Abstract
Malignant melanoma is characterized by the development of chronic inflammation in the tumor microenvironment, which leads to a strong immunosuppression associated with a rapid tumor progression. Adenosine is considered as one of the main immunosuppressive factors in the tumor environment. It is produced via enzymatic hydrolysis of extracellular ATP by ectonucleotidases CD39 and CD73 localized on cell surface. Using the ret transgenic mouse melanoma model that closely mimics human melanoma, we demonstrated an increased frequency of ectonucleotidase-positive myeloid-derived suppressor cells (MDSCs) in melanoma lesions and lymphoid organs. Furthermore, we observed that conventional CD4+FoxP3− and CD8+ T cells infiltrating melanoma lesions of ret transgenic mice were distinctly enriched in the CD39+CD73+ subpopulation that co-expressed also PD-1. Ectonucleotidase expression was also up-regulated in CD4+ and CD8+ T cells upon activation. In addition, these ectoenzymes were largely found to be expressed on memory T cell compartment (in particular, on effector memory cells). Our data suggest that extracellular adenosine produced by regulatory T cells (Tregs) and MDSCs can suppress T cell effector functions through paracrine signaling. Another mechanism involves its production also by effector T cells and an inhibition of their anti-tumor reactivity via autocrine signaling as a part of the negative feedback loop. This mode of adenosine signaling could be also used by Tregs and MDSCs to enhance their immunosuppressive activity.
Keywords: Immunosuppression, Adenosine, Myeloid-derived suppressor cells, T cells, Melanoma, PIVAC 13
Introduction
Malignant melanoma is an extremely aggressive form of skin cancer characterized by a rapid growth and metastasis to regional lymph nodes and distant organs [1–3]. Its incidence and death rates have been increasing in different countries faster than those of other cancers [1, 2]. The observed immunogenicity of melanoma has made this disease a preferred target for application of various immunotherapeutic approaches, dealing with tumor antigen-specific and -nonspecific immunostimulation or adoptive transfer of melanoma-specific activated T lymphocytes [4–6]. However, despite some positive reports, the overall results of immunotherapeutic clinical studies are not satisfactory. These paradoxical observations could be due to a profound immunosuppression mediated by tumor and stroma cells and strongly supported by chronic inflammation developing in the tumor microenvironment and accelerating tumor progression [7–11]. In addition to the expansion and recruitment of various immunosuppressive cells in the tumor microenvironment such as regulatory T cells (Tregs) [12, 13], myeloid-derived suppressor cells (MDSCs) [14, 15], tumor-associated M2 macrophages (TAMs) [16], Tie2-expressing monocytes [17], tumor-associated N2 neutrophils (TANs) [18] and regulatory/tolerogenic dendritic cells (DCs) [19], tumor and stroma cells secrete a variety of soluble immunosuppressive factors and metabolites. These include tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, interleukin (IL)-1β, IL-6, vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), prostaglandin E2, lactate, glutamate and adenosine [20–24].
Adenosine is a purine nucleoside produced via enzymatic hydrolysis of extracellular ATP by the nucleoside triphosphate diphosphohydrolase CD39 and the ecto-5′-nucleotidase CD73 localized on cell surface. The key role of adenosine in lymphocyte development and regulation was initially highlighted by the discovery of inherited deficiency of adenosine deaminase (ADA) that resulted in the most profound depletion of T, B, and natural killer (NK) lymphocytes found in any form of severe combined immunodeficiency disease [25, 26]. Potential mechanisms of such defects have been linked to the ADA substrate adenosine generated in large amounts from apoptotic cells in the thymus, bone marrow and lymph nodes [27]. More recently, adenosine has been described to be produced by Tregs and to mediate their ability to inhibit the activity of various immune cell subpopulations both in vitro and in vivo [28–30]. Moreover, adenosine can be also generated by tumor cells, strongly contributing to the immunosuppressive pattern of the tumor microenvironment [31, 32]. In this review, we discuss the role of extracellular adenosine metabolism in different immune cells during melanoma progression and its possible involvement in the autocrine regulation of activities of non-regulatory T cells in the tumor microenvironment.
