Abstract
Activated T cells from patients with chronic lymphocytic leukemia (CLL) provide survival and proliferative signals to the leukemic clone within lymphoid tissues. Recruitment of both, CLL cells and T lymphocytes, to this supportive microenvironment greatly depends on CXCL12 production by stromal and myeloid cells. CXCL12 also supplies survival stimuli to leukemic B cells, but whether it exerts stimulatory effects on T lymphocytes from CLL patients is unknown. In order to evaluate the capacity of CXCL12 to increase CD4+ T cell activation and proliferation in CLL patients, peripheral blood mononuclear cells were cultured with or without recombinant human CXCL12 or autologous nurse-like cells, and then T cell activation was induced by anti-CD3 mAb. CXCL12 increases the proliferation and the expression of CD25, CD69, CD154, and IFNγ on CD3-stimulated CD4+ T cells from CLL patients, similarly in T cells from ZAP-70+ to ZAP-70− patients. Autologous nurse-like cells establish a close contact with CD4+ T cells and increase their activation and proliferation partially through a CXCR4-dependent mechanism. In addition, we found that activated T cells in the presence of CXCL12 enhance the activation and proliferation of the leukemic clone. In conclusion, CXCL12 production by lymphoid tissue microenvironment in CLL patients might play a key dual role on T cell physiology, functioning not only as a chemoattractant but also as a costimulatory factor for activated T cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1320-7) contains supplementary material, which is available to authorized users.
Keywords: Chronic lymphocytic leukemia, T cell activation, CXCL12, Nurse-like cells
Introduction
B cell chronic lymphocytic leukemia (CLL) is characterized by a progressive accumulation of long-lived mature monoclonal B lymphocytes. Proliferating leukemic cells are found in lymph nodes, bone marrow and other lymphoid tissues disposed in particular areas termed proliferation centers in close contact with activated T cells, stromal cells and accessory myeloid cells [1–4]. Although the T cell compartment in CLL patients presents numerous qualitative and quantitative abnormalities [5–8], several in vitro findings suggest that these cells, mainly CD4+CD40L+, provide a short-term support that enhances malignant B cell proliferation through cytokine secretion (i.e., IL-4 or IFNγ) and CD40/CD40L interactions [4, 9, 10]. Moreover, the in vivo importance of T cells in CLL has been recently highlighted by Bagnara et al. [11] who described a murine model in which transferred CLL cells proliferate in lymphoid tissues depending on the activation of autologous CD4+ T cells. On the other hand, it was shown that stromal cells and myeloid cells, such as nurse-like cells (NLC), present in the lymphoid tissue microenvironment provide a long-term support that favors the extended survival and accumulation of leukemic cells through cell–cell contact and/or the production of different soluble factors [12–15].
CXCL12 is a highly conserved chemokine, which seems to be essential for the recirculation of CLL cells between blood and the lymphoid organs [16]. NLC produce high levels of CXCL12 that not only play a key role in leukemic cell migration but also provides important survival stimuli to CLL cells [12, 17] protecting them from spontaneous and drug-induced apoptosis in vitro and likely in vivo [13]. In fact, using a phosphoproteomics approach to identify and compare phosphopeptides in unstimulated and CXCL12-stimulated CLL cells, it was recently reported that CXCL12 preferentially activates survival signaling rather than those involved in cell migration [18]. CXCR4, the main CXCL12 receptor, is expressed not only by CLL cells [16] but also by non-malignant T cells [19]. We have previously reported that T cells from CLL patients are able to migrate toward CXCL12 [19]. Considering that both CXCL12 and activated T lymphocytes participate in the scenario that favors leukemic progression in lymphoid tissues [14], the aim of the present work was to evaluate whether CXCL12 may enhance the activation and proliferation of T cells from CLL patients.
Methods
Reagents and antibodies
RPMI 1640 was purchased from Life Technologies (Grand Island, NY, USA). Fetal calf serum (FCS), penicillin and streptomycin were obtained from GIBCO Laboratories (Grand Island, NY, USA). The human recombinant chemokine CXCL12/SDF-1 was purchased from PeproTech (DF, Mexico). Bovine serum albumin (BSA) was obtained from Weiner Laboratorios (Santa Fé, Argentina). The Fix&Perm Kit for intra cellular staining was purchased from Caltag Laboratories (Burlingan, CA, USA). Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or Peridinin Chlorophyll Protein Complex (PerCP-Cy™5.5)-conjugated monoclonal antibodies (mAbs) specific for CD3 (clone SK7), CD4 (clone RPA-T4 and clone SK3), CD8 (clone HIT8a), CD19 (clone SJ25C1), CXCR4 (clone 12G5), IFNγ (clone 4S.B3), CD40L (clone TRAP1), ZAP-70 (clone 1E7.2), Ig kappa light chain (clone G20-193), Ig lambda light chain (clone JDC-12) and control antibodies with irrelevant specificities (isotype control) were purchased from BD Biosciences, Pharmigen (CA, USA). PE-conjugated mAb specific for CD56 (clone N901) was obtained from Immunotech (Marseille, France) and FITC-conjugated mAb specific for CD19 (clone HIB19) was obtained from BioLegend (San Diego, CA, USA). Carboxyfluorescein succinimidyl ester (CFSE) was purchased from Invitrogen Argentina Ltd (Bs. As. Argentine). For T cell purification, purified mAbs specific for CD19 (clone HIB19), CD14 (clone M5E2) and CD56 (clone B159) were purchased from Immunotech and magnetic beads (MagnaBind Beads) from Pierce (Rockford, IL, USA). Anti-CXCR4 blocking antibody (clone 12G5) was kindly provided by Dr. Ana Ceballos from the Centro Nacional de Referencia para SIDA, Facultad de Medicina, Universidad de Buenos Aires (Argentine). For T cell activation, we employed the mAb specific for human CD3 (clone UCHT1), which was kindly provided by Dr. Claire Hivroz from Institut Curie (Paris, France). For nucleus staining, we used TO-PRO®-3 from Invitrogen (Bs. As. Argentine) and propidium iodide from Sigma Aldrich (Bs. As. Argentine). Brefeldin A (BD GolgiPlug™) and monensin (BD GolgiStop™) were purchased from BD Biosciences.
Chronic lymphocytic leukemia patients
Sterile heparinized peripheral blood was obtained from CLL patients after informed consent in accordance with the Declaration of Helsinki and with Institutional Review Board approval from the National Academy of Medicine, Buenos Aires. CLL was diagnosed according to standard clinical and laboratory criteria. At the time of the analysis, all patients were free from clinically relevant infectious complications and were either untreated or had not received treatment for a period of at least 6 months before investigation.
Cell separation procedures
Peripheral blood mononuclear cells (PBMC) were isolated from fresh blood samples as previously described [19] and were immediately used or cryopreservated in FCS 20 % Dimethylsulphoxide for further experiments with nurse-like cells (see below). Purified T cells (pT) from CLL patients were obtained by negative selection as previously reported [19]. The purity of T cell population was checked by using a flow cytometer (BD, Immunocytometry System, San Jose, California) analysis using anti-CD3 mAb and was found to be always more than 90 %.
