Abstract
The tumor-specific Thomsen-Friedenreich antigen (TFα, CD176) is an attractive target for a cancer vaccine, especially as TF-directed antibodies play an important role in cancer immunosurveillance. However, synthetic TF vaccines have not overcome the low intrinsic immunogenicity of TF. Since natural TF-directed antibodies present in human sera are generated in response to microbes found in the gastrointestinal tract, microbial TF structures are obviously more immunogenic than synthetic TF. We recently isolated a new strain (D-6) of the human gut bacterium Bacteroides ovatus, which carries the true TFα antigen. Here, we present experimental data on the immunogenicity of this strain. Mice immunized with B. ovatus D-6 in the absence of adjuvants developed specific anti-TFα IgM and IgG antibodies which also bound to human cancer cells carrying TFα. Our data suggest that B. ovatus D-6 presents a unique TFα-specific immunogenicity based on a combination of several inherent properties including: expression of the true TFα antigen, clustering and accessible presentation of TFα as repetitive side chains on a capsular polysaccharide, and intrinsic adjuvant properties. Therefore, B. ovatus strain D-6 is an almost perfect candidate for the development of the first adjuvant-free TFα-specific anti-tumor vaccine.
Keywords: Thomsen-Friedenreich antigen, Bacteroides ovatus, Cancer, Vaccine, Immune response, Commensal bacteria
Introduction
Changes in cell surface glycans are a prominent feature of the oncogenic transformation of cells. Disturbances in the glycosylation machinery lead to the appearance of tumor-associated carbohydrate antigens (TACA) that have been perceived as highly suitable targets for the development of cancer vaccines. Among TACAs, the Thomsen-Friedenreich antigen (TFα, CD176) has attracted much attention because of its unique tumor specificity, prevalence, role in metastasis and potential immunogenicity [1–8]. TFα occurs only in cryptic form on normal cells but is exposed on tumor cells of many types of carcinomas, including those of breast, colon, lung, prostate and bladder [3, 7]. It is widely accepted that natural anti-TFα antibodies present in sera of healthy individuals play a crucial role in immunosurveillance [5, 6, 8–10]. Initial attempts to induce a TF-specific therapeutic response in humans were undertaken by Georg F. Springer, who successfully immunized breast cancer patients with a TFα-positive vaccine containing asialoglycophorin (derived from red blood cell membranes), which resulted in an impressive improvement of survival [10].
More recent approaches using synthetic glycoconjugates failed to show significant clinical effects because of the poor inherent immunogenicity of single TF epitopes [11–13]. Several strategies for enhancing the immune response to this carbohydrate antigen were attempted: carbohydrate-protein conjugation, modified linkers, clustered epitopes, peptide mimetics, molecular rotation and the inclusion of adjuvants and/or other functional groups [14–17]. Interesting parallel developments are MUC1-targeting vaccines employing TF containing glycopeptides [18–20]. However, in contrast to Springer’s vaccine, none of these approaches have so far resulted in a significant clinical benefit for the patients.
“Natural” anti-TF antibodies are found in the sera of healthy adult individuals [21, 22] and are thought to be induced by gastrointestinal microorganisms which carry TF or TF-like structures [23–27]. Microorganisms may present the TF structures in a configuration more closely related to a tumor cell and may therefore surpass synthetic structures in terms of their ability to elicit a TFα-specific, tumor-directed immune response.
In a recent study, we used a novel combination of TF-specific antibodies and found that most of the bacterial strains previously reported to express TF in fact expressed TF-related or cryptic TF antigens rather than an exposed true TFα structure [28]. We also identified two novel bacterial strains that express the true (immunochemically identical) and immune-accessible TFα antigen that apparently corresponds to the TF structure specifically found on human tumors.
In the present study, we examined and compared the ability of commensal bacterial strains that express either the true TFα antigen, cryptic TFα antigen, a TF-related antigen, or no detectable TF antigen at all to elicit a TFα-specific immune response in vivo. We developed an analysis approach that offsets the interfering effect of natural carbohydrate-directed poly-reactive antibodies and thereby allows the detection of TFα-specific immune responses.
Our results are the first that unambiguously demonstrate that the bacterial strain B. ovatus D-6, which expresses a true and immune-accessible TFα-antigen, has substantial TFα-specific immunogenic potential. Therefore, B. ovatus D-6 presents an attractive opportunity for the development of a TFα-specific anti-tumor vaccine.
Materials and methods
Cultivation of cell lines and bacteria
The TFα-positive human acute myelogenous leukemia cell line KG-1 [43] and two sublines derived from it were cultured in Dulbecco’s MEM (DMEM) medium containing 10 % fetal bovine serum (Biochrom, Berlin, Germany) in tissue culture flasks (TPP, Trasadingen, Switzerland) in a humidified atmosphere of 8 % CO2 in air at 37 °C. Cultures were split 1:3 every 2–3 days. The original cell line contained around 50 % TFα-positive cells. We selected TFα-positive and TFα-negative sublines by means of magnetic beads (Dynabeads M-450, Deutsche Dynal GmbH, Hamburg, Germany) coated with the anti-TFα antibody, NM-TF2 (Glycotope). The TFα-positive subline remained stable, whereas the TFα-negative subline developed slowly (during many passages) back to the original state of around 50 % positivity (unpublished data).
Bacteria were grown overnight at 37 °C as described previously [28]. Bacteroides ovatus strain D-6 (TFα antigen positive) was cultivated in a CO2 atmosphere (Anaerogen, Oxoid, Wesel, Germany) in Wilkins-Chalgren (WC) broth (Oxoid, Wesel, Germany), whereas Escherichia coli strains DSM 8697 (cryptic TF antigen), D-3 (no detectable TF antigen) and G-2 (TF-related antigen) were cultivated under aerobic conditions in Luria–Bertani broth (LB) (Carl Roth, Karlsruhe, Germany). Bacteria were harvested by centrifugation (8,000×g, 15 min, 4 °C). Total cell numbers were determined with a 0.01 mm depth Thoma counting chamber. Bacteria were either heat-inactivated by pasteurization (30 min in a 75 °C water bath with brief vortexing every 5–10 min) or fixed with 4 % paraformaldehyde, washed and suspended in one volume of Maniatis phosphate-buffered saline (PBS) (9 g/l NaCl, 0.528 g/l Na2HPO4·2H2O, 0.144 g/l KH2PO4, pH 7.4), followed by addition of one volume of ice-cold ethanol [28] and stored at 4 °C.
Bacterial ELISA
Inactivated bacterial cells were adjusted to a cell concentration of 1 × 106 or 1 × 108 cells/ml with PBS. Of this suspension, 50 μl was applied in duplicate to the wells of a PolySorp microtitre plate (Nunc, Wiesbaden, Germany) and coated overnight at 37 °C. Prior to all further incubation steps, the plates were washed three times with 200 μl Tris-buffered saline containing Tween 20 (8.78 g/l NaCl, 6.06 g/l Tris, 0.05 % [v/v] Tween-20, pH 7.6) and blocked by incubating the wells with 200 μl PBS containing 2 % bovine serum albumin (BSA) for 60 min.
