Abstract
Purpose
Melanoma patients with a high risk of recurrence may benefit from immunotherapy with mRNA-electroporated autologous monocyte-derived dendritic cells (DCs). Further benefit may be found in combining DC-therapy with interferon alfa-2b.
Patients and methods
The long-term clinical outcome of AJCC stage III/IV melanoma patients who had no evidence of disease at the time of treatment with autologous mRNA-electroporated DCs in a single-center pilot clinical trial was analyzed. Antigen loading was accomplished by co-electroporation of mRNA encoding a fusion protein between MAGE-A1, -A3, -C2, Tyrosinase, MelanA/MART-1, or gp100, and an HLA class II-targeting sequence. DCs were administered by 4–6 bi-weekly intradermal injections. IFN-α-2b (5 MIU TIW) was initiated either at recurrence (cohort 1), concomitant with DCs (cohorts 2 and 3), or following the fourth DC administration (cohort 4).
Results
Thirty melanoma patients were recruited between April 2006 and June 2009. DC-related adverse events included grade 2 local injection site reactions in all patients, grade 2 fever and flu-like symptoms in one patient, and skin depigmentation in seven patients. After a median follow-up of over 6 years, the median relapse-free survival is 22 months (95 % CI 12–32 months). Twelve patients have died. The median overall survival has not been reached; the 2-year and 4-year survival rates are 93 and 70 %, respectively.
Conclusions
Adjuvant therapy following the resection of melanoma metastases with autologous mRNA-electroporated DCs, combined with interferon alfa-2b, is tolerable and results in encouraging long-term overall survival rates justifying further evaluation in a randomized clinical trial.
Keywords: Dendritic cell, Interferon alpha-2b, mRNA, Immunotherapy, Melanoma
Introduction
Patients diagnosed with a small number of macrometastases, either locoregionally to the skin and lymph nodes or to distant sites, are considered candidates for surgical resection but will be facing a high risk for disease recurrence (e.g., stage III melanoma is associated with a 5-year survival rate between 39 and 70 %) [1]. The largest subpopulation with resectable metastases concerns patients with metastases to the locoregional lymph nodes, and these patients have been the subject of numerous prospective randomized clinical trials investigating the possibility of medical adjuvant therapy to improve their relapse-free and overall survival (OS). Cytokine therapy with interferon alfa-2b (IFN-α-2b) or peginterferon alfa-2b is the only adjuvant therapy for high-risk melanoma that is approved by the US Food and Drug Administration [2, 3]. However, the impact of interferon alfa-2b on overall survival is limited (<5 % gain in overall survival), underlining the need for more effective adjuvant melanoma treatment [4]. Over the past few years both small molecule inhibitors of the MAPK pathway and the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)-blocking monoclonal antibody ipilimumab demonstrated to improve the overall survival of patients with metastatic melanoma (with the activity of BRAF and MEK inhibitors being restricted to BRAF V600-mutant melanoma) [5–9]. Also in the adjuvant setting, randomized clinical trials have been initiated. Very recently, ipilimumab, administered at a dose of 10 mg/kg, significantly improved the relapse-free survival [median RFS of 26.1 h 17.2 months (placebo)] for patients with high-risk stage III completely resected melanoma [10]. Although ipilimumab and BRAF inhibitors might improve the overall survival of patients treated in the adjuvant setting, these treatments are all associated with class-specific toxicities and new, less toxic alternatives will still be useful to complement the therapeutic armamentarium.
It is well established that spontaneous anti-melanoma immune responses are directed in part against melanocyte differentiation antigens (e.g., tyrosinase, gp100, MART-1/MelanA) and cancer-testis antigens (e.g., MAGE-A3, NY-ESO-1, PRAME) [11]. Several different therapeutic strategies are under evaluation, which aim to enhance the cellular anti‐tumor immunity against these melanoma-associated antigens (MAAs) in patients [12]. One such strategy is to use autologous dendritic cells (DCs) loaded with MAA. DCs are the most potent antigen-presenting cells of the immune system, capable of inducing antigen‐specific T cell and B cell responses. Driven by the expanding knowledge on the immunogenicity of MAAs, the biology of DCs, and ex vivo generation of autologous DC-therapy products derived from autologous monocytes, several clinical DC‐based immunotherapy protocols have been conducted in recent years [13]. These studies have demonstrated the potential of DC-therapy to stimulate antigen‐specific T cell responses, both cytotoxic CD8+ T cell responses and CD4+ Th1 responses. These responses are functionally different from those observed following vaccination with peptides or recombinant MAA‐expressing viruses [14]. Notwithstanding the well‐documented clinical tumor responses in some melanoma patients, the activity of currently used DC therapy for the treatment of advanced melanoma remains limited in patients with advanced disease, and further improvement of the DC‐therapy strategy is needed.
