Abstract
Adoptive cell therapy employing gene-modified T-cells expressing chimeric antigen receptors (CARs) has shown promising preclinical activity in a range of model systems and is now being tested in the clinical setting. The manufacture of CAR T-cells requires compliance with national and European regulations for the production of medicinal products. We established such a compliant process to produce T-cells armed with a first-generation CAR specific for carcinoembryonic antigen (CEA). CAR T-cells were successfully generated for 14 patients with advanced CEA+ malignancy. Of note, in the majority of patients, the defined procedure generated predominantly CD4+ CAR T-cells with the general T-cell population bearing an effector–memory phenotype and high in vitro effector function. Thus, improving the process to generate less-differentiated T-cells would be more desirable in the future for effective adoptive gene-modified T-cell therapy. However, these results confirm that CAR T-cells can be generated in a manner compliant with regulations governing medicinal products in the European Union.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1492-9) contains supplementary material, which is available to authorized users.
Keywords: Adoptive cell therapy, Chimeric antigen receptor, Bioprocessing, T-cell, Cell culture, Good manufacturing process
Introduction
Impressive early-phase clinical results have been reported with adoptive cell therapies using antibody-based chimeric antigen receptors (CAR) targeting B cell malignancies [1–4]. These demonstrate the potential of this approach in tackling advanced cancer. However, CAR T-cell therapy to treat solid tumors has a lower profile with few trials currently reported (as recently reviewed in [5, 6]).
Carcinoembryonic antigen (CEA) is a tumor-associated antigen, highly expressed on a broad range of gastrointestinal and other tumors [7]. The CEA-specific MFE23 single-chain antibody fragment (scFv) was isolated from a phage display library [8] and has been used clinically in imaging studies [9] and as a part of an enzyme pro-drug therapy [10]. The excellent safety profile of the MFE23 scFv in these clinical studies prompted its further development in a CAR strategy. A first-generation CAR consisting of the MFE23 scFv fused to the CD3ζ chain of the T-cell receptor/CD3 complex (MFEζ, Fig. 1a) was functional in normal donor T-cells [11] and also redirected the effector function of patient T-cells in an antigen-specific manner [12]. Interestingly, the MFE scFv appears to function optimally in the absence of an extracellular spacer region unlike other scFv specific for CEA [13] and other similar tumor-associated antigens [14]. Based upon these studies and the background of extensive vaccine and antibody-directed immune therapy targeting of CEA [15], a phase I clinical protocol was opened in November 2007 based upon first-generation MFEζ CAR technology to treat patients with advanced CEA+ malignancy (NCT 01212887, EUDRACT-2005-004085-16).
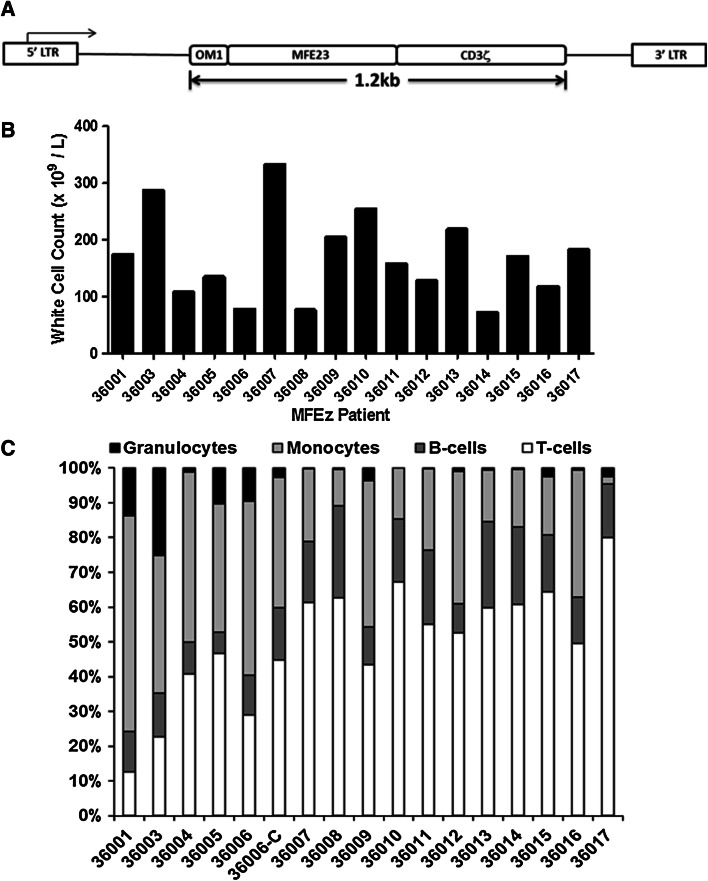
Fig. 1.
The MFEζ vector and white cell count and composition of MFEζ patient leukapheresis products prior to cell processing. a Schematic representation of the integrated pMP71 retroviral vector encoding the MFEζ CAR. Gene expression is driven by the 5′ long-terminal repeat (LTR) with the 1.2 Kb MFEζ transgene cassette comprising the oncostatin M leader sequence and the MFE23 scFv fused to amino acids 19–166 of the human CD3ζ receptor. Amino acids 19–23 were modified from the original TEAQ sequence to TDGA as described [11]. b White cell counts for each leukapheresis product collected. c Relative frequency of granulocytes, monocytes, B-, and T-cells within the leukapheresis product of each patient. 36006-C refers to the analysis of the cryopreserved leukapheresis product for that particular patient
To generate CAR T-cells for this protocol, compliance with good manufacturing process (GMP) was essential in order to adhere to the 2004 European Union Clinical directive and associated legislature (i.e., 2003/94/EC, 2001/20/EC, 1968 UK Medicines Act and SI 2004 1031, EU Guide to GMP and Eudralex Volume IV, and subsequent ATMP regulations). Here, we document a fully GMP-compliant process used in the UK to generate large numbers and report product characteristics of gene-modified CAR T-cells for administration to patients in a clinical trial setting.