For general (bacterial strain-specific), antibody binding animal sera were appropriately diluted (1:500–1:1000) with PBS containing 1 % BSA, and 50 μl was applied in duplicate and incubated for 1 h at room temperature. The plates were washed with 200 μl Tris-buffered saline containing Tween 20 and incubated for 1 h at room temperature with 50 μl of the secondary antibody (rabbit-anti-mouse, polyclonal, DAKO, Hamburg, Germany; goat-anti-mouse IgM, Jackson ImmunoResearch, Suffolk, UK; goat-anti-mouse IgG Fc, Jackson; goat-anti-mouse IgA, Bethyl, Montgomery, TX, USA), all peroxidase (POD)-conjugated diluted 1:2,000 and 1:5,000, respectively, in PBS containing 1 % BSA.
To determine the TFα expression of bacterial strains, anti-TFα monoclonal antibody NM-TF1 (Glycotope GmbH, Berlin, Germany) was diluted in PBS to concentrations of 20–100 ng/ml, and 50 μl was applied in duplicate to wells and incubated for 1 h at room temperature. The plates were washed with 200 μl Tris-buffered saline containing Tween 20 and incubated for 1 h at room temperature with 50 μl of the secondary antibody (goat-anti-mouse IgM-POD) diluted 1:5000 in PBS containing 1 % BSA. In both cases, the microtiter plates were washed with Tris-buffered saline containing Tween 20 and developed for 5–20 min in the dark after adding 100 μl of developing solution (TMB Substrate One Component, Tebu-Bio, Offenbach, Germany) to each well. Subsequently, 50 μl of 2.5 M H2SO4 was added to stop the reaction, and the absorbance (E450/630 nm) was measured in an ELISA reader (Dynex Technologies Inc., Chantilly, USA). The assays were performed in duplicate on at least two separate occasions.
Carbohydrate ELISA
Polyacrylamide [PAA]-glycoconjugates (Lectinity, Moscow, Russia) were adjusted to a concentration of 5 μg/ml in coating buffer (4.2 g/l NaHCO3, 1.78 g/l Na2CO3, pH 9.6), and 50 μl was applied in duplicate to the wells of a MaxiSorp microtiter plate (Nunc, Wiesbaden, Germany) and kept overnight at 4 °C. Prior to further incubation steps, the plates were washed and blocked as described above. Animal sera were appropriately diluted (1:50–1:200) with PBS containing 1 % BSA [or, in inhibition experiments, with PBS containing 1 % BSA and 20 μg/ml AGP (asialoglycophorin) or GP (glycophorin)], and 50 μl was applied in duplicate on coated wells and incubated for 1 h at room temperature. Further incubations, washings and developing steps were performed as described above. The relative level of TFα-specific antibodies (TFαR) was expressed as ratio of absorbances (A): A 450/630 nm on TFα-PAA/A 450/630 nm on Lec-PAA. To facilitate the interpretation, the pre-immunization ratio was set at 1 and the post-immunization proportionally adapted. Pre- and post-immunization sera from each single mouse were analyzed on the same microtiter plate. Comparing the ratio before and after immunization allowed to specifically analyze the changes in the TFα-specific antibody titer. Post-immunization sera presenting an increase of ≥15 % in the ratio were evaluated as positive, and those with an increase in ratio below 15 % were considered negative. Positive control antibodies were two mouse monoclonals from Glycotope: NM-TF1 and NM-TF2, both of the IgM κ isotype [3]. Their specificity was tested against more than 80 PAA-coupled synthetic mono- and oligosaccharides as well as with purified glycoproteins such as AGP and GP as negative control. Both antibodies are specific for TFα with a slight cross-reactivity with TFβ, which is common among anti-TFα antibodies because of the structural similarity of both anomers. There is evidence that these two antibodies bind to the TFα disaccharide from different angles.
Incubation with synthetic stomach and intestine juices
To simulate gastrointestinal passage, harvested B. ovatus D-6 cells were split into three equal parts. One part was fixed with paraformaldehyde, two parts were washed with reduced PBS (PBSred) (8.5 g/l NaCl, 0.3 g/l KH2PO4, 0.6 g/l Na2HPO4, 0.1 g/l peptone, 0.25 g/l cysteine·HCl, pH 7.0), suspended in synthetic stomach juice (2.9 g/l NaCl, 0.7 g/l KCl, 0.27 g/l KH2PO4, 1 g/l pepsin, pH 2) and incubated at 37 °C for 1.5 h while shaking at 100 rpm. The two parts exposed to stomach juice were harvested and washed with PBSred. One part was fixed with paraformaldehyde, the other was suspended in synthetic intestinal juice (0.3 g/l KCl, 0.5 g/l CaCl2, 0.2 g/l MgCl2, 1 g/l NaHCO3, 0.3 g/l trypsin, 9 g/l pancreatin, 9 g/l bile, 0.3 g/l urea, pH 6.8) prepared as described [44], and incubated for 4 h at 37 °C while shaking at 100 rpm. The cells exposed to intestinal juice were then harvested, washed and fixed. The stability of TFα expression of strain B. ovatus D-6 was examined by ELISA as described previously.
Animal experiments
Preparation of bacteria for animal experiments
Stationary phase cultures were harvested (8,000×g, 15 min, 4 °C), washed once and inactivated either through fixation with paraformaldehyde or through pasteurization. Prior to application, fixed cells were washed to remove paraformaldehyde and lyophilized to remove ethanol and suspended in 0.9 % NaCl to the required cell density. Successful inactivation and the absence of contaminants in bacterial preparations were assessed by inoculating Wilkins-Chalgren (WC) broth and plating on WC agar under aerobic and anaerobic conditions, and incubating the plates for at least 1 week to ensure the absence of growth. Total bacterial numbers were determined with a counting chamber.
Animals
Female C3H mice at 8 weeks of age were obtained from Charles River (Sulzendorf, Germany) and randomly divided into groups. Experiments were carried out in the animal facility of Experimental Pharmacology and Oncology (EPO) GmbH (Berlin-Buch, Germany) under specific pathogen-free conditions. The mice were fed with a sterilized standard diet (Ssniff Spezialdiäten, Soest, Germany). Water was offered ad libitum. All experiments were run in accordance with the German Animal Protection Law and approved by the Ministries of Nutrition, Agriculture and Forestry in Brandenburg and Berlin, Germany (permissions 32-44457 + 28 and G0221/03, respectively).
Intraperitoneal immunization
Inactivated bacteria were suspended in PBS, and 200 μl containing 2 × 108 cells was injected intraperitoneally into mice on days 0, 7 and 13. Serum samples were collected on day -1 (prior to immunization) and on days 14 and 21. In each experiment, at least four mice per group were used.
Oral immunization
Twenty-seven C3H female mice were orally immunized with daily doses of 1010, 109, 108, 107, or 106 pasteurized B. ovatus D-6 cells on 5 days a week for 8 weeks. Pasteurized D-6 cells were suspended in 200 μl of PBS. Serum samples were collected at days -1 (pre-immunization day), 13 and 21 (early stage), 35, 49 and 56 (late stage), and after recovery (day 77).
