Abstract
Melanomas in Chinese patients show relatively higher rates of acral and mucosal types than in other populations. However, the efficacy of checkpoint inhibitor therapies against these melanoma subtypes is not well defined. We analyzed 52 patients treated with ipilimumab, pembrolizumab, or a combination of both to evaluate the efficacy and safety of checkpoint inhibitors in Chinese patients with advanced melanoma, particularly those with acral and mucosal types. The objective response rates (ORRs) were 0, 25, and 20% for ipilimumab, pembrolizumab, and pembrolizumab plus ipilimumab, respectively. Pembrolizumab contained therapy was as effective in acral and mucosal melanoma patients (ORR 26.7 and 20%, respectively) as in non-acral cutaneous melanoma patients (ORR 26.7%). Baseline lactate dehydrogenase levels and relative lymphocyte counts were independent prognostic factors for PFS and OS. The incidences of grade 3–4 adverse events were 14% in the two monotherapy groups and 30% in the combined therapy group. The most frequent adverse events were elevation of aminotransferase, skin toxicity, thyroid dysfunction, pyrexia, and fatigue. Treatment-related rash or vitiligo was associated with a better prognosis. In summary, pembrolizumab-based therapy resulted in meaningful efficacy and good tolerability in Chinese patients with melanoma, including those with acral and mucosal types.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1989-8) contains supplementary material, which is available to authorized users.
Keywords: Ipilimumab, Pembrolizumab, Chinese patients, Acral melanoma, Mucosal melanoma, Biomarkers
Introduction
Malignant melanoma is one of the most aggressive types of cancer, and its incidence is increasing worldwide. The median survival of advanced melanoma patients ranges from 6 to 9 months with chemotherapy, and the 5-year survival rate is under 10% [1]. Checkpoint inhibitors against cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death 1 (PD-1) proteins have resulted in a paradigm shift in the management of melanoma due to their substantial antitumor activity and durable survival benefit [2–4]. Ipilimumab, a monoclonal antibody directed against CTLA-4, was first approved by the United States Food and Drug Administration (FDA) for the treatment of patients with advanced melanoma in 2011. Pembrolizumab and nivolumab are both anti-PD-1 monoclonal antibodies, and were approved by the FDA in 2014. Combination therapies of anti-PD-1 plus anti-CTLA-4 antibodies showed an even higher efficacy and longer survival time than either single agent alone [5–7]. The FDA granted accelerated approval to nivolumab in combination with ipilimumab for the treatment of patients with unresectable or metastatic melanoma in 2015.
Melanoma patients in China show a higher proportion of acral and mucosal types. The incidence of melanoma in China was about 0.48 per 100,000. Among them, more than 60% of the patients belong to acral or mucosal types [8]. Acral and mucosal melanomas have distinct genetic and clinical characteristics, lower somatic mutational burdens, and poorer prognoses [9–11]. However, few Chinese patients were involved in the previous clinical trials, and the outcome of patients with acral and mucosal melanoma treated with checkpoint inhibitors has not been well defined [12]. To that end, we conducted a retrospective analysis of 52 patients with metastatic melanoma who received ipilimumab or pembrolizumab therapy at our center to investigate the efficacy and safety of CTLA-4 and PD-1 blockade in metastatic melanoma patients in China, particularly those with acral and mucosal type tumors. In addition, we analyzed the association of baseline factors and treatment-related toxicities with response rates and survival in patients receiving pembrolizumab contained therapy.
Materials and methods
Patient population
Patients with histologically confirmed metastatic melanoma derived from skin and non-skin sections were included in this study. Patients with brain metastasis required radiotherapy for their brain lesions before commencing checkpoint inhibitor therapy. Furthermore, patients in this study had adequate reserved organ function with no history of autoimmune disease. Those who received at least one infusion of checkpoint inhibitors were included.
Study design and treatment
For ipilimumab monotherapy, patients received four cycles of 3 mg/kg ipilimumab every 3 weeks, unless severe adverse events or rapid progression of disease occurred. Patients in the pembrolizumab-based therapy group received 2 mg/kg pembrolizumab every 3 weeks for four cycles or 3 mg/kg of ipilimumab combined with 1 mg/kg of pembrolizumab every 3 weeks for four cycles as induction therapy. Patients without disease progression additionally received pembrolizumab 2 mg/kg every 6 weeks as maintenance therapy.
Assessments
Radiological evaluation was performed at baseline and week 12 (i.e., 3 weeks after the fourth cycle of induction therapy), then every 3 months thereafter. Response was assessed according to the RECIST version 1.1; assessments included complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease. The objective response rate (ORR; CR + PR) and disease control rate (DCR; CR + PR + SD) were also calculated. Treatment-related toxicities were continuously monitored and graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) in all treated patients. Progression-free survival (PFS) was defined as the interval between the start of the treatment and disease progression or death. Overall survival (OS) was defined as the interval between the start of the treatment and death due to any reason. Patients who did not progress or were still alive on the last follow-up date were censored.