Methods
Materials
Where possible, materials used for the manufacture of MFEζ T-cells were sourced from medical device manufacturers or preference given to materials that were CE marked for that purpose. Where materials were not manufactured specifically for the purpose, the materials were evaluated within process quantification (PQ) manufacturing runs to demonstrate that the products met the release requirements. All cell culture media and additives were sourced from Life Technologies (Paisley, UK) except where stated. Fetal bovine serum (FBS, PAA laboratories, Pasching, Austria) was certified according to recommendations of the European Directorate for the Quality of Medicines & Healthcare. GMP-compliant T-cell-mixed media composed of 20 % Roswell Park Memorial Institute (RPMI) media and 80 % AIM V were produced by Life Technologies (Paisley, Scotland). Pooled AB serum was supplied by Valley Biomedical (Winchester, VA, USA) and complied with Directive 2001/83/EC for the selection of donors and sourced from a region of low transmissible spongiform encephalitis (TSE) exposure. Interleukin-2 (IL-2; Proleukin) was obtained from Novartis, Camberley, UK, and frozen in aliquots at a concentration of 106 IU/mL based upon reported stability studies [16].
Flow cytometric analysis
About 100 μl samples of cells or blood were stained using the combination of either anti-CD45-FITC (clone J33, Beckman Coulter, High Wycombe, UK), anti-CD3-PE (clone UCHT-1), and 7-AAD or anti-CD3-FITC, Annexin-V-PE, and 7-AAD (all reagents were from BD Biosciences unless stated) in an additional 100 μl of 2× Annexin-V binding buffer for 20 min in the dark before the addition of 1 mL of 1× Lysis solution (Pharm Lyse) and 100 μl of Flowcount beads (Beckman Coulter) and incubation for a further 10 min. After staining and lysis, the cells were analyzed and enumerated using a FC500 flow cytometer (Beckman Coulter) based upon the following phenotypes:
CD45+ white blood cells;
CD45+ CD3+ (T-cells from all white blood cells);
CD45+ CD3− (Non-T-cell from white blood cells);
SSC/CD45+ (granulocyte, lymphocyte, and monocyte populations).
In each sample, 7-AAD was included to discriminate dead cell populations. To identify MFEζ CAR expressing T-cells, a two-layer stain of biotinylated CEA (biotinylated “in house” using Amersham Biosciences ECL Protein Biotinylation Module, Amersham, UK) and strepavidin-PE (Sigma-Aldrich, Dorset, UK) was used with the cells analyzed using the FC500 flow cytometer. The activity of the biotinylated CEA was confirmed prior to use by staining of a clonal Jurkat cell line expressing the MFEζ CAR.
Patient leukapheresis
Patient leukapheresis collection was performed using a COBE® Spectra Apheresis System (TerumoBCT Zaventem, Belgium) with a standard stem cell collection protocol in the Stem Cell Laboratory, Pathology Department, Christie Hospital Foundation Trust, Manchester, UK, before transport to the cell-processing laboratory.
Post-leukapheresis processing
The cell-processing laboratory was based at NHSBT, Plymouth Grove, Manchester, UK, which included culture hoods that maintained a grade A environment for open processing residing within grade B cleanrooms. Upon arrival, the fresh leukapheresis product was either used immediately to generate MFEζ T-cells or cryopreserved in Cryobags (Baxter Healthcare, Northampton, UK) in 20–100-mL aliquots using equal volumes of 20 % DMSO (Quest Biomedical, Solihull, UK) and either donor serum or 4.5 % human serum albumin (HSA; BPL, Elstree, UK).
Cryopreserved leukapheresis product was harvested by thawing on a digital hot plate (34.9 ± 0.8 °C, VWR, Lutterworth, UK) until all ice had thawed and then diluted in approximately 10 volumes of cold (2–8 °C) T-cell-mixed media. The cells were then washed using a CytoMate™ (Baxter Healthcare, Northampton, UK) with T-cell-mixed media as the washing medium employing a washing program with a residual fold reduction of 25 and a final volume of 150–250 mL as previously described [17]. Some of the leukapheresis products were subjected to Ficoll-density gradient centrifugation by layering of 0.75 × 109 white blood cells diluted in T-cell-mixed media to a final volume of 20 mL onto 20 mL of Ficoll-Paque™ PREMIUM (1.078 g/mL GE Healthcare, Amersham, UK) in a 50-mL tube (BD Biosciences, Oxford, UK) in a grade A environment, and the sealed tube then was centrifuged within the grade B environment. After centrifugation (400xg, 25 min, room temperature with no brake applied), the sealed tubes were returned to the grade A environment after alcohol wiping and peripheral blood mononuclear cells residing at the interphase were collected, washed, and resuspended in T-cell-mixed media.
T-cell activation
Approximately, 109 total CD45+/7-AAD− cells were diluted to 1-liter (1 × 106/mL) final volume with T-cell-mixed media containing 8 % heat inactivated AB donor serum (Valley Biomedical, Winchester, VA, USA), 100 IU/mL IL-2, and 10 ng/mL anti-CD3ε monoclonal antibody (OKT-3, Janssen-Cilag, High Wycombe, UK) and transferred to a 3L Lifecell Tissue culture bag (3L LCB, Baxter Healthcare, Northampton, UK) and cultured for 48 h at 37 °C, 5 % CO2 in a humidified incubator.
MFEζ retroviral supernatant
The first-generation MFEζ CAR (MFE23 scFv fused to the CD3ζ chain [11, 14], Fig. 1a) was cloned into the pMP71 retroviral vector [18]. A PG13 producer cell clone was generated and used to produce a master and working cell bank under GMP-compliant conditions at Eufets (Idar-Oberstein, Germany, see Supplementary table 1 for quality control testing of the master cell bank and end-of-production cells). GMP-compliant retroviral supernatant (see Supplementary tables 2 and 3 for test criteria) was supplied at a titer of 2 × 106 infectious units/mL (titer determined by transduction on HT1080 cells) in glass vials with crimp-sealed rubber septums.
Retroviral transduction
A 500-mL dendritic cell (DC) differentiation bag [19] (Miltenyi Biotec, Bisley, UK) was loaded with 1.2 mg of RetroNectin™ (final concentration 3 μg/cm2, Takara Bio, Japan) in 120 mL of sterile saline (0.9 % sodium chloride, Baxter Healthcare, Northampton, UK) using previously described methods [20] and incubated either at 2–8 °C for 16–48 h or at 37 °C for 2 h to coat the bag.