Flow cytometry
Fluorescence-activated cell sorting [FACS] was performed as previously described [45]. KG-1+ (TFα-positive) and KG-1− (TFα-negative) cells were harvested, counted, and diluted to a concentration of 250,000 cells in 50 μl per well seeded in 96-well U-bottom plates (Cotech, Berlin, Germany). Pre- and post-immunization sera were diluted 1:50 or 1:100 with PBS containing 1 % BSA or with PBS containing 1 % BSA and 20 μg/ml AGP (asialoglycophorin) or GP (glycophorin), and incubated with KG-1+ (TFα-positive) or KG-1− (TFα-negative) cells on ice for 30 min. The cells were washed, treated with goat-anti-mouse IgM-Cy3 or goat-anti-mouse IgG-FITC (Jackson) and analyzed by flow cytometry (Coulter Epics XL, Beckman Coulter, Krefeld, Germany) for mean percent TFα-positive cells as described previously [42]. Anti-TFα monoclonal antibody NM-TF1 served as positive control. For each mouse, the signal obtained with the pre-immunization sera was gated to reactivity of 10 % positive cells and compared with the post-immunization reactivity as described [13].
Statistical analysis
Statistical significance was calculated using GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA, USA). For each animal group (composed of at least four animals), means and standard errors of the means of pre- and post-immunization parametric variables were obtained and compared by one-tailed Student’s paired t test. p < 0.05 was considered statistically significant.
Results
Lec (Galß1-3GlcNAc-PAA) as a reference antigen to offset the effect of natural polyreactive antibodies
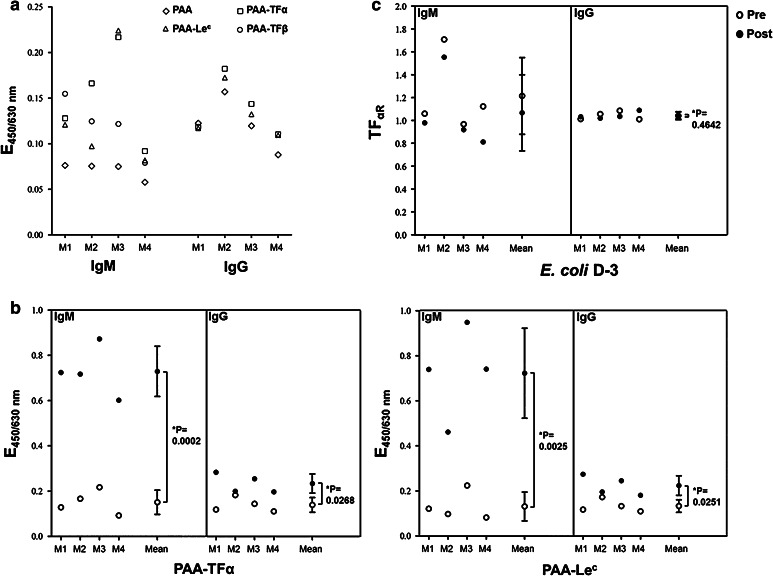
The ELISA data revealed that pre-immune mice sera already contained natural IgM antibodies that reacted with different polyacrylamide PAA-glycoconjugates including TFα-PAA (Fig. 1a). Each serum displayed a distinct binding pattern to different PAA-glycoconjugates (Fig. 1a), and higher reactivity to PAA-glycoconjugates than to unconjugated PAA. Pre-immune sera showed weak IgG binding to TFα-PAA, Lec-PAA (Fig. 1a), and to other PAA-glycoconjugates (data not shown). However, IgG binding to PAA-glycoconjugates did not differ from its binding to unconjugated (control) PAA. Independent of the bacterial strain used for the immunization, a dramatic increase in carbohydrate-directed polyreactive serum IgMs was observed following immunization. To a lesser extent, this effect was also seen for IgG antibodies. This effect is illustrated for TFα-PAA and Lec-PAA in mice immunized with E. coli D-3 (Fig. 1b), a strain that does not express TFα or Lec on its surface (data not shown).
Fig. 1.
Methodical details: influence of “natural” polyreactive antibodies in mouse sera and its elimination. The binding of serum antibodies to carbohydrate antigens was analyzed by ELISA with PAA-glycoconjugates in four mice (M1 to M4) before and after immunization with the TFα non-expressing strain E. coli D-3. a Natural IgM and IgG antibody binding of pre-immune sera to several PAA-glycoconjugates. b IgM and IgG antibody binding of pre- and post–immunization (day 21) sera to TFα and Lec (both absent at the bacterial strain E. coli D-3). c relative level of IgM and IgG TFα specific antibodies (TFαR) of pre- and post-immunization sera. Sera were diluted 1/50–1/100. Single points are either means of duplicates or means of all mice. * p < 0.05 was considered statistically significant. Pre: preimmune serum; post: post-immune serum
Previous studies used the unconjugated PAA molecule as a reference and expressed the level of TFα-specific signals as ratio “APAA-TFα/APAA” [29]. Since immunization with whole bacteria increased multiple carbohydrate-directed polyreactive antibody titers rather than PAA-directed antibody titers, we examined whether a PAA-glycoconjugate would be a better reference to offset false-positive binding by carbohydrate-directed polyreactive antibodies. Lec-PAA (Galß1-3GlcNAc-PAA) was selected as a reference because of a certain sequence homology to TFα and its absence from the surface of the bacterial strains used in our study (data not shown). The TFα-specific serum antibody immune response was expressed as the ratio of the absorbance (A) obtained for antibody binding to TFα-PAA in ELISA over that obtained for Lec-PAA 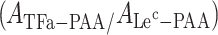 abbreviated as TFαR (relative TFα level). An increase of at least 15 % in the TFαR value following immunization was chosen as cut off for a TFα-specific immune response.
abbreviated as TFαR (relative TFα level). An increase of at least 15 % in the TFαR value following immunization was chosen as cut off for a TFα-specific immune response.
As shown in Fig. 1c, mice immunized with E. coli D-3 (with no detectable TFα) revealed no increase in IgM TFαR values. A single mouse presented a slightly increased IgG TFαR value following immunization (far below 15 %). Considering the mean values for all mice, no significant increase of the IgG TFαR could be detected. These results confirmed that the ratio analysis approach with Lec-PAA as a reference antigen indeed circumvented the effect of carbohydrate-directed polyreactive antibodies.
Only B. ovatus D-6, which expresses the true TFα antigen, elicits a TFα-specific immune response
Mice were intraperitoneally immunized with inactivated bacterial cells differing with respect to TF expression. Each mouse developed a general (strain-specific) anti-bacterial humoral immune response of comparable strength (data not shown). A different picture was seen for specific anti-TFα antibodies. As depicted in Fig. 2, no significant increase in the mean values for IgM and IgG TFαR could be detected following immunization with the E. coli strains DSM 8697 (carrying cryptic TFα antigen) or G-2 (carrying a TF-related antigen). In contrast, immunization with the strain B. ovatus D-6 (carrying the genuine and immunoaccessible TFα antigen on its surface) resulted in a significant increase in mean IgM and IgG TFαR values. All individual mice displayed an increase in the TFαR values of more than 15 % (Fig. 2). Furthermore, for each single mouse immunized with the strain B. ovatus D-6, the addition of AGP to the sera resulted in a strong decrease in both the IgM and the IgG TFαR values, confirming the TFα specificity of the induced antibodies (Fig. 2). Glycophorin (GP, carrying only cryptic TFα) did not inhibit the increase in TFαR values (data not shown).
Fig. 2.