Adenosine as an immunosuppressive metabolite
Adenosine is normally present in body fluids at low concentrations [33]. It is produced from pro-inflammatory ATP in a two-step process: CD39 hydrolyses ATP or ADP into AMP and CD73, in turn, cleaves AMP yielding adenosine. Interestingly, other extracellular enzymes adenylate kinase and nucleoside diphosphate kinase can phosphorylate AMP and ADP, respectively, thus antagonizing CD39 activity [34]. It has been demonstrated that adenosine can be accumulated in hypoxic tissues, including the tumor microenvironment [35–37], through a strong activation of the ectonucleotidase pathway and inhibition of adenosine kinase [38]. Furthermore, an ATP release from dying tumor cells together with high ectonucleotidase activity on the surface of tumor and infiltrating immune cells favors additional adenosine accumulation [34].
Adenosine interacts with four distinct cell surface G-protein-linked receptors: A1, A2A, A2B and A3 [39]. A1 and A3 adenosine receptors are coupled to the Gi/o subunit, and their activation leads to the inhibition of adenylate cyclase, cyclic AMP (cAMP) production, and as a consequence to the protein kinase A activation [39, 40]. Stimulation of A2A and A2B receptors has been reported to be accounted for immunosuppressive effects of adenosine [40]. A2A receptors (A2AR) are expressed on a variety of immune cells, including T lymphocytes, NK cells, DCs, macrophages and granulocytes [39, 41]. The A2AR-triggered cAMP elevation inhibits effector functions of all these cell populations. In T cells, adenosine mediates the blocking of the NF-κB pathway [42] that results in a potent suppression of (1) cell proliferation [43, 44]; (2) synthesis of cytokines IL-2 IFN-γ and TNF-α [45, 46]; (3) expression of perforin and CD95 ligand [47, 48]; (4) cytotoxic T cell adhesion to tumor target cells and granule exocytosis [47, 49]; and cell survival [23, 50]. Moreover, adenosine inhibits some of the earliest steps in T cell activation associated with signal transduction through the T cell receptor and costimulatory CD28 molecules [35].
Adenosine has also been demonstrated to restrain the maturation and proinflammatory cytokine production by DCs, impairing their ability to stimulate T cells [51]. Furthermore, it could impede the phagocytic and antigen-presenting capacity of macrophages as well as induce the differentiation of immunosuppressive alternatively activated macrophages [52]. Similar to T cells, adenosine has been reported to decrease cytotoxic functions of NK cells and their IFN-γ production [35, 39, 53].
Ectonucleotidases and adenosine metabolism in the tumor microenvironment
Ectonucleotidases CD39 and CD73, which are expressed on various immune cell populations as well as on tumor cells, have been reported to be responsible for adenosine accumulation outside the cell [54]. In contrast to many other enzymes, both CD39 and CD73 lack regulatory domains. Therefore, their net activity depends on their expression and the concentrations of the substrate, product and bivalent cations [55, 56]. Interestingly, CD73 is inhibited by high concentrations of ATP or ADP, underscoring the highly coordinated action of CD39 and CD73 [57]. Ectonucleotidases play an important role in the regulation of the balance between the proinflammatory ATP and immunosuppressive adenosine in the tumor microenvironment. It has been clearly demonstrated that the inhibition of adenosine production and signaling significantly reduces the tumor progression by enhancing anti-tumor immune responses [23, 55, 58–61]. For example, the deficiency of A2AR promoted the rejection of established immunogenic tumors, and the administration of A2AR antagonists resulted in the CD8 T cell-mediated inhibition of tumor growth and metastasis [23]. Furthermore, in a transplantable mouse model of breast cancer, Stagg et al. [59] demonstrated an efficient suppression of tumor development and metastasis upon the administration of anti-CD73 monoclonal antibodies (mAbs). This study together with several other reports [23, 60] showed an importance of CD73 expressed on cells in the tumor microenvironment in hindering the anti-tumor immune reactivity.