Activated T-cells were transferred from the activation culture to two 0.6-L blood bags (MacoPharma, Tourcoing, France) and centrifuged at 1,000xg for 15 min at 22 °C (HERAEUS Multi 4KR Centrifuge, Fisher Scientific UK Ltd, Loughborough, UK). Excess media were eliminated by the use of a plasma squeezer (Baxter Healthcare, Northampton, UK) into a waste blood bag without disturbing the cell pellet. The cell pellets along with the remaining minimal quantity of culture media were pooled, and a small sample was taken to determine the CD45+/7AAD− cell number.
The contents of the RetroNectin-DC bag were transferred to a connected 0.6-L blood bag; the RetroNectin-DC bag was washed with sterile saline before filling with 24–40 mL of MFEζ retroviral supernatant. This was achieved by thawing the glass vials of virus and subsequent transfer into the grade A environment where the virus was removed and transferred to the transduction bag using a syringe and needle with an air dart to reduce back pressure. Approximately, 2.6 × 108 CD45+/7AAD− activated T-cells were added to the RetroNectin-DC bag with additional T-cell-mixed media containing 8 % heat inactivated AB serum and 100 IU/mL IL-2 to a final volume of 120 mL. After a six-hour incubation period in a 37 °C/5 % CO2 humidified incubator, an additional 230 mL of T-cell-mixed media/8 % AB serum/100 IU/mL IL-2 was added to the RetroNectin-DC bag prior to overnight incubation (37 °C/5 % CO2).
The following day, the contents of the Retronectin-DC bag were transferred to a 0.6-L blood bag containing 250 mL T-cell-mixed media/8 % AB serum and cells pelleted by centrifugation at 1,000×g for 15 min at 22 °C. These cells were then returned to the same Retronectin-DC bag with fresh virus, media, and IL-2 for a second round of transduction [19].
MFEζ T-cell expansion
After transduction, cells were transferred from the Retronectin-DC bag to a 0.6-L blood bag, pelleted, excess media removed, and the cells adjusted to achieve a final cell concentration of 0.5 × 106 CD45+/7AAD− cells per mL with T-cell-mixed media/8 % AB serum supplemented with 360 IU/mL of IL-2 in a sterile 3L LCB culture bag and incubated in a humidified incubator (37 °C/5 % CO2). On day 7 post-activation, the cultures were readjusted to a cell concentration of 0.5 × 106 cells/mL with T-cell-mixed media supplemented with 8 % AB serum and IL-2 replenished to 360 IU/mL of total culture volume. On day 9 and every 2–3 days until final harvest, cultures were adjusted to a cell number of 0.5 × 106/mL with T-cell-mixed media and 360 IU/mL IL-2 until a maximum culture volume of 16 L’s was achieved, at which point, only additional IL-2 was added until the day of harvest. When necessary, cultures were divided between additional 3L LCB bags to maintain a culture volume of 0.5–2 L per bag.
Day 7 functionality assessment: CD25 up-regulation assay
Samples of MFEζ T-cells were collected on day 7 of culture and rested for 16 h in the absence of IL-2; 105 T-cells were incubated on plates pre-coated with 0.5 μg/mL CEA (Sigma-Aldrich, Dorset, UK). About 48–72 h later, T-cells were collected and stained with anti-CD4 or anti-CD8 FITC (clone RPA-T4 and clone RPA-T8, respectively), anti-CD25 APC (Clone M-A251BD Biosciences), and biotinylated CEA/strepavidin-PE to determine the relative expression of CD25 on activated MFEζ T-cells. Control levels of CD25 were determined on MFEζ T-cells cultured on plates which had been incubated on plates blocked with T-cell media alone.
Post-culture processing
The T-cell cultures were washed and reduced to a maximum volume of 500 mL by means of a Cytomate cell washer (Baxter Healthcare, Northampton, UK) according to the manufacturer’s instructions and transferred to a final product bag ready for infusion. Sterility during the process was assessed by BacT/ALERT ™ (Biomerieux, Hampshire, UK). On the last day of GMP manipulation prior to harvest, a gram stain (BD Biosciences, Aylesbury, UK) and mycoplasma analysis (VenorGeM® PCR test system, Minerva Biolabs, Berlin, Germany) were performed to confirm that the product was sterile and mycoplasma-free immediately prior to infusion into the patient (in 4.5 % HSA/phosphate-buffered saline (PBS)).
T-cell differentiation assays
Samples of the final product which had been cryopreserved and stored in liquid nitrogen were thawed and rested at 5 × 106/mL for 24 h at 37 °C in RPMI-1640 medium (Lonza, Basel, Switzerland) supplemented with 10 % FBS, 50 μM 2-mercaptoethanol, 1U/mL penicillin, 100 μg/mL streptomycin, and 25 mM Hepes buffer (Sigma-Aldrich, Dorset, UK); 105 T-cells were then resuspended in 1 %FBS/PBS and incubated with anti-CD8-FITC (2:100; clone HIT8a) and anti-CD4-APC (2:100; clone SK3, both from BD Bioscience) for 30 min on ice before fixation in 1 % paraformaldehyde, and 105 T-cells were also stained with antibodies at 1:100 dilution in 1 %FBS/PBS for 30 min on ice with the following antibodies: anti-CD8-FITC (clone HIT8a), anti-CD45RA-PE (clone HI100), anti-CD27-PE (clone M-T27I), αCD28-PE (clone 28.2; all from BD biosciences), anti-CD62L-PECy7 (clone Dreg 56, eBioscience, UK), and anti-CCR7-APC (clone FR 11-11E8, Miltenyi Biotec, Bisley, UK). Cells were then fixed in 1 % paraformaldehyde before data capture on FACScalibur (BD Bioscience) and analyzed using FloJo software (Tree Star, Ashland, Oregon, USA).
Functional assay
To determine interferon gamma secretion from the CAR T-cells, CEA protein (1 μg/mL) resuspended in borate buffer was used to coat 96-well non-tissue culture-treated plate for 12 h at 4 °C. Plates were blocked for 30 min at 37 °C in T-cell media prior to the addition of T-cells at 5 × 105/mL. PMA (500 ng/mL) and ionomycin (50 ng/mL) (Sigma-Aldrich, Dorset UK) were added to control T-cell wells. T-cells were incubated for 24 h at 37 °C before the supernatant was removed and frozen at −20 °C prior to use in IFN-γ ELISA. Samples were analyzed using Diaclone IFNγ ELISA (Gen Probe, San Diego, California, USA) performed in accordance with the manufacturer’s protocol.
