Abstract
Purpose
To determine whether abagovomab induces protective immune responses in ovarian cancer patients in first clinical remission. The present analysis is a substudy of monoclonal antibody immunotherapy for malignancies of the ovary by subcutaneous abagovomab trial (NCT00418574).
Methods
The study included 129 patients, 91 in the abagovomab arm and 38 in the placebo arm. Circulating CA125-specific cytotoxic T lymphocytes (CTL) were measured by a flow cytometry-based interferon-γ producing assay. Human antimouse antibody and anti-anti-idiotypic (Ab3) were assessed by ELISA. Patients were evaluated before starting the treatment and at different time points during induction and maintenance phases.
Results
A similar percentage of patients in both the placebo and abagovomab arms had CA125-specific CTL (26.3 and 31.8 %, respectively; p = 0.673 by Fisher’s exact test). Patients with CA125-specific CTL in both arms tended to have an increased relapse-free survival (RFS, log-rank test p = 0.095) compared to patients without. Patients (n = 27) in the abagovomab arm without CA125-specific CTL but that developed Ab3 above the cutoff (defined as median Ab3 level at week 22) had a prolonged RFS compared to patients (n = 24) that did not develop Ab3 above the cutoff (log-rank test p = 0.019).
Conclusion
Abagovomab does not induce CA125-specific CTL. However, patients with CA125-specific CTL perform better than patients without, irrespective of abagovomab treatment. Abagovomab-induced Ab3 associate with prolonged RFS in patients without CA125-specific CTL. Further studies are needed to confirm these data and to assess the potential utility of these immunological findings as a tool for patient selection in clinical trial.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1569-0) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer vaccine, CA125-specific cytotoxic T lymphocytes, HAMA, Ab3, MIMOSA trial
Introduction
Ovarian cancer (OC) is the leading cause of mortality from gynecologic malignancies in the USA and Western Europe. Because the disease tends to be asymptomatic in early stages, or associated with vague, nonspecific symptoms, the majority of patients are diagnosed with advanced stage disease, for which standard treatment consists in surgery usually followed by chemotherapy with a taxane and platinum. Despite the achievements of high response rates, the majority of patients eventually relapse. Second- and third-line multiagent chemotherapy usually yield low response rates, and most of these patients ultimately die of progressive disease [1, 2]. Given the palliative purpose of any medical treatment of recurrent OC [3], their substitution or integration with non-cytotoxic drugs is currently being investigated to increase response rates.
Among the novel OC treatment strategies, targeted molecular therapies are the most appealing, as by interfering with specific molecules required for tumor development and growth they display a greater selectivity and lower toxicities than traditional cytotoxic drugs. The most promising therapies are therapies aimed at disrupting tumor angiogenesis and inhibiting poly-(ADP) ribose polymerase (PARP) function [4]. Bevacizumab, a recombinant, fully humanized monoclonal IgG antibody that binds and inactivates the biologic activity of vascular endothelial growth factors has already provided convincing results in phase III trials either as monotherapy or in combination with chemotherapy for treatment of solid tumors of epithelial origin, including OC (for a comprehensive review, see Miyake et al. [5]). Up to now, the most investigated PARP inhibitor in OC is olaparib (AZD2281, KU-0059436) that has shown good promises in BRCA-related and certain histological subtypes of OC. However, data from phase III studies are still immature (for a comprehensive review, see Liu et al. [6] è ancora solo on line Gynecol Oncol). None of these drugs have been approved by the United States Food and Drug Administration (US FDA) for the treatment of OC. However, bevacizumab has been approved in the European Union (EU) for the treatment of OC, and AstraZeneca, which manufactures olaparib, has initiated two Phase III trials on olaparib in OC. Despite this remarkable progress in OC-drug discovery ameliorated the dismal clinical course of the disease, the accumulated knowledge in cancer immunology indicates that the immune system plays an essential role in host defense against OC [7] and can be exploited for an effective therapy.
Encouraging results have been obtained in OC treatment using monoclonal antibodies to CA125 [8–12], a high molecular weight protein that is highly expressed in most OC. However, stimulating an efficient immune response to CA125 remains a challenge, as this protein is also expressed on normal cells and the immune system of OC patients is usually tolerant to this antigen. Thus, effective cancer immunotherapy against CA125 must generate a destructive immune response despite tolerizing mechanisms that are in place to limit self-specific immunity. According to the idiotype network theory [13], an anti-idiotypic (anti-Id) monoclonal antibody (mAb) represents the mirror image of the original antibody (known as Ab1) formed against specific antigens. Thus, anti-Id mAb themselves (also known as Ab2) can act as antigens and elicit an immune response against the nominal antigen [14]. The murine anti-Id mAb abagovomab has been developed to mimic the epitope of CA125 defined by the mAb OC125 [15] and used as a surrogate for CA125 in tumor vaccination: In the pivotal phase Ib/II study, abagovomab stimulated human anti-mouse antibodies (HAMA) and anti-Ab2 (Ab3) production in most patients [16]. HAMA and Ab3 level was correlated with improved outcome [16]. The ability of abagovomab to induce HAMA and Ab3, and generate a CA125-specific cellular immune response was then confirmed in two additional phase I studies [17, 18], in which, however, clinical efficacy was not an endpoint. These studies supported the purpose of a phase 2/3 randomized, double-blind, placebo-controlled trial (Monoclonal Antibody Immunotherapy for Malignancies of the Ovary by Subcutaneous Abagovomab, MIMOSA). The conclusion of the trial was that abagovomab treatment did not translate into a prolonged relapse-free survival (RFS) and overall survival, although abagovomab induced HAMA and Ab3 in all patients [19].