Optimization of DC‐therapy characteristics can be achieved by electroporation of full-length MAA-encoding mRNA allowing for the cellular processing and presentation of the full range of antigenic peptides within the MAA protein. This offers the potential advantage for immunization regardless of the HLA type of the patient and overcomes the HLA‐type restrictions imposed by peptide vaccines. Additionally, MAA presentation in both HLA class I and class II molecules can be achieved by genetic fusion of the MAA‐encoding sequence with a HLA class II-targeting sequence [15]. A complementary improvement involves enhancing the immuno‐stimulatory capacity of the DCs. We have shown that the T cell stimulatory capacity of both peptide pulsed or MAA–mRNA-electroporated DCs can be enhanced either by (co‐)electroporation of poly I:poly C12U (Ampligen®) or with CD40L, CD70, and constitutively active TLR4 (caTLR4)-encoding mRNAs (so‐called TriMix mRNA) [16, 17]. Antigen-specific immunotherapies such as DC-therapy could potentially be complementary to antigen nonspecific immuno‐stimulating therapies that are dependent for their anti‐tumor activity on the presence of immune effector cells. Concurrent or sequential administration of IFN-α-2b with melanoma vaccines has been reported to result in an activation of anti‐tumor effector cells and to enhance anti‐tumor response in small studies [18–20]. However, no advantage could be demonstrated in combining IFN-α-2b with an allogeneic melanoma lysate vaccine (Melacine) or a peptide vaccine in two randomized controlled clinical trials [21, 22]. More recently, combination of the gp100: 209–217 (210 M) peptide vaccine with high-dose interleukin‐2 (IL‐2) improved survival as compared to IL‐2 alone in a randomized phase III clinical trial [23]. CTLA‐4 blockade by the monoclonal antibody ipilimumab has been reported to enhance polyfunctional NY‐ESO‐1-specific T cell responses in metastatic melanoma patients with an associated clinical benefit [24]. Also in a pilot clinical trial on the combination of MART‐1 peptide–pulsed DC and tremelimumab, higher range of durable tumor responses were observed as compared to each agent alone [25].
We here report on the feasibility, safety, and long-term clinical outcome of adjuvant autologous mRNA-electroporated DC-therapy in the subgroup of high-risk (resected stage IIIB/C or stage IV) melanoma patients who were sequentially treated with IFN-α-2b in different cohorts of a single-center pilot clinical trial. The outcome of the total population (resected and metastasis bearing patients) treated in these pilot studies has been reported previously [26–28].
Patients and methods
Patients
Patients with histologically confirmed melanoma American Joint Committee on Cancer (AJCC) stage III or IV were eligible in this single‐institution (UZ Brussel), single‐arm, multi-cohort clinical trial that aimed to document the feasibility, safety, and immunogenicity of therapeutic vaccination with mRNA-electroporated autologous DCs. Additional inclusion criteria included an age of 18 years or older, World Health Organization Performance Status (WHO-PS) score below 3, normal liver and renal function tests, baseline lactate dehydrogenase (LDH) value below the laboratory upper limit of normal, and ability to provide informed consent. Negative serological tests for HIV, hepatitis B, and hepatitis C were required. Exclusion criteria included primary ocular melanoma, central nervous system metastasis, and prior treatment with more than one regimen of systemic chemotherapy. Fertile patients were requested to use adequate anti‐conceptive measures throughout the study period. Screening procedures included baseline blood analyses (hematological, chemical, viral, and bacterial serology) and computed tomography (CT) and/or magnetic resonance imaging (MRI) of the entire body including the brain. The pilot study was conducted following approval by our institutional ethics committee, and written informed consent was obtained from all patients.
In this analysis, we investigate the long-term clinical outcome of stage III or stage IV melanoma patients, without measurable disease following the resection of melanoma metastases, who were included in this clinical trial.