Relative IgM and IgG anti-TF levels following immunization with bacterial strains expressing different TF-related antigens at their surface. Twelve mice allocated to three experimental groups were intraperitoneally immunized either with B. ovatus D-6 (expressing true TFα antigen, mice M1 to M4), E. coli G-2 (expressing a TF-related antigen, mice M5 to M8), or E. coli DSM 8697 (expressing cryptic TF antigen only, mice M9 to M12). Pre-immune (day -1) and post-immune sera (day 21) were analyzed in ELISA for IgM and IgG antibody binding to PAA-TFα and PAA-Lec in the presence or absence of AGP (or GP as control). Numbers shown at the bottom of each graph represent percent increase of the TFαR value following immunization. An increase of at least 15 % of the TFαR following immunization was set as limit for a serum to be positive. Sera were diluted 1/50–1/100. * p < 0.05 was considered statistically significant. Pre: preimmune serum; post: post-immune serum
We further looked at the relative TFß level (TFßR, which refers to the ratio of the ELISA absorbance (A) obtained for binding to TFß-PAA over that for Lec-PAA). From the four mice immunized with the strain B. ovatus D-6, three presented no change in the TFßR value. One mouse displayed an increase in the TFßR values of more than 15 % following immunization. However, this increase could be completely inhibited by adding AGP, demonstrating that the induced antibodies are, in fact, TFα-specific and do only cross-react with TFß. Again, co-incubation of the sera with GP did not inhibit the TFßR increase in this mouse (data not shown).
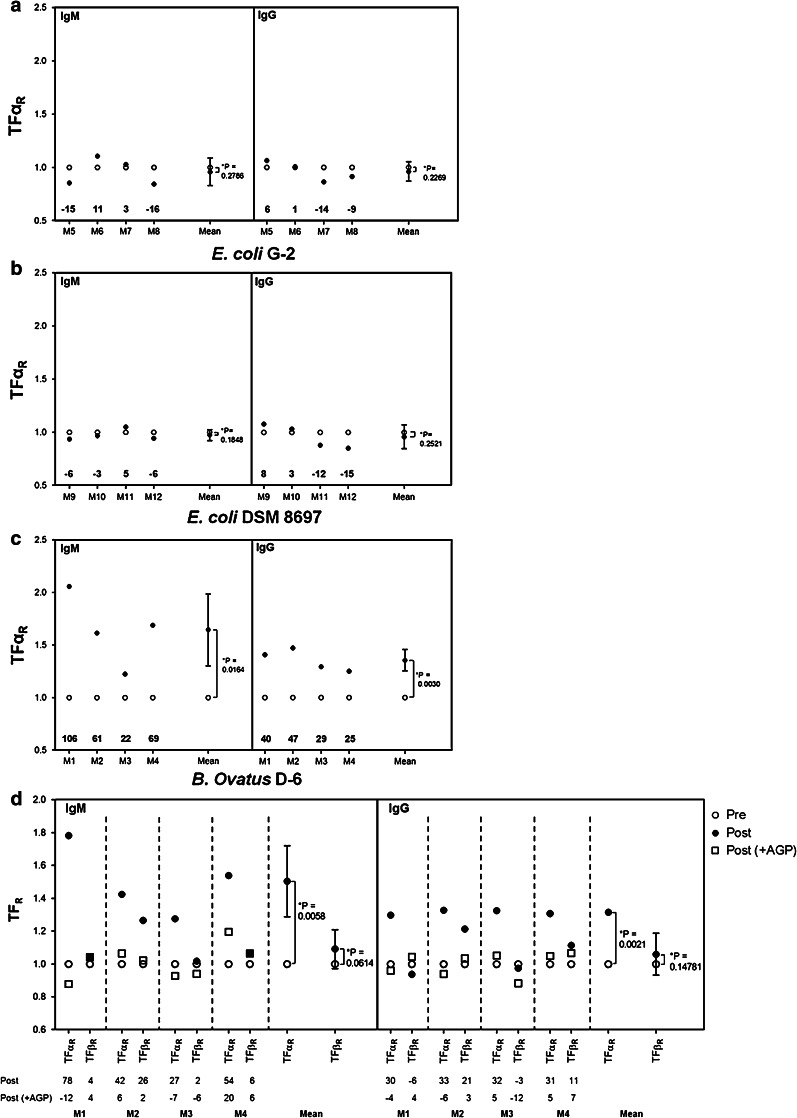
The TFα-specific immunogenicity of B. ovatus D-6 correlates with the epitope density
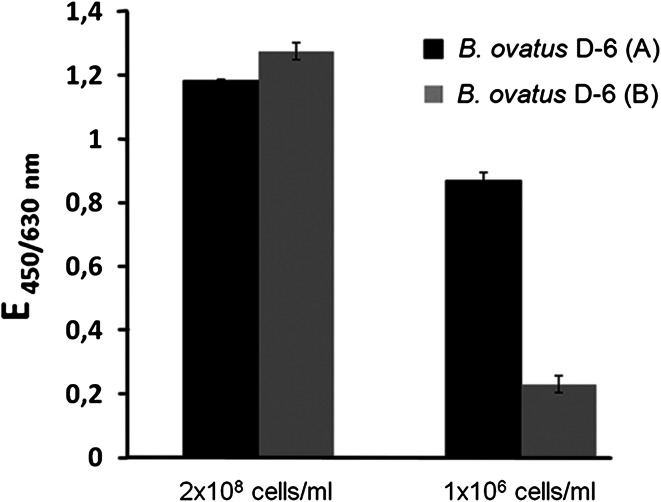
Batches of B. ovatus D-6 prepared on different dates varied in their ability to induce a TFα-specific immune response in vivo (data not shown). For example, the immune response to TFα was much higher in mice immunized with batch A than in mice immunized with batch B (data not shown). Therefore, the TFα-density of both batches of B. ovatus D-6 cells was compared in ELISA with the monoclonal antibody NM-TF1. When a low coating concentration (1 × 106 cells/ml) was used, it became evident that batch B had a TFα density that was only half that of batch A (Fig. 3). This result demonstrated a strong dependency of the immunogenicity on the epitope density and of a particular batch. It also revealed some instability in the TFα expression by B. ovatus D-6. As a consequence, in the following experiments, it was ensured that all B. ovatus D-6 preparations used had a TFα density comparable to batch A.
Fig. 3.
