib, temsirolimus, everolimus, pazopanib and bevacizumab. Despite these advances, the management of metastatic RCC remains a challenge, and the lack of defined antigens in RCC has hindered the development of targeted vaccine approaches. TroVax® (MVA-5T4) is a novel vaccine based on a modified vaccinia virus Ankara (MVA) vector engineered to express the 5T4 tumor-associated antigen. 5T4 is an oncofetal antigen which is expressed at high levels in the placenta [1, 2] and in the most common tumors, typically in >80 % of carcinomas of the kidney, breast, gastrointestinal tract, colon, prostate and ovaries. [2, 3] In addition, 5T4 has recently been shown to be a marker of tumor-initiating cells in NSCLC [4].
MVA-5T4 has been tested in multiple phase I, II and III studies in renal (n = 5 [5–9]), colorectal (n = 4 [10–13]) and prostate (n = 1 [14]) cancer patients. A correlation between 5T4-specific cellular and/or antibody response and enhanced patient survival was detected in 7 of the 9 phase I/II studies [15]; this observation was extended in a large phase III study in patients with RCC in which a 5T4 antibody response was shown to correlate with enhanced survival. Furthermore, an “immune response surrogate” was identified, which predicted both the magnitude of the 5T4 antibody response and treatment benefit [16], thus establishing the existence of a subset of patients receiving benefit from TroVax. Despite these encouraging results, the phase III study did not meet its primary endpoint of a significant increase in overall survival in the ITT population. Understanding why this vaccine appeared to be active in some patients but not in the overall population would help the ongoing clinical development of MVA-5T4 and potentially other similar treatment modalities.
It is widely accepted that immunotherapy approaches are best suited to patients with a relatively small tumor load and earlier-stage disease [17–19] although conventionally, new cancer therapies are still trialed in late-stage patients. Here, we hypothesized that MVA-5T4 would be more efficacious in patients with smaller tumor burdens and better prognosis. The availability of a large data set from a phase III study of MVA-5T4 in renal cancer patients in combination with data from multiple phase II studies has enabled us to identify and analyze potential biomarkers predictive of treatment benefit. These results extend previous observations by identifying a simple pre-treatment biomarker that is associated with tumor burden and predictive of treatment benefit.
Materials and methods
Study design
A detailed description of the phase III trial design has been published elsewhere [5]. In brief, patients with advanced or metastatic clear cell renal cancer who had undergone prior nephrectomy and had a good or intermediate prognosis (MSKCC score 0–2), Karnofsky performance status >80 % and life expectancy of >12 weeks were eligible. Patients were randomized 1:1 to receive MVA-5T4 (1 × 109 TCID50/mL) or placebo (at weeks 1, 3, 6, 9, 13, 17, 21, 25, 33, 41, 49, 57 and 65) plus local standard of care (interferon-α, IL-2 or sunitinib).
Analytes
A standard panel of demographic metrics, blood biochemistry, hematological tests and tumor measurements were performed on each patient prior to treatment. A number of additional analytes were measured separately at baseline and included C-reactive protein (CRP), ferritin, hepcidin, IL-6, IL-10 and VEGF. Hepcidin analysis was performed by SELDI-TOF–MS using synthetic stable isotope–labeled hepcidin as an internal standard. Commercially available ELISA kits were used for the analysis of IL-6 and IL-10 (eBioscience, Hatfield, UK) and VEGF (R+D Systems, Abingdon, UK). CRP was quantified in plasma using a wide-range latex-enhanced immunoturbidimetric assay on an ADVIA 2400 analyzer (Siemens Healthcare Diagnostics, Frimley, UK), with a quoted method linearity of 0.03–160 mg/L. Plasma ferritin was analyzed using chemiluminescence immunoassay on an ADVIA Centaur XP analyzer (Siemens Healthcare Diagnostics, Frimley, UK), working range 0.5–1,650 μg/L.
During the course of the study, plasma samples were obtained from patients prior to treatment and following the 3rd and 4th MVA-5T4/placebo vaccinations (weeks 7 and 10 post-treatment initiation, respectively) for the assessment of MVA- and 5T4-specific antibody responses as described previously [16].
Analysis data sets
The full phase III analysis set included 731 of the 733 randomized subjects. Two subjects were excluded from the full analysis set because they were randomized in error.
An immunologically evaluable analysis set was also defined and comprised all subjects from the full phase III analysis set who had a complete set of baseline and week 10 assessments of 5T4- and MVA-specific antibody responses. The immunologically evaluable analysis set included 590 subjects (288 in the MVA-5T4-treated group and 302 in the placebo-treated group)—of the 141 full analysis set subjects excluded, one had an outlying high baseline 5T4 level, 40 subjects (20 in each treatment group) had died by day 70, 10 subjects (five in each group) had been lost to follow-up by day 70, and the remainder had missing antibody data for that assessment but remained on study.
In addition to the phase III data set in renal cancer patients, two additional independent data sets were utilized to investigate whether the relationships found in the phase III study could also be partially validated in very different contexts: (1) pooled results from nine phase I or II clinical studies of MVA-5T4 in colorectal cancer (n = 4), prostate cancer (n = 1) and renal cancer (n = 4) patients (n = 189 patients in total) and (2) data from 38 melanoma patients treated with Hi8-MEL, a peptide vaccine given in a DNA prime, MVA boost schedule [20].
An immunological analysis set for the MVA-5T4 phase I/II studies (subjects with antibody response data at baseline and after the 4th MVA-5T4 vaccination) comprised 52 patients with renal cancer, 32 with colorectal cancer and 24 with prostate cancer. An immunological analysis set for the Hi8-MEL phase II study (subjects with MVA antibody response data at baseline and 16 weeks post-treatment initiation) comprised 32 patients with melanoma. It should be noted that the Hi8-MEL study was initiated by a different company who scheduled the vaccinations and the immunological analyses at different time points from those used for MVA-5T4.