PD-L1 expression analysis
Formalin-fixed, paraffin-embedded tissues were sliced to 4-μm thickness and baked for 1 h at 65 °C. Then, the sections were dewaxed, rehydrated, and blocked with hydrogen peroxide. The sections were immersed in EDTA antigen retrieval buffer (pH 9.0), placed under high pressure for 3 min for antigen retrieval, and then allowed to cool to room temperature. After blocking with sheep serum, the sections were incubated overnight at 4 °C with a rabbit anti-human PD-L1 mAb at a dilution of 1:200 (E1L3N, Cell Signaling Technology). Subsequently, biotinylated secondary antibodies and streptavidin-biotinylated horseradish peroxidase complexes were used. Diaminobenzidine tetrahydrochloride (DAB) was used to develop positive signals, and sections were counter-stained with hematoxylin. PD-L1 expression was evaluated in tumor cells and tumor-infiltrating immune cells. Samples were categorized to be PD-L1 positive or negative with a cut-off value of 5% as most studies defined [13].
Statistical analysis
PFS and OS were estimated by the Kaplan–Meier method. Baseline characteristics, relative lymphocyte count in peripheral blood (RLC, defined as the percentage of absolute value of lymphocyte count divided by absolute value of white blood cell count), and relative eosinophil count in peripheral blood (REC, defined as the percentage of absolute value of eosinophil count divided by absolute value of white blood cell count) before treatment were also analyzed. The Wilcoxon rank sum test was used to compare the median values of RLC and REC between groups. To transform the RLC and REC into categorical variables, their optimal cut-off values were assessed by receiver-operating characteristic analyses. The log-rank test was used to compare the correlations of the baseline characteristics with PFS and OS. Cox proportional hazard regression analyses were used to determine the relative impact of confirmed prognostic factors. Associations of baseline factors with tumor response rates were analyzed by the Chi-square test or Fisher exact test. A two-tailed p value <0.05 was considered to be statistically significant. All the statistical analyses were performed using the IBM SPSS 19.0 software.
Results
Patients and treatment
Fifty-two advanced melanoma patients treated at our center between August 2012 and March 2016 were included in this study; 14 received ipilimumab monotherapy, 28 received pembrolizumab monotherapy, and the remaining 10 received pembrolizumab combined with ipilimumab therapy. The median number of doses was 4 (range 2–4) in the ipilimumab group and 4 (range 1–12) in the pembrolizumab group. In the combination pembrolizumab/ipilimumab group, the median number of doses was 6 (range 1–12) of pembrolizumab and 4 (range 1–4) of ipilimumab. Thirteen patients (25%) did not complete the four courses of therapy (five in the ipilimumab group, six in the pembrolizumab group, and two in the combination group). The most common reason for drug discontinuation was rapid disease progression.
Patient characteristics at baseline
The median patient age was 53 years; 59.6% were men. In terms of primary lesion, 22 (42.3%) were acral melanomas that arose from palms, soles, and subungual sites; 22 (42.3%) were chronic sun-derived (CSD) or non-CSD melanomas that arose in the skin other than in acral sites, 5 (9.6%) were mucosal melanomas, and 3 (5.8%) were uveal melanomas. There were seven patients with brain metastases (13.5%); these patients received radiotherapy for brain lesions before the infusion of checkpoint inhibitors. The baseline characteristics of patients in the two pembrolizumab-containing groups were homogeneous (all p > 0.05). Baseline characteristics of patients in the different treatment groups are summarized in Table 1.
Table 1.