Moreover, it has been demonstrated that CD73-deficiency enhanced anti-tumor immune responses and resistance to experimental metastasis in four different transplantable mouse tumor models [58]. This anti-tumor effect was associated with an expansion of antigen-specific IFN-γ-producing CD8 T cells in the peripheral blood and tumor microenvironment. Experiments with the bone marrow chimeras indicated that CD73 expressed on both hematopoietic and non-hematopoietic cell populations non-redundantly contribute to the tumor immune escape. In addition, CD73 expression at least partially accounted for the tumor-promoting effects of Tregs. Another publication reported that the enzymatic activity of CD73 on non-hematopoietic cells hindered leukocyte migration into the tumor site, whereas CD73 on hematopoietic cells suppressed a systemic anti-tumor T cell expansion and their effector functions [55]. Both groups demonstrated anti-tumor and anti-metastatic effects of the CD73 targeting using its selective inhibitor α,β-methylene adenosine diphosphate or respective mAbs [55, 58]. Moreover, Wang et al. [55] showed that anti-CD73 mAbs could significantly augment the efficacy of adoptive T cell therapy in tumor-bearing mice. The translational significance of these findings is underscored by the fact that various adenosine receptor antagonists are already used in clinical settings for other indications [62].
Role of CD39 and CD73 in the function of immunosuppressive cells
It has been well documented that Tregs coexpress ectoenzymes CD39 and CD73 and can produce adenosine in the tumor-bearing hosts strongly contributing to their immunosuppressive activity [13, 29, 30, 34, 40, 63, 64]. Under physiological conditions, Tregs has been shown to play a critical role in the maintenance of peripheral tolerance by suppressing immune responses against “self” antigens [65]. However, in tumor-bearing hosts, these cells have been described as one of the key mediators of tumor resistance against effector immune cells [12, 13, 29, 30, 66]. Importantly, adenosine producing Tregs have been shown to inhibit not only the proliferation of effector cells but also their activities [29, 64].
The significance of ectonucleotidases was underlined by the observation that Tregs from CD39-deficient mice failed to suppress effector T cell proliferation [50]. Furthermore, in CD73−/− mice, activated CD4 T cells produced increased amounts of pro-inflammatory cytokines (IFN-γ, IL-2 and TNF-α), and Tregs displayed no suppressive activity [67]. It has been suggested that CD73-derived adenosine ensures a tonic inhibition of NF-κB in CD4+ T cells, limiting thereby the development of effector T cell responses [67]. Therefore, CD39 and CD73 expressed on Tregs can play an important role in regulating the balance between the pro-inflammatory ATP and immunosuppressive adenosine (Fig. 1).
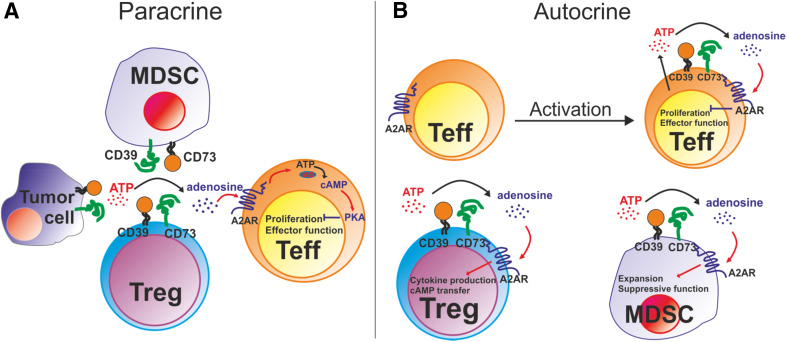
Fig. 1.