Results
MFEζ patients: Cell manipulation, T-cell transduction, and expansion characteristics
Fourteen patients enrolled into the MFEζ clinical trial were leukapheresed at the Christie Hospital, Manchester, UK, with the apheresis material and then transferred fresh to the NHS BT Cell Production Facility, Plymouth Grove, Manchester, UK. However, for two patients (36001 and 36016), production failures (described below) meant that a second apheresis was collected (36003 and 36017) from which a suitable T-cell product was generated; thus, 16 leukapheresis products were collected in total for processing.
Absolute white cell counts varied among all 16 leukapheresis products (average cell count 169 ± 77 × 109 cells/L; range 333–72 × 109 cells/L; Fig. 1b) as did the relative frequency of T-cells in the apheresis material (50 ± 17 % of the CD45+ compartment; range 77.6–12.6 %) as well as the relative frequency of B-cells, monocytes, and granulocytes (Fig. 1c). The relative increase in T-cell frequency within the leukapheresis product in later patients reflected an increased level of experience with the technology and refining of the collection gates to enrich the lymphocyte fraction.
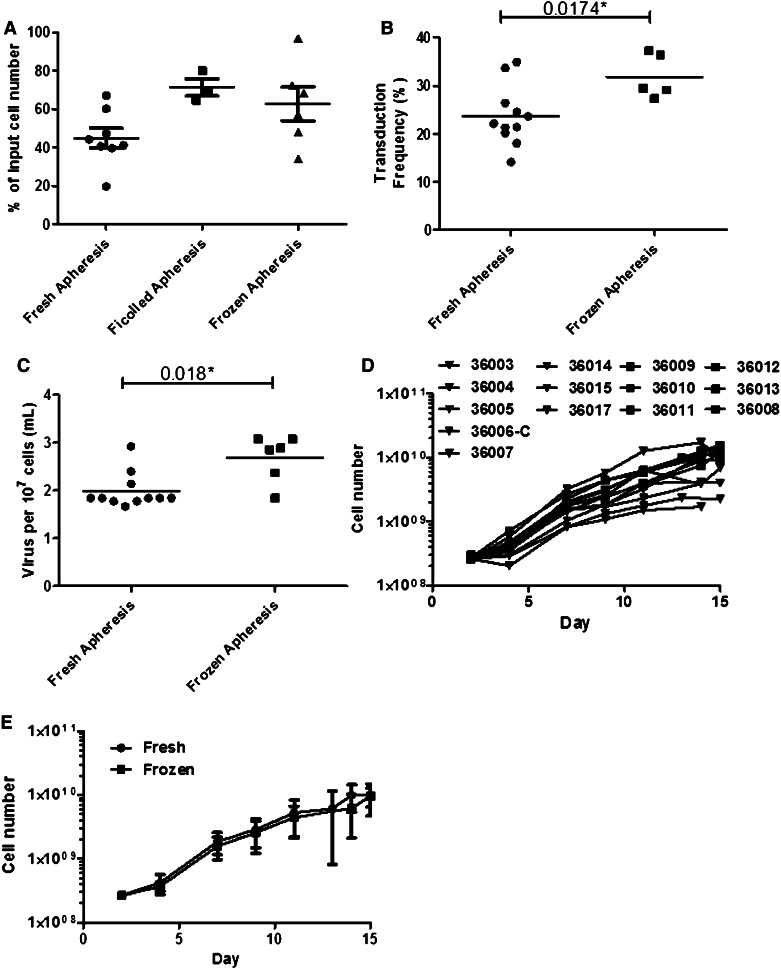
T-cell cultures were initiated either from the fresh leukapheresis product directly, after ficoll-density centrifugation, or from a cryopreserved product (summarized in Table 1). Approximately, 109 total CD45+ cells were used to initiate the T-cell activation culture using a 3-L culture bag, including anti-CD3ε mAb and IL-2. After 2 days of culture, there was no significant difference in the relative recovery of viable cells between fresh, frozen, and ficolled leukapheresis starting products (Fig. 2a).
Table 1.
Culture cell counts (viable CD45+ cells × 109)
| Product | 36001a | 36003a | 36004 | 36005 | 36006b | 36006-Cb | 36007 | 36008 | 36009 | 36010 | 36011 | 36012 | 36013 | 36014 | 36015 | 36016c | 36017c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh (F) Ficoll (FIC) Frozen (C) | F | F | F | F | F | C | F | F | F | FIC | FIC | FIC | C | C | C | C | C |
| Time (Days) | |||||||||||||||||
| 0 | 0.96 | 0.95 | 0.97 | 0.88 | 1.02 | 1.98 | 0.94 | 0.91 | 0.96 | 1.05 | 1.55 | 0.75 | 1.00 | 1.00 | 1.01 | 1.00 | 1.01 |
| 2d | 0.38 | 0.39 | 0.43 | 0.53 | 0.2 | 0.91 | 0.63 | 0.43 | 0.39 | 0.73 | 1.00 | 0.60 | 0.57 | 0.48 | 0.73 | 0.34 | 0.69 |
| 2e | 0.26 | 0.26 | 0.26 | 0.26 | 0.20 | 0.26 | 0.26 | 0.27 | 0.28 | 0.27 | 0.30 | 0.26 | 0.27 | 0.27 | 0.26 | 0.28 | 0.26 |
| 4 | 0.13 | 0.36 | 0.30 | 0.20 | 0.53 | 0.57 | 0.39 | 0.34 | 0.40 | 0.72 | 0.44 | 0.48 | 0.42 | 0.36 | 0.41 | 0.40 | 0.29 |
| 7 | 0.22 | 1.53 | 1.03 | 0.82 | 1.50 | 3.20 | 1.62 | 1.89 | 2.00 | 2.67 | 1.79 | 2.05 | 1.79 | 2.26 | 1.41 | 0.80 | 0.82 |
| 9 | Endf | 1.70 | 1.85 | 1.33 | Endf | 5.80 | 1.80 | 3.03 | 3.12 | 4.41 | 2.56 | 3.15 | 2.36 | 4.35 | 2.49 | Endf | 1.08 |
| 11 | 2.31 | 3.22 | 1.76 | 12.70 | 4.00 | 6.30 | 5.88 | 6.36 | 3.70 | 5.96 | 3.99 | 5.85 | 6.34 | 1.50 | |||
| 13 | 2.35 | 9.82 | |||||||||||||||
| 14 | 3.87 | 10.60 | 17.20 | 4.12 | 12.50 | 11.50 | 11.80 | 7.53 | 9.00 | 10.20 | 3.83 | 1.69 | |||||
| 15 | 2.24 | 9.68 | 4.01 | 15.50 | 14.80 | 10.10 | 12.10 | 9.12 | 13.20 | 6.80 | |||||||
17 individual T-cell cultures were initiated. Three cultures (36001, 36006, and 36016) failed at the day 7 assessment and were terminated. Further cultures (36003, 36006-C, and 36017) were initiated for these patients which generated sufficient MFEζ T-cells. Flow cytometric cell counts were performed at the time points indicated
a36001 and 36003 are the same individual using two different leukaphaeresis collections; 36001 failed expansion and transduction criteria
bProcess carried out with fresh and cryopreserved cells from the same leukaphaeresis collection. The process performed with fresh leukaphaeresis failed minimum transduction criteria
c36016 and 36017 are the same individual using two different aphaeresis collections; 36016 failed expansion and transduction criteria
dNumber of viable CD45 cells (×109) harvested from original activation bag after centrifugation based concentration to remove excess media
eNumber of viable CD45 cells (×109) used to initiate cultures for transduction
fThese T-cell cultures were halted as the product had failed to meet day 7 pre-conditioning release criteria
Fig. 2.