The aim of the present study was to assess the generation of CA125-specific cytotoxic T lymphocytes (CTLs) and its relationship with clinical outcome in a study sample that was a cohort of the MIMOSA trial.
Materials and methods
Study design
Present study was designed by the authors, in consultation with the study sponsor (Menarini Ricerche s.r.l. Pomezia, Rome, Italy), as a prospective substudy of the MIMOSA trial (NCT00418574) [19]. MIMOSA was a randomized, double-blind multicenter trial of abagovomab maintenance therapy versus placebo. Abagovomab was administered subcutaneously in a 1-ml suspension once every two weeks for three injections (induction phase) and then once every four weeks for up to 21 months after random assignment of the last patient (maintenance phase). The MIMOSA trial was conducted in about 120 study centers distributed in Europe and USA and included 888 patients (n = 593 in the abagovomab arm and n = 295 in the placebo arm, ratio 2.01) with epithelial ovarian, primary peritoneal, or fallopian tube cancer in first complete clinical remission. The primary endpoint was RFS; secondary endpoints were OS and immunologic response. The present substudy included all the patients enrolled at the 18 Italian study centers (n = 129 patients, 91 in the abagovomab arm and 38 in the placebo arm, ratio 2.31). The endpoint of the substudy was to assess the ability of abagovomab to induce CA125-specific IFN-γ producing CD8+ T cells (thereafter referred to as CA125-specific CTL) and the association of the immunologic response to RFS (calculated as time from randomization to documented recurrence). A total of 579 samples (407 from the abagovomab arm and 172 from the placebo arm) were analyzed. Characteristics of patients included in this substudy mirrored those of patients in the MIMOSA study [19].
CA125-specific CTL
CA125-specific CTL were assessed before starting the treatment and then at weeks 4, 10, 22, 34, 58, and 94, and at final visit. Blood (5–12 ml) was collected at each study site by venipuncture in Na-heparin tubes and stored at room temperature, and the length of time from blood draw to sample delivery to the central lab never exceeded 48 h (median 24 h). Samples were immediately processed upon delivery to optimize cell viability and response to the activation. IFN-γ production was assessed by following the protocol originally delineated by Waldrop with modifications [20, 21], and the procedure detailed in the data sheet of the FastImmune CD8 Intracellular Cytokine Detection Kit (BD Biosciences, Mountain View, CA). Briefly, peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation [21]. Median yield was ~1 × 106 PBMC/ml of whole blood. Cell counting was performed using a Z2 cell counter (Beckman Coulter, Hialeah, FL). At least 5 × 105 cells were stimulated with CA125 (5,000U/ml) or Staphylococcal Enterotoxin B (SEB, 200 ng/ml; Sigma) and the costimulatory monoclonal antibodies (mAbs) anti-CD28 and anti-CD49d (FastImmune CD28/CD49d, BD Biosciences) for an initial 2-h period in complete RPMI-1640 medium supplemented with 1 % pooled human AB serum in a humidified incubator at 37 °C, 5 % CO2 (Sigma). AB serum was from a single lot pretested for toxicity on SEB-stimulated PBMC. A sample was stimulated with CD28/CD49d cocktail alone. Brefeldin A (Sigma) was then added (10 μg/ml) to inhibit the secretion of newly synthesized cytokine, and incubation was continued for an additional 4 h. Next, activated PBMC (about 4 × 105 cells in each experimental condition) were fixed with BD Cytofix (BD Biosciences) and cryopreserved in 100 μl of the BD Cytofix buffer at −80 °C for up to 5 weeks. This allowed for the postponement of staining and flow cytometry run, while avoiding the freezing and thawing of cells before activation. With this approach, it was possible to improve functional preservation of cells and measure cell response to the various stimuli under comparable terms and conditions in each sample at a given time point. As detailed below, this strategy was essential in obtaining the clearest possible definition of positive and negative signals in the flow cytometry analyses. Due to the labor- and time-intensive step of IFN-γ determination, the various time points of a given patient could not be analyzed in one single experiment.
Activated PBMC were thawed, washed, and permeabilized using BD FACS Permeabilizing Solution (BD Biosciences). PBMC were then stained for membrane and intracellular antigens. MAbs used were: FITC-labeled anti-CD69, PE-labeled anti-IFN-γ, PeCy5-labeled anti-CD8 (all from BD Biosciences), and ECD-labeled anti-CD3 (Beckman Coulter). Each lot of mAbs was titrated to optimize signal-to-noise ratio and improve consistency. Flow cytometer was a 6-parameter (2 scatter and 4 fluorescence signals) EPICS-XL (Beckman Coulter). The PMT voltages were adjusted using single-color-stained cells. For PE-fluorescence signal, a PE-labeled CD8 instead of the anti-IFN-γ mAb was used. The mean background fluorescence was set at ~the middle of the first logarithmic decade. The assays were performed by two trained technicians throughout the course of the study. Flow cytometry analyses were conducted by two independent observers to limit the impact of the investigator’s interpretation on flow data. List mode data were analyzed using Expo 32™ (Beckman Coulter) software as follows:
1. Forward and side scatter signals served to establish the anchor gate on lymphocytes and exclude debris and aggregates.