Cell culture and T cell activation
After PBMC isolation or T cell purification, cells were cultured (1 × 106 cells/ml) for 2 h in complete medium (RPMI 1640 supplemented with 10 % FCS, 100 U/ml penicillin and 100 μg/ml streptomycin) at 37 °C to recover CXCR4 expression [19, 20]. Afterward, cells were treated with or without the optimal concentration of CXCL12 (1 μg/ml), which was previously determined by evaluating the T cell migratory response toward the chemokine (see Supplementary Fig. 1) [19]. After 2 h of culture, cells were transferred to 48-well cell culture plate with immobilized anti-CD3 mAb (500 ng/well) or the corresponding isotype control antibody. For CXCR4 blocking experiments, PBMC cells were pre-incubated with 50 μg/ml of mAb anti-CXCR4 (12G5) at 37 °C for 1 h before CXCL12 treatment [20].
Nurse-like cell (NLC) differentiation was performed as previously described [13]. Briefly, 5 × 106 cells PBMC from CLL patients were cultured in 1 ml of complete medium in 24-well cell culture plate, and after 14 days of culture, non-adherent cells were removed and NLC (large, round, adherent cells) were used for co-stimulation experiments. To this aim, cryopreservated PBMC from CLL patients were thawed and cultured for 2 h with or without autologous NLC. Then, polyestyrene beads coated with anti-CD3 mAb or the corresponding control antibody were added and CD25 and CD69 expression was measured on CD4+ T cells after 24 h of culture. For CXCR4 blocking experiments, PBMC cells were pre-incubated with 50 μg/ml of mAb anti-CXCR4 (12G5) at 37 °C for 1 h before NLC coculture. Viability of thawed PBMC included in the experiments was always more than 85 %, as determined by fluorescence microscopy using acridine orange and ethidium bromide [21].
Flow cytometry analysis
Evaluation of ZAP-70 expression in CLL cells by flow cytometry
ZAP-70 expression was evaluated by flow cytometry as previously described [19]. Patients were considered ZAP-70+ when 20 % or more of the CD19+ cells express ZAP-70 [22].
Analysis of CD69, CD25, CD154, IFNγ, CD3 and CXCR4 expression in CD4+ T cells by flow cytometry
The expression of CD25 and CD69 on CD4+ T cells were evaluated by flow cytometry after 24 h of culture. To this aim, PBMC or pT cells were stained with PE-conjugated mAb specific for CD25, CD69 or isotype control, and FITC- mAb specific for CD4.
CD154 expression was measured as previously described [23]. Briefly, FITC-conjugated anti-CD154 mAb and monensin (2 μM) were added to the culture during the stimulation, cells were collected after 24 h of culture and CD154 expression was analyzed by flow cytometry on CD4+ T cells.
Intracellular IFNγ expression was measured by adding brefeldin A (10 μg/ml) during the last 6 h of culture in order to inhibit cytokine release. IFNγ producing cells were detected by flow cytometry at 24 h of culture using antibodies against IFNγ (PE) and CD4 (FITC).
The expression of CD3 on CD4+ T cells treated or not with CXCL12 (1 μg/ml) for 2 h was evaluated by using mAb specifics for CD3 or isotype control (PerCP), CD4 (FITC) and CD56 (PE).
CXCR4 expression on CD4+ T cells was analyzed by flow cytometry using mAbs specific for CXCR4 (PE), CD3 (PerCP) and CD4 (FITC). The basal expression of CXCR4 was evaluated after PBMC isolation, as we have previously detailed [19]. PBMC were then cultured for 14 days in compete medium for NLC differentiation as described above. Afterward, non-adherent cells were collected, washed and plated back onto the adherent NLC (NLC coculture) or plated alone in complete medium (24 h alone after NLC coculture). After 24 h of culture, CXCR4 expression on CD4+ T cells was analyzed by flow cytometry.
Assessment of CD19+ and CD4+ T cell proliferation by flow cytometry
To evaluate CD19+ and CD4+ T cell proliferation, the CFSE dilution assay was used [24, 25]. To this aim, PBMC were labeled with CFSE 5 μM for 20 min at 37 °C and then washed three times with RPMI 1640 5 %. Afterward, cells were pre-treated with CXCL12 (1 μg/ml) and then transfer to a 48-well culture plate with immobilized anti-CD3 mAb or isotype control (1 μg/well). In the case of NLC cocultures, thawed PBMC from CLL patients were labeled with CFSE and cultured as described above. After 5 days of culture, cells were collected, washed and stained with mAb specific for CD19 or CD4 (PerCP). CFSE dilution was measured on CD19+ or CD4+ T cells population by flow cytometry. Viable cells were gated according to FSC and SSC parameters criteria. The number of cells that had proliferated was determined by gating on the CD19+ or CD4+ CFSElow subset.
Confocal microscopy
PBMC from CLL patients were cultured on chambered microscope slide (1 × 106 cells PBMC in 0.3 ml of complete medium). After 14 days of culture, non-adherent cells were removed and adherent NLC were cocultured with thawed autologous PBMC for 24 h. Then, cell were gently washed with PBS, fixed with paraformaldehyde 3 % in PBS for 30 min, treated with glycyne 0.1 M for 10 min, washed twice with PBS and stained with FITC- or PE- mAb specific for CD19, CD3 or CD4 and TOPRO-3 or Propidium Iodure for nuclei staining. Coverslips were mounted on the microscope slide using Fluoromount G. Immunofluorescence images were acquired with a FluoView FV1000 confocal microscope (Olympus, Tokio, Japan) using a Plapon 60 X 1.42 NA oil immersion objective and images were analyzed using the Olympus FV10-ASW software.
Statistics
Statistical significance was determined using the nonparametric tests: Wilcoxon’s signed rank test or Mann–Whitney test. In all cases, p < 0.05 was considered statistically significant.