Baseline characteristics of the patients
| Characteristics | Patients no. (%) | |||
|---|---|---|---|---|
| Ipi (n = 14) | Pem (n = 28) | Ipi + Pem (n = 10) | Total (n = 52) | |
| Age, mean (range) | 52 (23–77) | 52 (20–78) | 47 (31–77) | 53 (20–78) |
| Gender | ||||
| Male | 9 (64.3) | 16 (57.9) | 6 (60.0) | 31 (59.6) |
| Female | 5 (35.7) | 12 (42.1) | 4 (40.0) | 21 (40.4) |
| ECOG status | ||||
| 0–1 | 13 (92.9) | 24 (85.7) | 8 (80.0) | 15 (86.5) |
| ≥2 | 1 (7.1) | 4 (14.3) | 2 (20.0) | 7 (13.5) |
| Primary site | ||||
| Acral | 7 (50) | 13 (46.4) | 2 (20.0) | 22 (42.3) |
| CSD/non-CSD | 7 (50) | 11 (39.3) | 4 (40.0) | 22 (42.3) |
| Mucosal | 0 (0) | 3 (10.7) | 2 (20.0) | 5 (9.6) |
| Uveal | 0 (0) | 1 (3.6) | 2 (20.0) | 3 (5.8) |
| Metastasis stagea | ||||
| M1a | 0 (0) | 7 (25.0) | 0 (0) | 7 (13.5) |
| M1b | 3 (21.4) | 6 (21.4) | 3 (30.0) | 12 (23.1) |
| M1c | 11 (78.6) | 15 (53.6) | 7 (70.0) | 33 (63.5) |
| LDH level | ||||
| ≤UNL | 6 (42.9) | 19 (67.9) | 7 (70.0) | 32 (61.5) |
| >UNL | 8 (57.1) | 9 (32.1) | 3 (30.0) | 20 (38.5) |
| Brain metastasis | ||||
| Yes | 4 (28.6) | 1 (3.6) | 2 (20.0) | 7 (13.5) |
| No | 10 (71.4) | 27 (96.4) | 8 (80.0) | 45 (86.5) |
| Liver metastasis | ||||
| Yes | 4 (28.6) | 9 (32.1) | 4 (40.0) | 17 (32.7) |
| No | 10 (71.4) | 19 (67.9) | 6 (60.0) | 35 (67.3) |
| BRAF V600E status | ||||
| Mutation | 5 (35.7) | 5 (17.9) | 3 (30.0) | 13 (25.0) |
| Wild-type | 9 (64.3) | 23 (82.1) | 7 (70.0) | 39 (75.0) |
| Prior therapyb | ||||
| Yes | 11 (78.6) | 18 (64.3) | 5 (50.0) | 34 (65.4) |
| No | 3 (21.4) | 10 (35.7) | 5 (50.0) | 18 (34.6) |
ECOG Eastern Cooperative Oncology Group, CSD chronic sun-derived, LDH Lactate dehydrogenase, ULN upper limit of normal
aAccording to the 7th edition of the AJCC staging manual
bIncluding chemotherapy or BRAF inhibitors for patients with BRAF mutation
Efficacy
Response rate
None of the patients responded to ipilimumab monotherapy except for 1 who achieved SD for 2 months. Among patients who received pembrolizumab monotherapy, none achieved CR, while 7 (25.0%) showed PR and 3 (10.7%) achieved SD. The ORRs and DCRs were 25.0 and 35.7%, respectively. In the pembrolizumab/ipilimumab combination group, two patients (20%) achieved CR (one with acral lesions, the other with CSD lesions) after six cycles of treatment. Two other patients (20.0%) achieved SD. The ORRs and DCRs for combined therapy were 20.0 and 40.0%, respectively. Due to the limited sample size, the difference in the response rate between the pembrolizumab monotherapy group and pembrolizumab/ipilimumab combination group in Chinese melanoma patients needs to be further studied. The response rate of ipilimumab monotherapy was significantly lower than those of the pembrolizumab monotherapy and combination regimens (p = 0.04). The efficacy data for the different treatment groups are summarized in Table 2. Of the nine patients who achieved PR or CR, there were eight patients with lung, lymph node or subcutaneous metastases, and the other one patient manifested as simultaneously lung, lymph node, brain, and bone metastases. The effective sites were lymph node, subcutaneous, and lung lesions. The details about these nine patients are listed in Supplementary Table 1.
Table 2.