Baseline variables
The baseline (pre-treatment) variables assessed as potential predictors of tumor burden, overall survival and treatment benefit are listed in the left panel of Fig. 2 (the ‘standard set’). The square root or natural logarithm of each variable was taken, if appropriate, to reduce skewness. Values below a lower limit of quantitation were analyzed as if they were at the lower limit; additional analyses were undertaken using an indicator variable to mark whether the value was below or above the lower limit. Total tumor burden was calculated for each patient by summing the longest diameter for all target tumor lesions (as defined by RECIST).
Fig. 2.
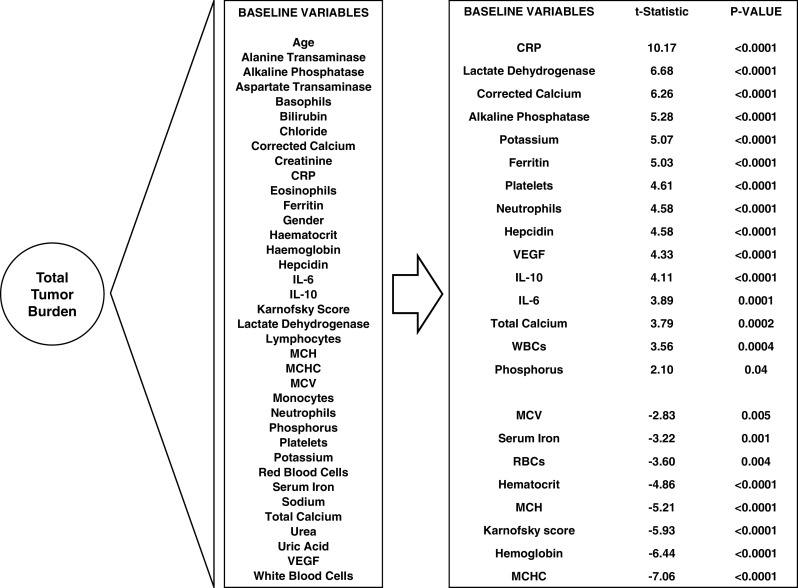
Association between total tumor burden and pre-treatment demographic, blood biochemical and hematological factors. Factors showing a significant association (P < 0.05) are tabulated in order of the strength and direction of the association
Immunological response variables
In all MVA-5T4 clinical studies, antibody responses against 5T4 and MVA were assessed in the same manner. The 5T4 immunological response to vaccination was defined in all MVA-5T4 studies as the natural logarithm of the ratio of the 5T4-specific antibody level after 4 vaccinations to the pre-treatment antibody level. A similar definition was used for the MVA immunological response to vaccination in these studies. In the Hi8-MEL melanoma data set, MVA response was defined as the logarithm of the ratio of the MVA antibody level 16 weeks post-vaccination to the pre-treatment antibody level.
Statistical analysis strategy
There were many baseline variables which could be candidate predictors of treatment benefit. Setting a conventional P value threshold of 0.05 could result in a number of false positives. However, studies that are adequately powered to detect a treatment difference or to evaluate a prognostic marker are usually underpowered to evaluate a predictor of treatment benefit; therefore, setting a lower P value threshold would raise the false-negative risk unrealistically for an exploratory analysis. For these reasons, the candidate predictors of treatment benefit had to satisfy stringent inclusion criteria designed to eliminate variables with weak scientific plausibility. Firstly, we required strong evidence (P < 0.0001) that the candidate variable was associated with baseline tumor burden. Baseline tumor burden is a reflection (albeit imperfect) of how advanced the tumor is; the rationale for this filter was that an immunotherapy will have more chance of success when the cancer is less advanced and the lesions are smaller. Secondly, we required that there was strong evidence (P < 0.0001) that the candidate variable was associated with overall survival. The rationale for this filter was that a candidate variable associated with tumor burden may be associated in a clinically irrelevant way: by requiring that the candidate variable is also associated with survival, we are more confident of clinical relevance. As markers were pre-selected using only data from patients of the placebo arm, the filter was not biased in favor of variables predictive of treatment benefit, but was—intentionally—biased in favor of markers that were prognostic for survival under standard of care only.
Candidate variables that satisfied both these criteria were assessed as predictors of treatment benefit in the immunologically evaluable analysis set. The immunologically evaluable analysis set included all subjects with 5T4-specific antibody levels measured at baseline and at week 10. This analysis set was used partly because it enables the results to be related to the magnitude of the antibody response but mainly because it is not expected that an immunotherapy for cancer would have an effect until after several vaccinations.
Statistical methods
Statistical modeling was performed within SAS® (version 9.2, SAS Institute, Cary, NC, USA 2002–2008). PROC GLM was used for linear regression with intercept, and diagnostic plots were inspected for model adequacy before and after fitting. PROC PHREG was used for proportional hazards modeling.
The standard baseline variables (see Fig. 2) were used to predict tumor burden in the phase III full analysis set. A univariate regression model was used with a single predictor and square root of total tumor burden as the response variable: the t-statistic (the estimate of the regression coefficient divided by its standard error) and the P value were reported for each regression. Variables that were significant predictors at P < 0.0001 were taken through to the next stage of the analysis: modeling survival in the placebo group. The same set of standard baseline variables were used to predict placebo overall survival in the phase III full analysis set. A univariate proportional hazards model was used, stratified by standard of care.