Results
CXCL12 increases the expression of activation markers on CD3-stimulated CD4+ T cells from CLL patients
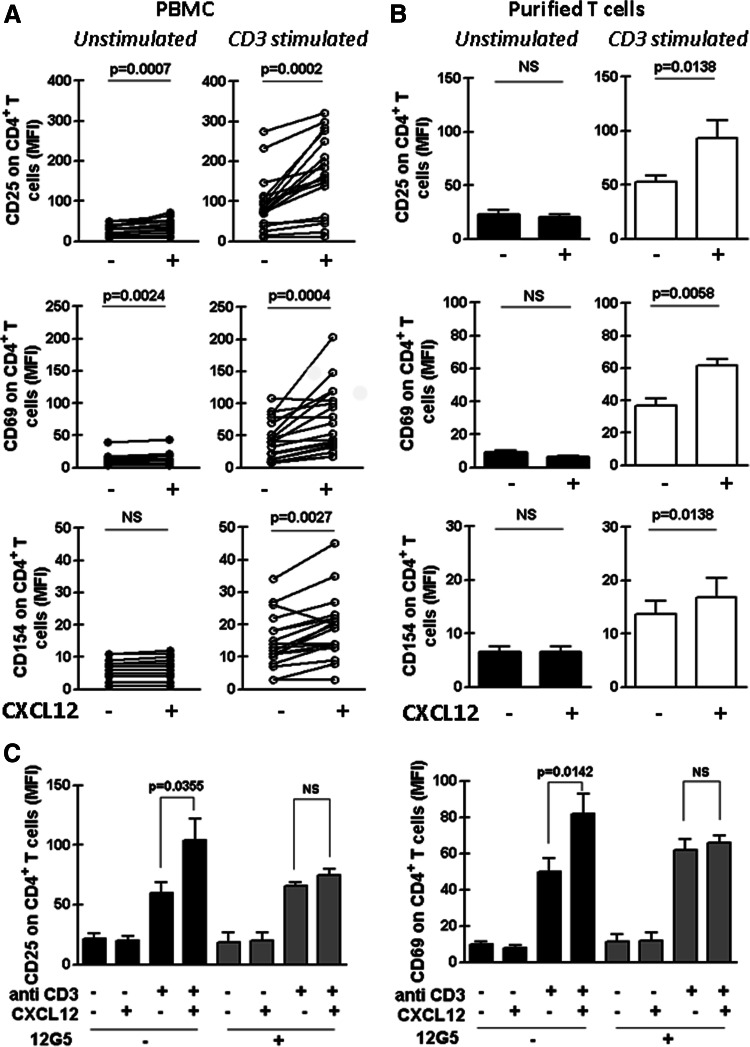
In order to evaluate the capacity of CXCL12 to increase CD4+ T cell activation in CLL patients, PBMC from these patients were cultured for 2 h with or without the optimal concentration of recombinant human CXCL12 (see “Methods” section) [19] and then transferred to 48-well cell culture plates with immobilized anti-CD3 mAb or the corresponding isotype control antibody. After 24 h of culture, the expression of the activation markers CD25, CD69 and CD154 on CD4+ T cells was evaluated by flow cytometry. As expected, stimulation of PBMC with anti-CD3 increased the basal mean fluorescence intensity (MFI) of CD25, CD69 and CD154 on CD4+ T cells (p = 0.0002; p = 0.0002 and p = 0.0003, respectively, Wilcoxon’s signed rank test). As it is shown in Fig. 1a (left panels), CXCL12 alone did not modify or slightly augmented the MFI of the activation markers on unstimulated CD4+ T cells. Notably, the combination of anti-CD3 and CXCL12 significantly increased the expression of all activation markers beyond the one induced by anti-CD3 alone (Fig. 1a, right panels). Similar results were obtained when the percentages of CD4+ T cells expressing CD25, CD69 and CD154 were analyzed (Supplementary Fig. 2a). Representative histograms are shown in Supplementary Fig. 3. The CXCL12-mediated enhancement of CD4+ T cell activation was also evident when we performed the experiments with purified T cells (Fig. 1b and Supplementary Fig. 2b) and was blocked by the anti-CXCR4 mAb, 12G5 (Fig. 1c and Supplementary Fig. 2c). In addition, the costimulatory capacity of CXCL12 depended on its concentration since the lower the concentration of CXCL12 employed, the less obvious the T cell costimulation was (Supplementary Fig. 4a) and was not related to an increment in surface CD3 molecules on T cells upon CXCL12 treatment since the chemokine did not modify the CD3 surface expression (Supplementary Fig. 4b).
Fig. 1.
CXCL12 increases the expression of CD25, CD69 and CD154 on CD3-stimulated CD4+ T cells from CLL patients. PBMC from 18 CLL patients (a) or purified T cells from 9 CLL patients (b) were pre-treated or not with CXCL12 (1 μg/ml) for 2 h and then transferred to a well with immobilized anti-CD3 mAb (CD3 stimulated) or the corresponding isotype control antibody (Unstimulated). After 24 h of culture, CD25, CD69 and CD154 expression on CD4+ T cells was analyzed by flow cytometry. The figure shows the values of the mean fluorescence intensity (MFI) of CD25, CD69 and CD154 on CD4+ T cells of each patient obtained in PBMC cultures (a) or with purified T cell (b). c PBMC from 8 CLL patients were pre-treated anti CXCR4 mAb (12G5) or the corresponding isotype control antibody for 1 h before the co-stimulation assay. The bars represent the mean values ± SEM for the MFI of the activation marker on CD4+ T cells. The p values for the statistical analysis with the Wilcoxon’s signed rank test are shown in the figure. NS not statistically significant
CLL patients with poor prognosis typically display leukemic cells with B cell receptors encoded by unmutated immunoglobulin variable heavy-chain genes (IgVH) express the protein tyrosine kinase ZAP-70 and the type II transmembrane glycoprotein CD38 [26–29]. By contrast, mutated IgVH genes and the absence of ZAP-70 and CD38 are mostly found in leukemic cells from good prognostic patients, who exhibit a more stable, indolent disease, with no benefit from palliative chemotherapy [30]. We have previously reported that T cells from ZAP-70− samples showed significantly less migratory capacity compared to T cells from ZAP-70+ patients although they showed similar CXCR4 expression. This observation prompted us to compare the costimulatory capacity of CXCL12 in CD4+ T cells from ZAP-70 positive (n = 10) and negative (n = 8) CLL patients. As it is shown in Supplementary Fig. 5, there was no difference between groups in the expression of CD25, CD69 or CD154 on activated CD4+ T cells incubated in the presence or absence of CXCL12.