Transduction and expansion characteristics of MFEζ patient T-cells. a The relative number of cells collected after 2 days of activation expressed as a percentage of the initial number of cells seeded into the culture. There was no significant difference in the relative yield of cells between fresh, ficolled, and frozen apheresis (p > 0.05; Kruskal–Wallis test). b MFEζ transduction frequency was significantly different between T-cells initiated from a fresh and frozen apheresis product (Mann–Whitney U test). c However, the relative level of viral vector used in T-cells (determined as volume of MFEζ retroviral supernatant used per 107 target cells) in the frozen apheresis product was higher than that of the fresh product suggesting that this may explain the increase in observed transduction frequency (Mann–Whitney U test). d Cell counts of each patient sample during culture. e The mean (±standard deviation) expansion of fresh (n = 10) and frozen cells (n = 4) was not significantly different at each time point of culture
About 2.6 × 108 CD45+ cells (median 2.60 × 108, range 2.76–2.00 × 108; Table 2) were then seeded into a RetroNectin-coated DC bag with 24–40 mL of GMP-compliant, GALV-pseudotyped MFEζ retroviral supernatant (Table 2) and cultured overnight. The following day, the T-cells were collected and subjected to a second round of transduction using the same RetroNectin-coated DC bag but with fresh retroviral supernatant. After this second overnight culture, T-cells were collected and transferred to three-liter culture bags with fresh media and IL-2.
Table 2.
Cell dose, transduction, functionality, and expansion characteristics of MFEζ T-cell cultures
| Product | 36001a | 36003a | 36004 | 36005 | 36006b | 36006-Cb | 36007 | 36008 | 36009 | 36010 | 36011 | 36012 | 36013 | 36014 | 36015 | 36016c | 36017c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosed | 1.00 | 1.00 | 1.00 | 9.68 | 4.10 | 10.00 | 10.00 | 10.01 | 12.10 | 9.82 | 9.00 | 13.20 | 6.80 | 1.65 | |||
| Virus (mL)e | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 48 | 60 | 48 | 50 | 76 | 78 | 64 | 80 | 80 | 80 |
| Day 7 Expnf | 1.8 | 4 | 3.6 | 4.1 | 5.1 | 5.5 | 4.2 | 5.5 | 5 | 3.8 | 4.1 | 4.40 | 4.4 | 6.4 | 4 | 2 | 2.8 |
| % CD25g | 61.0 | 65.9 | 83.0 | 70.9 | 89.2 | 59.5 | 69.5 | 70.4 | 51.3 | 54.0 | 30.6 | 59.5 | 58.0 | 73.6 | |||
| % CARh | 18 | 21.3 | 21.4 | 22.1 | 14.1 | 27.3 | 33.7 | 26.4 | 24.5 | 20.1 | 23.6 | 34.90 | 37.3 | 29.4 | 36.4 | 9 | 29.1 |
Summary of key characteristics of the individual MFEζ T-cell populations
a36001 and 36003 are the same individual using two different leukaphaeresis collections; 36001 failed expansion and transduction criteria
bProcess carried out with fresh and cryopreserved cells from the same leukaphaeresis collection. The process performed with fresh leukaphaeresis failed minimum transduction criteria
c36016 and 36017 are the same individual using two different aphaeresis collections; 36016 failed expansion and transduction criteria
dCell dose administered to patient (×109 viable cells)
eRetronectin-assisted transduction was carried out twice. The volume presented here represents the combined volume of virus used over both transductions
fFold expansion of cell number between day 4 and 7. An expansion of ≥2.5 was set as the criterion to determine whether to continue or halt the culture
gPercentage of MFEζ T-cell CEA-specific CD25 up-regulation compared to T-cells cultured in the absence of antigen
hMFEζ transduction level. Day 7 criteria required a transduction level of ≥20 %. Italicized numbers represent products that failed this criterion
After 7 days, each culture was analyzed for transduction efficiency, and expansion with those that achieved a transduction level of less than 20 % or expansion of less than 2.5-fold over initial cell number deemed to be a production failures and the culture then was stopped. Cultures 36001 and 36016 failed on both criteria which required the patient to undergo a second leukapheresis with the resulting cultures (36003 and 36017), achieving a suitable level of transduction and expansion at this time point (Tables 1 and 2). One further culture (36006) failed with a transduction level of 14 %; however, a frozen aliquot of leukapheresis product was available from this patient, and a culture was initiated from this source (36006-C; Tables 1 and 2), which passed transduction and expansion requirements after 7 days of culture. The reasons for these three production failures remain unclear though for each patient, a MFEζ T-cell product was successfully generated for use.