Two mechanisms of adenosine signaling. a Paracrine signaling. Extracellular adenosine, which is produced via ectonucleotidases CD39 and CD73 expressed on Tregs, MDSCs or tumor cells can interact with A2A receptors (A2AR) on non-regulatory T cells (Teff). This stimulates the synthesis of cAMP in Teff leading to a significant suppression of their proliferation and effector functions. b Autocrine signaling. A pronounced up-regulation of the ectonucleotidase expression on activated Teff results in an autocrine adenosine signaling and the cAMP accumulation. This can limit the activation of T cells and induce their anergy. In addition, an increased expression of CD39 and CD73 on Tregs and MDSCs enable an autocrine adenosine signaling in these cells, which might serve to sustain or enhance their immunosuppressive activity
Myeloid-derived suppressor cells are considered as other key players mediating immunosuppression in the tumor microenvironment. This heterogeneous population consists of immature myeloid cells that fail to terminally differentiate into granulocytes, macrophages or DCs upon chronic inflammatory conditions in tumor-bearing hosts [14, 68, 69]. In mice, MDSCs express both CD11b and Gr1 markers and can be divided on two major subsets: granulocytic CD11b+Ly6G+Ly6Clo and monocytic CD11b+Ly6G−Ly6Chi cells [14, 69, 70]. In human MDSCs, granulocytic subpopulation is defined as Lin−HLA-DR−/loCD11b+CD33+CD14−CD15+ and monocytic one as CD11b+CD14+HLA-DR−/lo [14, 15, 68]. MDSCs derive from bone marrow hematopoietic precursors due to the altering of myelopoiesis by chronic inflammatory mediators [10, 14]. They can remarkably inhibit anti-tumor reactivity of T and NK cells by the deprivation of arginine and cysteine, increased production of nitric oxide and reactive oxygen species, an expression of membrane-bound TGF-β and a down-regulation of the TCR ζ-chain expression [10, 14, 68, 71, 72].
It has been recently demonstrated that the frequency and function of MDSCs could be modulated by extracellular adenosine through the receptor A2B [73]. Furthermore, the authors detected high CD73 levels on the surface of granulocytic MDSCs and showed that the MDSC immunosuppressive activity in vitro was enhanced in the presence of AMP. This suggests that adenosine produced by MDSCs contributes to their suppressive properties by the paracrine modulation of other cells (e.g., the inhibition of effector T cell anti-tumor activities). On the other hand, autocrine adenosine production can increase the numbers and immunosuppressive activity of MDSCs (Fig. 1).
To elucidate the role of CD39 and CD73 on MDSCs, we studied their expression on these cells isolated from tumor-bearing ret transgenic mice and on CD11b+Gr1+ immature myeloid cells (IMCs) from healthy animals. This transgenic model mimics human melanoma with respect to etiology, tumor genetics, histopathology and clinical development [74, 75]. Animals develop spontaneously skin melanoma metastasizing to lymph nodes and distant organs. We observed that both MDSCs and IMCs constitutively express CD39, indicating the capacity of these cells to hydrolyze ATP [76]. This is in agreement with reports describing an important role of CD39 in myeloid cell migration and function [54, 77, 78]. In contrast, CD73 expression showed considerable heterogeneity. Analyzing the granulocytic subset of CD11b+Gr1+ cells, we found that IMCs from the spleen and bone marrow contained a substantial population of CD73-expressing cells [76]. Moreover, granulocytic MDSCs from respective lymphoid organs and melanoma lesions harbored distinctly higher percentages of CD73+ cells. This could be linked to the observed MDSC expansion in this tumor model [79]. Although only a minor proportion of monocytic MDSCs expressed CD73, the number of CD73-positive cells was elevated in melanoma lesions [76]. Since TGF-β alone [80] or in combination with IL-6 [81] has been shown to induce CD73 expression on T cells through the down-regulation of the transcription factor Gfi-1 [81], the up-regulation of CD73 on MDSCs could also be mediated by these factors that were accumulated in the tumor microenvironment of ret transgenic mice [82].