Dendritic cellular therapy production
Enrichment of monocytes following leukapheresis was achieved by plastic adherence and cell culture in a semi‐closed culture system for all patients except for the 3 latest patients recruited on this study. In these 3 patients, elutriation followed by cell culture in Cell‐Max GmbH bags (Beldico, Belgium) was used. Monocytes were cultured for 6 days in the presence of 1,000 U/ml GM‐CSF and 500 U/ml IL‐4 [29]. Resulting immature DCs were harvested and co-electroporated with either poly I:poly C12U (Ampligen®, Hemispherx BioPharma Inc., Philadelphia, USA) and CD40L (cohort 1, 2 and 3) or CD40L, CD70 and caTLR4 (TriMix mRNA) (cohort 4) for maturation as described previously [17].
Poly I:poly C12U/CD40L-matured DCs were co-electroporated with 1 out of 6 MAAs (MAGE-A1, MAGE-A3, MAGE-C2, MelanA/MART-1, tyrosinase, or gp100) linked to an HLA class II-targeting sequence. TriMix-DCs were co-electroporated with 1 out of 4 MAAs (MAGE‐A3, MAGE‐C2, tyrosinase, or gp100) linked to an HLA class II-targeting sequence [30, 31]. After electroporation, the different cellular constituents (i.e., DCs expressing one of the MAAs) were mixed at equal ratios.
An in-process, as well as quality control of the final product, was performed for the TriMix-matured DCs, as reported previously [26].
Treatment schedule
DCs (±24.106) were administered intradermally at two different injection sites (axillar and/or inguinal region, 2 injections at each site), for a total of 4–6 administrations every 2 weeks. After the 4–6th administration, a disease evaluation was performed. In the absence of progressive disease, additional administrations were performed every 6 weeks (cohorts 1 and 2) or every 8 weeks (cohort 3) as availability permitted, or a single fifth administration (8 weeks after the fourth) in cohort 4. IFN‐α-2b (Intron A, Merck 1 Co., Inc., Whitehouse Station, NJ) at a dose of 5 million units, 3 times per week (5 MIU TIW) by subcutaneous injection was initiated at progression (cohort 1), concomitant with DC-therapy initiation (cohorts 2 and 3) or following the fourth administration (cohort 4).
The different patient cohorts, DC maturation, and administration protocols are summarized in Table 1.
Table 1.
Overview of patient cohorts: DC characteristics and treatment schedule
| Cohort | Number of patients | DC maturation by electroporation | Antigen loading by electroporation of MAAs linked to an HLA class II-targeting sequence | DC administrations | Interferon alfa-2b (5 MU, 3x/week) |
|---|---|---|---|---|---|
| 1 | 1 | poly I:poly C12U and CD40L | MAGE-A1, MelanA/MART-1, MAGE-A3, MAGE-C2, gp100, tyrosinase |
6 administrations: weeks 1, 3, 5, 7, 9, and 11 In absence of PD: additional admin q6 weeks |
Initiated at progression |
| 2 | 11 | poly I:poly C12U and CD40L | MAGE-A1, MelanA/MART-1, MAGE-A3, MAGE-C2, gp100, tyrosinase |
6 administrations: weeks 1, 3, 5, 7, 9, and 11 In absence of PD: additional admin q6 weeks |
Concomitant |
| 3 | 4 | poly I:poly C12U and CD40L | MAGE-A1, MelanA/MART-1, MAGE-A3, MAGE-C2, gp100, tyrosinase |
4 administrations: weeks 1, 3, 5, and 7 In absence of PD: additional admin q8 weeks |
Concomitant |
| 4 | 14 | CD40L, CD70 and caTLR4 (TriMix) | MAGE-A3, MAGE-C2, gp100, tyrosinase | 5 administrations: weeks 1, 3, 5, 7, and 15 | Initiated at week 7 |
Immunomonitoring
Immunomonitoring of the peripheral blood and DTH skin biopsies of the patients was performed in a portion of patients and reported previously [32–34].
Statistics
Overall survival was calculated from the date of first DC-administration to the date of death and analyzed by Kaplan–Meier estimation. The log‐rank test was used to compare the survival between subgroups. (SPSS 20.0 software, Chicago, Illinois, USA).