Overall, there was an apparent increase in the transduction frequency of T-cells generated from frozen apheresis harvests when measured on day 7 of culture as compared to those derived from fresh leukapheresis harvests (Fig. 2b). This likely reflected a greater quantity of virus used in those samples rather than an intrinsic difference in the T-cell populations (Table 2; Fig 2c). Across all 14 MFEζ T-cell products, the average level of transduction on day 7 was 27.7 ± 5.9 % (median 26.9 %; range 20.1–37.3 %) and expansion was 4.4 ± 0.9-fold above input (median 4.15; range 2.8–6.4). To confirm the functional activity of the expanding MFEζ T-cells, aliquots of cells collected from these day 7 cultures were incubated for twenty-four hours on plates coated with CEA protein and the level of up-regulation of the CD25 activation marker was assessed by flow cytometry. All MFEζ T-cells up-regulated the CD25 expression (average increase 64.0 ± 14.3 %; range 30.6–89.2 %; Table 2), confirming the functional activity of the MFEζ CAR.
The 14 T-cell cultures that satisfied the day 7 criteria were then expanded further by the addition of further mixed T-cell media and IL-2 every 2–3 days, maintaining a cell concentration of approximately 106 cells per mL of culture until the culture reached a maximum volume of 16 liters. There was a consistent level of cell expansion within the 14 T-cell products with no apparent difference among products started from fresh or frozen apheresis harvests (Fig. 2d, e), achieving an average of 8.8 ± 4.5 × 109 MFEζ T-cells (range 1.69–15.50 × 109; Table 1) within 13–15 days of culture. Importantly, sufficient MFEζ T-cells were produced for each patient to receive at least the lowest cell dose (1 × 109 cells) required for the trial.
T-cell product release
Each T-cell product was supplied as a fresh product ready for patient infusion. This presented issues with respect to confirmation of the sterility of the final infusion product. To counter this, the criteria for the release of the final T-cell products were dependent upon a two-stage process. The first stage involved the day 7 assessment of MFEζ T-cell functionality (CD25 up-regulation), transduction (MFEζ expression), minimum fold expansion (>twofold expansion), and sterility tests (Supplementary table 4). Once the T-cell culture satisfied these criteria, a pre-release certificate was signed by the Quality Specialist and the patient then started upon pre-conditioning chemotherapy prior to adoptive T-cell transfer. The second-stage release involved the final formulation of the T-cells for adoptive transfer. A gram stain was performed on a sample of the culture taken one day prior to final processing to ensure that no gross level of bacterial contamination was present. Once the final T-cell product had been generated, the cell dose, dose volume, and available microbiological reports were used to support a further certificate for the release of the T-cell product for infusion (Supplementary table 4).
Since the T-cell product was administered fresh, complete microbiological analysis was not available at the time of infusion. The product assessment was finalized upon the completion of this full sterility assessment of the product and associated environmental monitoring. No T-cell product failed these assessments. However, in the case where a microbial contaminant was detected, the patient would be given the appropriate antibiotic.
Transduction level and T-cell phenotype fail to correlate with the absolute levels of antigen-driven CAR T-cell interferon gamma secretion
We questioned whether the level of CAR expression in the final 14 T-cell products correlated with in vitro CAR T-cell effector response. Frozen aliquots of cells (except for cultures 36006-C and 36017 for which insufficient samples was available) were defrosted and batch analyzed for cell surface phenotype, MFEζ CAR expression, and interferon gamma secretion after a 24-hour culture on CEA antigen-coated plates.
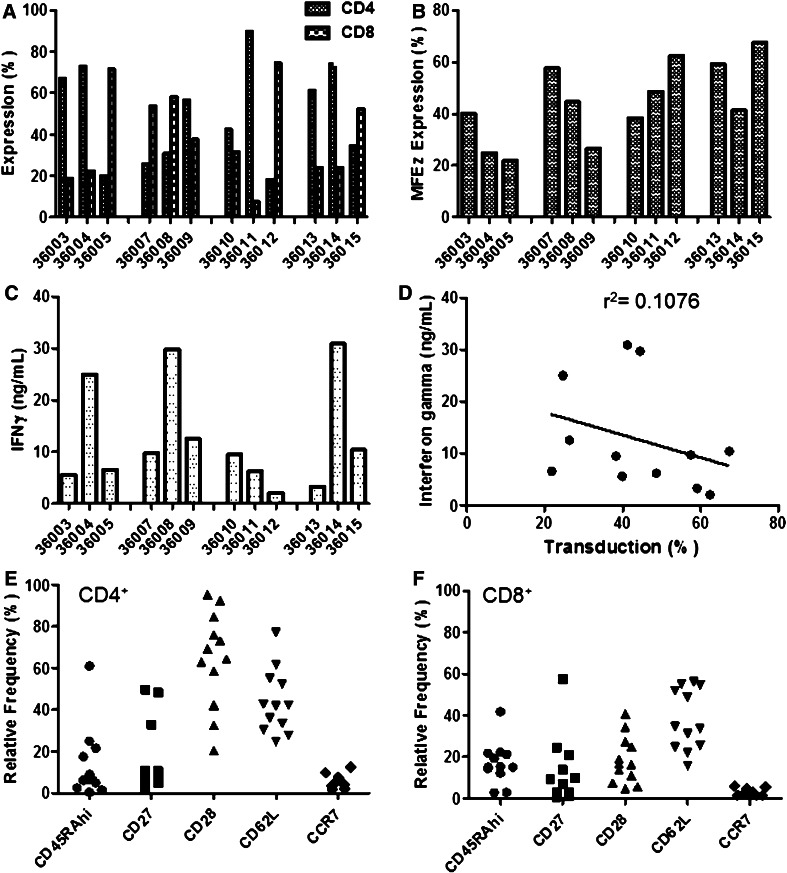
Interestingly, unlike the situation commonly observed with normal donors where CD8+ T-cells tend to expand relative to CD4+ T-cells (Supplementary Fig 1a), seven of the 12 (58 %) patient products had a greater frequency of CD4+ compared to CD8+ T-cells. In the case of patient 36011, 90 % of the expanded T-cells were CD4+ (Fig 3a). The range of CAR expression was 21.8–67.4 % (median 42.9 %, Fig. 3b). This was higher than observed in the fresh sample analysis for reasons that remain unclear. However, there was a reasonable correlation between both analyses, suggesting that the relative CAR expression level between patient samples was consistent (Supplementary Fig 1b).
Fig. 3.