Variables that were significant predictors at P < 0.0001 in both the tumor burden model and the placebo survival model were taken through to the next stage of the analysis: assessing treatment benefit. The ability of a baseline variable to predict treatment benefit in the phase III immunological analysis set was assessed using a proportional hazards model. The proportional hazards model was stratified by standard of care (with a separate treatment effect in each stratum) and included the baseline variable and its interaction with treatment as predictors. The baseline variable was deemed to be a predictor of treatment benefit if the P value for the interaction was <0.05.
Hazard ratios for continuous variables were presented as the ratio across the middle 80 % of the distribution of that variable, that is, the ratio of the hazard experienced by a subject at the 90th centile of the explanatory variable to the hazard experienced by a corresponding subject at the 10th centile. Correlation coefficients were calculated using Spearman’s rank method, unless otherwise stated. Comparisons of tumor burden over subgroups used the Mann–Whitney test, as did comparisons of baseline factors over dichotomized tumor burden.
Results
Total tumor burden and association with baseline variables
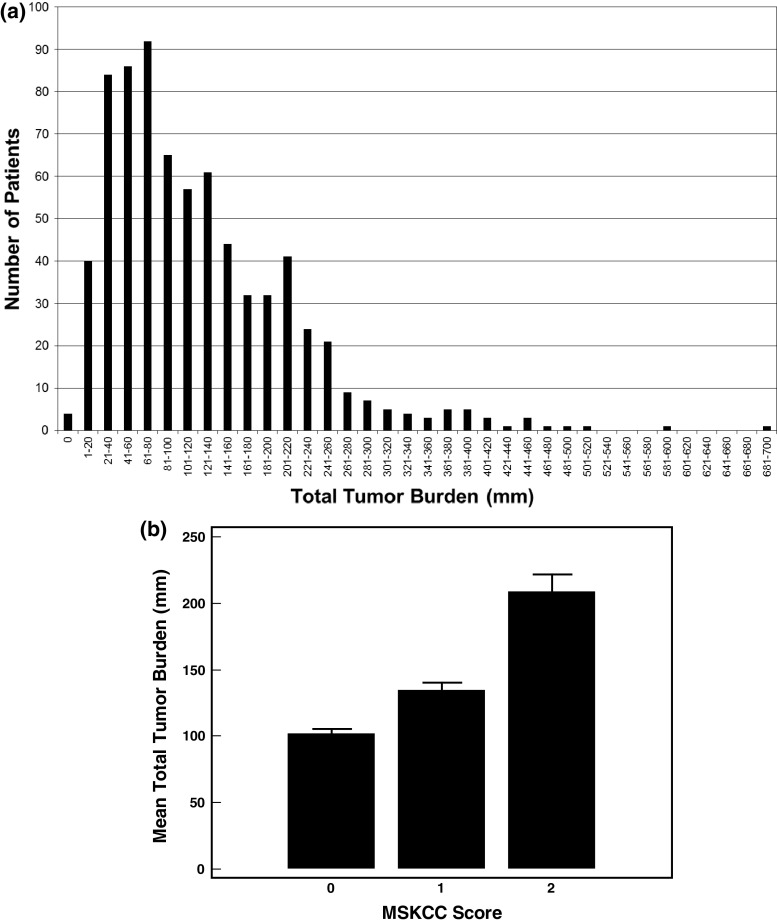
Patients enrolled in the TRIST phase III study had total tumor burdens (sum of the longest diameter of all target lesions) ranging from 10 mm to 690 mm (median = 100 mm) at trial entry (Fig. 1a). Patients classified as having a good prognosis (MSKCC score = 0) or intermediate prognosis (MSKCC score = 1 or 2) were included in the phase III study. Categorization of baseline tumor burden by MSKCC score showed significant differences between all three MSKCC categories (Mann–Whitney all P < 0.001; Fig. 1b), suggesting that even within the “intermediate” prognosis group (MSKCC score 1 or 2), there are a diverse range of patients.
Fig. 1.
Distribution of baseline total tumor burden in renal cancer patients enrolled in the phase III clinical trial (a) and mean tumor burden (±95 % CI) by MSKCC score (b)
Univariate regression was used to model the association between total tumor burden and the standard set of candidate pre-treatment explanatory variables. There was strong evidence (P < 0.0001) of a positive association between total tumor burden and baseline values of CRP, LDH, corrected calcium, alkaline phosphatase, potassium, ferritin, platelets, neutrophils, hepcidin, VEGF, IL-10 and IL-6 and a negative association between total tumor burden and baseline values of MCHC, hemoglobin, Karnofsky score, MCH and hematocrit (Fig. 2).