2. Starting with the anchor gate on scatter signals, CD8+ T cells were identified on a dual fluorescence bivariate dot plot and gated.
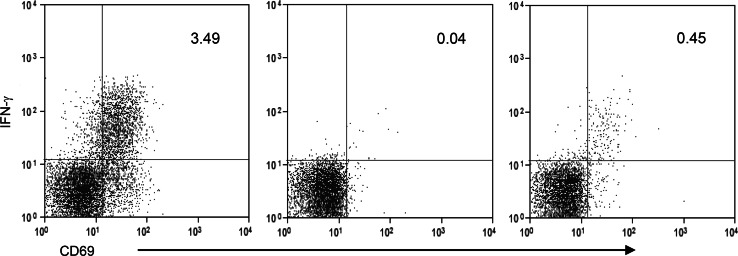
3. The proportion of CD8+ T cells that were CD69+IFN-γ+ was visualized on a dual fluorescence bivariate dot plot using the sample stimulated with CD28/CD49d cocktail and SEB. This is the sample that contains the highest number of CD69+IFN-γ+ cells and, therefore, the one in which the quadstat markers are most easily set by visual inspection (Fig. 1 left plot).
Fig. 1.
Representative dot plots depicting the method to identify CA125-specific CTL as CD8+ T cells staining positively for CD69 and IFNγ. Dot plots show CD69/IFNγ intracellular staining in CD8+ T cells stimulated with CD28/CD49d cocktail and SEB (left plot), CD28/CD49d cocktail alone (middle plot) and CD28/CD49d cocktail and CA125 (right plot). Quadstat cursors are set by visual inspection in the left plot and kept identical in the middle and right plots. The frequency of double-positive CD69/IFNγ CD8+ T cells is reported in the upper right quadrant in each plot
4. Points 1–3 are repeated using the sample stimulated with CD28/CD49d cocktail alone. The quadstat markers between CD69−IFN-γ−and CD69+IFN-γ+ CD8+ T cells are kept identical to those of the sample that was stimulated with CD28/CD49d cocktail and SEB. The proportion of CD69+IFN-γ+ CD8+ T cells is measured and taken as the background value (Fig. 1, middle plot).
5. Point 4 was repeated for the sample stimulated with CA125 so as to visualize the proportion of CD69+IFN-γ+ CD8+ T cells (Fig. 1 right plot).
6. The proportion of CD69+IFN-γ+ CD8+ T cells assessed as described in point 4 is subtracted from the proportion of CD69+IFN-γ+ CD8+ T in the CA125 stimulated sample (point 5), and the result taken as the final indicator of CA125-specific IFN-γ production.
Samples showing evident abnormalities in light-scattering characteristics and/or that produced a proportion of CD69+IFN-γ+ cells upon stimulation with CD28/CD49d cocktail and SEB ≤ to that produced upon stimulation with CD28/CD49d cocktail alone were rejected. Absolute numbers of peripheral blood CD69+IFN-γ+ CD8+ T cells were calculated using the total lymphocyte count determined with an automated hematology analyzer.
The intracellular cytokine assay was an investigative assay that was performed using internal standard operating procedures (SOPs) and conducted by well-trained personnel with more than 4 years’ experience in a laboratory that operated under the principles of good laboratory practices. Standardized SOPs describing assay performance, data evaluation and storage were applied.
Humoral immune response
HAMA and Ab3 assessment has been described in a previous publication [19]. Sera collected at baseline and various time intervals after abagovomab treatment were assayed for HAMA and Ab3.
Statistical analysis
All analyses and tables were produced using Microsoft Excel 2010 and Statistica version 7.1. RFS curves were calculated using the Kaplan–Meier method. Statistical differences between curves were tested using the log-rank test. p values <0.05 were considered significant. The generated raw data can be provided on request.