Why is the expression of ectonucleotidase up-regulated on activated T cells?
Interestingly, ectonucleotidase expression has been found not only on immunosuppressive cells but also on non-regulatory T cells. We observed that conventional CD4+FoxP3− (Tcons) and CD8+ T cells infiltrating melanoma lesions of ret transgenic mice were distinctly enriched in CD39+CD73+ subpopulation. Moreover, these cells co-expressed also PD-1 suggesting that similar to CTLA-4 and PD-1 molecules, ectonucleotidases can represent an immune checkpoint maintaining tolerance and regulating the duration and intensity of the immune response [83]. In line with this assumption, we found that the CD25+ subset of Tcons, presumably representing activated T cells [84], contained significantly higher frequencies of CD39+ and CD73+ cells than the resting CD25− counterpart both in lymphoid organs (bone marrow, spleen, lymph nodes) and skin tumors.
To analyze the impact of T cell activation on the expression of ectonucleotidases, we stimulated splenocytes from healthy mice through the engagement of CD3 and CD28. It was found that the frequencies of CD39+ cells among CD4+ and CD8+ T cells significantly increased upon activation that is in agreement with the previous report, which demonstrated also a TGF-β-dependent up-regulation of CD73 expression on activated CD8+ T cells displayed [80]. Moreover, we found that CD4+ and CD8+ T cells that rapidly produced IFN-γ upon stimulation contained a higher frequency of ectonucleotidase expressing cells as compared to the subset unable to produce IFN-γ. Activated T cells have been demonstrated to release increased ATP amounts, promoting thereby their activation through autocrine purinergic signaling [54, 85]. The TCR stimulation resulted in the release of cellular ATP through pannexin-1 channels, which provides an autocrine positive feedback loop amplifying T cell activation [86]. On the other hand, high concentrations of extracellular ATP may induce T cell apoptosis [39]. Therefore, a rapid induction of CD39 upon the T cell activation may provide an effective tool for controlling extracellular ATP levels and preventing T cell apoptosis. Furthermore, CD73 induction in activated T cells may dampen their function through the accumulation of extracellular adenosine. Interestingly, a recent study on Jurkat T cell line in vitro has demonstrated that upon activation, adenosine could be produced also through the CD38/CD203a/CD73 ectoenzymatic pathway, bypassing the canonical catabolic pathway mediated by CD39 [87].
Comparing naïve and memory T cells, we demonstrated that the ectonucleotidase expression in T cells was largely associated with the memory compartment. Moreover, the effector memory subset contained a higher frequency of ectonucleotidase expressing cells than the central memory counterpart since effector memory cells are known to display a rapid effector function [88] and their inappropriate activation should be more strictly controlled. Thus, memory T cells might constitutively produce extracellular adenosine from ATP to prevent their adverse activation through autocrine adenosine signaling (Fig. 1).
Conclusion
Taken together, extracellular adenosine produced through the two-step hydrolysis of extracellular ATP by ectonucleotidases CD39 and CD73 is involved in the limitation of excessive T cell-mediated immune responses. Ectonucleotidases play a crucial role in the regulation of the balance between the proinflammatory ATP and immunosuppressive adenosine. However, this mechanism can be hijacked by tumors (including melanoma) to subvert an anti-tumor immune reactivity, leading to the tumor progression. During the last decade, it has been well documented that Tregs coexpress CD39 and CD73 and produce extracellular adenosine in the tumor-bearing mice and cancer patients, representing one of the key mechanisms of Treg immunosuppressive activity. Moreover, it has been reported that MDSCs (another cell population critically contributing to the immunosuppression developing in tumor-bearing hosts) can also produce adenosine via ectonucleotidases expressed on their surface. This leads to an inhibition of T cell functions and an increase in MDSC frequencies and activities in the tumor microenvironment. Furthermore, recent observations on the up-regulation of ectonucleotidase expression on CD4+ and CD8+ T cells upon activation and on those cells infiltrating melanoma lesions in ret transgenic mice indicate a previously unappreciated role of ectonucleotidases expressed on effector T cells in the limitation of anti-tumor immune reactions through autocrine adenosine signaling mechanisms. We thus suggest two possible modes of adenosine signaling in the tumor microenvironment: (1) extracellular adenosine produced by CD39 and CD73 expressed on Tregs and MDSCs can inhibit proliferation and effector functions of non-regulatory T cells via paracrine signaling; (2) adenosine produced by ectonucleotidase on tumor-infiltrating T cells suppresses their activities and induces their anergy through negative feedback mechanisms. In addition, an enhancement of Treg and MDSC immunosuppressive function can be also achieved by the autocrine adenosine signaling in these cells (Fig. 1).