Cell phenotype and in vitro functionality of patient MFEζ T-cells. Cryopreserved aliquots of MFEζ T-cells were defrosted, washed, and analyzed for a CD4/CD8 subset expression and b expression of the MFEζ receptor by flow cytometry. c Patient MFEζ T-cells were cultured on plates pre-coated with recombinant CEA protein (1 μg/mL). After 24 h, culture media were collected, and the levels of secreted interferon gamma (IFNγ) determined. Background levels of IFNγ were determined in MFEζ T-cells cultured on plates lacking antigen and were subtracted from the antigen-induced level of cytokine production. d There was no obvious correlation of IFNγ levels produced by MFEζ T-cells with transduction level (linear regression analysis). Flow cytometric analysis of the relative expression of T-cell markers in e CD4+ and f CD8+ T-cells. There was a significant difference in the relative level of CD28 expression between CD4+ and CD8+ T-cell subsets (p = 0.0002, Mann–Whitney U test), but no difference between other markers examined
When challenged with CEA protein antigen, all patient samples produced interferon gamma (2.0–30.9 ng/mL; median 9.5 ng/mL, Fig. 3c). However, there was no obvious correlation between interferon gamma secretion and transduction level (Fig. 3d). There was also no obvious correlation between cytokine secretion and the CD4/CD8 ratio, suggesting that the expanded CD4+ T-cells were not exhibiting an inhibitory effect upon CAR function and were therefore unlikely to be regulatory T-cells (data not shown). However, due to modulation of markers generally used to identify regulatory T-cells by activated non-regulatory T-cells (including CD25 and FoxP3), a formal confirmation of the absence of regulatory T-cells by flow cytometry in these expanded T-cell populations was not possible.
Cell surface phenotype analysis of the expanded T-cell products suggested that both CD4+ and CD8+ populations possessed a low relative frequency of CD45RAhi+, CD27+, and CCR7+ T-cells (Fig. 3e, f; by cohort, Supplementary Fig 1c-1f). However, while both T-cell subsets possessed higher frequencies of CD62L+ T-cells, there was a significantly higher frequency of CD28+ T-cells in the CD4+ (64.3 ± 23.1 %) than the CD8+ population (18 ± 11.4 %; p = 0.002, Mann–Whitney U test). Overall, the CD8+ T-cell population was predominantly composed of a differentiated, effector phenotype, while the CD4+ T-cell population was composed of a mixture of central and effector memory T-cell subtypes.
Discussion
The early positive results of clinical studies targeting the CD19 antigen with CAR T-cells [1–3] are prompting an expansion in the level of interest of this form of therapy for cancer and other diseases. Previous clinical trials using CAR T-cells to target non-hematological tumors have not been as successful either through lack of persistence [21] or on-target toxicity as a result of the expression of high levels of target antigen on normal tissue [22, 23]. Clearly, improved strategies are required in the design of the CAR to target non-hematological tumors. Nonetheless, delivering these therapies to the clinic remains a major issue with respect to technological development but also critically with respect to compliance with the legislature.
The initial application and eventual approval of the MFEζ trial proposal straddled the implementation of the European Union Clinical trials directive, the development of legislation for Advanced Therapy Medicinal Product (ATMP), and associated legislation, which meant that the production of the MFEζ CAR T-cells had to comply with the principle of GMP. At the time of the clinical trial application (CTA), the manufacturing process was designed to meet the UK Department of Health requirements (“A code of practice for tissue banks providing tissues of human origin for therapeutic purposes,” 2001). However, as the ATMP regulations were available for review prior to becoming EU legislation, the production process was already achieving the requirements other than where specific reagents were not available to the standards required, and hence, the impact of ATMP regulations was minimized through clear risk assessment processes which were available as a part of the CTA. Moreover, colleagues in the Netherlands had worked on a GMP-compliant process to generate CAR T-cells specific for the G250 antigen expressed on renal cell carcinoma [24], and this was used as a basis to develop the protocols used to produce MFEζ T-cells.
The overall process (Supplementary Fig 2) combined open processes which require a grade A environment on four days of the process (Days 0, 2, 3, and 4) and closed processes where all operations are carried out using culture bag technology and a sterile-connecting device during the T-cell expansion. Importantly, a T-cell product was successfully generated for each patient. There were three production failures due to poor transduction or expansion. The reasons for these production failures were not clear but potentially were due to variations in the composition of the starting material which result in poor T-cell stimulation and expansion or the presence of non T-cell binding sites for the retroviral vector resulting in low transduction frequencies. However, in each case, a second CAR T-cell culture was initiated and achieved the necessary criteria for release and treatment.
The final cell phenotype suggested that the T-cells were well-differentiated, and this was reflected in the high levels of interferon gamma produced during antigenic challenge. Moreover, over half of the patient products possessed >50 % CD4+ T-cells which was surprising in comparison with that seen in normal healthy donor T-cell cultures where CD8+ T-cells tend to predominate. Whether this is a feature associated with the specific patients recruited to this study remains unclear, and further investigation of the initial T-cell phenotype of patients and correlation with final T-cell product will be important in future studies. Moreover, whether CAR T-cell phenotype or transduction level impacts upon clinical response is awaited (Thistlethwaite et al.; manuscript in preparation). Nonetheless, recent studies strongly suggest that T-cells with a less-differentiated phenotype display properties that are optimal for adoptive cell therapies [25] with modulation of the cytokines used during ex vivo culture potentially impacting upon T-cell differentiation status and produce T-cells that offer improved adoptive cell therapy potential [26, 27].
The number of open processes requiring access to a grade A environment in a grade B clean room in this protocol remains restrictive in terms of potential patient throughput, i.e., one product per room for 14–16 days. Recent developments in technology such as WAVE bioreactors [28] and G-Rex gas-permeable rapid expansion culture ware [29] are providing closed system answers to some of the technical issues relating to the expansion of T-cells for adoptive T-cell therapy. However, these systems may not completely avoid the requirement for open processes that require a grade A environment which impacts upon the potential throughput of patient products and acts as a barrier to large-scale clinical trial development. To overcome this restriction, glove-box isolator technology is now being employed in several cell production facilities avoiding the requirement for large, expensive clean rooms. Further, automated systems such as the Prodigy (Miltenyi Biotec, Germany) are at the early stages of testing and may eventually provide solutions which avoid the requirement of clean rooms all together although the ability to work to scale (in terms of the large number of T-cells currently required for therapy) and high throughput for such systems remains to be determined.