Results
CA125-specific CTL and association with clinical outcome
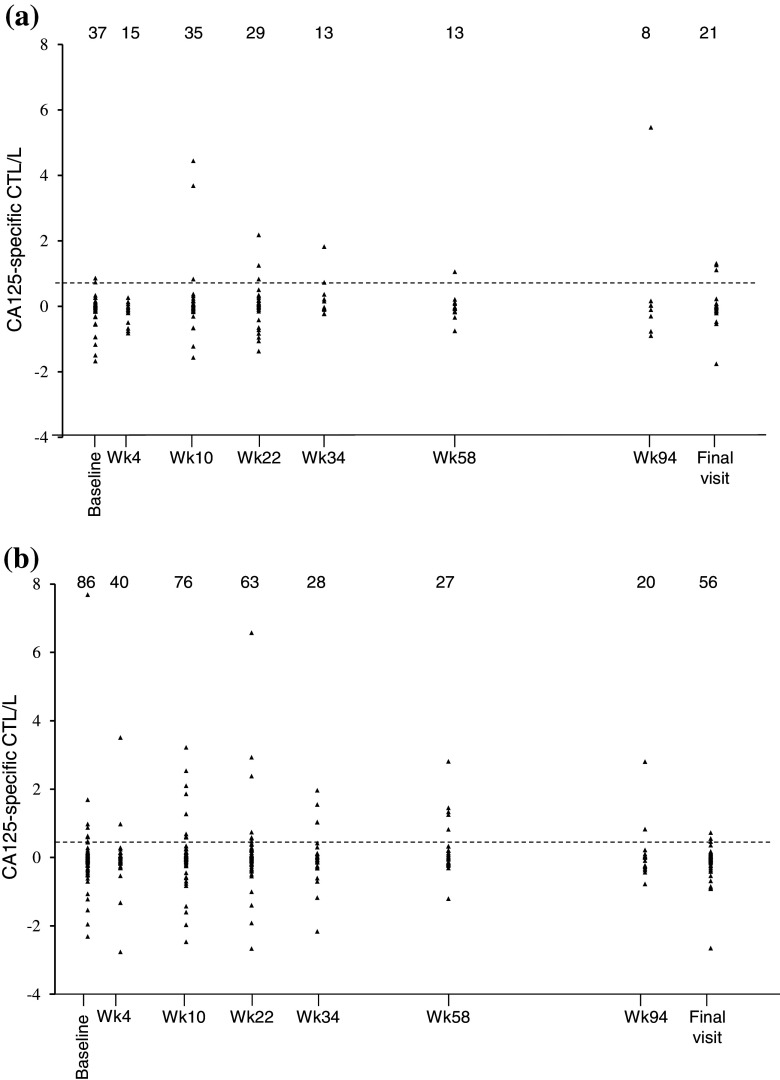
An empirical threshold for CA125-specific CTL was defined ad hoc at the 90th percentile level of CA125-specific CTL count distribution obtained from the cumulated measurements performed in patients from the abagovomab and the placebo arm (n = 129) at all time points (Fig. 2 a, b, respectively). Under this criterion, the threshold for CA125-specific CTL response was 0.410 × 106 cells/l. Patients with a CA125-specific CTL count above this value at least at one time point throughout the study were defined as having CA125-specific CTL.
Fig. 2.
Scatterplot of individual CA125-specific CTL number over time. a Patients in the placebo arm. b Patients in the abagovomab arm. The interrupted line depicts the cutoff, as defined in the text. Number of patients at each time point is indicated. Wk week
The frequency of patients having CA125-specific CTL was 31.8 % (28 out 88 patients analyzed) in the abagovomab arm and 26.3 %, in the placebo arm (10 out of 38 patients analyzed). The difference was not statistically significant (Fisher’s exact test; p = 0.673), indicating that abagovomab does not induce CA125-specific CTL.
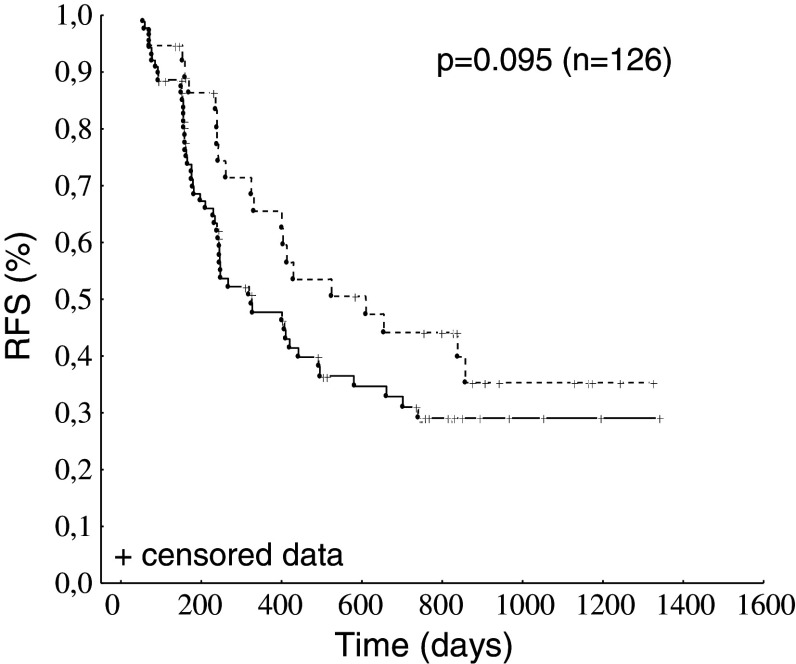
The observation that a sizeable proportion of patients had spontaneously arising CA125-specific CTL prompted us to explore whether CA125-specific CTL would associate with a favorable clinical outcome in patients, irrespective of abagovomab treatment. Thus, patients from both study arms were grouped and subdivided according to the presence/absence of CA125-specific CTL (patients with CA125-specific CTL n = 38; patients without n = 88). When we constructed Kaplan–Meier plot of the RFS of the groups classified by presence/absence of CA125-specific CTL, there was a survival difference, although this was of no statistical significance (Fig. 3, log-rank test p = 0.095).