Results
Patients
A total of 30 melanoma patients without measurable disease following the resection of melanoma metastases were recruited between April 2006 and June 2009. The baseline characteristics of the study population are summarized in Table 2. The median age at baseline was 47 years (range 28–74), and the majority of patients had been diagnosed with macrometastases: 21 patients with stage IIIB/C, 3 with IV-M1a, and 1 with stage MIV-M1c disease according to AJCC criteria. Five patients had been diagnosed with micrometastases (IIIA). One patient with a slightly elevated LDH (<1.5 × ULN) during screening, which was considered not-disease related, was included in this analysis.
Table 2.
Baseline characteristics of the patients
| Variable | Patients |
|---|---|
| Total (male/female)—no. | 30 (20/10) |
| Median age—years (range) | 47 (28–74) |
| WHO-PS—no. (%) | |
| 0 | 26 (87) |
| 1 | 4 (13) |
| AJCC stage—no. (%) | |
| IIIA/IIIB/IIIC | 5 (17)/4 (13)/17 (57) |
| IV-M1a/IV-M1b/IV-M1c | 3 (10)/0/1 (3) |
| LDH level—no. (%) | |
| ≤ULN | 29 (97) |
| >ULN | 1 (3) |
| Primary tumor site—no. (%) | |
| Head and neck | 5 (17) |
| Extremities | 11 (37) |
| Trunk | 10 (33) |
| Acral | 3 (10) |
| Unknown | 1 (3) |
| Ulcerated primary—no. (%) | |
| Yes | 7 (23.3) |
| No | 5 (17) |
| Unknown | 18 (60) |
| Prior therapy—no. (%) | |
| Surgery | 30 (100) |
| Adjuvant radiotherapy | 9 (30) |
| Immunotherapy | |
| Adjuvant high-dose IFN-α-2b | 4 (13.3) |
| DC-vaccination | 1 (3.3) |
AJCC American Joint Committee on Cancer, WHO-PS World Health Organization Performance Status, LDH lactate dehydrogenase, ULN laboratory upper limit of normal, IFN-α-2b interferon alfa-2b, DC dendritic cell
Treatment disposition and toxicity
A total of 189 DC‐vaccines were administered (median/patient: 5, range 3–12). DC-related adverse events (AEs) were limited to transient local injection site reactions (grade 2 itching or tenderness with swelling and erythema) in all patients, and grade 2 fever in 1 patient.
IFN-α-2b was initiated in 29 patients. One patient refused such adjuvant treatment after completing DC-therapy. According to the different treatment protocols, IFN-α-2b was initiated at progression in 1 patient, concomitant with DC in 15 patients, and at week 7 in 13 patients (Table 1). Median duration of IFN-α-2b treatment was 5.5 months (range 0.9–13.8 months). IFN-α-2b was discontinued in 4 patients because of treatment-related toxicity: grade 3 constitutional symptoms in 2 patients, grade 2 depression in 1 patient, and grade 2 hyperthyroidism in 1 patient. An additional 5 patients requested to stop IFN-α-2b treatment in the absence of grade ≥2 toxicity.
Skin depigmentation (vitiligo) and a sarcoidosis-like syndrome (18F-FDG-avid bilateral mediastinal and hilar lymphadenopathies on PET–CT, with complete normalization in size and metabolic activity upon cessation of IFN-α-2b) were observed in seven and one patients who were exposed to DC and IFN-α-2b. All treatment-related AEs are summarized in Table 3.
Table 3.
Treatment-related adverse events
| Adverse events | Patients—no. (%) | |
|---|---|---|
| Grade 1/2 | Grade 3 | |
| DC-related | ||
| Local injection site reactions (duration 2–3 days) | 30/30 (100) | 0 |
| Fever, myalgia (≤7 days) | 1/30 (3) | 0 |
| Interferon alfa-2b related | ||
| Constitutional symptoms | 29/29 (100) | 2/29 (7) |
| Depression | 1/29 (3) | 0 |
| Hyperthyroidism | 1/29 (3) | 0 |
| DC/IFN-α-2b | ||
| Skin depigmentation | 7/29 (24) | 0 |
| Sarcoidosis-like syndrome | 1/29 (3) | 0 |
Immunomonitoring
In 10 of the 30 patients, immunological responses were analyzed in DTH biopsies. In 2 out of these 10 patients, peripheral blood prior- and post-DC-therapy was additionally screened. An immune response against at least one MAA was demonstrated in 4/10 patients (in the DTH biopsies) and could be confirmed in the peripheral blood in 1 out of the 2 patients tested. These results were reported previously [26, 34].