CXCL12 enhances IFNγ production and proliferation of activated CD4+ T cells from CLL patients
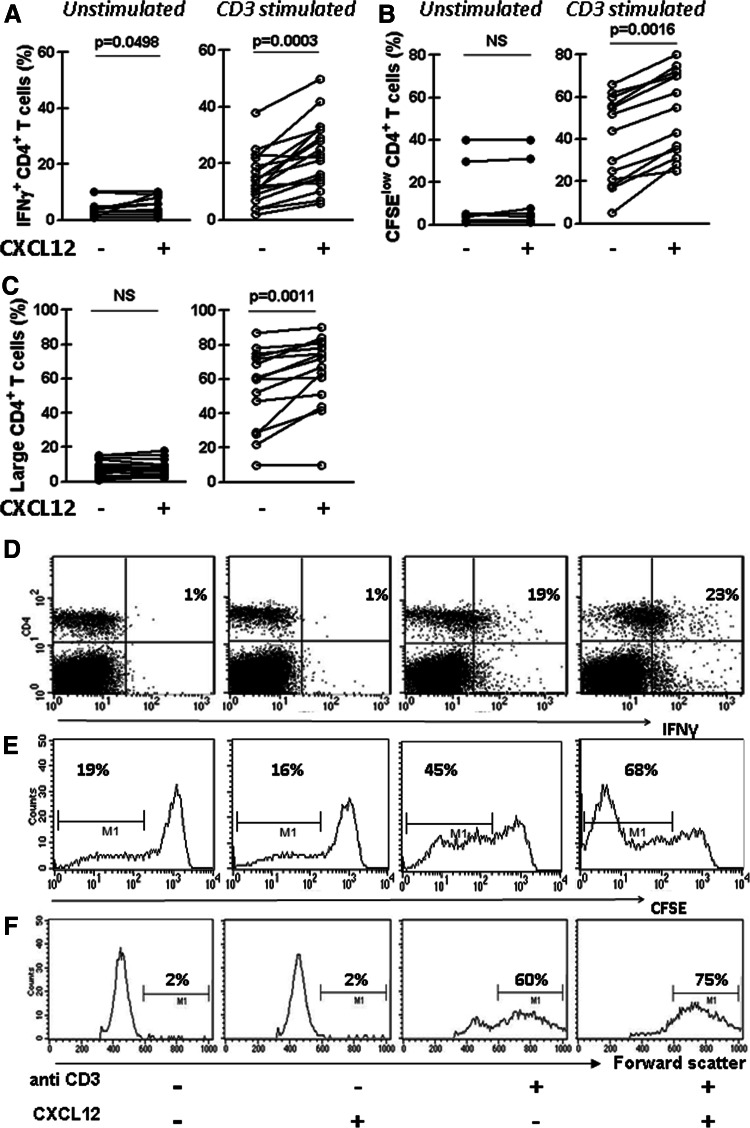
The cytokine network between CLL cells and T lymphocytes could sustain the survival and/or the expansion of the leukemic clone. Among them, the IFNγ network may play an important role since leukemic cells express high levels of IFNγ receptors whose signaling was shown to inhibit their spontaneous and drug-induced apoptosis [31–33]. In order to evaluate the capacity of CXCL12 to increase the production of IFNγ by activated CD4+ T cells from CLL patients, PBMC were treated as mentioned above and intracellular IFNγ was measured in CD4+ T cells by flow cytometry. We found that stimulation of these cells with plate-bound anti-CD3 mAb increased the intracellular expression of IFNγ by CD4+ T cells (p = 0.0002, Wilcoxon’s signed rank test), which was significantly higher when CXCL12 is also present during the activation (Fig. 2a). The results obtained with a representative CLL patient are depicted in Fig. 2d. We next evaluated the capacity of CXCL12 to increase anti-CD3 induced proliferation of CD4+ T cells from CLL patients by using the CFSE dilution assay [24]. To this aim, CFSE-labeled PBMC from CLL patients were cultured for 5 days with or without plate-bound anti-CD3 mAb in the presence or absence of CXCL12, and then the percentage of CD4+ T cells with low CFSE label was assessed by flow cytometry. We found that CXCL12 in combination with anti-CD3 significantly enhanced the percentage of CD4+ T cells with low CFSE (Fig. 2b) showing that CXCL12 was also able to costimulate the anti-CD3 induced proliferation of CD4+ T cells from CLL patients. The results obtained with a representative CLL case are depicted in Fig. 2e. As expected, when we performed a forward/side scatter analysis (which reflects cell size and can be a measure for activation) of the cultured PBMC from CLL patients, we observed that the presence of anti-CD3 with recombinant CXCL12 in the culture induced the highest accumulation of enlarged CD4+ T cells (Fig. 2c). A representative CLL case is shown in Fig. 2f.
Fig. 2.
CXCL12 enhances IFNγ production and proliferation of activated CD4+ T cells from CLL patients. PBMC from CLL patients were cultured as described in Fig. 1. a After 24 h of culture, intracellular IFNγ in CD4+ T cells was analyzed by flow cytometry. The figure shows the percentage of CD4+ T cells expressing IFNγ of each patient (n = 18). b CFSE-labeled PBMC from 13 CLL patients were cultured as mentioned above, and after 5 days of culture, cells were collected, stained with specific mAb for CD4 (PerCP) and then analyzed by flow cytometry. The figure shows the percentage of CD4+ CFSElow of the total CD4+ viable lymphocytes of each patient. c PBMC from 15 CLL patients were cultured as mentioned above and after 5 days of culture CD4+ T cell enlargement was evaluated by performing a forward scatter analysis of the CD4+ cells. The figure shows the percentage of large CD4+ T cells determinate as the percentage of the total of CD4+ viable lymphocytes with forward scatter values over 600. d–f Representative dot plots for IFNγ production by CD4+ cells (d), histograms for CFSE staining in CD4+ cells (e) and for forward scatter analysis of CD4+ cells (f) are shown. The percentages of positive cells in each case are depicted. The p values for the statistical analysis with the Wilcoxon’s signed rank test are shown in the figure. NS not statistically significant
Activated T cells in the presence of CXCL12 enhance the activation and proliferation of the leukemic clone
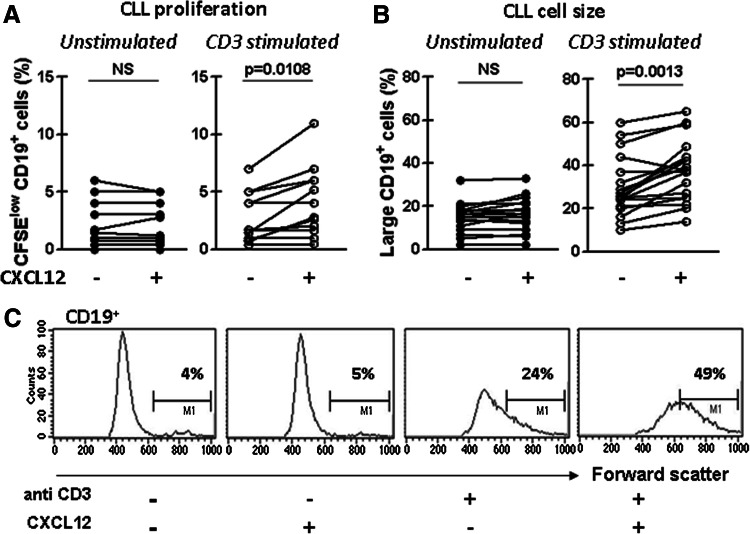
Since activated T cells are known to contact proliferating tumor cells in proliferation centers [34], we investigated the effect of coculture with activated autologous T cells in the presence or absence of CXCL12 on the proliferation status of CLL cells. Thus, CFSE-labeled PBMC of CLL patients were cultured as mentioned above and the percentage of CD19+ cells expressing low CFSE was evaluated. As it was previously reported [35], we found that coculture of CLL cells with activated autologous T cells results in leukemic cell division as indicated by the higher proportion of CD19+ cells expressing low CFSE in CD3-stimulated cultures compared to unstimulated cultures (p = 0.0091, Wilcoxon’s signed rank test). Interestingly, while CXCL12 alone did only induce CLL cell proliferation, the highest proportion of CD19+ cells displaying low CFSE was observed when anti-CD3 and recombinant CXCL12 are both present in the culture (Fig. 3a). Light chain restriction was maintained at the end of the coculture period, confirming the expansion of the CLL clone and not residual polyclonal normal B cells (data not shown). As expected, when we evaluated the size of CD19+ cells by performing a forward/side scatter analysis, we observed that the presence of anti-CD3 with recombinant CXCL12 in the culture induced the highest accumulation of enlarged CD19+ cells (Fig. 3b). Representative histograms showing the proportion of CD19+ enlarged cells within each culture condition are shown in Fig. 3c. Altogether, these results show that leukemic cell activation and proliferation were enhanced by activated T cells in the presence of CXCL12.