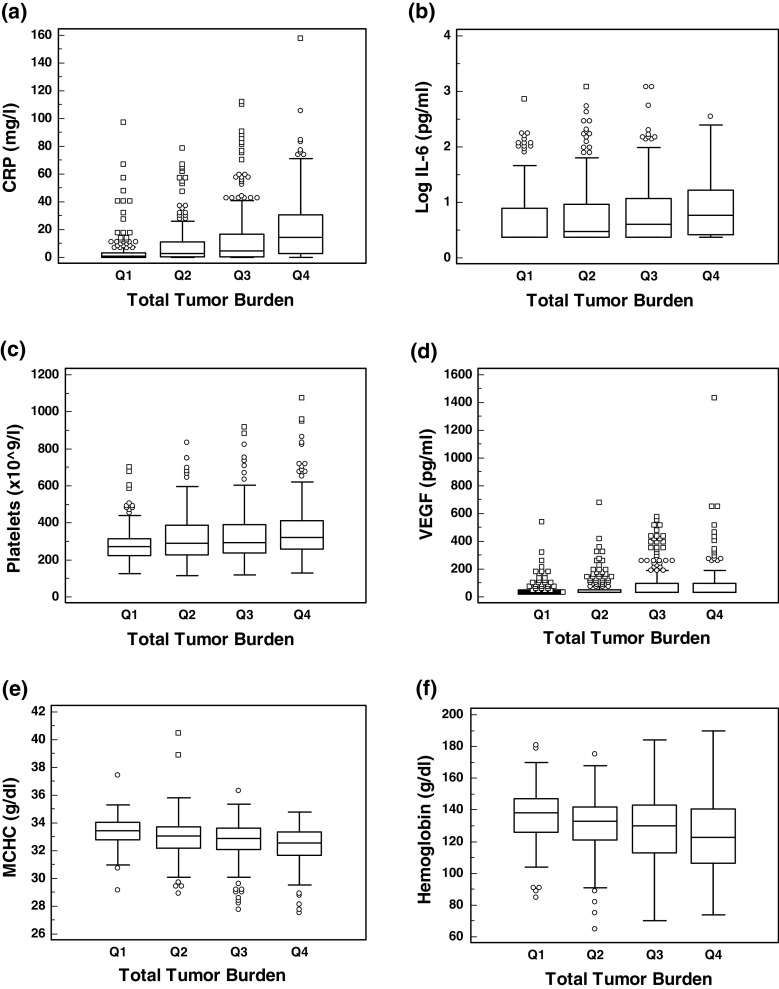
The median concentration of various analytes categorized by pre-treatment tumor burden quartile [<50 mm (Q1), 51–100 mm (Q2), 101–200 mm (Q3) and >200 mm (Q4)] is presented in Fig. 3. It is clear that CRP (Fig. 3a), IL-6 (Fig. 3b), platelets (Fig. 3c) and VEGF (Fig. 3d) increase in concentration as the tumor burden increases, whereas MCHC (Fig. 3e) and hemoglobin (Fig. 3f) levels decrease. Compared to patients with a pre-treatment tumor burden <200 mm, those with a tumor burden >200 mm had significantly elevated levels of CRP (P < 0.001), ferritin (P < 0.001), hepcidin (P < 0.05), platelets (P < 0.001), IL-6 (P < 0.001) and VEGF (P < 0.001) and significantly reduced levels of MCHC (P < 0.001), hematocrit (P < 0.001) and hemoglobin (P < 0.001) (Mann–Whitney; data not shown). All of these factors are associated with inflammatory anemia (also called anemia of chronic disease).
Fig. 3.
Box and whisker plot of analyte concentration by baseline tumor burden category (Q1 = 1–50 mm, Q2 = 51–100 mm, Q3 = 101–200 mm, Q4 > 200 mm). a CRP, b IL-6, c Platelets, d VEGF, e MCHC, f Hemoglobin
Predictors of survival of placebo-treated patients
The standard set of pre-treatment explanatory variables were assessed in univariate proportional hazards models of overall survival in placebo-treated patients (within the full analysis set, stratified by standard of care). There was strong evidence of a positive association (P < 0.0001; hazard ratio < 1) between survival and the pre-treatment values of hemoglobin, hematocrit, Karnofsky score, RBCs and MCHC and a negative association (hazard ratio > 1) with CRP, platelets, alkaline phosphatase, hepcidin, neutrophils, ferritin, VEGF, corrected calcium and LDH. CRP was a particularly strong negative predictor of survival, the hazard increasing more than eightfold from the 10th centile of the distribution of CRP (0.1 mg/L) to the 90th centile (37.2 mg/L).
Obtaining a predictor of treatment benefit
Those candidate pre-treatment variables that were strongly associated with both baseline tumor burden and survival of placebo-treated patients (P < 0.0001) were considered as potential predictors of treatment benefit. Proportional hazards models for overall survival were fitted and stratified by standard of care. Treatment benefit was assessed by dividing the hazard ratio for the explanatory variable in the MVA-5T4 group by the corresponding hazard ratio in the placebo group (Table 1). A ratio of ratios <1 means that increased levels of the explanatory variable are associated with treatment benefit in favor of MVA-5T4. Of the pre-treatment explanatory variables, MCHC emerged as a predictor of treatment benefit (P = 0.013) after being established both as a predictor of tumor burden (P < 0.0001, R 2 = 6.4 %) and as a predictor of placebo survival (P < 0.0001).
Table 1.
Baseline factors associated with the survival of patients in the immunologically evaluable analysis set treated with either placebo or MVA-5T4 and predictors of treatment benefit (HR < 1 favors the MVA-5T4 treatment group). The table lists only those factors which achieved P < 0.0001 in predicting within the full analysis set both tumor burden and placebo survival
| Baseline factors | Placebo | MVA-5T4 | Treatment benefit | |||
|---|---|---|---|---|---|---|
| HR | P value | HR | P value | HR | P value | |
| MCHC | 0.65 | 0.0407 | 0.34 | <0.0001 | 0.52 | 0.0132 |
| Neutrophils | 2.17 | 0.0002 | 1.73 | 0.0072 | 0.79 | NS |
| Hemoglobin | 0.31 | <0.0001 | 0.27 | <0.0001 | 0.88 | NS |
| Corrected calcium | 2.03 | <0.0001 | 1.98 | <0.0001 | 0.97 | NS |
| Alkaline phosphatase | 2.48 | <0.0001 | 2.48 | <0.0001 | 0.99 | NS |
| LDH | 1.56 | 0.004 | 1.61 | 0.0027 | 1.03 | NS |
| Hematocrit | 0.32 | <0.0001 | 0.34 | <0.0001 | 1.06 | NS |
| Hepcidin | 2.57 | <0.0001 | 2.75 | <0.0001 | 1.07 | NS |
| Karnofsky | 0.38 | <0.0001 | 0.42 | 0.0005 | 1.09 | NS |
| VEGF | 1.94 | <0.0001 | 2.33 | <0.0001 | 1.20 | NS |
| Ferritin | 2.20 | 0.0003 | 2.81 | <0.0001 | 1.28 | NS |
| CRP | 6.35 | <0.0001 | 8.64 | <0.0001 | 1.36 | NS |
| Platelets | 2.26 | <0.0001 | 3.26 | <0.0001 | 1.44 | NS |
The magnitude of the treatment benefit is striking: the MVA-5T4/placebo hazard ratio at the 90th centile of MCHC (342.5 g/L) is 52 % of the ratio at the 10th centile (314.1 g/L). This treatment benefit is also apparent when the immunological analysis set is extended to the full analysis set: the MVA-5T4/placebo hazard ratio at the 90th centile of MCHC is 61 % of that at the 10th centile (P = 0.026).