Clinical outcome
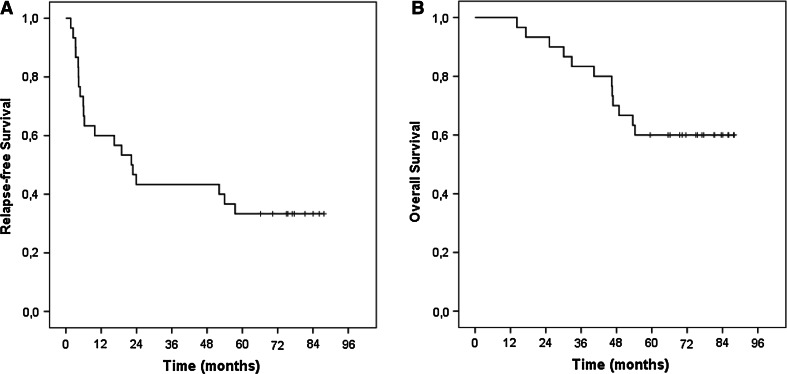
After a median follow-up of 6.4 years (range 4.7–7.9), 10 patients (33.3 %) remain disease free, and twenty patients (66.6 %) have experienced a recurrence of their melanoma. The Kaplan–Meier estimate for the relapse-free survival is shown in Fig. 1a. At first relapse, 11 patients had locoregional disease only, while 9 patients developed distant metastases. Five patients who experienced a recurrence (respectively, 1.6, 4.8, 6.2, 53.9, and 57.5 months following the first DC-administration) were salvaged by surgery or radiotherapy, and they all remain free from disease up to the latest follow-up, respectively, after 4.9, 5.7, 5.9, 6.7, and 7.1 years. The median relapse-free survival of the total study population (30 patients) is 22 months [95 % confidence interval (CI) 12–32 months], and 18 months (95 % CI 2–35) for the subgroup of 24 patients with macrometastases. No significant correlation with relapse-free survival was found for the baseline co‐variables that were analyzed: gender, age, AJCC stage, primary tumor site, ulceration of the primary tumor, adjuvant radiation therapy, DC maturation.
Fig. 1.
Kaplan–Meier probability curves for relapse-free survival (a) and overall survival (b) in melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases
Twelve patients have died, 11 from metastatic melanoma and one patient’s death was not melanoma-related. Consequently, the median overall survival has not yet been reached. The 2-year and 4-year survival rates are 93, 70, 91, and 75 % for the total study population and for patients with macrometastases, respectively. Figure 1b illustrates the Kaplan–Meier estimates for overall survival. No significant correlation with overall survival was found for the baseline co‐variables that were analyzed.
Discussion
A major breakthrough for cancer immunotherapy was achieved when both an antigen nonspecific immunotherapy with ipilimumab, a CTLA-4 receptor-blocking monoclonal antibody, and an antigen-specific immunotherapy with sipuleucel-T, an autologous dendritic cell vaccine against prostatic acid phosphatase, demonstrated to improve the overall survival of patients with advanced melanoma and castration-resistant prostate cancer, respectively [8, 9, 35].
Notwithstanding the known susceptibility of melanoma for immunotherapy, so far no randomized clinical trial with a cancer vaccine has been able to convincingly demonstrate a survival benefit for melanoma. Hypothetically, melanoma vaccines such as peptide, protein vaccines, or dendritic cell therapies, may have an optimal therapeutic effect in patients with low burden of disease. This relates to the absence of strong tumor-related systemic immunosuppression, to the longer life expectancy of such a population allowing for sufficient time to induce a protective anti-tumor immune response, and with respect to dendritic cell therapy, the possibility to manufacture an optimal DC-product [36].
We recently reported on the development of new and improved DC-based cell therapy formulations using the mRNA electroporation technology [15, 17, 30, 33, 34]. We previously reported that the immunostimulatory capacity of DCs can be enhanced by co-electroporation of DCs with mRNA encoding CD40L, CD70 and caTLR4 (TriMix-DC) and that these DCs co-electroporated with mRNA encoding a melanoma-associated antigen linked to an HLA class II-targeting sequence are safe and immunogenic in patients with advanced melanoma when injected intradermally [26]. However, in patients with evaluable macrometastatic disease, no objective tumor responses could be objectivated. In order to assess the potentially superior effect of DC-therapy when offered to patients with only a microscopic disease burden, we analyzed the long-term clinical outcome of 30 disease-free melanoma patients who were treated in the adjuvant setting with intradermally injected autologous mRNA-electroporated DC and concomitantly or sequentially administered IFN-α-2b in a sequential-cohort pilot clinical trial.