Fig. 3.
Kaplan–Meier estimates for RFS according to the presence (interrupted line) or absence (continuous line) of CA125-specific CTL
Humoral immune response and association with clinical outcome
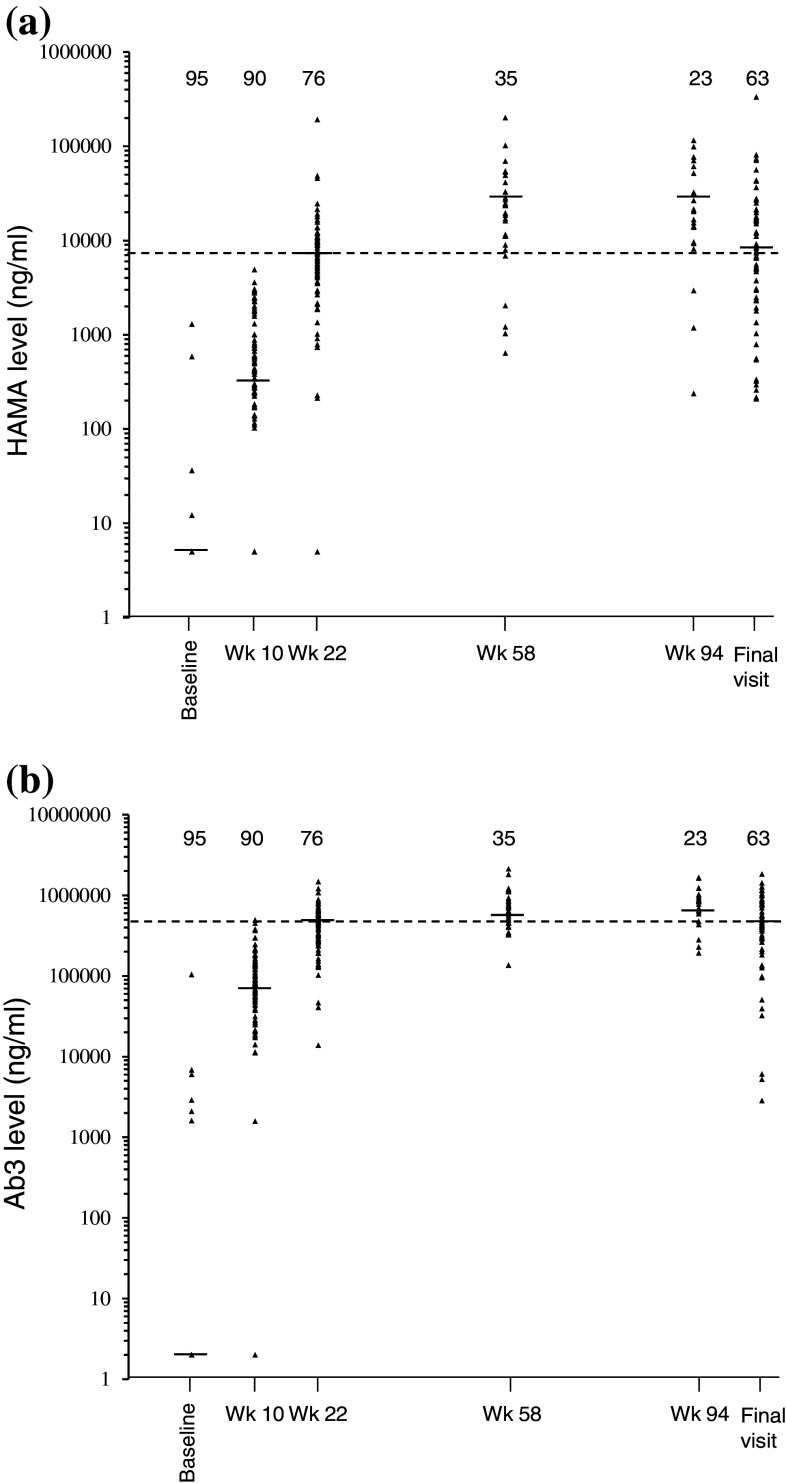
Median baseline value (0 ng/ml) of HAMA in patients in the abagovomab (Fig. 4a) and the placebo arm (not shown) was identical. All patients in the abagovomab arm (n = 91) showed at least one posttreatment value higher than baseline, whereas only 2 of the 38 patients (5.2 %) evaluated in the placebo arm showed detectable HAMA levels, which did not exceed 300 ng/ml at any visit. HAMA levels increased in most abagovomab-treated patients by the first visit of immunologic evaluation (week 10) and plateaued by week 58 in all patients (Fig. 4a). In previous publications [8–10], a HAMA cutoff level greater than 5,000 ng/ml defined a clinically relevant immune response. Here the cutoff level was set at 7,455 ng/ml. This cutoff was selected because it was the median value observed at week 22, the first visit after the start of the maintenance phase (Fig 4a). HAMA levels above the cutoff did not identify patients with a favorable clinical outcome (log-rank test, p = 0.718; data not shown).
Fig. 4.