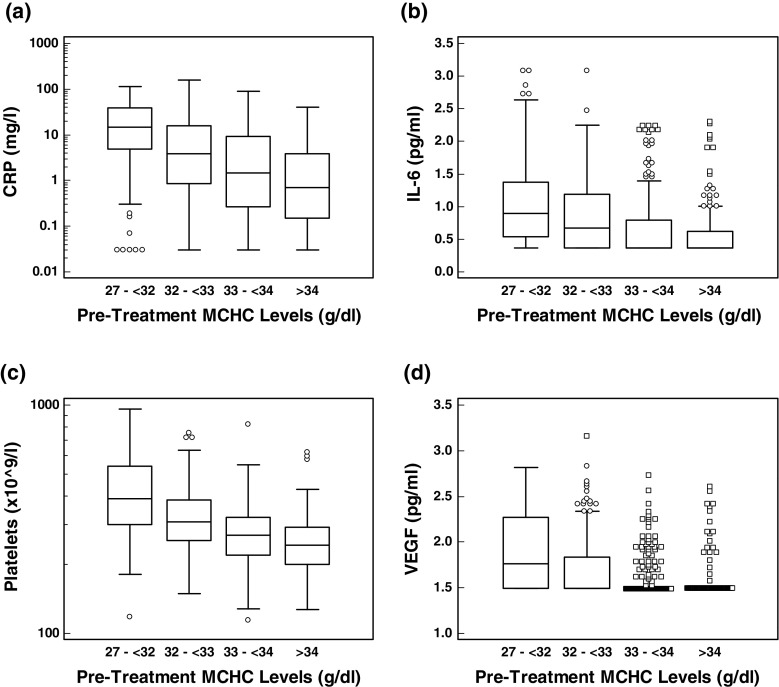
It is interesting to note that patients with higher pre-treatment MCHC levels presented with lower average tumor burdens and lower levels of CRP (Fig. 4a), IL-6 (Fig. 4b), platelets (Fig. 4c) and VEGF (Fig. 4d) all of which are associated with inflammatory anemia. Indeed, MCHC levels showed significant negative correlations (Spearman’s rank) with platelets (−0.434; P < 0.0001), CRP (−0.419; P < 0.0001), IL-6 (−0.329; P < 0.0001), VEGF (−0.329; P < 0.0001), IL-10 (−0.21; P < 0.0001) and hepcidin (−0.1; P = 0.008). (The only predictor of treatment benefit that was more significant than MCHC was total calcium: negatively, with P = 0.009. Total calcium was not a candidate variable as it did not predict survival in the placebo group: P = 0.85).
Fig. 4.
Box and whisker plot of analyte concentration by baseline MCHC category. a CRP, b IL-6, c Platelets, d VEGF
The predictor of treatment benefit (MCHC) as a predictor of 5T4 antibody responses
Previously, 5T4 antibody responses were shown to predict enhanced survival in patients treated with MVA-5T4 in multiple clinical studies, and a surrogate for 5T4 antibody response has been derived [16]. The immune response surrogate (IRS) is a linear combination of baseline 5T4 antibody level, hemoglobin (positively) and hematocrit (negatively). Given the constituents of the IRS, it is not surprising that it correlates strongly with MCHC (Pearson’s correlation coefficient = 0.52). Here, we demonstrate that pre-treatment MCHC levels show a significant association with 5T4 antibody response induced following vaccination with MVA-5T4 in the phase III study in RCC patients (P = 0.014, incremental R 2 of 1.8 %, adjusted for baseline 5T4 antibody level and standard of care). For example, a subject at the 90th centile of MCHC (342.5 g/L) has a predicted 5T4 immune response 23 % higher than a corresponding subject at the 10th centile (314.1 g/L). The only candidate pre-treatment explanatory variable that achieved a smaller P value was CRP, with P = 0.009 and a negative association.
The association of MCHC with 5T4 antibody response was investigated in an independent data set comprising pooled results from nine phase I and phase II studies of MVA-5T4 in colorectal cancer (n = 4), prostate cancer (n = 1) and renal cancer (n = 4). Encouragingly, MCHC was found to be a positive predictor of 5T4 antibody response over the whole data set (P = 0.0019).
MCHC as a predictor of MVA antibody response
These analyses were extended further by investigating whether MCHC levels were associated with MVA antibody response (fold increase after 4th vaccination) in patients treated with MVA-5T4 in the phase III trial. Surprisingly, baseline MCHC level was a negative predictor of MVA antibody response (P = 0.021; adjusted for standard of care and baseline MVA level), which is in contrast to MCHC being a positive predictor of 5T4 antibody response. The strongest single predictor of both MVA and 5T4 antibody response was CRP with the direction of prediction being positive and negative, respectively. It is of considerable interest that while MVA and 5T4 response are positively correlated (Pearson’s correlation coefficient = 0.37, P < 0.0001), the best predictors of 5T4 response also predict MVA response but with opposite sign.
