Abstract
Background
Antibody-dependent cell-mediated cytotoxicity (ADCC) may contribute to the antitumor activity of cetuximab. However, the extent of this contribution is unclear. In this study, we investigated the impact of baseline ADCC on the outcome of patients with locally advanced squamous cell carcinoma treated with cetuximab and radiotherapy.
Methods
We determined baseline ADCC in 28 patients treated with cetuximab and radiotherapy and in 15 patients treated with chemoradiation. We linked the values observed with complete response and with overall survival. We also considered the role of epidermal growth factor receptor (EGFR) expression and studied the combined effect of EGFR and ADCC.
Results
We observed a wide range of baseline values of ADCC. Complete response did not correlate with either ADCC or EGFR expression. However, when ADCC and EGFR were considered together using a mixed score, they significantly correlated with achieving a complete response (p = 0.04). High baseline ADCC significantly correlated with outcome compared to low (p = 0.03), but not in patients treated without cetuximab. Patients showing high baseline levels of both ADCC and EGFR3+ achieved the best outcome compared to the others (p = 0.02).
Conclusions
In this study, patients treated with cetuximab and radiotherapy, showing high baseline of both ADCC and EGFR3+, have significant higher probability of achieving a complete response and a long overall survival compared to the others.
Keywords: ADCC, Cetuximab, EGFR, Head and neck cancer, Outcome
Introduction
Therapeutic use of monoclonal antibodies (mAbs) over the last quarter of the past century has achieved impressive results in some tumors. For example, trastuzumab changed the prognosis of patients (pts) with Her2-positive breast cancer [1]. However, their role in direct targeting tumours includes a second, immunological, mechanism of action, namely, at least for IgG1 and IgG3 mAbs, their well-known capacity to trigger ADCC. However, this has been poorly investigated in the clinic setting. Existing data support a significant contribution of ADCC to the overall activity of trastuzumab [2]. Similarly, rituximab induces ADCC in clinical settings and this contributes to its clinical activity [3]. Other mechanisms of action of mAbs relate to their effects via natural killer (NK) cells. Data from colon cancer suggest that cetuximab is significantly more effective in patients with NK cell-infiltrated tumours, which is not the case in patients treated without cetuximab [4]. In oropharyngeal cancer, the presence of NK infiltration is associated with a significant amelioration of overall survival (OS) compared to tumours lacking infiltration in either the whole population and in human papilloma virus (HPV) negative subgroup [5]. However, the magnitude of the ADCC effect in tumours treated with IgG1 mAbs remains unclear.
The present paper reports results from part of a larger project devoted to investigate mAb-related ADCC in cancer therapy. In a previous paper, we described a simple method to measure baseline ADCC from blood of patients with solid cancers [6]. In a following paper, we analysed the interplay between invariant natural killer T cells (iNKT) and ADCC in patients with colon cancer on a cetuximab-based therapy [7]. The present paper considers the role of ADCC in head and neck cancer patients treated with bio-radiotherapy (cetuximab-based), its relationship with epidermal growth factor receptor (EGFR) expression, and the iNKT—ADCC interplay.
Materials and methods
Patients
We considered for this study 43 patients with stage III–IV squamous cell carcinoma of the head and neck. All patients were treated at our Institution between January 2009 and May 2014 according to daily clinical practice. Twenty-eight patients considered unfit for chemoradiation, received cetuximab plus radiotherapy (bio-radiation) according to the Bonner’s regimen [8], and were considered as the study group; 15 patients received standard chemoradiation according to Adelstein et al. [9] and were considered as cancer control group. Patients were considered unfit for chemoradiation on the basis of clinical evaluation, including medical contraindications to the use of cisplatin (pre-existing neuropathy, border-line renal function, history of coagulation disorders, and severe hearing loss) or the general conditions which preclude, in the opinion of the physician, the complete and correct delivery of the chemoradiation program. All subjects signed an informed consent for blood collection and analysis. Table 1 summarizes the main patient and tumor characteristics, correlated with clinical outcome and ADCC basal activity.