Dendritic cell administration was well tolerated without any grade 3/4 treatment-related adverse events. As anticipated, constitutional symptoms were experienced by all patients who initiated IFN-α-2b (grade 3 in 2/29 patients). There were no unexpected adverse events in patients exposed to both DC and IFN-α-2b, although the incidence of vitiligo (23 %) may be higher than what is expected with either treatment alone.
The survival of our patient cohort, and especially for the 24 patients who were diagnosed with AJCC stage IIIB/C and IV disease (macrometastases), compares favorably with the outcome of IFN-α-2b-treated patients in large randomized trials (Table 4). The difference with large historical series on adjuvant therapy of patients with macrometastases is more remarkable with respect to OS as compared to RFS. In part, this favorable long-term OS observed in our series relates to the 5 out of 20 patients who experienced a melanoma recurrence and who were successfully salvaged by local therapy (surgery or radiotherapy) and remain free from disease recurrence after a minimal follow-up of more than 4 years. This may indicate that DC-therapy changed the aggressiveness of their disease offering the potential for curative salvage therapy. Moreover, in some of these patients the recurrence was isolated and diagnosed within the first months following the first administration of the DC-therapy, possibly within the time-frame where protective immunity was not yet acquired. The discrepancy between the RFS, which is more comparable with historical controls, and the seemingly improved overall survival rates resembles to some extent the observation made with ipilimumab and sipuleucel-T phase III studies where OS was improved in the absence of a significant improvement in progression-free survival [8, 35]. This pattern may relate to the different mode of action of immunotherapy compared with conventional cytotoxic chemotherapy. Anti-tumor responses with immunotherapy are associated with a latency period of 8–12 weeks, and the observed atypical patterns of response with delayed slow tumor responses, and progressive disease prior to tumor response observed with ipilimumab treatment, strengthens this hypothesis [37].
Table 4.
Clinical outcome following adjuvant DC-therapy and historical data from randomized trials
| Adjuvant mRNA DC-therapy | Historical data | |||
|---|---|---|---|---|
| 18991 [2] | HeCOG [40] | |||
| Patient population | All (n = 30) | Stages IIIB/C and IV (n = 24) | Stage III (n = 627) | Stages IIB, IIC, and III (n = 353) |
| mRFS (months, 95 % CI) | 22 (12–32) | 18 (2–35) | 36 | 24–27 |
| mOS | Not reached | Not reached | 6.2 years | 5.3 years |
| Survival rate | ||||
| 2 years | 93 % (95 % CI 84–100) | 91 % (95 % CI 80–100) | ||
| 3 years | 83 % (95 % CI 70–97) | 83 % (95 % CI 68–98) | 63–70 % | |
| 4 years | 70 % (95 % CI 54–86) | 75 % (95 % CI 58–92) | 56.8 % | |
mRFS median relapse-free survival, mOS median overall survival, CI confidence interval
As our results are obtained from a single-institution, single-arm, small patient study with 4 cohorts treated with a number of different DC-formulations, no definitive conclusions can be drawn. The observed survival difference with historical control data, however, legitimates the further evaluation of mRNA-electroporated DC-formulations for the adjuvant therapy of patients with high-risk melanoma.
We recently reported the results of a phase-I study, with combined intradermal and dose-escalated intravenous DC-administration and observed objective clinical tumor responses [38, 39]. These results underline the importance of the route of administration for the anti-tumor activity of TriMix-DC-MEL therapy and indicate that intravenous administration might also provide a higher activity in adjuvantly treated patients.
In conclusion, this pilot trial demonstrates that adjuvant therapy with autologous mRNA-electroporated DCs is feasible and safe and results in encouraging long-term overall survival rates.