Acknowledgments
This project has been funded by a grant from the Cooperation Program in Cancer Research between German Cancer Research Center (DKFZ) and Ministry of Science and Technology of Israel (MOST) (CA157, to Viktor Umansky) and by a grant from Else-Kröner Fresenius Foundation (2010.A124, to Viktor Umansky and Alexandr V. Bazhin).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Thirteenth International Conference on Progress in Vaccination against Cancer (PIVAC 13), held in Amsterdam, the Netherlands, October 2nd–4th, 2013. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–vi7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflugfelder A, Kochs C, Blum A, Capellaro M, Czeschik C, Dettenborn T, et al. Malignant melanoma S3-guideline “diagnosis, therapy and follow-up of melanoma”. J Dtsch Dermatol Ges Suppl. 2013;6:1–116. doi: 10.1111/ddg.12113_suppl. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer–what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogas H, Polyzos A, Kirkwood J. Immunotherapy for advanced melanoma: fulfilling the promise. Cancer Treat Rev. 2013;39:879–885. doi: 10.1016/j.ctrv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22:307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 13.Whiteside TL. Disarming suppressor cells to improve immunotherapy. Cancer Immunol Immunother. 2012;61:283–288. doi: 10.1007/s00262-011-1171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother. 2012;61:255–263. doi: 10.1007/s00262-011-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 18.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Shurin GV, Gutkin DW, Shurin MR. Tumor associated regulatory dendritic cells. Semin Cancer Biol. 2012;22:298–306. doi: 10.1016/j.semcancer.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 21.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang X, Caldwell S, Liu K, Smith P, Chen J-F, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carson DA, Iizasa T, Seto S, Carrera CJ, Kubota M, Willis EH, Wasson DB, Kajander O. Metabolic basis for immune dysfunction in adenosine deaminase deficiency. Ann N Y Acad Sci. 1985;451:34–41. doi: 10.1111/j.1749-6632.1985.tb27094.x. [DOI] [PubMed] [Google Scholar]
- 26.Hershfield MS. New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing immunodeficiency associated with adenosine deaminase deficiency. Eur J Immunol. 2005;35:25–30. doi: 10.1002/eji.200425738. [DOI] [PubMed] [Google Scholar]
- 27.Apasov SG, Blackburn MR, Kellems RE, Smith PR, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. J Clin Invest. 2001;108:131–141. doi: 10.1172/JCI200110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteside TL, Mandapathil M, Schuler P. The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg) Curr Med Chem. 2011;18:5217–5223. doi: 10.2174/092986711798184334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteside TL. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother. 2014;63:67–72. doi: 10.1007/s00262-013-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 32.Ghiringhelli F, Bruchard M, Chalmin F, Rébé C. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol. 2012;2012:473712. doi: 10.1155/2012/473712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 34.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int J Oncol. 2008;32:527–535. [PubMed] [Google Scholar]
- 36.Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22:335–341. doi: 10.1016/j.semcancer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 38.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.RES.81.2.154. [DOI] [PubMed] [Google Scholar]
- 39.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 40.Allard B, Turcotte M, Stagg J. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol. 2012;2012:485156. doi: 10.1155/2012/485156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Majumdar S, Aggarwal BB. Adenosine suppresses activation of nuclear factor-kappaB selectively induced by tumor necrosis factor in different cell types. Oncogene. 2003;22:1206–1218. doi: 10.1038/sj.onc.1206184. [DOI] [PubMed] [Google Scholar]