This study demonstrates that it is feasible to generate gene-modified T-cells compliant with the standards required for phase I/II clinical trial within the EU. However, scientific developments and the rapid pace of technological innovation are providing the adoptive T-cell therapy field with the ability to move forward exciting initial clinical observations and ultimately will enable larger-scale randomized studies that can be completed in a timely manner and at realistic cost.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors wish to acknowledge the support of Cancer Research UK for this work. This work was also supported by funding from the Kay Kendall Leukaemia Fund, the FP6 Program ATTACK, the NHSBT, Christie Hospital NHS Trust, and the BBSRC (HG).
Conflict of interest
Ryan D Guest, Robert E Hawkins, and David E Gilham are cofounders of Cellular Therapeutics Ltd. All other authors do not have any conflict of interest to declare.
Abbreviations
- 7-AAD
7-Aminoactinomycin D
- ATMP
Advanced therapy medicinal product
- CAR
Chimeric antigen receptor
- CEA
Carcinoembryonic antigen
- CTA
Clinical trials authorization
- DC
Dendritic cell
- DMSO
Dimethyl sulfoxide
- FBS
Fetal bovine serum
- GMP
Good manufacturing process
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HSA
Human serum albumin
- PBS
Phosphate-buffered saline
- PQ
Process quantification
- scFv
Single-chain variable fragment
- TSE
Transmissible spongiform encephalitis
- UK
United Kingdom
Footnotes
Robert E. Hawkins and David E. Gilham are Joint Senior Authors.
References
- 1.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18(7):377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunol Immunother. 2012;61(7):953–962. doi: 10.1007/s00262-012-1254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann W, Weber B, Ortlieb B, Rudert F, Schempp W, Fiebig HH, Shively JE, von Kleist S, Thompson JA. Chromosomal localization of the carcinoembryonic antigen gene family and differential expression in various tumors. Cancer Res. 1988;48(9):2550–2554. [PubMed] [Google Scholar]
- 8.Verhaar MJ, Chester KA, Keep PA, Robson L, Pedley RB, Boden JA, Hawkins RE, Begent RH. A single chain Fv derived from a filamentous phage library has distinct tumor targeting advantages over one derived from a hybridoma. Int J Cancer. 1995;61(4):497–501. doi: 10.1002/ijc.2910610412. [DOI] [PubMed] [Google Scholar]
- 9.Begent RH, Verhaar MJ, Chester KA, Casey JL, Green AJ, Napier MP, Hope-Stone LD, Cushen N, Keep PA, Johnson CJ, Hawkins RE, Hilson AJ, Robson L. Clinical evidence of efficient tumor targeting based on single-chain Fv antibody selected from a combinatorial library. Nat Med. 1996;2(9):979–984. doi: 10.1038/nm0996-979. [DOI] [PubMed] [Google Scholar]
- 10.Mayer A, Francis RJ, Sharma SK, Tolner B, Springer CJ, Martin J, Boxer GM, Bell J, Green AJ, Hartley JA, Cruickshank C, Wren J, Chester KA, Begent RH. A phase I study of single administration of antibody-directed enzyme prodrug therapy with the recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin Cancer Res. 2006;12(21):6509–6516. doi: 10.1158/1078-0432.CCR-06-0769. [DOI] [PubMed] [Google Scholar]
- 11.Gilham DE, O’Neil A, Hughes C, Guest RD, Kirillova N, Lehane M, Hawkins RE. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002;25(2):139–151. doi: 10.1097/00002371-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Sheen AJ, Sherlock DJ, Irlam J, Hawkins RE, Gilham DE. T lymphocytes isolated from patients with advanced colorectal cancer are suitable for gene immunotherapy approaches. Br J Cancer. 2003;88(7):1119–1127. doi: 10.1038/sj.bjc.6600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178(7):4650–4657. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 14.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, Embleton MJ, Stern PL, Gilham DE. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28(3):203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 15.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39(3):296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safar M, Junghans RP. Interleukin 2 maintains biologic stability and sterility over prolonged time. Immunopharmacology. 2000;49(3):419–423. doi: 10.1016/S0162-3109(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 17.Calmels B, Houze P, Hengesse JC, Ducrot T, Malenfant C, Chabannon C. Preclinical evaluation of an automated closed fluid management device: Cytomate, for washing out DMSO from hematopoietic stem cell grafts after thawing. Bone Marrow Transplant. 2003;31(9):823–828. doi: 10.1038/sj.bmt.1703905. [DOI] [PubMed] [Google Scholar]
- 18.Fehse B, Kustikova OS, Li Z, Wahlers A, Bohn W, Beyer WR, Chalmers D, Tiberghien P, Kuhlcke K, Zander AR, Baum C. A novel ‘sort-suicide’ fusion gene vector for T cell manipulation. Gene Ther. 2002;9(23):1633–1638. doi: 10.1038/sj.gt.3301828. [DOI] [PubMed] [Google Scholar]
- 19.Lamers CH, van Elzakker P, van Steenbergen SC, Sleijfer S, Debets R, Gratama JW. Retronectin-assisted retroviral transduction of primary human T lymphocytes under good manufacturing practice conditions: tissue culture bag critically determines cell yield. Cytotherapy. 2008;10(4):406–416. doi: 10.1080/14653240801982961. [DOI] [PubMed] [Google Scholar]
- 20.Lamers CH, Willemsen RA, Luider BA, Debets R, Bolhuis RL. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Ther. 2002;9(7):613–623. doi: 10.1038/sj.cgt.7700477. [DOI] [PubMed] [Google Scholar]
- 21.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, Gratama JW. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamers CH, van Elzakker P, Langeveld SC, Sleijfer S, Gratama JW. Process validation and clinical evaluation of a protocol to generate gene-modified T lymphocytes for imunogene therapy for metastatic renal cell carcinoma: GMP-controlled transduction and expansion of patient’s T lymphocytes using a carboxy anhydrase IX-specific scFv transgene. Cytotherapy. 2006;8(6):542–553. doi: 10.1080/14653240601056396. [DOI] [PubMed] [Google Scholar]
- 25.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12(10):671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Ji Y, Gattinoni L, Zhang L, Yu Z, Restifo NP, Rosenberg SA, Morgan RA. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother. 2013;62(4):727–736. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somerville RP, Devillier L, Parkhurst MR, Rosenberg SA, Dudley ME. Clinical scale rapid expansion of lymphocytes for adoptive cell transfer therapy in the WAVE(R) bioreactor. J Transl Med. 2012;10:69. doi: 10.1186/1479-5876-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, Wilson J, Dotti G, Heslop HE, Leen AM, Rooney CM. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33(3):305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.