Table 1.
Patient and tumor characteristics
| Characteristics | No. of patients (N = 43) | ADCC high (N = 22/43) | ADCC low (N = 21/43) | Complete responders (CR, N = 27/43) | Non-complete responders (no-CR, N = 16/43) |
|---|---|---|---|---|---|
| Age | |||||
| Group 1 (36–52 years) | 10 (23.25) | 3 (13.6) | 7 (33.4) | 7 (25.8) | 3 (18.7) |
| Group 2 (53–69 years) | 23 (53.5) | 15 (68.2) | 8 (38) | 13 (48.4) | 7 (43.8) |
| Group 3 (70–87 years) | 10 (23.25) | 4 (18.2) | 6 (28.6) | 7 (25.8) | 6 (37.5) |
| Gender | |||||
| Female | 8 (18.6) | 5 (22.7) | 3 (14.3) | 5 (18.5) | 3 (18.7) |
| Male | 35 (81.4) | 17 (77.3) | 18 (85.7) | 22 (81.5) | 13 (81.3) |
| Cancer location | |||||
| Oropharynx | 20 (46.5) | 12 (54.5) | 8 (38) | 17 (62.9) | 3 (18.7) |
| Oral cavity | 5 (11.6) | 2 (9) | 3 (14.3) | 0 (0) | 5 (31.3) |
| Hypopharynx | 8 (18.6) | 5 (22.7) | 3 (14.3) | 6 (22.3) | 2 (12.6) |
| Larynx | 5 (11.6) | 3 (13.8) | 2 (9.5) | 2 (7.4) | 3 (18.7) |
| Others | 5 (11.6) | 0 (0) | 5 (23.9) | 2 (7.4) | 3 (18.7) |
| Type of treatment | |||||
| Bio-radiation | 28 (65.1) | 14 (63.7) | 14 (66.7) | 19 (70.3) | 9 (56.2) |
| Chemoradiation | 15 (34.9) | 8 (36.3) | 7 (33.3) | 8 (29.7) | 7 (43.8) |
| p16 (oropharynx) | 20 | 12 | 8 | 17 | 3 |
| Pos | 9 (45) | 6 (50) | 3 (37.5) | 8 (47) | 1 (33.3) |
| Neg | 11 (55) | 6 (50) | 5 (62.5) | 9 (53) | 2 (66.7) |
| Smoke | |||||
| Non-smokers | 6 (13.9) | 3 (13.7) | 3 (14.4) | 4 (14.8) | 2 (12.6) |
| Smokers <10 pack/year | 4 (9.3) | 3 (13.7) | 1 (4.8) | 4 (14.8) | 0 (0) |
| Smokers >10 pack/year | 16 (37.2) | 8 (36.2) | 8 (38) | 12 (44.4) | 4 (25) |
| Smokers (pack/year unknown) | 10 (23.3) | 3 (13.7) | 1 (4.8) | 2 (7.4) | 2 (12.6) |
| Past smokers (more than 10 years) | 3 (7) | 3 (13.7) | 0 (0) | 2 (7.4) | 1 (6.1) |
| Unknown | 4 (9.3) | 2 (9) | 8 (38) | 3 (11.2) | 7 (43.7) |
| EGFR | |||||
| Neg; 1+; 2+ | 18 (41.8) | 7 (31.8) | 11 (52.4) | 11 (40.7) | 7 (43.7) |
| 3+ | 25 (58.2) | 15 (68.2) | 10 (47.6) | 16 (59.3) | 9 (56.3) |
All data are presented as No (%). ADCC antibody-dependent cell-mediated cytotoxicity, EGFR epidermal growth factor receptor. p16 status was considered only in oropharyngeal cancer. ADCC high, ≥64.7%; ADCC low, <64.7% (ADCC median value in the whole pts group, N = 43)
Blood sample collection and methods
We collected 15 ml of peripheral blood on the day of starting treatment. Samples were immediately processed. Intrinsic ADCC was evaluated from ex vivo NK-dependent activity measuring LDH release using the Cytotoxic 96® cytotoxicity assay, previously standardized in our laboratory [6]. We divided the patient population into four groups according to treatment (bio-radiation or chemoradiation) and ADCC value (high and low ADCC using the median value observed in each group as cutoff).