In order to pursue this observation further, a potential relationship between MCHC and MVA antibody response was investigated in two independent data sets: (i) pooled results from 9 phase I and phase II trials of MVA-5T4 in RCC, CRC and prostate cancer patients and (ii) data from melanoma patients treated with Hi8-MEL, a peptide vaccine given in a DNA prime, MVA boost schedule [20]. MVA antibody levels from the phase I/II MVA-5T4 trials were quantified following the 4th vaccination in the same way as for the phase III trial. However, MVA antibody levels in the Hi8-MEL melanoma trial were quantified using a different ELISA format. Despite such profound differences in data sets, there was also evidence that MCHC was a negative predictor of MVA antibody response over the whole MVA-5T4 phase II data set (P = 0.0007, adjusted for baseline) and in the Hi8-MEL data set (P = 0.031, adjusted for baseline).
The interaction between MCHC, tumor burden and treatment benefit
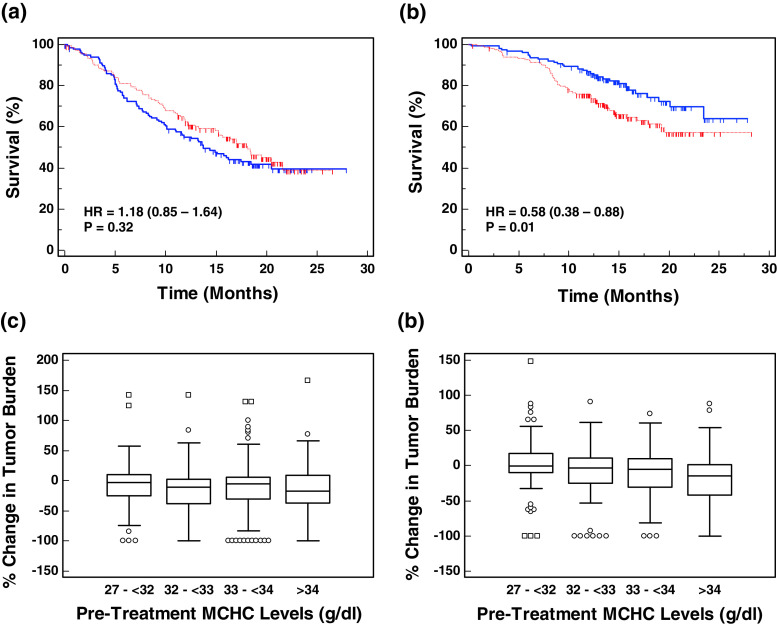
Exemplification of the impact of tumor burden and MCHC levels on the relative efficacy of MVA-5T4 in RCC patients is shown in Fig. 5. Patients with very large pre-treatment tumor burdens (>200 mm; n = 138 patients) do not appear to benefit from MVA-5T4 irrespective of MCHC levels (data not shown). Patients with a pre-treatment tumor burden < 200 mm and low MCHC levels (n = 271 patients) do not show a significant difference in survival (Fig. 5a). However, patients with a pre-treatment tumor burden < 200 mm and normal MCHC levels (n = 317 patients) show a significant survival advantage in favor of MVA-5T4 (Fig. 5b; HR = 0.58; P = 0.01).
Fig. 5.
Impact of pre-treatment tumor burden and MCHC levels on the relative efficacy of MVA-5T4. Kaplan–Meier survival plots of patients treated with MVA-5T4 (blue solid line) or placebo (red dashed line) who have a pre-treatment tumor burden <200 mm and MCHC levels < 33 or >33 are shown in (a) and (b), respectively. c and d show box and whisker plots of the change in tumor burden (percent change from week 26 to baseline) in patients treated with placebo (c) or MVA-5T4 (d) categorized by their pre-treatment MCHC levels
Having identified MCHC levels as the best single predictor of treatment benefit, further analyses were undertaken to determine whether MCHC was associated with other markers of clinical efficacy. During the phase III trial, total tumor burden was measured prior to treatment and 26 weeks after treatment. The percentage change in tumor burden between baseline and week 26 was calculated and correlated with pre-treatment MCHC levels. Patients treated with placebo showed no correlation between MCHC and tumor shrinkage (Spearman’s correlation coefficient = −0.01; P = 0.91; n = 293 patients); however, patients treated with MVA-5T4 showed a significant correlation (Spearman’s correlation coefficient = −0.2; P = 0.0008; n = 273 patients). The change in tumor burden in patients treated with placebo or MVA-5T4 and categorized by pre-treatment MCHC levels are shown in Fig. 5c, d respectively.
Discussion
Although new paradigms for the development of immunotherapies are being discussed [21], few studies have directly addressed the link between tumor burden, “immune health” and treatment benefit from the immunotherapy being tested. The results reported here support the general consensus that cancer vaccines, such as MVA-5T4, are more likely to demonstrate benefit in patients with lower tumor burdens. A mechanistic explanation for this observation is likely to include the physical challenge of eliminating a large solid tumor but also physiological changes related to disease progress, which may impact on the quantity and/or quality of the induced immune response. Our results demonstrate a strong association (but not necessarily causation) between increasing tumor burden and changes in various blood biochemical factors, which may impact on the relative efficacy of immunotherapy compounds (e.g., CRP, VEGF, IL-6, platelets). Many of the biochemical factors identified as being associated with tumor burden, patient survival and immune response induced by MVA-5T4 are known markers of inflammatory anemia. A case in point is MCHC, which not only was a significant predictor of treatment benefit but also showed significant associations with tumor burden, increased patient survival, tumor shrinkage and 5T4-specific antibody response in MVA-5T4-treated patients.