Influence of TFα density on two batches of B. ovatus D6. 50 μl of a suspension of 2 × 108 and 1 × 106 bacteria/ml of two batches of B. ovatus D-6 (A and B) were coated on ELISA plates. The binding of the TFα-specific antibody NM-TF1 was measured in ELISA. Results are presented as means of duplicates ± SE)
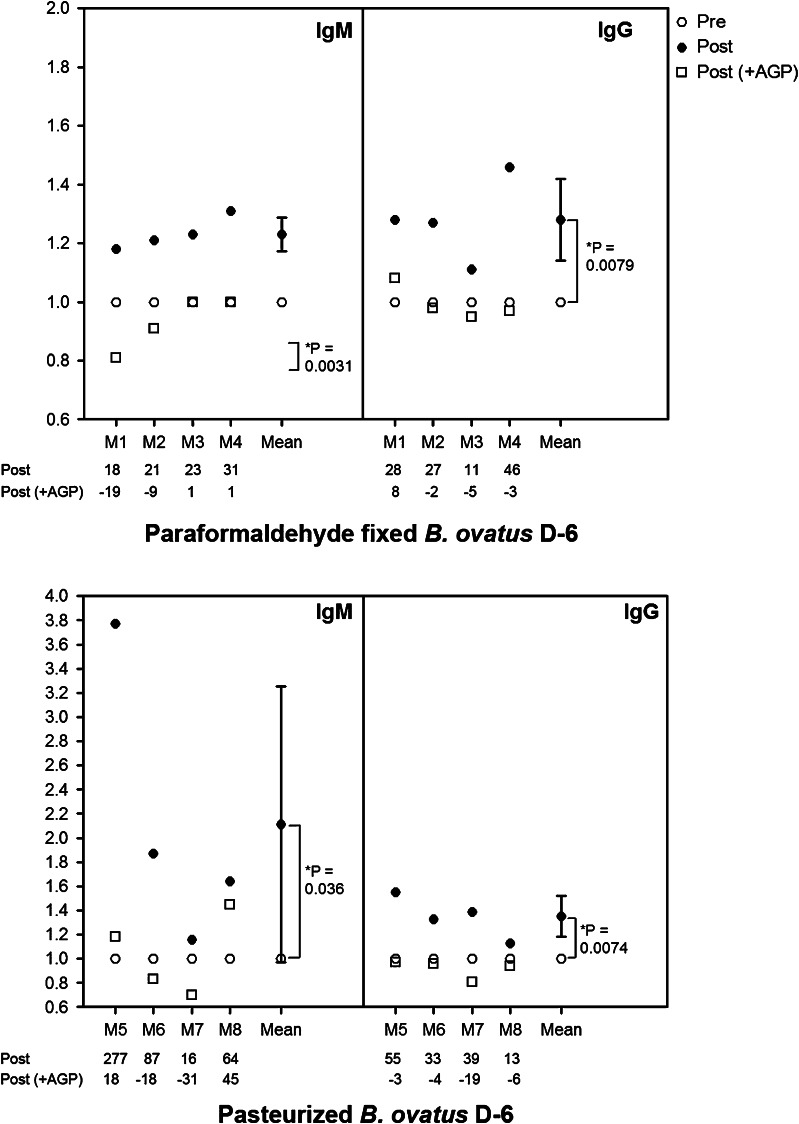
Heat inactivation does not alter the TFα-specific immunogenicity of B. ovatus D-6
Having established the TFα-specific immunogenic potential of B. ovatus D-6 after systemic application, we were interested in determining its immunogenic potential following oral application. To exclude complications from a potentially varying TFα expression during the intestinal passage, dead cells were used. We decided to use heat-inactivated (pasteurized) bacteria, as these are more suitable for oral application.
We first established that pasteurization had no negative impact on the expression of TFα (data not shown). We then examined the immunogenic potential of heat-inactivated and, in addition, of paraformaldehyde-treated bacteria after i. p. application. Both preparations induced an increase of at least 15 % in the IgM TFαR in all mice, and in the IgG TFαR in three and four mice, respectively (Fig. 4). Furthermore, this increase observed in TFαR could be strongly inhibited in all cases through co-incubation with AGP, but not with GP (GP data not shown), suggesting that pasteurization (or fixation) did not affect TFα antigenicity.
Fig. 4.
Relative levels (TFαR) of IgM and IgG TFα-specific antibodies following immunization with paraformaldehyde-fixed or pasteurized B. ovatus D6, respectively. Eight mice were allocated to two groups and intraperitoneally immunized either with paraformaldehyde-fixed (mice M1– M4) or with pasteurized B. ovatus D-6 (mice M5–M8). IgM and IgG antibody binding to PAA-TFα and PAA-Lec was determined in ELISA in the presence or absence of AGP or GP (GP data not shown). Relative levels of IgM and IgG TFα-specific antibodies (TFαR) for pre- and post-immunization sera were calculated. The numbers at the bottom of each graph show the percent increase or decrease of TFαR following immunization. Sera were diluted 1/50–1/100. Goat-anti-mouse IgM-POD was used as blank. * p < 0.05 was considered statistically significant
TFα-specific antibodies induced by B. ovatus D-6 bind to TFα on human cancer cells
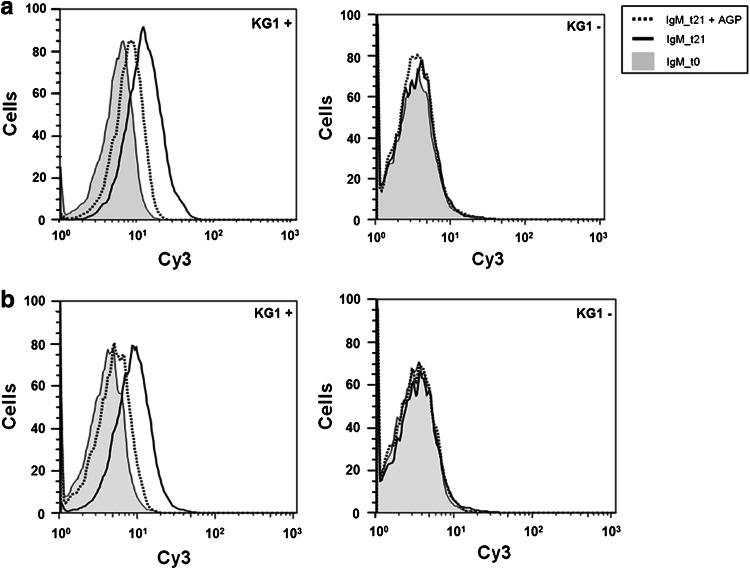
The binding properties of sera from immunized mice to the TFα-positive and TFα-negative sublines of the human myelogenous leukemia cell line KG-1 were analyzed using flow cytometry. Figure 5 shows the results obtained from one mouse of each group. Following immunization with fixed and heat-inactivated B. ovatus D-6 bacteria, an increase in IgM binding to the TFα-positive, but not to the TFα-negative KG-1 subline, could be identified. This increase could be inhibited by the addition of AGP, confirming the TFα specificity of the induced antibodies. No IgG binding could be detected in this case (data not shown).
Fig. 5.
IgM TFα-specific binding of pre- (t0) and post- (t21) immunization sera to TFα-positive and -negative KG-1 cells determined by flow cytometry in the presence or absence of AGP or GP. a mouse immunized with paraformaldehyde-fixed B. ovatus D-6 cells. b mouse immunized with pasteurized B. ovatus D-6 cells. Sera were diluted 1/50 to 1/100
Effects of oral immunization with B. ovatus D-6
Mice were fed 1010, 109, 108, 107, or 106 pasteurized B. ovatus D-6 cells (groups A-E, respectively) on 5 days a week for 8 weeks. All doses induced the development of IgM, IgG and IgA antibodies against the B. ovatus D-6 bacterium (data not shown). The intensity of the strain-specific response was dose-dependent (correlation coefficient 0.958).