EGFR was detected by immunohistochemistry using the monoclonal mouse anti-human epidermal growth factor receptor Clone E30 (DAKO, Denmark) on a Ventana Benchmark ULTRA automated Immunostainer (Ventana Medical System Inc., Tucson, AZ, USA), and quantified according to FDA guidelines on a scale from 0 to 3+. NK cells were defined by cytofluorimetry as CD56+/CD3− and iNKT by co-expression of CD3, TCR Vα24, and TCR Vβ11.
Statistics
Complete response (CR) was defined as the complete disappearance of any clinical or instrumental evidence of disease 3 months after treatment end. Clinical assessment was based on physical examination, fiberoscopy, CT scan, and PET scan when clinically indicated. All these procedures are part of our standard clinical practice. All patients who did not match the criteria for CR were considered failures (no-CR). Statistical analyses were performed using the Graph Pad Prism 5 (San Diego, CA, USA) and SPSS version 13 (SPSS, Chicago, IL, USA) programs. OS was the time from treatment start to the last follow-up or death. Life status of patients lost to follow-up was obtained by census. OS was analysed using the Kaplan–Meier method, with log-rank test for statistical significance. A p value < 0.05 was considered statistically significant.
Results
ADCC
Basal ADCC was measured in patients treated with cetuximab and radiotherapy and in patients treated with chemoradiation. Median basal ADCC for all 43 patients was 64.7%. Patients deemed unfit for chemotherapy and treated with cetuximab and radiation (N = 28) showed a median ADCC value of 62.63%; patients receiving standard chemotherapy (control group, N = 15) presented a similar median ADCC value of 65.2%.
Bio-radiation treatment response
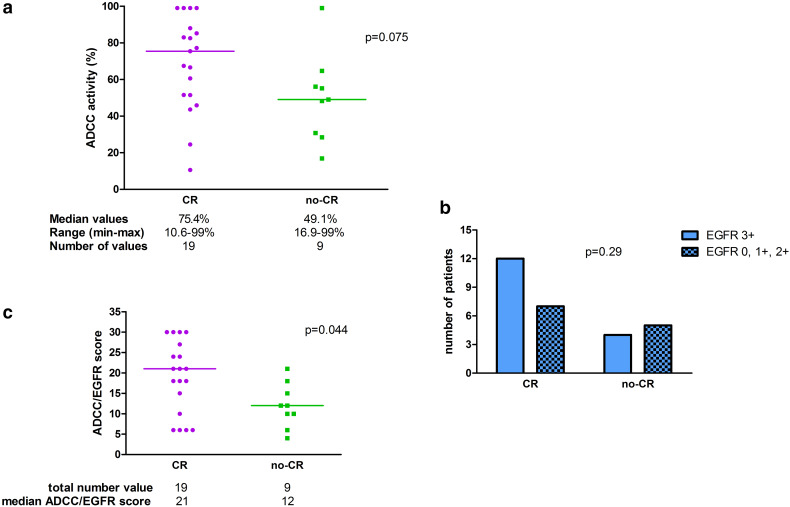
In Table 2, ADCC activity, EGFR status, clinical response, and iNKT level are reported for each patient of the group treated with bio-radiation (N = 28). First, we considered the distribution of complete responses and failures according to individual baseline ADCC value in patients treated with bio-radiation. The median value of ADCC in patients achieving CR was 75.4% compared to 49.1% of those who failed. This difference shows a trend toward statistical significance (p = 0.075) (Fig. 1a). Next, we considered the distribution of CR and failures according to EGFR status, compared with Fisher´s Exact probability test (EGFR 3+ versus EGFR 0, 1+, 2+). The difference according to EGFR status was not significant (p = 0.29) (Fig. 1b). We also performed an analysis considering both basal ADCC and EGFR expression. To do so, we created a mixed ADCC/EGFR score: ADCC values were grouped in deciles and one point was attributed to each decile. We then graded EGFR expression assigning one point to each “+” given to the expression (“0” status was considered as “+”). Finally, we multiplied the points given to ADCC values with those given to EGFR expression, to obtain a final individual mixed score. Comparing complete responders (N = 19, 67.8%) and failures (N = 9, 32.2%) using the mixed score, the difference reached the statistical significance (p = 0.044) (Fig. 1c).