Scatterplots of individual and median humoral immune response overtime. a HAMA levels. b Ab3 levels. The interrupted line depicts the cutoff, as defined in the text. Number of patients at each time point is indicated. Wk week
Median baseline value (0 ng/ml) of Ab3 in patients in the abagovomab (Fig. 4b) and the placebo arm (not shown) was identical. All patients (n = 91) in the abagovomab arm showed at least one posttreatment value higher than baseline (Fig. 4b), whereas low level Ab3 (below 15,600 ng/ml at any visit) were detected in 7 of the 38 patients (18.4 %) in the placebo arm. Ab3 levels increased in most abagovomab-treated patients by the first immunologic evaluation (week 10) and plateaued by week 58 in all patients (Fig. 4b). There is not an established consensus in defining a cutoff for the Ab3 response [10, 11]. Thus, the cutoff was set at the median Ab3 level observed at week 22 (447,500 ng/ml, Fig. 4b), in analogy with HAMA. Ab3 levels above the cutoff did not identify patients with a favorable clinical outcome (log-rank test p = 0.430; not shown).
Association of immune responses and clinical outcome
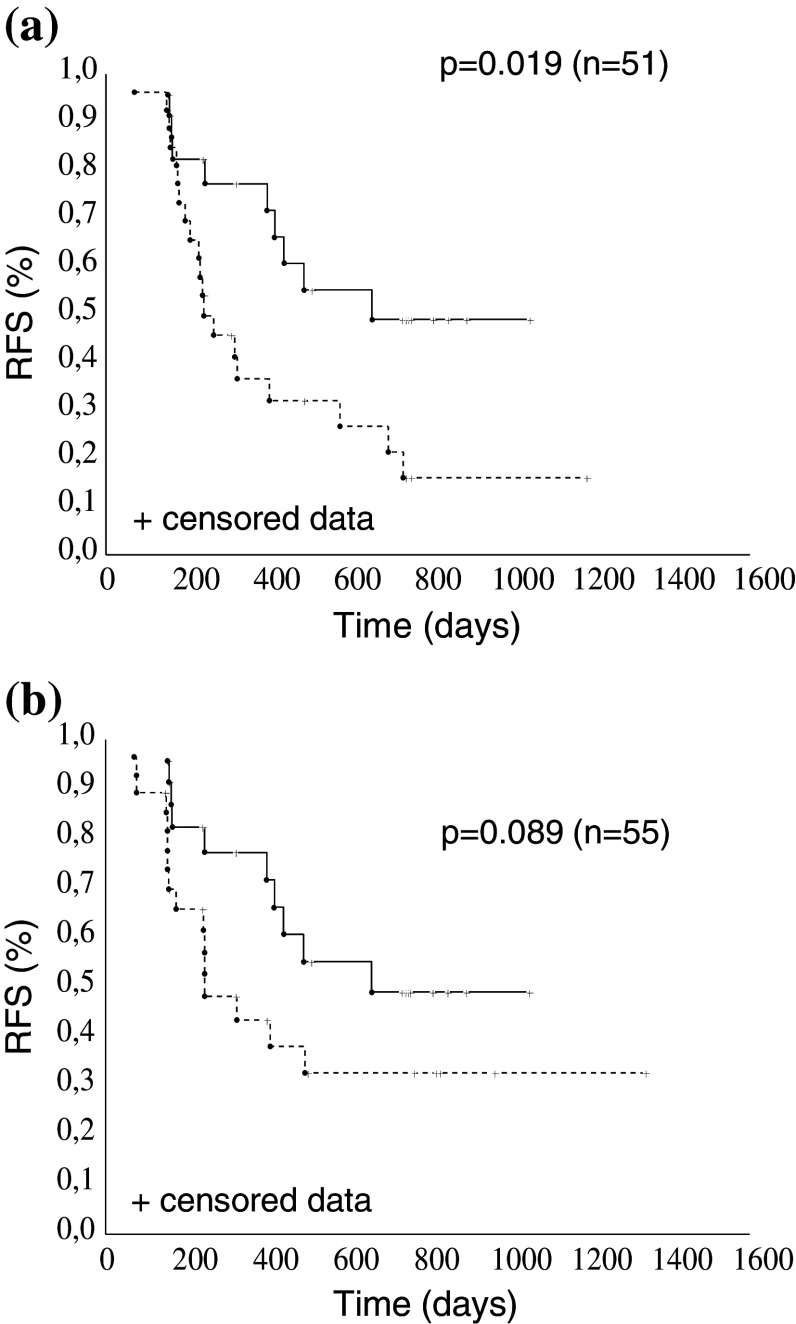
As alluded to above, about 30 % of patients had CA125-specific CTL, irrespective of abagovomab treatment, and tended to perform better than patients without (Fig. 3). Thus, we hypothesized the induction and potential benefit of humoral immune response should be best examined in patients that are homogenous in terms of CA125-specific CTL. By this approach, we observed that the frequency of patients with HAMA levels above cutoff was 37.5 % (n = 9) and 54.9 % (n = 28) in the group with (n = 24) and without (n = 51) CA125-specific CTL, respectively, and HAMA levels above cutoff were not associated to the outcome in either group (log-rank test p = 0.228 in patients with CA125-specific CTL; log-rank test p = 0.226 in patients without CA125-specific CTL, not shown). The frequency of patients with Ab3 levels above the cutoff was 50.0 % (14 out of 28) and 52.9 % (27 out of 51) in the group with and without CA125-specific CTL, respectively. In analogy with HAMA, patients with CA125-specific CTL that developed Ab3 levels above the cutoff did not show a survival advantage compared to patients that developed Ab3 levels below the cutoff (log-rank test p = 0.11, not shown). Conversely, patients without CA125-specific CTL that developed Ab3 levels above cutoff had a significantly improved RFS compared to patients that developed Ab3 levels below the cutoff (log-rank test p = 0.019, Fig. 5a). Patients without CA125-specific CTL that developed Ab3 levels above cutoff also tended to perform better than patients without CA125-specific CTL in the placebo arm (log-rank test; p = 0.089; Fig. 5b). Collectively, these data suggested a protective effect of a robust abagovomab-induced Ab3 response when CA125-specific CTL are absent. However, a robust abagovomab-induced Ab3 response did not confer a survival advantage in patients with CA125-specific CTL (log-rank test p = 0.110, not shown), possibly reflecting insufficient statistical power to detect statistically significant improvements in patients with a relatively good clinical outcome.