Summary of efficacy data for different treatment groups
| Variable | P1 | Ipilimumab | Pembrolizumab-based regimen | ||
|---|---|---|---|---|---|
| (n = 52) | (n = 14) | Ipi + Pem (n = 10) | Pem (n = 28) | P2 | |
| CR, n (%) | 0.031 | 0 (0) | 2 (20.0) | 0 (0) | 0.064 |
| PR, n (%) | 0.008 | 0 (0) | 0 (0) | 7 (25.0) | 0.156 |
| SD, n (%) | 0.56 | 1 (7.1) | 2 (20.0) | 3 (10.7) | 0.59 |
| ORR, % (95% CI) | 0.04 | 0 (0) | 20.0 (0–50.0) | 25.0 (9.5–42.3) | 1.0 |
| DCR, % (95% CI) | 0.033 | 7.1 (0–25.0) | 40.0 (10.0–75.0) | 35.7 (19.2–53.8) | 1.0 |
| mPFSa (95% CI) | – | 3.0 (2.6–3.4) | 3.0 (1.5–4.6) | 3.0 (2.6–3.4) | – |
| 6-month PFS,% | 0.028 | 0 | 40.0 | 31.7 | 0.967 |
| mOSa (95% CI) | 0.167 | 8.0 (5.5–10.5) | 16.0b | 10.0b | 0.507 |
| 1-year survival rate, % | 0.167 | 23.8 | 66.7 | 46.2 | 0.507 |
All p was two-tailed
Ipi ipilimumab, Pem pembrolizumab, ORR objective response rate, DCR disease control rate, P 1 comparison between three groups; P 2 comparison between Ipi + Pem and Pem
aThe unit for median survival time was month
b95% CI of mOS for these groups were unavailable due to the limited follow-up time
Only patients who received pembrolizumab-based regimens were subjected to univariate analysis, as none of the patients who received ipilimumab monotherapy responded to it. The ORR was significantly lower in patients with liver metastasis and in those with elevated lactate dehydrogenase (LDH) or M1c stage. As M stage is related to liver metastasis (p = 0.001) and elevation of LDH (p = 0.002), it was not included in the subsequent survival analysis. Pretreatment median RLCs and RECs were significantly higher in patients with controlled disease compared to those with progressive disease (29.1 vs. 18.8%, p = 0.037 for RLC; 3.0 vs. 1.3%, p = 0.039 for REC, respectively). The optimal cut-off values were 25.2% for RLC (area under the curve [AUC] = 0.703, 95% confidence interval [CI] = 0.533–0.84, p = 0.0248) and 2.2% for REC (AUC = 0.7, 95% CI = 0.53–0.838, p = 0.031), respectively. High baseline RLC was associated with a higher rate of response, but this was not the case for REC. Treatment response did not correlate with patient age, sex, BRAF V600E status, the presence of brain metastasis, Eastern Cooperative Oncology Group (ECOG) performance status (PS), or the number of prior therapies (Supplementary Table 2).
There were 15 acral and 5 mucosal melanomas in the pembrolizumab-containing groups. The ORRs for acral, mucosal, and non-acral cutaneous melanoma were 26.7, 20, and 26.7% respectively. There were no differences in CR, PR, SD, ORR, and DCR according to melanoma subtypes (all p > 0.05). The efficacy data with respect to different melanoma subtypes are detailed in Supplementary Table 3.
PFS and OS
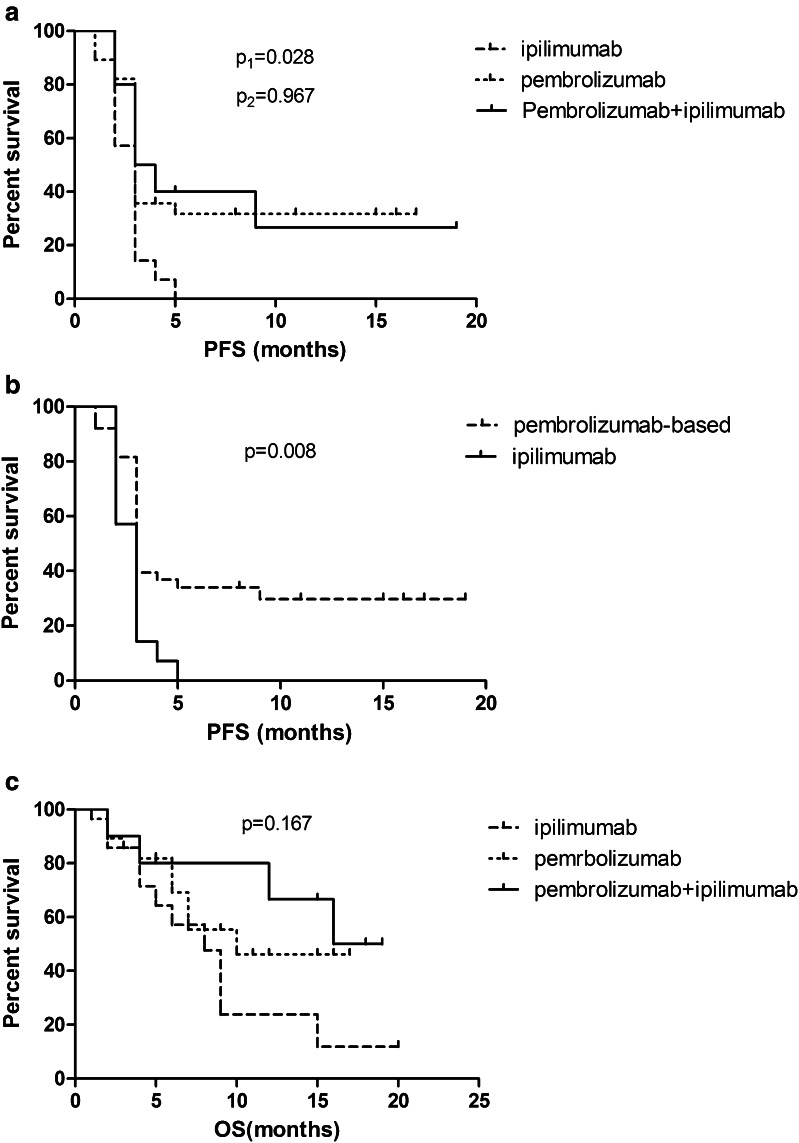
The median PFS in each group was 3 months. However, the survival curve for ipilimumab was significantly different from the two pembrolizumab-treated groups by the fifth month, with no difference between the pembrolizumab monotherapy and pembrolizumab/ipilimumab combination group (p = 0.967). The estimated 6-month PFS rate was 0% for ipilimumab, 31.7% for pembrolizumab, and 40% for the combined regimen; this rate was significantly improved with pembrolizumab-based regimens (hazard ratio = 0.27, 95% CI = 0.10–0.70, p = 0.008) (Fig. 1a, b; Table 2).