Table 2.
ADCC activity, EGFR status, clinical response, and iNKT level in 28 patients treated with bio-radiation
| Patient # | ADCC basal activity (%) | EGFR status | iNKT number (cells/microl) | Clinical response |
|---|---|---|---|---|
| 1 | 99.00 | 3+ | 0.083 | CR |
| 2 | 51.44 | 1+ | 0.094 | CR |
| 3 | 82.5 | 2+ | 1.500 | CR |
| 4 | 99.0 | 0 | 2.710 | NV |
| 5 | 88.0 | 3+ | 0.658 | CR |
| 6 | 75.4 | 3+ | 0.750 | CR |
| 7 | 43.6 | 2+ | 0.303 | CR |
| 8 | 66.55 | 3+ | 4.496 | CR |
| 9 | 28.4 | 2+ | 0.427 | PD |
| 10 | 64.65 | 3+ | 3.867 | PD |
| 11 | 85.2 | 2+ | 1.185 | CR |
| 12 | 30.78 | 3+ | 0.498 | PD |
| 13 | 49.08 | 3+ | 1.675 | TD |
| 14 | 48.3 | 2+ | 0.061 | NV |
| 15 | 83.0 | 2+ | 0.219 | CR |
| 16 | 77.2 | 3+ | 1.913 | CR |
| 17 | 55.15 | 3+ | 0.292 | PR |
| 18 | 56.1 | 2+ | 1.696 | PR |
| 19 | 51.55 | 1+ | 0.3305 | CR |
| 20 | 67.4 | 3+ | 1.676 | CR |
| 21 | 16.9 | 2+ | 0.254 | PR |
| 22 | 99.00 | 3+ | 0.298 | CR |
| 23 | 60.61 | 3+ | 1.334 | CR |
| 24 | 99.00 | 3+ | 0.085 | CR |
| 25 | 24.5 | 2+ | 0.292 | CR |
| 26 | 99.0 | 3+ | 0.602 | CR |
| 27 | 45.88 | 3+ | 1.381 | CR |
| 28 | 10.59 | 3+ | 0.551 | CR |
ADCC antibody-dependent cell-mediated cytotoxicity, EGFR, epidermal growth factor receptor, CR complete response, NV not valuable, PD progressive desease, TD toxic death, PR partial response, iNKT invariant natural killer T cells
Fig. 1.