The TFαR-specific response was calculated for each mouse and for the isotypes IgM and IgG. This was done for pre-immunization sera as well as for sera at an early stage (day 13 or 21), late stage (day 49 or 56), and after recovery (day 77). All mice (n = 6) in group A showed an increase in IgM TFαR values, indicating that TFα-specific IgM antibodies were formed (Table 1). The proportion of responding mice (showing at least a 15 % increase in TFαR values following immunization) was dose-dependent (Table 1). However, the dose of the immunogen did not seem to influence the strength of the TFα-specific immune response. Mice which showed a strong increase (over 100 % in TFαR values) were identified in almost all dose groups (Table 1). Cessation of the daily intake led to a decrease in the level of TFα-specific IgM antibodies, and in some cases to their complete disappearance (Table 1), demonstrating that the TFα-specific IgM immune response is reversible. As was the case after systemic immunization, the increase in IgM TFαR values was strongly inhibited through addition of AGP, confirming the TFα specificity of the binding (data not shown). The IgG response as detected in ELISA after oral immunization was generally weak. We only considered sera with an absorbance reading ≥0.1 to TFα-PAA as relevant for the calculation of TFαR. TFα-specific IgG antibodies were exclusively detected in sera from mice which also developed IgM anti-TFα antibodies (Table 1), but not vice versa.
Table 1.
Relative increase in TFα levels after oral intake of B. ovatus D-6
| Sera samples | Increase in TFαR from pre-immunization TFαR | |||||
|---|---|---|---|---|---|---|
| Early | Late | Recovery | ||||
| IgM | IgG | IgM | IgG | IgM | IgG | |
| Group A (1010 bacteria/day) | ||||||
| A1 | + | – | ++ | – | + | – |
| A2 | – | – | +++ | +++ | + | + |
| A3 | +++ | +++ | +++ | +++ | +++ | +++ |
| A4 | + | – | + | + | – | – |
| A5 | +++ | – | +++ | + | +++ | +++ |
| A6 | +++ | – | +++ | ++ | – | +++ |
| Group B (109 bacteria/day) | ||||||
| B1 | + | – | + | + | ++ | + |
| B2 | +++ | – | ++ | – | ++ | – |
| B3 | – | – | + | + | + | + |
| B4 | – | – | – | – | – | – |
| B5 | +++ | – | +++ | – | +++ | – |
| B6 | ++ | – | – | – | – | – |
| Group C (108 bacteria/day) | ||||||
| C1 | – | – | – | – | – | – |
| C2 | – | – | – | – | – | – |
| C3 | – | – | – | – | – | – |
| C4 | +++ | +++ | +++ | +++ | +++ | ++ |
| C5 | ++ | – | + | – | – | – |
| Group D (107 bacteria/day) | ||||||
| D1 | – | – | – | – | – | – |
| D2 | – | – | – | – | – | – |
| D3 | – | – | ++ | – | – | – |
| D4 | + | – | + | +++ | – | ++ |
| D5 | – | – | – | – | – | – |
| Group E (106 bacteria/day) | ||||||
| E1 | – | – | – | – | – | – |
| E2 | + | – | +++ | + | – | ++ |
| E3 | – | – | – | – | – | – |
| E4 | – | – | + | – | + | – |
| E5 | – | – | – | – | – | – |
Serum samples were taken before (day -1), 14 or 21 days after first intake (early point), 49 or 56 days after first intake (late point), and beyond 77 days (recovery; 3 weeks after the last intake). Serum samples were analyzed by ELISA for IgM and IgG antibody binding to PAA-TFα and PAA-Lec. The relative TFα level (TFαR) was calculated, and the percent change relative to the pre-immunization TFαR value was determined for each time point. An increase between 15 and 50 % was designated as (+), between 51 and 100 % as (++), and over 100 % as (+++). Percent values lower than 15 % were considered negative (−)
Of the 16 mice that developed TFα-specific IgM antibodies, nine also developed detectable TFα-specific IgG antibodies. As shown in Table 1, the development of TFα-specific IgG appeared delayed compared with the IgM response. Also, the strength of the bacteria-specific IgM response did not predict whether a TFα-specific IgG would occur. In addition, no clear correlation was found between the strength of the TFα-specific IgM immune response and the development of TFα-specific IgG antibodies (correlation coefficient 0.218). TFα-specific IgA antibodies were not detected after oral immunization.
Discussion
In the present study, we investigated the ability of the B. ovatus strain D-6 to induce TFα-specific antibodies in mice. This strain was recently described to express a true and immune-accessible TFα antigen on its surface [28]. In order to detect the de novo generation of TFα-specific antibodies in sera of immunized mice, the presence of natural antibodies had to be considered. Animal and human sera contain polyreactive IgM, IgA and IgG antibodies [30]. Most polyreactive IgM antibodies are reportedly directed toward carbohydrate antigens [31]. More importantly, the titer of natural polyreactive antibodies may increase after immunization [31–33], which hampers the identification of antigen-specific antibodies, and may lead to a false-positive interpretation of immunization results. In accordance with this, we found that pre-immune sera of mice contained a level of anti–carbohydrate IgM antibodies. Following intraperitoneal immunization with bacteria, we observed that binding even to oligosaccharide structures not present on the bacteria (including TFα) increased drastically. Surprisingly, an increase in such antibodies was also observed in the IgG fraction.
These results clearly illustrate that, when taken as sole indicator, an increase in antibodies binding to TFα after immunization with whole bacteria cannot be used to draw conclusions as to whether a TFα-antigen-specific immune response did occur. The repertoire of specificities of polyreactive antibodies is supposed to be largely unaffected by the specificity of the external stimulation [30]. Therefore, under the assumption that an immunization with whole bacteria induced a similar activation of all natural polyreactive antibody specificities, we hypothesized that the signal increase observed on a carbohydrate not present on the surface of the bacterial strain used for immunization could be used as a reference for the “polyreactivity background” (i.e., the background of carbohydrate-directed polyreactive antibodies).
In this study, the Lec antigen (lacto-N-tetraose, Galß1-3GlcNAcß1-, conjugated to PAA) was chosen as reference antigen. This disaccharide has a certain similarity to TFα and could not be detected on any of the bacterial strains used in this study (data not shown). Therefore, the level of specific anti-TFα IgM and IgG antibodies was expressed as the ratio of absorbances of TFα over Lec, also referred to as the “relative TFα level” or “TFαR”. Analyses of pre- and post-immunization sera from mice immunized with the TFα non-expressing strain E. coli D-3 confirmed the accuracy of our approach. Employing this approach, we found that bacterial strains expressing TF-related or cryptic TFα antigens, or strains not expressing any TF antigen, did not induce the formation of TFα-specific antibodies. In contrast, the strain B. ovatus D-6, which expressed the true and exposed TFα antigen, induced the development of a TFα-specific IgM and IgG humoral immune response.
The generation of TFα-specific IgG antibodies is thought to be a key element of an effective carbohydrate cancer vaccine [34], and its absence is a major drawback of many synthetic TFα vaccination studies. IgM antibodies are known to have a higher affinity than IgG antibodies for small disaccharide antigens like TFα. Therefore, despite the fact that the TFα-specific IgG signals obtained were much weaker than those of their IgM counterparts, our results suggest that TFα-expressing bacterial strains such as B. ovatus D-6 may be able to overcome some drawbacks encountered by most synthetic vaccination approaches. We do at present not know, however, whether this property is restricted to this particular strain. The localization of the TFα antigen at the capsular polysaccharide [28] may confer an advantage. As capsular polysaccharides consist of highly repetitive subunits, a high epitope density will be presented to the immune system. Antigen clustering is known to be an essential factor for the immunogenicity of tumor-associated carbohydrate antigens [13]. This is supported by our own observation that a reduction in the TF epitope density was associated with a loss of immunogenicity.