Although it is not immediately obvious why factors associated with anemia may impact on the relative efficacy of an immunotherapy product, such as MVA-5T4, anemia is a common co-morbidity in patients with solid tumors. The cause of anemia can be multifactorial and may occur either as a direct effect of the cancer (e.g., blood loss, bone marrow infiltration), by factors produced by the cancer or by therapeutic agents given to treat the cancer. Furthermore, anemia is often a marker of poor prognosis in cancer patients [22, 23]; indeed, hemoglobin is one of the factors included in the MSKCC (Motzer) prognostic algorithm, which classifies renal cancer patients as having good, moderate or poor prognosis [24]. Here, we have demonstrated that patients who have higher tumor burdens also show increases in factors that are associated with inflammatory anemia (also known as the anemia of chronic disease). Inflammatory anemia is often difficult to differentiate from iron deficiency anemia; patients have hemoglobin levels in the low-to-normal range (7–12 g/dL) but, unlike patients with iron deficiency anemia, show normal-to-high levels of serum ferritin [25] along with elevated levels of inflammatory cytokines and acute-phase reactants (e.g., IL-6, IL-1β, TNFα and CRP).
We have demonstrated that numerous factors that are associated with inflammatory anemia appear to impact on the relative clinical and immunological efficacy of the cancer vaccine MVA-5T4. In patients with inflammatory anemia, CRP levels are elevated and MCHC levels reduced. Results from our analyses showed that pre-treatment levels of MCHC and CRP were the two factors most strongly associated with tumor burden, survival of placebo-treated patients and the magnitude of 5T4 and MVA antibody responses. The association of CRP and MCHC with the output measurement was in opposite directions in all cases. We suspect that MCHC is not the key causative factor modulating the relative efficacy of MVA-5T4 but is simply a robust measurement, which is indicative of an underlying inflammatory state and tumor burden. Indeed, it has been suggested that determination of the percentage of hypochromic red blood cells or reticulocyte hemoglobin content can help differentiate iron deficiency anemia from anemia of chronic disease [26, 27]. Our data provide further support for the link between MCHC levels and other factors associated with inflammatory anemia.
Pre-clinical work by Chiarella et al. [28] has also noted a link between tumor burden, inflammation and immune responsiveness. Chiarella et al. studied the effect of tumor burden on immune responsiveness and subsequent tumor rejection in a murine fibrosarcoma model. The authors demonstrated that immunological non-responsiveness to tumor antigens was observed when the tumor exceeded 500 mm3; this coincided with the onset of a “systemic inflammatory condition,” which included the detection of increased levels of neutrophils and pro-inflammatory cytokines including IL-1β, TNF-α, IL-6 and CRP. Treatment of tumor-bearing mice with dexamethasone successfully reduced all systemic inflammatory parameters and restored the ability to mount an anti-tumor immune response. Interestingly, the authors also demonstrated that the vaccination of mice that had a tumor burden exceeding 500 mm3 actually accelerated tumor growth.
The conflicting roles of inflammation in protection against cancer or the progression of cancer appear paradoxical. Acute inflammation usually precedes the induction of an adaptive immune response, whereas chronic inflammation has been shown to contribute to tumorigenesis. Schreiber et al. [29] postulate that “tumor-promoting inflammation and protective tumor immunity are dynamically interconnected processes that vie for dominance as tumor cells develop and transit through cancer immunoediting.” Inflammatory mediators such as IL-6, IL-10 and VEGF not only support tumor cell survival but suppress the function of immune cells, most notably dendritic cells, which are critical to the induction of tumor-specific immune responses [30]. High levels of one or more of such inflammatory mediators could blunt the immune response to 5T4 following vaccination with MVA-5T4 through suppression of antigen presentation by dendritic cells. We have demonstrated that patients with higher pre-treatment MCHC levels have lower levels of numerous inflammatory mediators such as IL-6, IL-10, CRP and VEGF. Therefore, MCHC may represent a simple, single measurement that could be used to exclude patients who have an “inflammatory signature” and who may not benefit from treatment with a cancer vaccine.
One particularly surprising observation from this study was the identification of a positive association between MCHC and 5T4 antibody levels but a negative association between MCHC and MVA antibody levels. The latter correlation was evident in 3 independent data sets which included different indications and two different MVA-based vaccines. The reason for this remains unclear, and we do not know whether this observation is specific to MVA or all viral vector delivery systems used in immunotherapy approaches. Although speculation, it is possible that a highly inflammatory environment helps to drive a very strong MVA-specific immune response following 1–2 vaccinations. Subsequent vaccinations may result in a further expansion of the MVA-specific T-cell population at the expense of the much rarer 5T4-specific T-cell population. It would be very interesting to know whether other investigators in the field detect a similar relationship. Such studies may confirm factors that could indicate whether a patient is suitable/unsuitable for a particular immunotherapeutic vaccine.