a ADCC distribution between complete (CR) and non-complete responders (no-CR) in 28 pts treated with bio-radiation; b EGFR status distribution in complete (CR) and non-complete responders (no-CR) in 28 pts treated with bio-radiation; c ADCC/EGFR score calculated as described in Methods section in complete (CR) and non-complete responders (no-CR) in 28 pts treated with bio-radiation
Outcome
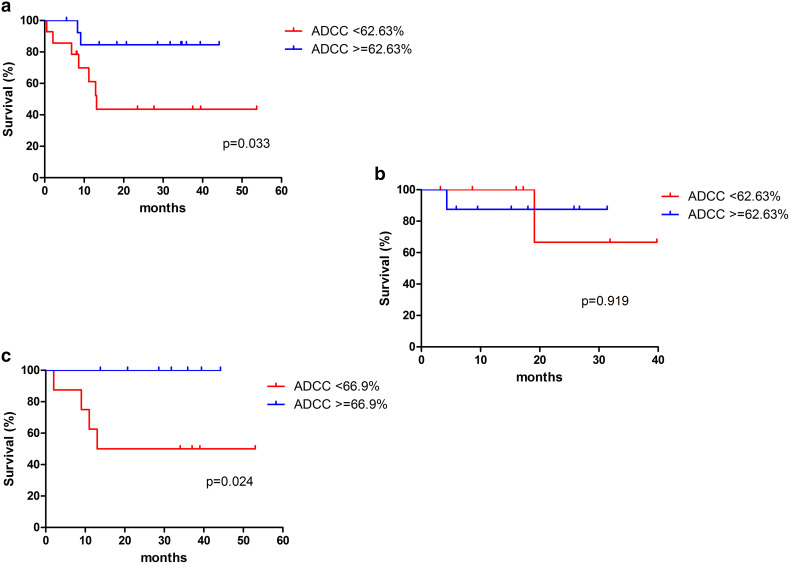
We performed an analysis of ADCC high vs low in the group receiving bio-radiation and in the group receiving chemoradiation. High ADCC correlates with a significant gain in OS compared to low ADCC in patients receiving cetuximab and radiation but not in those treated with chemoradiation (Fig. 2). In particular, patients treated with cetuximab and showing high ADCC achieved 85% OS at 44 months compared to a prospective OS of 43% at 53 months in pts with low ADCC (p = 0.03) (Fig. 2a). In contrast, patients receiving chemoradiation had similar outcomes regardless of basal ADCC value (3-year OS 86 vs 63% in high vs low ADCC, respectively, p = 0.9) (Fig. 2b). We performed a univariate analysis in patients receiving bio-radiation (N = 28) considering the main biological parameters: gender, HPV status, smoking, alcohol use, performance status, stage, T, N, grading, EGFR, and ADCC. The results showed that only T (p = 0.025) and ADCC (p = 0.034) were significant predictors of survival, while tumour grading reached a trend (p = 0.07). We then performed multivariate analysis considering factors achieving either significance or a trend in the univariate analysis (ADCC, T, grading); only ADCC emerged as a significant factor affecting OS (p = 0.02).
Fig. 2.
Role of ADCC high vs low in a 28 pts treated with bio-radiation, (b 15 pts treated with chemoradiation without cetuximab and c 16 pts treated with bio-radiation with high EGFR expression (3+). ADCC median value in 28 patients group (62.63%) and 16 patients group (66.9%) was used as cutoff
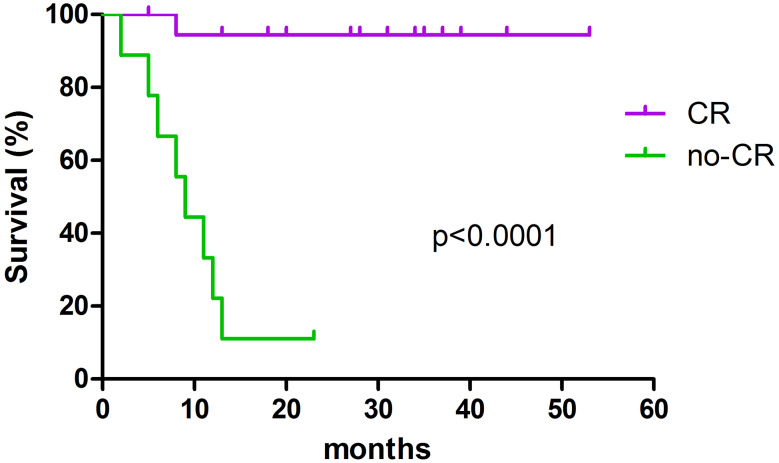
We analysed OS of complete responders in the bio-radiation group according to high or low ADCC (19 pts). Only 1 patient died (group with low ADCC) and the difference was not significant. Overall, achieving CR, as expected, is the most important prognostic factor for survival, overcoming any pre-treatment factor (Fig. 3). Indeed, adding complete response in the multivariate analysis, only CR emerges as significant (p = 0.005).
Fig. 3.