Fig. 3.
Activated T cells in the presence of CXCL12 enhance the proliferation and enlargement of CD19+ cells from CLL patients. a CFSE-labeled PBMC from 12 CLL patients were pre-treated with CXCL12 (1 μg/ml) for 2 h and then transferred to a well with immobilized anti-CD3 mAb or the corresponding isotype control antibody. After 7 days of culture, cells were collected, stained with specific mAb for CD19 (PerCP) and then analyzed by flow cytometry. The figure shows the percentage of CD19+ CFSElow cells of the total of CD19+ viable lymphocytes of each patient. b PBMC from 18 CLL patients were cultured as mentioned above, and after 7 days of culture, cells were washed and stained with specific mAb for CD19 (PerCP), and the forward scatter (size) of the CD19+ cell population was analyzed by flow cytometry. Large CD19+ cells were determinate as the CD19+ cells with forward scatter values over 600. The figure shows the percentage of large CD19+ cells of the total of CD19+ viable lymphocytes. c Representative histograms for forward scatter analysis of CD19+ are shown. The p values for the statistical analysis with the Wilcoxon’s signed rank test are shown in the figure. NS not statistically significant
Autologous nurse-like cells increase the activation and proliferation of CD4+ T cells from CLL patients partially through a CXCR4-dependent mechanism
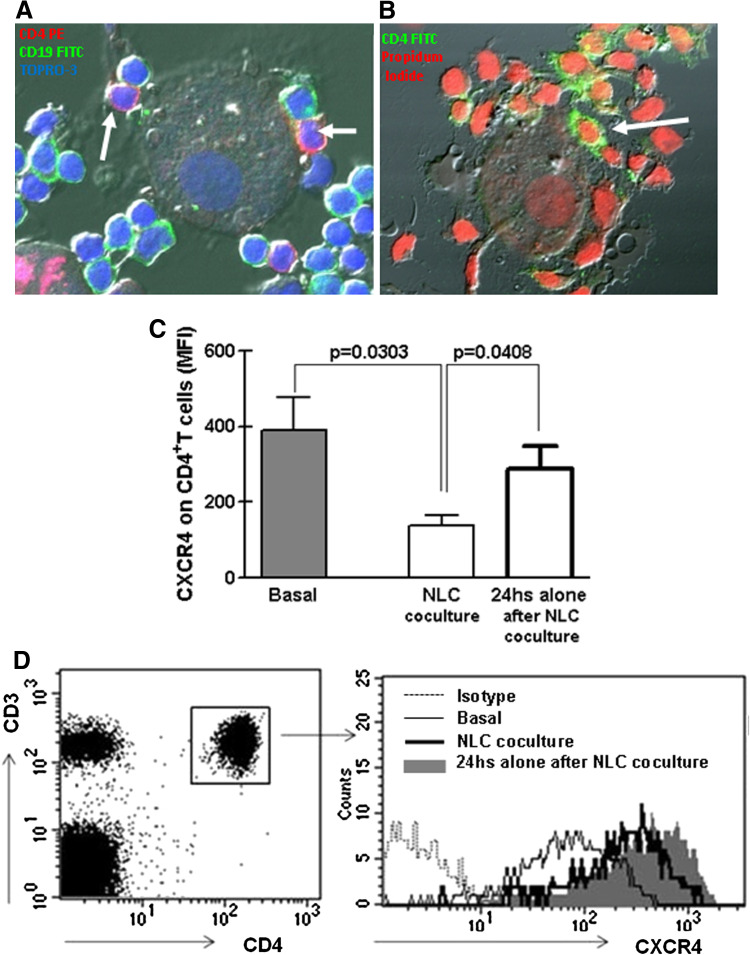
The main sources of CXCL12 in vivo are stromal and myeloid cells [16]. In vitro, CXCL12-producing cells can be obtained by differentiation of CD14+ mononuclear leukocytes into large, round and adherent cells called nurse-like cells [13]. NLC are in close contact with leukemic cells and protect them from apoptosis, partially through a CXCR4-dependent mechanism [13]. There is no information regarding the interaction of T lymphocytes with NLC or the role of CXCL12 released by NLC on these cells. To gain insight into NLC-T cells crosstalk in CLL, we first differentiated NLC and then added autologous peripheral blood cells (CLL plus T lymphocytes) to culture plates. As depicted in Fig. 4a and b, NLC were in close contact not only with CD19+ cells but also with CD4+ lymphocytes. To demonstrate that CXCL12 released by NLC interacted with CXCR4 expressed by T cells, we assessed whether CXCR4 was downregulated during coculture of T cells and NLC, as chemokine receptor internalization by endocytosis is a normal step upon chemokine–chemokine receptor interaction [13, 36]. Compared with basal expression, there was a marked reduction in the staining of CXCR4 on CD4+ T cells cultured with NLC (Fig. 4c). The expression was recovered when T cells were separated from NLC and cultured in complete medium alone for 24 h (Fig. 4c). A representative case is shown in Fig. 4d.
Fig. 4.