Fig. 5.
Kaplan–Meier estimates for RFS. a Abagovomab-treated patients without CA125-specific CTL with Ab3 levels above cutoff (continuous line) and below cutoff (interrupted line). b Abagovomab-treated patients without CA125-specific CTL and with Ab3 levels above cutoff (continuous line) and patients without CA125-specific CTL in the placebo arm (interrupted line)
Discussion
The main purpose of this study was to assess the occurrence and clinical relevance of treatment-emergent protective CA125-specific CTL in a subgroup of OC patients included in the MIMOSA trial, a randomized, double-blind multicenter trial of abagovomab maintenance therapy versus placebo, including patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer in first complete clinical remission [19]. We found that an almost identical proportion of patients in the abagovomab and placebo arms (~30 %) had CA125-specific CTL, demonstrating that abagovomab treatment fails to induce CA125-specific CTL. This observation contrasts with the conclusions of original phase I studies in which abagovomab did evoke CA125-specific CTL [17, 18]. We cannot explain the basis for differences between our study and those of others. Small sample size, patient heterogeneity, presence of comorbidities, and variation in sampling might have contributed to the inconsistency. We believe, however, that the most probable reason for the discordance is the lack of discrimination between treatment-emergent and spontaneously arising CA125-specific CTL, as those earlier studies were not placebo-controlled.
The presence of a sizeable proportion of OC patients with CA125-specific CTL irrespective of abagovomab treatment indicated that OC induces a measurable cellular immune response. These spontaneously arising CA125-specific CTL confer a certain degree of protection from relapse, as patients with CA125-specific CTL overall had a better RFS than patients without, in line with the notion that CTL found in tumor lesions in OC patients represents a favorable prognostic factor [7].
In accord with previous work, we report that abagovomab is able to induce HAMA and Ab3 in all patients [19]. The clinical relevance of abagovomab-induced HAMA and Ab3 is a topic of some controversy: HAMA and Ab3 have been deemed able to identify patients with an improved clinical outcome [16] and, conversely, have been considered as a mere biomarker of ability to mount a humoral immune response [18, 19]. Thus, we hypothesized that part of the controversy might reflect the inability of detecting a protective role of abagovomab-induced humoral immune response in cohorts of patients containing individuals with different risks of recurrence, i.e., patients with and without spontaneously arising CA125-specific CTL. Thus, we stratified patients in the abagovomab arm according to the presence/absence of CA125-specific CTL and examined the relationship between humoral immune response and outcome in these two groups. Using this strategy, we demonstrated that patients without CA125-specific CTL who met the predefined criteria for robust, treatment-emergent Ab3 response had a better outcome. Remarkably, the outcome of these patients was similar to that of patients with CA125-specific CTL in the abagovomab and placebo arms, suggesting that a robust Ab3 response is in fact protective in the absence of CA125-specific CTL. Ab3 represent the antibody response to idiotypes within the variable region of abagovomab, including the primary target, i.e., CA125 [15]. We may infer that Ab3 may have mediated complement- and cell-dependent lysis of target cells [13], resulting in a direct tumor destruction independent of CTL activity. Additionally, Ab3-mediated tumor cell destruction may also have induced CTL that specifically recognized tumor epitopes other than CA125-derived ones, as proposed in other clinical settings [22] thereby minimizing immune escape. These CTL with a broad antitumor activity went undetected in our test that was designed to exclusively recognize CA125-specific CTL.
In conclusion, this study does not confirm that abagovomab treatment elicits a cellular immune response to CA125, contrary to the expectations [17, 18]. However, our study suggests that patients who are not protected by a spontaneous CA125-specific CTL might have some advantage from abagovomab treatment through the development of a robust Ab3 response. The present study has some limitations due to the small sample size and the absence of covariate analysis for the OC validated prognostic factors; therefore, these data have to be confirmed in a larger population. In addition, further evaluations are needed to elucidate the role of the specific humoral response in this setting and to make these immunological findings a selection tool for future clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
HAMA and Ab3 data were provided by Menarini Ricerche, Pomezia, Italy.