Outcome of complete (CR) and non-complete responders (no-CR) in 28 pts treated with bio-radiation
Finally, we selected within the bio-radiation group, patients harbouring tumours with EGFR 3+ expression (16 pts), and compared high and low ADCC in this subgroup (Fig. 2c). We observed a dramatic difference between the two groups: 100 vs 41% OS at 48 months in high and low ADCC, respectively (p = 0.02). Moreover, in this group of 16 patients treated with bio-radiation and expressing high EGFR level (3+), we analysed the relationship between iNKT and ADCC as we previously did in colon cancer [7]. We assigned patients to two groups: group A, high ADCC, and high iNKT infiltration and group B, all the others. As observed in colon cancer, patients in the former group tended to show benefit compared to the latter group (Fig. 4), albeit the limited number of cases precludes the achievement of statistical significance (p = 0.3).
Fig. 4.
Relationship between high iNKT and high ADCC compared to others in 16 pts treated with bio-radiation with high EGFR expression (3+)
HPV and oropharyngeal cancer
Our series included 20 oropharyngeal cancers (OPC). Considering only the bio-radiation group, we recorded 16 OPC, but only seven of them were p16-positive, in line with the epidemiology of HPV-positivity OPC in the south of Europe. The outcome of p16-positive patients was better, but not significantly so, compared to the p16-negative group (p = 0.08). The lack of significant difference between the two groups may be due to the small number of patients, but it must also be considered that all our patients were heavy smokers.
Discussion
In this study, high basal ADCC correlated with a significant gain in OS in patients with head and neck cancer treated with cetuximab and radiotherapy, compared to similar patients with low ADCC. Moreover, patients with both high expression of EGFR and high baseline ADCC experienced an additional advantage. In contrast, high ADCC did not significantly impact on the outcome of patients treated without cetuximab. Noteworthy, we observed a similar ADCC median value in patients treated with bio-radiation and chemoradiation.
Mickel et al. in the late eighties suggested that NK function suppression might occur in head and neck cancer patients [8]. Other researchers reported similar data in different tumours [9], suggesting that ADCC suppression is not related to specific cancers. Nonetheless, our present data and recent reports from our group and from others suggest that at least some ADCC activity remains and might carry clinical implications. For example, Wagner et al. [5] observed that the infiltration of oropharyngeal tumours by NK cells positively affects outcome regardless HPV status. Taylor et al. [10] and Lo Nigro et al. [7] showed that patients with high ADCC, in head and neck cancer and in colon cancer, respectively, are more likely to benefit from cetuximab. It is already known that mAbs may trigger ADCC in the clinical setting. This has been demonstrated in Herceptin-treated breast cancer patients amongst others [2]. However, our data suggest that an elevated basal ADCC activity seems to be necessary to exploit this potential of mAbs. Indeed, there is a significant difference between high and low ADCC on clinical outcome, in the presence of cetuximab. The lack of benefit attributable to basal ADCC in patients treated without cetuximab that we observed in our control group is indirectly supported by similar results in in colon cancer. Indeed, Wagner et al. [4] observed that the infiltration of CD56+ cells correlates with a significantly better outcome in patients treated with cetuximab, but not in those treated without it.
We also observed that a second prerequisite is necessary to optimize results of the ADCC-related, cetuximab-induced benefit: the high expression of EGFR, as might be expected. Indeed, ligation of the Fab fragment of cetuximab and EGFR, as well as between Fc and FcγR, is a stochastic event, so higher abundance of receptors will favour contact. Consistent with this, patients with both high ADCC and EGFR expression experienced the best outcome in this study. However, as expected, the achievement of CR is the strongest predictor of survival, overcoming any other factor, including ADCC. Nonetheless, on the basis of the observed data, we postulate that, in our series, ADCC corrected according to EGFR expression (as we did with the ADCC/EGFR score) can predict the achievement of CR, linking complete response, ADCC, and outcome.