CD4+ T cells from CLL patients are in close contact with nurse-like cells. PBMC from CLL patients were cultured for 14 days in complete medium in a chambered microscope slide. Then, non-adherent cells were removed and NLC were cocultured with autologuous thawed PBMC for 24 h as detailed in materials and methods. Then, cell were washed with PBS, fixed and stained with FITC-labeled mAb specific for CD19, PE-labeled mAb specific for CD4 (clone SK3) and TOPRO-3 in a and with FITC-labeled mAb specific for CD4 (clone RPA-T4) and Propidium Iodide in b. The images were acquired with a FluoView FV1000 confocal microscope and analyzed using the Olympus FV10-ASW software. The arrows indicate CD4+ T cells attached to NLC from a representative CLL case (n = 5). c CXCR4 expression on CD4+ T cells from 6 CLL patients was analyzed by flow cytometry after PBMC isolation (basal). Then, PBMC were cultured for 14 days in complete medium for NLC differentiation, and afterward, non-adherent cells were collected, washed and cultured back with autologous NLC (NLC coculture) or alone for another 24 h (24 h alone after NLC coculture). Then, the expression of CXCR4 on CD4+ T cells was determined by flow cytometry. The bars represent the mean values ± SEM for the MFI of CXCR4 expression on CD4 + T cells. The p values for the statistical analysis with the Mann–Whitney test are shown in the figure. d The results obtained with a representative CLL patient are shown
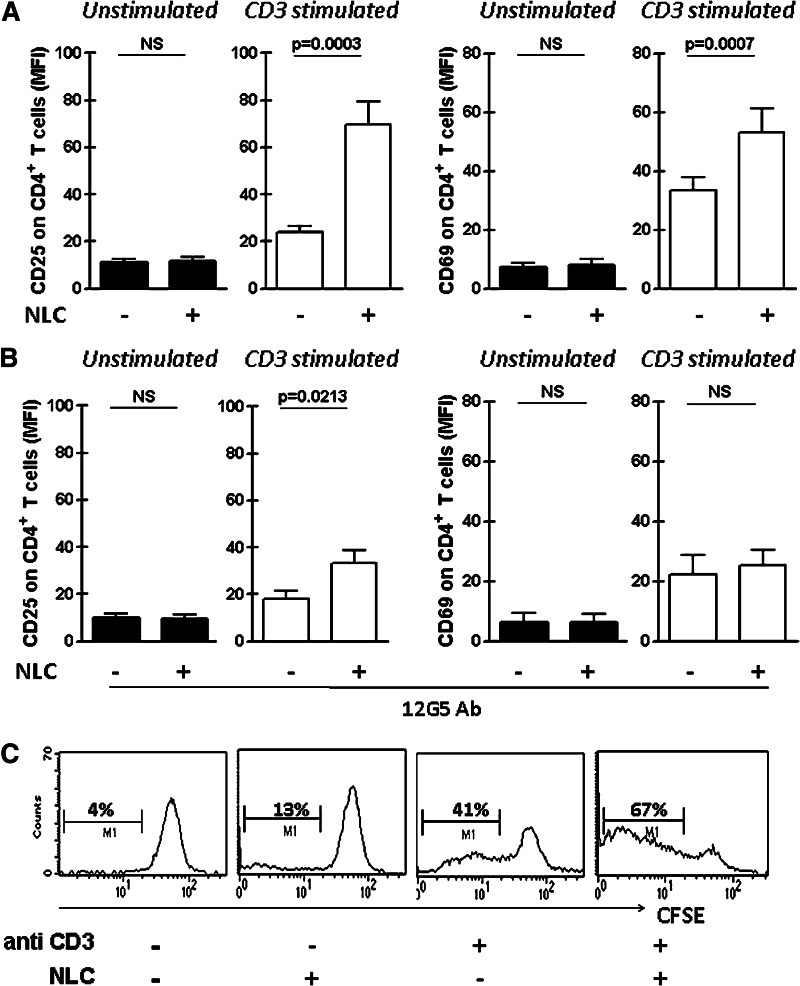
Then, we evaluated whether the presence of NLC could increase the activation of CD4+ T cells from CLL patients and to that aim we stimulated T lymphocytes with anti-CD3 coated polysterene beads in the presence or absence of autologous NLC. After 24 h, CD25 and CD69 expression on CD4+ T cells was analyzed by flow cytometry. We observed a significantly higher expression of CD25 and CD69 in CD4+ T cells activated in the presence of NLC (Fig. 5a). Similarly, we found that CD4+ T cell proliferation was enhanced in the presence of NLC (CFSClowCD4+ T cells (%): 6 ± 2 vs 8 ± 2 vs 35 ± 6 vs 62 ± 5, mean ± SEM for control vs NLC vs anti-CD3 vs NLC + anti-CD3 cultures, n = 8, *p < 0.01 for anti-CD3 vs NLC+ anti-CD3, Wilcoxon’s signed rank test). A representative case is shown in Fig. 5c. By comparing the costimulatory capacity of NLC in the absence or presence of the anti-CXCR4 blocking mAb (12G5), we observed that the pre-treatment of PBMC with 12G5 partially impaired its costimulatory capacity (Fig. 5b). Altogether, our results suggest that CXCL12 production by NLC and its interaction with CXCR4 on T cells is partially responsible for the enhanced activation and proliferation of CD4+ T cells from CLL patients.
Fig. 5.
Autologous nurse-like cells increase the expression of activation markers on CD3-stimulated CD4+ T cells from CLL patients partially through a CXCR4-dependent mechanism. NLC were obtained by culturing PBMC from CLL patients in complete medium for 14 days. Then, thawed PBMC were cultured in the presence or absence of autologous NLC and then activated with anti-CD3 (or isotype control antibody) coated polystyrene beads. After 24 h of culture, CD25 and CD69 expression was analyzed on CD4+ T cells by flow cytometry. a The bars represent the mean values ± SEM for the mean fluorescence intensity (MFI) of CD25 and CD69 on CD4+ T cells (n = 17). b For blocking experiments, PBMC from 7 CLL patients were pre-treated with 50 μg/ml of anti CXCR4 (12G5) or the corresponding isotype control for 1 h and then activated as described above. After 24 h of culture, CD25 and CD69 expression on CD4+ T cells was analyzed by flow cytometry. The bars represent the mean values ± SEM for the mean fluorescence intensity (MFI) of CD25 and CD69 on CD4+ T cells. The p values for the statistical analysis with the Wilcoxon’s signed rank test are shown in the figure. NS not statistically significant. c Proliferation of CD4+ T cells was analyzed using the dilution CFSE assay. To this aim, thawed CFSE-labeled PBMC were cultured in the presence or absence of autologous NLC and then activated with anti-CD3 (or isotype control antibody) coated polystyrene beads. After 5 days of culture, non-adherent cells were collected, stained with specific mAb for CD4 (PerCP) and then analyzed by flow cytometry. Proliferation of CD4+ T cells was calculated as the percentage of CD4+ CFSElow of the total CD4+ viable cells. Representative histograms are shown (n = 8)
Discussion
The classical view of chemokine receptor signaling and function proposes that when engaged by their specific ligands, chemokine receptors induce cell migration via a Gi-mediated signaling pathway. However, in CLL patients, CXCL12 secretion in particular tissue microenvironment seems to be a key step not only for leukemic cell recirculation from blood to lymphoid organs but also for CLL cell survival. Along this line, it was reported that CXCL12 provides survival stimuli to CLL cells and partially protects them from spontaneous and drug-induced apoptosis [13] and may preferentially activate survival signaling pathways rather than those involved in cell migration in CLL cells [18]. Not only leukemic cells from CLL patients but also T lymphocytes express CXCR4 and are capable to respond to CXCL12 stimulus. We have previously demonstrated that T cells from CLL patients migrate toward CXCL12 [19]. However, besides its role in chemoattraction, there is no information about CXCL12 effect on CLL T cells. We here show that recombinant CXCL12 increases the expression of the activation markers CD25, CD69 and CD154, the proliferation and the production of the anti-apoptotic cytokine IFNγ by activated CD4+ T cells from CLL patients, suggesting that CXCL12 may play an important role not only in CLL T cell migration [19] but also in the effective activation of T lymphocytes. Our results are in line with those obtained with T cells from healthy individuals which described that CXCL12 costimulates various TCR/CD3 mediated responses [20, 37, 38]. Our interest was focused on CD4+ T cells because this subpopulation was shown to participate in the proliferation centers [1–4, 35] and plays a pivotal role for in vivo CLL cells expansion [11]. However, in some experiments (n = 7), we also evaluated the CD8+ T cells and found that recombinant CXCL12 increases the expression of the activation markers CD25 and CD69 by activated CD8+ T cells from CLL patients (p < 0.05), slightly increases the production of IFNγ (p = 0.0489) and does not modify CD8+ T cell proliferation (p > 0.05), suggesting that CXCL12 might also regulate some aspects of CD8+ T cell physiology.