Fig. 1.
Kaplan–Meier curves for all patients. a Progression-free survival (PFS)for the three groups (p1, comparison between three groups; P2, Pembrolizumab vs. Pembrolizumab + Ipilimumab). b PFS for ipilimumab monotherapy and pembrolizumab-based therapy. c Overall survival (OS) for three groups
The estimated median OS of the patients in the ipilimumab, pembrolizumab, and combination treatment groups were 8, 10, and 16 months, respectively, after a median follow-up period of 11 months. The differences were not significant (p = 0.167) (Fig. 1c).
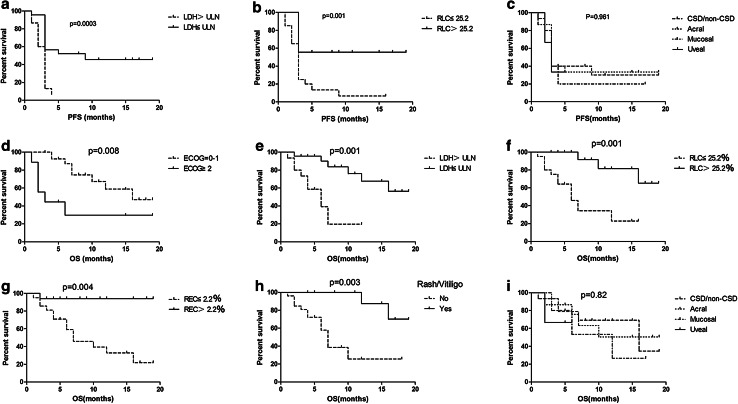
Since no tumor shrinkage was observed among patients in the ipilimumab group, all of whom progressed within 5 months, we analyzed the association between baseline characteristics and treatment-related toxicities and survival only for patients who received pembrolizumab-based regimens. On univariate analysis, ECOG PS, liver metastasis, LDH, RLC, REL, and treatment-related rash/vitiligo were significantly associated with PFS and OS. No correlation with either OS or PFS was observed for sex, age, brain metastasis, BRAF mutation status, and the number of previous therapies (all p ≥ 0.05). On multivariate analysis, LDH level and RLC were independent prognostic factors for PFS, while ECOG PS, LDH level, RLC, REC, and treatment-related rash/vitiligo were independent prognostic factors for OS. There was no difference in PFS and OS between different melanoma subtypes (Table 3; Fig. 2).
Table 3.
Univariate and multivariate analyses of survival for pembrolizumab-based therapy
| Variables | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| mPFS (months) | p | HR for PFS | p | mOS (months) | p | HR for OS | p | |
| Age | ||||||||
| ≤60 years | 3.0 | 0.845 | 12.0 | 0.381 | ||||
| >60 years | 3.0 | NR (>18) | ||||||
| Sex | ||||||||
| Male | 3.0 | 0.95 | 16.0 | 0.957 | ||||
| Female | 3.0 | 7.0 | ||||||
| Melanoma subtype | ||||||||
| CSD/non-CSD | 3.0 | 0.961 | 16.0 | 0.82 | ||||
| Acral | 3.0 | NR (>19) | ||||||
| Mucosal | 3.0 | 12.0 | ||||||
| Uveal | 3.0 | NR (>6) | ||||||
| ECOG | ||||||||
| 0–1 | 3.0 | 0.009 | 1 | 0.167 | 16.0 | 0.008 | 1 | 0.013 |
| 2 | 2.0 | 3.2 | 3.0 | 6.4 | ||||
| LDH level | ||||||||
| ≤ULN | 9.0 | 0.0003 | 1 | 0.029 | 16.0 | 0.013 | 1 | 0.025 |
| >ULN | 3.0 | 2.5 | 7.0 | 6.3 | ||||
| Liver Metastasis | ||||||||
| No | 3.0 | 0.008 | 0.81 | NR (>19) | 0.001 | 0.348 | ||
| Yes | 3.0 | 6.0 | ||||||
| Brain metastasis | ||||||||
| No | 3.0 | 0.9 | 12.0 | 0.613 | ||||
| Yes | 9.0 | NR (>16) | ||||||
| BRAF mutation | ||||||||
| Yes | 3.0 | 0.927 | 16.0 | 0.282 | ||||
| No | 3.0 | 10.0 | ||||||
| Previous treatment | ||||||||
| No | 3.0 | 0.96 | 16.0 | 0.901 | ||||
| Yes | 3.0 | NR (>19) | ||||||
| Relative lymphocyte count | ||||||||
| ≤25.2% | 3.0 | 0.001 | 2.6 | 0.034 | 6.0 | 0.001 | 7.8 | 0.06 |
| >25.2% | NR (>19) | 1 | NR (>19) | 1 | ||||
| Relative eosinophil count | ||||||||
| ≤2.2% | 3.0 | 0.041 | 1 | 0.227 | 7.0 | 0.004 | 3.2 | 0.016 |
| >2.2% | 5.0 | 0.4 | NR (>19) | 1 | ||||
| Treatment-related rash/vitiligo | ||||||||
| No | 3.0 | 0.001 | 0.077 | 7.0 | 0.003 | 66.8 | 0.007 | |
| Yes | NR (>19) | NR (>19) | 1 | |||||
NR not reached
Fig. 2.