An additional observation in this study regards the interplay between iNKT and ADCC in patients expressing high EGFR levels treated with bio-radiation. Only three patients showed both high iNKT and high ADCC, so we cannot derive any unequivocal conclusions. However, all three patients are alive at a median follow-up of 31+ months (range 13+ to 34+), compared to 69% alive at a median follow-up of 20+ months (range 2–53+). These data are in line with a previous observation by our group in colon cancer [7] and in our opinion can be considered a hypothesis-generating observation. It would be of interest to evaluate ADCC and iNKT numbers at different time points during treatment and follow-up. This is the objective of an on-going study in our group.
The importance of iNKT in the activation of NK, CD8+, and dendritic cells, through the production of IFNγ and other cytokines [11], is in favour of the clinical importance of iNKT as already observed by others [12]. Interestingly, IFNγ is a powerful inducer of PD-L1 [13] and the confirmation of our observation could support the combination of cetuximab with inhibitors of the PD-1 PD-L1 axis.
Finally, a major limitation of this study is its limited size. Thus, we cannot derive any definitive conclusions on the clinical relevance of ADCC induced by cetuximab in head and neck cancer, but our study adds additional data to those available data favouring of this hypothesis.
Acknowledgements
This study has been partially supported by Associazione Ricerca Clinica Oncologica (ARCO) Foundation, Cuneo, Italy.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- CR
Complete response
- IgG
Immunoglobulin G
- INFγ
Interferon gamma
- iNKT
Invariant natural killer T cells
- mAbs
Monoclonal antibodies
- N
Lymph node status
- OPC
Oropharyngeal cancers
- pts
Patients
- T
Tumor size
Compliance with ethical standards
Conflict of interest
Marco Merlano took part in Merck Serono and MSD advisory boards, and has speaker role in Merck Serono promoted meetings. All other authors declare no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2009;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boero S, Morabito A, Banelli B, et al. Analysis of in vitro ADCC and clinical response to trastuzumab: possible relevance of FcyRIIIA/FcyRIIA gene polymorphism and HER-2 expression levels on breast cancer cell lines. J Transl Med. 2015;13:324. doi: 10.1186/s12967-015-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 4.Maréchal R, De Schutter J, Nagy N, Demetter P, Lemmers A, Devière J, Salmon I, Tejpar S, Van Laethem JL. Putative contribution of CD56 + positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer. 2010;10:340. doi: 10.1186/1471-2407-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner S, Wittekindt C, Reuschenbach M, Hennig B, Thevarajah M, Würdemann N, Prigge ES, von Knebel Doeberitz M, Dreyer T, Gattenlöhner S, Klussmann JP. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;138:2263–2273. doi: 10.1002/ijc.29962. [DOI] [PubMed] [Google Scholar]
- 6.Monteverde M, Milano G, Strola G, Maffi M, Lattanzio L, Vivenza D, Tonissi F, Merlano M, Lo Nigro C. The relevance of ADCC for EFGR targeting: a review of the literature and a clinically applicable method of assessment in patients. Crit Rev Oncol Hematol. 2015;95(2):179–190. doi: 10.1016/j.critrevonc.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Lo Nigro C, Ricci V, Vivenza D, Monteverde M, Strola G, Lucio F, Tonissi F, Miraglio E, Granetto C, Fortunato M, Merlano MC. Evaluation of antibody-dependent cell-mediated cytotoxicity activity and cetuximab response in KRAS wilde-type colorectal cancer patients. World J Gastrointest Oncol. 2016;8:222–230. doi: 10.4251/wjgo.v8.i2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mickel RA, Kessler DJ, Taylor JM, Lichtenstein A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer Res. 1988;48(17):5017–5022. [PubMed] [Google Scholar]
- 9.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62(20):5813–5817. [PubMed] [Google Scholar]
- 10.Taylor RJ, Saloura V, Jain A, Goloubeva O, Wong S, Kronsberg S, Nagilla M, Silpino L, de Souza J, Seiwert T, Vokes E, Villaflor V, Cohen EE. Ex vivo antibody-dependent cellular cytotoxicity inducibility predicts efficacy of cetuximab. Cancer Immunol Res. 2015;3(5):567–574. doi: 10.1158/2326-6066.CIR-14-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molling JW, Langius JAE, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, Zhang P. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217(4):385–393. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]