Although we have previously found that CXCL12-induced T cell chemotaxis is impaired in T cells from ZAP-70 negative compared to ZAP-70 positive CLL patients [19], when we compared the capacity of CXCL12 to enhance the expression of CD25, CD69 and CD154 on activated CD4+ T cells from ZAP-70 positive and negative CLL patients, no significant differences were found between groups. This observation suggests that the signaling pathways of CXCR4 that increases CD3-induced cell activation and proliferation seems to be different to those involved in CXCR4-mediated T cell chemotaxis. Given that the cells were first incubated for 2 h with CXCL12 and then transferred to anti-CD3 coated plates to activate T lymphocytes, a simple explanation to the costimulatory capacity of CXCL12 could be that the chemokine enhanced the expression of CD3 on the surface of T lymphocytes. However, this was not the case since CD3 expression did not significantly change during the 2 h of culture with CXCL12. While in T cells from healthy donors, CXCL12 was shown to stimulate the physical association of CXCR4 and the TCR/CD3 [37, 39] and utilizes the ZAP-70 binding ITAM domains of the TCR/CD3 for signal transduction [39] and its costimulatory capacity involved a robust AP-1 transcriptional activity [39], it remains to be determined whether these signaling pathways are involved in the CXCL12 costimulation of activated CD4+ T cells from CLL patients. In addition, although it was clearly stated that CXCR4 is the main CXCL12 receptor, it was reported that CXCL12 could associate with heparan sulfate [40] and also binds to a non-CXCR4 receptor [41] which is expressed on T cells from CLL patients [19], suggesting that CXCR4 may not be a unique ligand for this chemokine. However, the current data showed that anti-CXCR4 mAb (12G5) blocked the costimulation of anti-CD3-activated CD4+ T cells by recombinant CXCL12, establishing that this receptor is necessary for the T cell costimulation by CXCL12.
Several in vitro and in vivo evidences indicate that T cell activation and proliferation is crucial to support the growth of leukemic cells [11, 14, 42]. Since we found that CXCL12 enhanced the in vitro activation and proliferation of T cells from CLL patients, we evaluated the activation and proliferation status of the CLL cells present is these cultures. We found that the combination of anti-CD3 and recombinant CXCL12 significantly increased the proportion of enlarged and proliferating CD19+ cells beyond the one induced by anti-CD3 alone. Thus, it is tempting to speculate that the highest CD19+ cell activation and proliferation observed in anti-CD3 and CXCL12 cultures was due to the presence of much more activated T cells. However, we cannot exclude the possibility that the presence of activated T cells and the interaction of CXCL12 with CXCR4 expressed by CD19+ cells may account for these results. Whatever happens, these results support the notion that activated T cells and CXCL12 directly affecting CLL cells and/or indirectly costimulating T lymphocytes favors the activation and proliferation of the leukemic clone.
We have previously shown that circulating monocytes from CLL patients play an important role in leukemic cell survival [43]. In addition, monocytes can differentiate in vitro into NLC that protect leukemic cells from spontaneous and drug-induced apoptosis partially through a CXCL12-dependent mechanism [13]. Since NLC from CLL patients were shown to produce high amounts of CXCL12, we wondered whether these cells may interact with T cells favoring its activation. We consistently observed that NLC undoubtedly attached not only to leukemic cells but also to CD4+ T cells from CLL patients, suggesting that NLC might be a suitable area to favor the crosstalk between B and T lymphocytes in CLL patients. We also found that CXCL12 produced by NLC was able to interact with T cells from CLL patients since it induced the expected CXCR4 internalization by endocytosis. Notably, the presence of NLC enhanced the activation and proliferation of autologous CD4+ T, suggesting that NLC may also be important for T cell physiology in CLL patients. The results obtained with the anti-CXCR4 blocking antibody (12G5) showed that the costimulatory capacity of NLC was partially mediated by a CXCR4 dependent mechanism.
Altogether, our results prompted us to hypothesize that CXCL2 production by lymphoid tissue microenvironment in CLL patients may play key dual role for T cell physiology, functioning not only as a chemoattractant but also as a costimulatory factor for activated T cells. Since it was clearly demonstrated that activated T cells are crucial in the disease, identifying the mechanism(s) whereby T cells respond to microenvironmental activating and proliferating signals could reveal novel targets for therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Beatriz Loria and Edit Mabel Horvat for technical assistance. We also thank CLL patients who participated in the study. This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica, CONICET and Fundación Roemmers. M. B. designed and did experiments and created the figures; P. R. N. and P. E. M contributed in the analysis and interpretation of the data; C. J. contributed in the analysis and interpretation of the data and collaborated in confocal microscopy assay; R. F. B. and A. B. provided patients samples and advice; M. G. participated in the project conception and critically reviewed the manuscript, which was written by M. B. and R. G.; R. G. designed and supervised the study.
Conflict of interest
The authors reported no potential conflicts of interest.