Previously, analysis of data from the phase III clinical trial in RCC patients identified a pre-treatment biomarker (termed the immune response surrogate; IRS), which predicted 5T4 antibody response and was also associated with enhanced survival of MVA-5T4-treated patients. The IRS consisted of three pre-treatment factors: 5T4 antibody, hemoglobin and hematocrit in a linear combination, which predicted treatment benefit to the extent that the MVA-5T4/placebo hazard ratio at the 90th centile of the IRS is 45 % of that at the 10th centile (P = 0.0017). The study reported here aimed to identify a pre-treatment predictor of treatment benefit without going through an intermediary step of predicting 5T4 antibody response. The best single predictor of treatment benefit for RCC patients treated with MVA-5T4 was the pre-treatment MCHC level. It is very encouraging that independent statistical modeling undertaken to address two different questions (identification of predictors of immunological and clinical efficacy) identified common factors. As with all analyses of this type, the results should be interpreted with some caution since they were performed retrospectively and applied primarily to patients with renal cancer. However, it is encouraging that MCHC levels showed significant associations with different output measures (tumor shrinkage, 5T4 and MVA antibody response) and in independent data sets. Furthermore, MCHC levels are measured routinely in patients with cancer; therefore, the targeted selection of patients who have favorable biomarker levels is much easier based on the measurement of MCHC levels than for 3 factors included in the immune response surrogate. (The ratio of hazard ratios across the population shows that the IRS is a slightly better predictor (45 vs. 52 %) of treatment benefit; this advantage would be more than offset by the ease of use of MCHC.) Ongoing and future clinical studies of MVA-5T4 will enrich for patients who we believe are more likely to receive treatment benefit based on the observations reported here.
The ultimate goal of this work will be in the setting of a cutoff or cutoffs for the predictor of treatment benefit: the decision to admit a subject to a trial or to treat a patient is discrete, either ‘yes’ or ‘no’. However, the formal setting of a cutoff is beyond the scope of this paper. It is well established in the field of diagnostic tests that to avoid over-optimistic assessment of performance, at least three independent data sets should be used: one to establish the form of the test (in this instance, the selection of a baseline characteristic such as MCHC), one to set the cutoff and one to validate the cutoff. Unfortunately, the phase III data set was not large enough to permit random subdivision while maintaining power. The scope of this paper is therefore to put forward a predictor, with statistically and scientific justification, to be validated in a future study. If MCHC’s value as a predictor of treatment benefit is confirmed, then the next step will be to derive and validate a cutoff.
In conclusion, we have shown that renal cancer patients with very large tumor burdens and markers of inflammatory anemia receive little or no benefit from treatment with MVA-5T4; in contrast, patients with lower tumor burdens and no overt signs of inflammatory anemia may receive considerable benefit. This finding has potentially important implications for MVA-5T4 and other cancer vaccines which rely on the induction of a potent immune response. Confirmation of these observations in future studies and in different cancer types is ongoing.
Conflict of interest
RH, JdB, MK, GB, SN and WS wish to disclose that they are employees of Oxford BioMedica, the manufacturer and developer of TroVax®. PT wishes to disclose that he has been paid consulting fees by Oxford BioMedica.
References
- 1.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239–246. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Southall PJ, Boxer GM, Bagshawe KD, et al. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61:89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzynska T, Marsh PJ, Schofield PF, et al. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damelin M, Geles KG, Follettie MT, et al. Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells. Cancer Res. 2011;71:4236–4246. doi: 10.1158/0008-5472.CAN-10-3919. [DOI] [PubMed] [Google Scholar]
- 5.Amato RJ, Hawkins RE, Kaufman HL, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–5547. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 6.Amato RJ, Shingler W, Goonewardena M, et al. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32:765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 7.Amato RJ, Shingler W, Naylor S, et al. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res. 2008;14:7504–7510. doi: 10.1158/1078-0432.CCR-08-0668. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins RE, Macdermott C, Shablak A, et al. Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother. 2009;32:424–429. doi: 10.1097/CJI.0b013e31819d297e. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman HL, Taback B, Sherman W, et al. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med. 2009;7:2. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkord E, Dangoor A, Drury NL, et al. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008;31:820–829. doi: 10.1097/CJI.0b013e3181876ab3. [DOI] [PubMed] [Google Scholar]
- 11.Harrop R, Connolly N, Redchenko I, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 12.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 14.Amato RJ, Drury N, Naylor S, et al. Vaccination of prostate cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother. 2008;31:577–585. doi: 10.1097/CJI.0b013e31817deafd. [DOI] [PubMed] [Google Scholar]
- 15.Harrop R, Shingler W, Kelleher M, et al. Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother. 2010;33:999–1005. doi: 10.1097/CJI.0b013e3181f5dac7. [DOI] [PubMed] [Google Scholar]
- 16.Harrop R, Shingler WH, McDonald M, et al. MVA-5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother. 2011;60:829–837. doi: 10.1007/s00262-011-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17:3884–3891. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–e157. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dangoor A, Lorigan P, Keilholz U, et al. Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine. Cancer Immunol Immunother. 2010;59:863–873. doi: 10.1007/s00262-009-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoos A, Britten CM, Huber C, et al. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol. 2011;29:867–870. doi: 10.1038/nbt.2000. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol. 2011;61:549–559. doi: 10.1016/j.eururo.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm DU, Lebrecht A, Schmidt M, et al. Prognostic impact of haemoglobin levels in breast cancer. Anticancer Res. 2007;27:1223–1226. [PubMed] [Google Scholar]
- 24.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.20.1.289. [DOI] [PubMed] [Google Scholar]
- 25.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 27.Brugnara C. Iron deficiency and erythropoiesis: new diagnostic approaches. Clin Chem. 2003;49:1573–1578. doi: 10.1373/49.10.1573. [DOI] [PubMed] [Google Scholar]
- 28.Chiarella P, Vulcano M, Bruzzo J, et al. Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors. Cancer Immunol Immunother. 2008;57:701–718. doi: 10.1007/s00262-007-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 30.Sheng KC, Wright MD, Apostolopoulos V. Inflammatory mediators hold the key to dendritic cell suppression and tumor progression. Curr Med Chem. 2011;18:5507–5518. doi: 10.2174/092986711798347207. [DOI] [PubMed] [Google Scholar]