Additionally, capsular polysaccharides are type-2 antigens (TI-2) that are thought to be able to stimulate B cells without the participation of T cells [35–37]. Furthermore, several studies suggest that, in contrast to purified and/or hapten-coupled polysaccharides, the exposure of humans to capsular polysaccharides in the context of whole microorganisms induces the formation of capsular polysaccharide-specific antibodies that undergo both class switch and hypermutation [38–40]. Therefore, the exposure of glycotopes presented in the context of a bacterial surface may generate specific antibody production and memory development [38–40]. This was further confirmed in a recent paper indicating that intrinsic adjuvant properties of bacterial strains may enhance the TI-2 antibody response and promote the production of IgG by follicular B cells [41]. Taken together, it appears that the combination of (1) high antigen density in the capsular polysaccharide together with (2) the intrinsic adjuvant potency of B. ovatus D-6 offers a unique opportunity to develop a TFα-specific immune response that may induce the formation of IgG antibodies and memory cells.
A further critical need for any effective TF-specific vaccine is the generation of antibodies that not only recognize the TF-carrying immunogen but also the corresponding TF on tumor cells [12].
Therefore, a further positive aspect of our study is the fact that the TFα-specific IgM antibodies induced by immunization with B. ovatus D-6 did not only bind to the synthetic disaccharide, but also to naturally occurring TFα as, for instance, to cells of the TFα-expressing acute myelogenous leukemia cell line KG-1. Inhibition of binding by the TFα-carrying glycoprotein AGP (but not by GP) clearly demonstrates the specificity of the binding.
This may be based on an advantage that B. ovatus D-6 may have over synthetic vaccines. Since B. ovatus D-6 is naturally expressing the TF antigen, it may present it in a configuration more closely to that found at the surface of cancer cells.
Whereas IgM antibody binding to KG-1 cells occurred without doubt, the level of IgG binding was obviously below the detection limit. The presence of a high concentration of TFα-specific IgM antibodies at the analyzed time points (21 or 28 days after the first immunization), the low affinity of the IgG antibodies for the TFα-antigen, and/or the lower sensitivity of flow cytometry compared to ELISA may explain this. Further investigations are required to clarify this point. Our results demonstrate that immunization with the TFα-expressing strain B. ovatus D-6 may offer an attractive alternative to synthetic vaccines for the induction of a TFα-specific immune response.
Having established the systemic TFα-specific immunogenic potential of B. ovatus D-6, we started to investigate its immunogenic potential after oral application. We were able to demonstrate that mice, which were fed daily with heat-inactivated B. ovatus D-6, developed general, strain-specific serum antibodies of isotypes IgM, IgG, and IgA in a dose-dependent manner. In addition, we identified TFα-specific serum antibodies of the isotype IgM and in some cases a weak signal of the isotype IgG.
Interestingly, the intensity of the TFα-specific IgM response was not dose-dependent, and lowering the daily dose resulted only in a reduction of the percentage of responders. In support of the dose independency, the detection of TFα-specific IgGs was not restricted to mice receiving the highest daily dose. Since the development of an immune response to intestinal bacterial antigens requires sampling by the gut-associated lymphoid tissue (GALT), we assume that the TFα-specific immune response may depend on the sampling rate, which is related to the daily dose. Once the strain B. ovatus D-6 is sampled and the development of a specific immune response is initiated, the strength of this response appears to depend exclusively on the intrinsic immunogenicity of the TFα antigen in the context of the bacterial strain. It is noteworthy that the development of TFα-specific IgG antibodies appears to be delayed, which is suggestive of a classical two-phase immune response.
Our results demonstrate that oral immunization with the TFα-expressing strain B. ovatus D-6 may offer an attractive alternative to synthetic vaccines for the induction of a TFα-specific immune response. Indeed, oral application offers several advantages, starting with a lower risk of side effects. This is illustrated by a relatively small increase in carbohydrate-directed natural polyreactive antibodies observed after oral administration, which strongly contrasts with the strong increase observed following systemic application (data not shown). Furthermore, oral administration may reproduce the natural TFα-specific stimulation that is responsible for the natural TFα-antibody level identified in humans. This natural process may not be subject to tolerance induction and may therefore be an attractive alternative to induce or reinforce the development of TFα-antibodies.
In summary, by studying the immunogenicity of the TFα-expressing bacterial strain B. ovatus D-6 in mice, we have (1) developed a new analysis approach for the determination of carbohydrate-specific antibodies in sera which allows to circumvent the troublesome effect of polyreactive antibodies, (2) confirmed the causal relationship between TFα-expressing gut bacteria and the induction of natural TFα antibodies, (3) proven the TFα immunogenicity to be restricted to bacterial strains expressing a true and immune-accessible TFα antigen, and (4) shown that the strain B. ovatus D-6 possesses a remarkable TFα-specific immunogenic potential.
Taken together, our results suggest that it may be possible to induce the production of protective and/or therapeutic TFα-specific antibodies in humans by the systemic or oral intake of non-pathogenic (heat-inactivated) TFα-expressing cells of B. ovatus D-6, thus providing the basis for the development of an adjuvant-free TFα-specific anti-tumor vaccine.
Acknowledgments
We are grateful to Jenny Merz, Heike Schmidt, Sigrid Wiese, and Anke Gühler for technical assistance. This work was partially funded by the German Federal Ministry of Education and Research (BioProfile Nutrigenomik Project 1202-27).
Conflict of interest
The studies were sponsored by Glycotope GmbH. All authors except Michael Blaut and Gemma Henderson are employees of Glycotope GmbH. Michael Blaut and Gemma Henderson declare that they have no conflict of interest.