Kaplan–Meier curves for patients receiving pembrolizumab-based therapy. a Progression-free survival (PFS) according to lactate dehydrogenase (LDH) level. b PFS according to relative lymphocyte count (RLC) level. c PFS according to primary site. d Overall survival (OS) according to Eastern Cooperative Oncology Group performance status. e OS according to LDH level. f OS according to RLC level. g OS according to relative eosinophil count (REC) level. h OS according to treatment-related rash or vitiligo. i OS according to primary tumor site
Associations between PD-L1 expression and efficacy
Of these 38 patients who received pembrolizumab-based therapy, only 18 had pretreatment tumor specimens available for PD-L1 staining. Of these 18 patients, 6 (33.3%) were considered positive. In the PDL-1 (+) group, ORR was 66.7% (4/6) and DCR was 83.3%; for PDL-1 (−) group, ORR was 25.0% (3/12) and DCR was 33.3%. The median PFS was 3.0 months in PDL-1 (−) group but not reached in PD-L1 (+) group (p = 0.1). Tumor response and patients’ survival based on PD-L1 status were summarized in Supplementary Table 4 and Supplementary Fig. 1.
Toxicity
Ipilimumab and pembrolizumab were well tolerated by patients in this cohort. The most frequent treatment-related adverse events were elevation of aspartate transaminase (AST) and alanine transaminase (ALT), skin toxicity (including rash, pruritus, and vitiligo), pyrexia, fatigue, and hyper- or hypothyroidism. Treatment-related diarrhea was observed in only 1 patient who received the combination therapy. None of the patients developed treatment-related pneumonia. Grade 3–4 adverse events were observed in 14%, 14%, and 30% of patients receiving ipilimumab, pembrolizumab, and the combination therapy, respectively. Among the nine patients with grade 3–4 toxicity, eight exhibited elevated aminotransferases and one had psoriasis. The patient with psoriasis developed multiple erythema covered with silvery, white scales on the skin of the scalp, back, and limbs after three cycles of pembrolizumab infusion. The patient complained of itching and burning. Psoriasis vulgaris was diagnosed by the dermatologist. Pembrolizumab was withheld and topical corticosteroids was prescribed. The symptoms were controlled at grade 1 but persisted during the whole course of treatment. Only one patient discontinued treatment owing to immune-associated hepatitis. There were no treatment-related deaths. Treatment-related toxicities are listed in Table 4. The occurrence of rash or vitiligo after pembrolizumab-based therapy was associated with a higher response rate and longer OS (Table 3; Fig. 2).
Table 4.