Acknowledgments
We thank the patients who consented to participate in the clinical study, their families, and referring physicians. We are very much indebted to the data managers Katrien Van den Bossche and Kathleen Mooren, and to Elsy Vaeremans, Gwenny De Metter, Inge Betz, Chiraz Mahmoud for excellent technical assistance, and to Prof. Coomans for help with the statistical analyses. This work was supported by grants from the Interuniversity Attraction Poles Program—Belgian State—Belgian Science Policy, the Stichting tegen Kanker, an Integrated Project and a Network of Excellence sponsored by the EU, the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO-Vlaanderen) and the Willy Gepts Wetenschappelijk Fonds of the Universitair Ziekenhuis Brussel. Sofie Wilgenhof is a PhD student of the FWO-Vlaanderen.
Conflict of interest
The use of dendritic cells electroporated with tumor antigen mRNA and TriMix is the topic of a patent (W2009/034172) on which Dr. A. Bonehill and Prof. Dr. K. Thielemans are filed as inventors. None of the authors receive any support or remuneration related to this platform. No potential conflicts of interest were disclosed.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- AEs
Adverse events
- AJCC
American Joint Committee on Cancer
- caTLR4
Constitutively active Toll-like receptor 4
- CD40L
CD40 ligand
- CI
Confidence interval
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen 4
- CT
Computed tomography
- DC
Dendritic cell
- DTH
Delayed-type hypersensitivity
- GM-CSF
Granulocyte/macrophage colony-stimulating factor
- HLA
Human leucocyte antigen
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IFN-α-2b
Interferon alfa-2b
- LDH
Lactate dehydrogenase
- MAAs
Melanoma-associated antigens
- MAPK
Mitogen-activated protein kinase
- MIU
Million international units
- MRI
Magnetic resonance imaging
- mRNA
Messenger ribonucleic acid
- OS
Overall survival
- PET–CT
Positron emission tomography–computed tomography
- RFS
Relapse-free survival
- SKILs
Skin infiltrating lymphocytes
- Th1
T helper type 1
- TIW
Three times in a week
- WHO-PS
World Health Organization Performance Status
Contributor Information
Sofie Wilgenhof, Email: Sofie.Wilgenhof@vub.ac.be.
Bart Neyns, Phone: +3224776415, Email: Bart.Neyns@uzbrussel.be.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggermont AM, Suciu S, Testori A, et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 3.Sondak VK, Kudchadkar R. Pegylated interferon for the adjuvant treatment of melanoma: FDA approved, but what is its role? Oncologist. 2012;17:1223–1224. doi: 10.1634/theoncologist.2012-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 8.Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Ipilimumab versus placebo after complete resection of stage III melanoma: initial efficacy and safety results from the EORTC 18071 phase III trial. Alexandria: ASCO Meeting Abstracts; 2014. [Google Scholar]
- 11.Boon T, Coulie P, Van den Eynde B, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 12.Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 13.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connerotte T, Van Pel A, Godelaine D, Tartour E, Schuler-Thurner B, Lucas S, Thielemans K, Schuler G, Coulie P. Functions of Anti-MAGE T-cells induced in melanoma patients under different vaccination modalities. Cancer Res. 2008;68:3931–3940. doi: 10.1158/0008-5472.CAN-07-5898. [DOI] [PubMed] [Google Scholar]
- 15.Bonehill A, Heirman C, Tuyaerts S, Michiels A, Breckpot K, Brasseur F, Zhang Y, Van Der Bruggen P, Thielemans K. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172:6649–6657. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- 16.Michiels A, Breckpot K, Corthals J, Tuyaerts S, Bonehill A, Heirman C, Thielemans K, Aerts JL. Induction of antigen-specific CD8 + cytotoxic T cells by dendritic cells co-electroporated with a dsRNA analogue and tumor antigen mRNA. Gene Ther. 2006;13:1027–1036. doi: 10.1038/sj.gt.3302750. [DOI] [PubMed] [Google Scholar]
- 17.Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell M, Jakowatz J, Harel W, Dean G, Stevenson L, Boswell W, Groshen S. Increased effectiveness of interferon alfa-2b following active specific immunotherapy for melanoma. J Clin Oncol. 1994;12:402–411. doi: 10.1200/JCO.1994.12.2.402. [DOI] [PubMed] [Google Scholar]
- 19.Astsaturov I, Petrella T, Bagriacik E, et al. Amplification of virus-induced antimelanoma T-cell reactivity by high-dose interferon-alpha2b: implications for cancer vaccines. Clin Cancer Res. 2003;9:4347–4355. [PubMed] [Google Scholar]