- 43.Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer. 2002;99:386–395. doi: 10.1002/ijc.10325. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 45.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4 + T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 46.Raskovalova T, Lokshin A, Huang X, Su Y, Mandic M, Zarour HM, Jackson EK, Gorelik E. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8 + and CD4 + T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 2007;67:5949–5956. doi: 10.1158/0008-5472.CAN-06-4249. [DOI] [PubMed] [Google Scholar]
- 47.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 48.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 49.MacKenzie WM, Hoskin DW, Blay J. Adenosine suppresses alpha(4)beta(7) integrin-mediated adhesion of T lymphocytes to colon adenocarcinoma cells. Exp Cell Res. 2002;276:90–100. doi: 10.1006/excr.2002.5514. [DOI] [PubMed] [Google Scholar]
- 50.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Haskó G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–7765. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 54.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knapp K, Zebisch M, Pippel J, El-Tayeb A, Muller CE, Strater N. Crystal structure of the human ecto-5′-nucleotidase (CD73): insights into the regulation of purinergic signaling. Structure. 2012;20:2161–2173. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Strater N. Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 59.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 62.Pere H, Tanchot C, Bayry J, Terme M, Taieb J, Badoual C, Adotevi O, Merillon N, Marcheteau E, Quillien VR, Banissi C, Carpentier A, Sandoval F, Nizard M, Quintin-Colonna F, Kroemer G, Fridman WH, Zitvogel L, Oudard SP, Tartour E. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology. 2012;1:326–333. doi: 10.4161/onci.18852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4 + CD25highFOXP3 + regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandapathil M, Whiteside TL. Targeting human inducible regulatory T cells (Tr1) in patients with cancer: blocking of adenosine-prostaglandin E2 cooperation. Expert Opin Biol Ther. 2011;11:1203–1214. doi: 10.1517/14712598.2011.581225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakaguchi S. Naturally arising Foxp3-expressing CD25 + CD4 + regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 66.Shevach EM. Mechanisms of foxp3 + T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Romio M, Reinbeck B, Bongardt S, Huls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol Cell Physiol. 2011;301:C530–C539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 68.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu T, Gabrilovich DI. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin Cancer Res. 2012;18:4877–4882. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b + Gr1 + cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 75.Umansky V, Abschuetz O, Osen W, Ramacher M, Zhao F, Kato M, Schadendorf D. Melanoma-specific memory T cells are functionally active in Ret transgenic mice without macroscopic tumors. Cancer Res. 2008;68:9451–9458. doi: 10.1158/0008-5472.CAN-08-1464. [DOI] [PubMed] [Google Scholar]
- 76.Shevchenko I, Bazhin AV, Umansky V. Comment on “Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b(+)Gr1(+) cells”. J Immunol. 2012;188:2929–2930. doi: 10.4049/jimmunol.1290007. [DOI] [PubMed] [Google Scholar]
- 77.Hyman MC, Petrovic-Djergovic D, Visovatti SH, Liao H, Yanamadala S, Bouis D, Su EJ, Lawrence DA, Broekman MJ, Marcus AJ, Pinsky DJ. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 81.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC, Eberl G, Pallandre JR, Borg C, Ryffel B, Apetoh L, Rebe C, Ghiringhelli F. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 82.Umansky V, Sevko A. Overcoming immunosuppression in the melanoma microenvironment induced by chronic inflammation. Cancer Immunol Immunother. 2012;61:275–282. doi: 10.1007/s00262-011-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 87.Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013;2:e26246. doi: 10.4161/onci.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]