Adverse events considered to be drug-related by investigators (CTCAE v. 4.0)
| Number of patients with event (percent) | ||||||
|---|---|---|---|---|---|---|
| Adverse event | Pembrolizumab (n = 28) | Pembrolizumab + Ipilimumab (n = 10) | Ipilimumab (n = 14) | |||
| Total | Grade 3/4 | Total | Grade 3/4 | Total | Grade 3/4 | |
| Any | 17 (61) | 4 (14) | 8 (80) | 3 (30) | 10 (71) | 2 (14) |
| Fatigue | 4 (14) | 0 (0) | 6 (60) | 0 (0) | 4 (29) | 0 (0) |
| Pyrexia | 4 (14) | 0 (0) | 3 (30) | 0 (0) | 1 (7) | 0 (0) |
| Arthralgia/myalgia | 3 (11) | 0 (0) | 2 (20) | 0 (0) | 1 (7) | 0 (0) |
| Headache | 1 (4) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 3 (11) | 0 (0) | 5 (50) | 0 (0) | 4 (29) | 0 (0) |
| Rash | 3 (11) | 1 (4) | 4 (40) | 0 (0) | 3 (21) | 0 (0) |
| Vitiligo | 5 (18) | 0 (0) | 2 (20) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
| Hypothyroidism | 4 (14) | 0 (0) | 1 (10) | 0 (0) | 1 (7) | 0 (0) |
| Hyperthyroidism | 1 (4) | 0 (0) | 1 (10) | 0 (0) | 1 (7) | 0 (0) |
| Adrenal insufficiency | 1 (4) | 0 (0) | 2 (20) | 0 (0) | 1 (7) | 0 (0) |
| Elevated transaminase | 5 (18) | 3 (11) | 5 (50) | 3 (30) | 5 (36) | 2 (14) |
Discussion
In this study, pembrolizumab alone or in combination with ipilimumab improved the ORR and PFS in patients with advanced melanoma compared with ipilimumab monotherapy; these findings were consistent with the results of previous clinical trials [5–7]. The ORRs and DCRs following ipilimumab treatment were 0 and 7.1%, respectively, which were lower than those reported in previous studies. Patients receiving pembrolizumab therapy showed a consistent ORR compared to Western patients in clinical trials [14, 15].
The Checkmate 067 and Keynote 029 trials revealed that combining anti-PD-1 antibody with ipilimumab showed a numerically higher response rate and longer PFS than PD-1 blockade alone [6, 7]. However, we failed to support this result in our study. There may be several reasons for the discrepancy. First, the sample size in this study is small which cannot represent the overall situation. Second, the baseline characteristics of patients in our study were different from those in the other clinical trials. Both Checkmate 067 and Keynote 029 trials rarely included acral and mucosal melanoma patients, while ocular melanoma was excluded entirely. Conversely, more than 60% of the patients treated with pembrolizumab-containing regimens in our study belonged to one of the above subtypes (15 acral, 5 mucosal, and 3 uveal). There was no stage III patients in our study, while the clinical trials did include such patients. The Checkmate 067 trial only included previously untreated patients, while more than 50% of the patients in our study had failed previous therapies. In addition, the combination therapy doses we used were slightly different. In contrast to the Checkmate 067 study, we used pembrolizumab to block PD-1 instead of nivolumab. Moreover, we used pembrolizumab and ipilimumab at lower and higher doses than those used in the Keynote 029 study, respectively.
Acral and mucosal melanomas are the most common subtypes of this disease in China, and are characterized by aggressive histopathological features, lower somatic mutational burden, and poorer prognoses. However, there are very limited data on the role of immune checkpoint inhibitors in the management of acral and mucosal melanomas [10–12]. In this study, we included 22 patients (42.3% of the total) with acral melanoma; seven of them accepted ipilimumab therapy, 13 received pembrolizumab therapy, and two received pembrolizumab/ipilimumab combination therapy. Of the seven patients who received ipilimumab monotherapy, only one achieved SD, the rest all progressed. Anti-PD-1 treatment was effective against acral melanoma, with 1 patient (7.1%) achieving CR, 3 (21.4%) achieving PR, and 2 (15.4%) achieving SD in the pembrolizumab-based groups. The ORR and DCR for acral melanoma were 28.6 and 42.9%, respectively. We also included five mucosal melanoma patients, three of whom were treated with pembrolizumab alone and two with the pembrolizumab/ipilimumabcombination regimen. One patient in the pembrolizumab group achieved good PR and another in the combined group had SD. The ORR and DCR for mucosal melanoma were 20 and 40%, respectively. The response rates of acral and mucosal melanoma patients receiving pembrolizumab-based therapy were similar to patients with other subtypes in this study, suggesting that pembrolizumab can be a feasible treatment option for patients with advanced acral or mucosal melanoma.