References
- 1.Rosati S, Kluin PM. Chronic lymphocytic leukaemia: a review of the immuno-architecture. Curr Top Microbiol Immunol. 2005;294:91–107. [PubMed] [Google Scholar]
- 2.Soma LA, Craig FE, Swerdlow SH. The proliferation center microenvironment and prognostic markers in chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum Pathol. 2006;37(2):152–159. doi: 10.1016/j.humpath.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 3.Ghia P, Circosta P, Scielzo C, Vallario A, Camporeale A, Granziero L, Caligaris-Cappio F. Differential effects on CLL cell survival exerted by different microenvironmental elements. Curr Top Microbiol Immunol. 2005;294:135–145. doi: 10.1007/3-540-29933-5_8. [DOI] [PubMed] [Google Scholar]
- 4.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123(3):380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 5.Shanafelt T, Kay N. T-cell abnormalities in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47(7):1197–1198. doi: 10.1080/10428190600687976. [DOI] [PubMed] [Google Scholar]
- 6.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44(3):383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, O’Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55(2):197–209. doi: 10.1007/s00262-005-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115(7):1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fluckiger AC, Rossi JF, Bussel A, Bryon P, Banchereau J, Defrance T. Responsiveness of chronic lymphocytic leukemia B cells activated via surface Igs or CD40 to B-cell tropic factors. Blood. 1992;80(12):3173–3181. [PubMed] [Google Scholar]
- 10.Buske C, Gogowski G, Schreiber K, Rave-Frank M, Hiddemann W, Wormann B. Stimulation of B-chronic lymphocytic leukemia cells by murine fibroblasts, IL-4, anti-CD40 antibodies, and the soluble CD40 ligand. Exp Hematol. 1997;25(4):329–337. [PubMed] [Google Scholar]
- 11.Bagnara D, Kaufman MS, Calissano C, Marsilio S, Patten PE, Simone R, Chum P, Yan XJ, Allen SL, Kolitz JE, Baskar S, Rader C, Mellstedt H, Rabbani H, Lee A, Gregersen PK, Rai KR, Chiorazzi N. A novel adoptive transfer model of chronic lymphocytic leukemia suggests a key role for T lymphocytes in the disease. Blood. 2011;117(20):5463–5472. doi: 10.1182/blood-2010-12-324210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC, Zvaifler NJ, Kipps TJ. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106(3):1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. [PubMed] [Google Scholar]
- 14.Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? J Clin Oncol. 2008;26(27):4497–4503. doi: 10.1200/JCO.2007.15.4393. [DOI] [PubMed] [Google Scholar]
- 15.Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]
- 16.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 17.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99(3):1030–1037. doi: 10.1182/blood.V99.3.1030. [DOI] [PubMed] [Google Scholar]
- 18.O’Hayre M, Salanga CL, Kipps TJ, Messmer D, Dorrestein PC, Handel TM. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PLoS One. 2010;5(7):e11716. doi: 10.1371/journal.pone.0011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borge M, Nannini PR, Galletti JG, Morande PE, Avalos JS, Bezares RF, Giordano M, Gamberale R. CXCL12-induced chemotaxis is impaired in T cells from patients with ZAP-70-negative chronic lymphocytic leukemia. Haematologica. 2010;95(5):768–775. doi: 10.3324/haematol.2009.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanki T, Lipsky PE. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164(10):5010–5014. doi: 10.4049/jimmunol.164.10.5010. [DOI] [PubMed] [Google Scholar]
- 21.Gamberale R, Fernandez-Calotti P, Sanjurjo J, Arrossagaray G, Avalos JS, Geffner J, Giordano M. Signaling capacity of FcgammaRII isoforms in B-CLL cells. Leuk Res. 2005;29(11):1277–1284. doi: 10.1016/j.leukres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, Marce S, Lopez-Guillermo A, Campo E, Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay PK, Yu J, Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat Protoc. 2006;1(1):1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- 24.Crawford MP, Yan SX, Ortega SB, Mehta RS, Hewitt RE, Price DA, Stastny P, Douek DC, Koup RA, Racke MK, Karandikar NJ. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103(11):4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 25.Galletti J, Canones C, Morande P, Borge M, Oppezzo P, Geffner J, Bezares R, Gamberale R, Giordano M. Chronic lymphocytic leukemia cells bind and present the erythrocyte protein band 3: possible role as initiators of autoimmune hemolytic anemia. J Immunol. 2008;181(5):3674–3683. doi: 10.4049/jimmunol.181.5.3674. [DOI] [PubMed] [Google Scholar]
- 26.D’Arena G, Tarnani M, Rumi C, Vaisitti T, Aydin S, De Filippi R, Perrone F, Pinto A, Chiusolo P, Deaglio S, Malavasi F, Laurenti L. Prognostic significance of combined analysis of ZAP-70 and CD38 in chronic lymphocytic leukemia. Am J Hematol. 2007;82(9):787–791. doi: 10.1002/ajh.20936. [DOI] [PubMed] [Google Scholar]
- 27.Del Giudice I, Morilla A, Osuji N, Matutes E, Morilla R, Burford A, Maravelaki S, Owusu-Ankomah K, Swansbury J, A’Hern R, Brito-Babapulle V, Catovsky D. Zeta-chain associated protein 70 and CD38 combined predict the time to first treatment in patients with chronic lymphocytic leukemia. Cancer. 2005;104(10):2124–2132. doi: 10.1002/cncr.21437. [DOI] [PubMed] [Google Scholar]
- 28.Schroers R, Griesinger F, Trumper L, Haase D, Kulle B, Klein-Hitpass L, Sellmann L, Duhrsen U, Durig J. Combined analysis of ZAP-70 and CD38 expression as a predictor of disease progression in B-cell chronic lymphocytic leukemia. Leukemia. 2005;19(5):750–758. doi: 10.1038/sj.leu.2403707. [DOI] [PubMed] [Google Scholar]
- 29.Hus I, Podhorecka M, Bojarska-Junak A, Rolinski J, Schmitt M, Sieklucka M, Wasik-Szczepanek E, Dmoszynska A. The clinical significance of ZAP-70 and CD38 expression in B-cell chronic lymphocytic leukaemia. Ann Oncol. 2006;17(4):683–690. doi: 10.1093/annonc/mdj120. [DOI] [PubMed] [Google Scholar]
- 30.Dighiero G, Binet JL. When and how to treat chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1799–1801. doi: 10.1056/NEJM200012143432410. [DOI] [PubMed] [Google Scholar]
- 31.Tangye SG, Weston KM, Raison RL. Cytokines and cross-linking of sIgM augment PMA-induced activation of human leukaemic CD5+ B cells. Immunol Cell Biol. 1997;75(6):561–567. doi: 10.1038/icb.1997.87. [DOI] [PubMed] [Google Scholar]
- 32.Buschle M, Campana D, Carding SR, Richard C, Hoffbrand AV, Brenner MK. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J Exp Med. 1993;177(1):213–218. doi: 10.1084/jem.177.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamberale R, Geffner JR, Giordano M. Immune complexes and apoptosis in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(2):251–255. doi: 10.1080/10428190290006008. [DOI] [PubMed] [Google Scholar]
- 34.Ghia P, Strola G, Granziero L, Geuna M, Guida G, Sallusto F, Ruffing N, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Chronic lymphocytic leukemia B cells are endowed with the capacity to attract CD4+, CD40L+ T cells by producing CCL22. Eur J Immunol. 2002;32(5):1403–1413. doi: 10.1002/1521-4141(200205)32:5<1403::AID-IMMU1403>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Patten PE, Buggins AG, Richards J, Wotherspoon A, Salisbury J, Mufti GJ, Hamblin TJ, Devereux S. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111(10):5173–5181. doi: 10.1182/blood-2007-08-108605. [DOI] [PubMed] [Google Scholar]
- 36.Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065X.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- 37.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6(5):465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol. 2001;167(6):3064–3073. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25(2):213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274(34):23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 41.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 42.Granziero L, Ghia P, Circosta P, Gottardi D, Strola G, Geuna M, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97(9):2777–2783. doi: 10.1182/blood.V97.9.2777. [DOI] [PubMed] [Google Scholar]
- 43.Gamberale R, Geffner J, Arrosagaray G, Scolnik M, Salamone G, Trevani A, Vermeulen M, Giordano M. Non-malignant leukocytes delay spontaneous B-CLL cell apoptosis. Leukemia. 2001;15(12):1860–1867. doi: 10.1038/sj.leu.2402288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.