- 20.Di Pucchio T, Pilla L, Capone I, et al. Immunization of stage IV melanoma patients with Melan-A/MART-1 and gp100 peptides plus IFN-alpha results in the activation of specific CD8(+) T cells and monocyte/dendritic cell precursors. Cancer Res. 2006;66:4943–4951. doi: 10.1158/0008-5472.CAN-05-3396. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell M, Abrams J, Thompson J, et al. Randomized trial of an allogeneic melanoma lysate vaccine with low-dose interferon Alfa-2b compared with high-dose interferon Alfa-2b for Resected stage III cutaneous melanoma. J Clin Oncol. 2007;25:2078–2085. doi: 10.1200/JCO.2006.10.1709. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine ± granulocyte–monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas A, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilgenhof S, Van Nuffel AM, Corthals J, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011;34:448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
- 27.Neyns B, Corthals J, De Greve J, De Greef C, Coulie PG, Thielemans K (2006) A cohort pilot study on therapeutic vaccination of advanced melanoma patients with dendritic cells loaded with multiple peptides or electroporated with mRNA. ASCO Meeting Abstracts 24: 18011
- 28.Neyns B, Corthals J, Thielemans K (2007) Interferon alfa-2b treatment following therapeutic vaccination with mRNA electroporated dendritic cells results in skin depigmentation and tumor regression in patients with advanced melanoma. ASCO Meeting Abstracts 25: 8537
- 29.Tuyaerts S, Noppe S, Corthals J, Breckpot K, Heirman C, De Greef C, Van Riet I, Thielemans K. Generation of large numbers of dendritic cells in a closed system using cell factories. J Immunol Methods. 2002;264:135–151. doi: 10.1016/S0022-1759(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 30.Bonehill A, Van Nuffel A, Corthals J, et al. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res. 2009;15:3366–3375. doi: 10.1158/1078-0432.CCR-08-2982. [DOI] [PubMed] [Google Scholar]
- 31.Van Nuffel A, Corthals J, Neyns B, Heirman C, Thielemans K, Bonehill A. Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated through mRNA electroporation. Methods Mol Biol. 2010;629:405–452. doi: 10.1007/978-1-60761-657-3_27. [DOI] [PubMed] [Google Scholar]
- 32.Van Nuffel AM, Tuyaerts S, Benteyn D, Wilgenhof S, Corthals J, Heirman C, Neyns B, Thielemans K, Bonehill A. Epitope and HLA-type independent monitoring of antigen-specific T-cells after treatment with dendritic cells presenting full-length tumor antigens. J Immunol Methods. 2012;377:23–36. doi: 10.1016/j.jim.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Van Nuffel AM, Benteyn D, Wilgenhof S, et al. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4 + and CD8 + T cells in melanoma patients. Mol Ther. 2012;20:1063–1074. doi: 10.1038/mt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benteyn D, Van Nuffel AM, Wilgenhof S, Corthals J, Heirman C, Neyns B, Thielemans K, Bonehill A. Characterization of CD8(+) T-cell responses in the peripheral blood and skin injection sites of melanoma patients treated with mRNA electroporated autologous dendritic cells (TriMixDC–MEL) Biomed Res Int. 2013;2013:976383. doi: 10.1155/2013/976383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantoff P, Higano C, Shore N, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 36.Hasebe H, Nagayama H, Sato K, Enomoto M, Takeda Y, Takahashi TA, Hasumi K, Eriguchi M. Dysfunctional regulation of the development of monocyte-derived dendritic cells in cancer patients. Biomed Pharmacother. 2000;54:291–298. doi: 10.1016/S0753-3322(00)80050-5. [DOI] [PubMed] [Google Scholar]
- 37.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 38.Van Nuffel AM, Benteyn D, Wilgenhof S, Corthals J, Heirman C, Neyns B, Thielemans K, Bonehill A. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother. 2012;61:1033–1043. doi: 10.1007/s00262-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilgenhof S, Van Nuffel AM, Benteyn D, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 40.Pectasides D, Dafni U, Bafaloukos D, et al. Randomized phase III study of 1 month versus 1 year of adjuvant high-dose interferon alfa-2b in patients with resected high-risk melanoma. J Clin Oncol. 2009;27:939–944. doi: 10.1200/JCO.2008.16.3121. [DOI] [PubMed] [Google Scholar]