Biomarkers that can predict the efficacy of PD-1 blockers have widely been studied. Several studies have shown that expression of PD-L1 may be a potential predictive marker for response and outcome in patients with metastatic melanoma treated with anti-PD-1 antibody [16]. However, patients whose disease is PD-L1 negative can still achieve clinical benefit with anti-PD-1 therapy. In our study, ORR and median PFS were better in the positive PD-L1 expression group than those in negative PD-L1 expression group; however, there was no statistical significance. Although PD-L1 expression has been investigated most extensively; however, it is not an appropriate biomarker for patient selection, because its expression is dynamic and inducible, with limited assay standardization options [17, 18]. A number of blood-derived parameters have also been studied as they are easily obtainable. Diemet al. reported that patients with elevated baseline LDH had a significantly shorter OS compared to patients with normal LDH levels [19]. Weideet al. found that LDH level, the pattern of visceral involvement, RLC, and REC were independently associated with OS in melanoma patients treated with pembrolizumab [20]. Treatment-related cutaneous adverse events also hindered clinical benefits [21, 22]. However, elevated neutrophil count at baseline was associated with poor OS in melanoma patients undergoing ipilimumab therapy [23]. Different components of peripheral blood leukocyte may have different effects on the prognosis of melanoma. In order to take both good and bad factors in consideration, we choose RLC and REC as predictive factors instead of absolute value in this study. Consistent with previous studies, we observed that liver metastasis and elevated LDH level were associated with lack of response, but RLC > 25.2% was associated with good response in this study. Furthermore, LDH level and RLC were independent prognostic factors of PFS; ECOG PS, LDH level, RLC, and REL were independent prognostic factors of OS. Furthermore, treatment-related skin rash and vitiligo were associated with a higher response rate and longer OS. This is consistent with previous studies.
The incidence of treatment-related adverse events was lowest in the pembrolizumab group and highest in the combination group. The frequency of grade 3/4 toxicity was higher in the combination group than in either of the monotherapy groups, which was consistent with previous clinical trials [6, 7]. The safety profile was also consistent with previous data; there were no additional safety signals observed. In contrast to previous studies performed in Western countries, however, the incidence of treatment-related diarrhea in Chinese patients was much lower, while liver function damage was more common [24]. Safety data for a portion of the patients in this study have previously been reported [25].
This study has some limitations. The sample size was relatively small; moreover, patient selection bias was likely owing to its retrospective nature. For example, we included patients in poor medical condition in this study; there were 13 patients in the cohort who did not complete four doses of therapy, 12 of whom due to rapid tumor progression. However, our cohort may be more representative of actual clinical conditions.
In conclusion, pembrolizumab-based therapy was effective and well tolerated in Chinese patients with advanced melanoma, including those with acral and mucosal subtypes. For patients treated with pembrolizumab, liver metastasis and high LDH at baseline were associated with poor prognosis. On the contrary, high RLC was associated with a favorable prognosis. As this study was limited by its retrospective nature and small sample size, the application of immune checkpoint inhibitors for the treatment of melanoma patients in China requires appropriate verification in prospective clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ASTA
Spartate aminotransferase
- ALT
Alanine aminotransferase
- CSD
Chronic sun-derived
- CR
Completeremission
- CTCAE
National Cancer Institute Common Terminology Criteria for Adverse Events
- DCR
Disease control rate
- ECOG
Eastern Cooperative Group
- Ipi
Ipilimumab
- NR
Not reached
- OS
Overall survival
- ORR
Objective response rate
- Pem
Pembrolizumab
- PR
Partial remission
- PFS
Progression-free survival
- RECIST
Response evaluation criteria in solid tumors
- RLC
Relative lymphocyte count
- REC
Relative eosinophil count
- SD
Stable disease
- ULN
Upper limit of normal
Compliance with ethical standards
Funding
This study received support from the National Natural Science Foundation of China (Grant No. 81272341).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The institutional review board at our hospital approved this study.
Informed consent
All patients provided written informed consent for this study.
Footnotes
X. Wen and Y. Ding contributed equally to this work.
References
- 1.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16:5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumabin unresectable or metastatic melanoma. J ClinOncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J ClinOncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumaband ipilimumabor monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long GV, Atkinson V, Cebon JS, et al. Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: Results of the KEYNOTE-029 expansion cohort. J ClinOncol. 2016;34(suppl 15):9506. [Google Scholar]
- 8.Chi Z, Li S, Sheng X, Si L, Cui C, Han M, Guo J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. 2011;11:85. doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 10.Furney SJ, Turajlic S, Stamp G, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230:261–269. doi: 10.1002/path.4204. [DOI] [PubMed] [Google Scholar]
- 11.Furney SJ, Turajlic S, Stamp G, et al. The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell. Melanoma Res. 2014;27:835–838. doi: 10.1111/pcmr.12279. [DOI] [PubMed] [Google Scholar]
- 12.Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;22:3354–3362. doi: 10.1002/cncr.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 16.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, Melero I, Ascierto PA. Cancer treatment with anti-PD-1/PD-L1 agents: Is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76:925–945. doi: 10.1007/s40265-016-0588-x. [DOI] [PubMed] [Google Scholar]
- 18.Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell. Melanoma Res. 2015;28:245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 19.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 22.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S. Pembrolizumabcutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci PFC, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 24.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 25.Wen X, Wang Y, Ding Y, Li D, Li J, Guo Y, Peng R, Zhao J, Zhang X, Zhang XS. Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Res. 2016;26:284–289. doi: 10.1097/CMR.0000000000000256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.