References
- 1.Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76:1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- 3.Goletz S, Cao Y, Danielczyk A, Ravn P, Schoeber U, Karsten U. Thomsen-Friedenreich antigen: the “hidden” tumor antigen. Adv Exp Med Biol. 2003;535:147–162. doi: 10.1007/978-1-4615-0065-0_10. [DOI] [PubMed] [Google Scholar]
- 4.Irazoqui FJ, Jansson B, Lopez PH, Nores GA. Correlative fine specificity of several Thomsen-Friedenreich disaccharide-binding proteins with an effect on tumor cell proliferation. J Biochem. 2001;130:33–37. doi: 10.1093/oxfordjournals.jbchem.a002959. [DOI] [PubMed] [Google Scholar]
- 5.Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316–323. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 6.Shigeoka H, Karsten U, Okuno K, Yasutomi M. Inhibition of liver metastases from neuraminidase-treated colon 26 cells by an anti-Thomsen-Friedenreich-specific monoclonal antibody. Tumor Biol. 1999;20:139–146. doi: 10.1159/000030056. [DOI] [PubMed] [Google Scholar]
- 7.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 8.Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj J. 2007;224:411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 11.MacLean GD, Bowen-Yacyshyn MB, Samuel J, Meikle A, Stuart G, Nation J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen-Friedenreich) determinant using a synthetic carbohydrate antigen. J Immunother. 1992;11:292–305. doi: 10.1097/00002371-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Adluri S, Helling F, Ogata S, Zhang S, Itzkowitz SH, Lloyd KO, et al. Immunogenicity of synthetic TF-KLH (keyhole limpet hemocyanin) and sTn-KLH conjugates in colorectal carcinoma patients. Cancer Immunol Immunother. 1995;41:185–192. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irazoqui FJ, Lopez PHH, Vides MA, Nores GA. Novel immunogenicity of Thomsen-Friedenreich disaccharide obtained by a molecular rotation on its carrier linkage. Glycobiology. 2000;10:781–787. doi: 10.1093/glycob/10.8.781. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Gendler SJ, Franco A. Designer glycopeptides for cytotoxic T cell-based elimination of carcinomas. J Exp Med. 2004;199:707–716. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimburg-Molinaro J, Almogren A, Morey S, Glinskii OV, Roy R, Wilding GE, et al. Development, characterization, and immunotherapeutic use of peptide mimics of the Thomsen-Friedenreich carbohydrate antigen. Neoplasia. 2009;11:780–792. doi: 10.1593/neo.09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinas RP, Sundgren A, Sahoo P, Morey SM, Rittenhouse-Olson K, Wilding GE, et al. Design and synthesis of multifunctional gold nanoparticles bearing tumor-associated glycopeptide antigens as potential cancer vaccines. Bioconjug Chem. 2012;23:1513–1523. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann-Röder A, Kaiser A, Wagner S, Gaidzik N, Kowalczyk D, Westerlind U, et al. Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew Chem Int Ed. 2010;49:8498–8503. doi: 10.1002/anie.201003810. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson BL, Day S, Malins LR, Apostolopoulos V, Payne RJ. Self-adjuvanting multicomponent cancer vaccine candidates combining per-glycosylated MUC1 glycopeptides and the Toll-like receptor 2 agonist Pam3CysSer. Angew Chem Int Ed. 2011;50:1635–1639. doi: 10.1002/anie.201006115. [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Huang Z-H, Shi L, Sun Z-Y, Zhao Y-F, Kunz H, Li Y-M. Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew Chem Int Ed. 2012;51:1719–1723. doi: 10.1002/anie.201106396. [DOI] [PubMed] [Google Scholar]
- 21.Springer GF, Desai PR, Murthy MS, Scanlon EF. Human carcinoma-associated precursor antigens of the NM blood group system. J Surg Oncol. 1979;11:95–106. doi: 10.1002/jso.2930110204. [DOI] [PubMed] [Google Scholar]
- 22.Butschak G, Karsten U. Isolation and characterization of Thomsen-Friedenreich-specific antibodies from human serum. Tumor Biol. 2002;23:113–122. doi: 10.1159/000064026. [DOI] [PubMed] [Google Scholar]
- 23.Boccardi V, Attina D, Girelli G. Influence of orally administered antibiotics on anti-T agglutinin of normal subjects and of cirrhotic patients. Vox Sang. 1974;27:268–272. doi: 10.1111/j.1423-0410.1974.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 24.Springer GF, Tegtmeyer H. Origin of anti-Thomsen-Friedenreich (T) and Tn agglutinins in man and in White Leghorn chicks. Br J Haematol. 1981;47:453–460. doi: 10.1111/j.1365-2141.1981.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 25.Klaamas K, Kurtenkov O, Rittenhouse-Olson K, Brjalin V, Miljukhina L, Shljapnikova L, et al. Expression of tumor-associated Thomsen-Friedenreich antigen (T Ag) in Helicobacter pylori and modulation of T Ag specific immune response in infected individuals. Immunol Invest. 2002;31:191–204. doi: 10.1081/IMM-120016240. [DOI] [PubMed] [Google Scholar]
- 26.Thors C, Jansson B, Helin H, Linder E. Thomsen-Friedenreich oncofetal antigen in Schistosoma mansoni : localization and immunogenicity in experimental mouse infection. Parasitology. 2006;132:73–81. doi: 10.1017/S003118200500867X. [DOI] [PubMed] [Google Scholar]
- 27.Gilboa-Garber N, Sudakevitz D. Usage of Aplysia lectin interactions with T antigen and poly-N-acetyllactosamine for screening of E. coli strains which bear glycoforms cross-reacting with cancer-associated antigens. FEMS Immunol Med Microbiol. 2001;30:235–240. doi: 10.1111/j.1574-695X.2001.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 28.Henderson G, Ulsemer P, Schöber U, Löffler A, Alpert CA, Zimmermann-Kordmann M, et al. Occurrence of the human tumor-specific antigen structure Galß1-3GalNAc{alpha}- (Thomsen-Friedenreich) and related structures on gut bacteria: prevalence, immunochemical analysis and structural confirmation. Glycobiology. 2011;21:1277–1289. doi: 10.1093/glycob/cwr058. [DOI] [PubMed] [Google Scholar]
- 29.Smorodin EP, Kurtenkov OA, Sergeyev BL, Kodar KE, Chuzmarov VI, Afanasyev VP. Postoperative change of anti-Thomsen-Friedenreich and Tn IgG level: the follow-up study of gastrointestinal cancer patients. World J Gastroenterol. 2008;14:4352–4358. doi: 10.3748/wjg.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29:219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 32.Jones HE, Taylor PR, McGreal E, Zamze S, Wong SY. The contribution of naturally occurring IgM antibodies, IgM cross-reactivity and complement dependency in murine humoral responses to pneumococcal capsular polysaccharides. Vaccine. 2009;27:5806–5815. doi: 10.1016/j.vaccine.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 33.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bundle DR. A carbohydrate vaccine exceeds the sum of its parts. Nat Chem Biol. 2007;3:605–606. doi: 10.1038/nchembio1007-605. [DOI] [PubMed] [Google Scholar]
- 35.Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- 36.Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165:49–52. doi: 10.1093/infdis/165-Supplement_1-S49. [DOI] [PubMed] [Google Scholar]
- 37.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 38.Lucas AH, Apicella MA, Taylor CE. Carbohydrate moieties as vaccine candidates. Clin Infect Dis. 2005;41:705–712. doi: 10.1086/432582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas AH, Moulton KD, Tang VR, Reason DC. Combinatorial library cloning of human antibodies to Streptococcus pneumoniae capsular polysaccharides: variable region primary structures and evidence for somatic mutation of Fab fragments specific for capsular serotypes 6B, 14, and 23F. Infect Immun. 2001;69:853–864. doi: 10.1128/IAI.69.2.853-864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent variable region gene usage and somatic mutation in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae type 23F. Infect Immun. 2002;70:4083–4091. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koeffler HP, Golde DW. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978;200:1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- 44.Paetz A. Neue DIN 19738: 2000–05 (Entwurf): soil quality - Absorption availability of organic and inorganic pollutants from contaminated soil material. Umweltmedizin in Forschung und Praxis. 2000;5:319–320. [Google Scholar]
- 45.Chapman PB, Morrissey DM, Panageas KS, Hamilton WB, Zhan C, Destro AN, et al. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-keyhole limpet 701 hemocyanin + QS21 vaccine: a dose-response study. Clin Cancer Res. 2000;6:874–879. [PubMed] [Google Scholar]