Conflict of interest
Part of reagents (e.g., mAbs, CA125, staining buffers, etc.) and disposables (plasticwares) have been provided by Menarini Ricerche, Pomezia, Italy. The authors declare they have no financial or other interest that is relevant to the subject matter under consideration in this article with Menarini Ricerche.
Abbreviations
- Ab3
Anti-anti-idiotypic
- Anti-Id
Anti-idiotypic
- CTL
Cytotoxic T lymphocytes
- ECD
Proprietary name for Texas Red-conjugated phycoerythrin
- FITC
Fluorescein
- HAMA
Human antimouse antibody
- IFN
Interferon
- mAb
Monoclonal antibody
- MIATA
Minimal information about T cell assays
- MIMOSA
Monoclonal antibody immunotherapy for malignancies of the ovary by subcutaneous abagovomab
- OC
Ovarian cancer
- PE
Phycoerythrin
- PeCy5
Cy5-conjugated phycoerythrin
- PMT
Photomultiplier tube
- RFS
Relapse free survival
- SEB
Staphylococcal Enterotoxin B
Footnotes
Marco Fossati and Alexia Buzzonetti have contributed equally to this work.
Andrea Fattorossi and Alessandra Battaglia are joint senior authors.
References
- 1.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival—a review of the epidemiological literature. J Ovarian Res. 2009;2:13. doi: 10.1186/1757-2215-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legge F, Paglia A, D’Asta M, Fuoco G, Scambia G, Ferrandina G. Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients. BMC Cancer. 2011;11:214. doi: 10.1186/1471-2407-11-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Ziebarth AJ, Landen CN, Alvarez RD. Molecular/genetic therapies in ovarian cancer: future opportunities and challenges. Clin Obstet Gynecol. 2012;55:156–172. doi: 10.1097/GRF.0b013e31824b1699. [DOI] [PubMed] [Google Scholar]
- 5.Miyake TM, Sood AK, Coleman RL. Contemporary use of Bevacizumab in ovarian cancer. Exp Opin Biol Ther. 2013;13:283–294. doi: 10.1517/14712598.2012.745508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JF, Konstantinopoulos PA, Matulonis UA (2014) PARP inhibitors in ovarian cancer: Current status and future promise. Gynecol Oncol. doi:10.1016/j.ygyno.2014.02.039 [DOI] [PubMed]
- 7.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 8.Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J, Jr, Rivkin S, Schultes BC, Whiteside TL, Nicodemus CF. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22:3507–3516. doi: 10.1200/JCO.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Baum RP, Noujaim AA, Nanci A, Moebus V, Hertel A, Niesen A, Donnerstag B, Sykes T, Boniface G, Hör G. Clinical course of ovarian cancer patients under repeated stimulation of HAMA using MAb OC125 and B43.13. Hybridoma. 1993;12:583–589. doi: 10.1089/hyb.1993.12.583. [DOI] [PubMed] [Google Scholar]
- 10.Möbus VJ, Baum RP, Bolle M, Kreienberg R, Noujaim AA, Schultes BC, Nicodemus CF. Immune responses to murine monoclonal antibody-B43.13 correlate with prolonged survival of women with recurrent ovarian cancer. Am J Obstet Gynecol. 2003;189:28–36. doi: 10.1067/mob.2003.347. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AN, Schultes BC, Gallion H, Edwards R, Whiteside TL, Cermak JM, Nicodemus CF. CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients. Gynecol Oncol. 2004;94:340–351. doi: 10.1016/j.ygyno.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32:54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 13.Jerne NK. Towards a network theory of the immune system. Ann immunol. 1974;125C(1–2):373–389. [PubMed] [Google Scholar]
- 14.Gómez RE, Ardigo ML. Anti-idiotype antibodies in cancer treatment: the pharmaceutical industry perspective. Front Oncol. 2012;2:147. doi: 10.3389/fonc.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlebusch H, Wagner U, Grünn U, Schultes BA. Monoclonal antiidiotypic antibody ACA 125 mimicking the tumor-associated antigen CA 125 for immunotherapy of ovarian cancer. Hybridoma. 1995;14:167–174. doi: 10.1089/hyb.1995.14.167. [DOI] [PubMed] [Google Scholar]
- 16.Reinartz S, Köhler S, Schlebusch H, Krista K, Giffels P, Renke K, Huober J, Möbus V, Kreienberg R, DuBois A, Sabbatini P, Wagner U. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (phase Ib/II) Clin Cancer Res. 2004;10:1580–1587. doi: 10.1158/1078-0432.CCR-03-0056. [DOI] [PubMed] [Google Scholar]
- 17.Pfisterer J, du Bois A, Sehouli J, Loibl S, Reinartz S, Reuss A, Canzler U, Belau A, Jackisch C, Kimmig R, Wollschlaeger K, Heilmann V, Hilpert F. The anti-idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A phase I trial of the AGO-OVAR. Ann Oncol. 2006;17:1568–1577. doi: 10.1093/annonc/mdl357. [DOI] [PubMed] [Google Scholar]
- 18.Sabbatini P, Dupont J, Aghajanian C, Derosa F, Poynor E, Anderson S, Hensley M, Livingston P, Iasonos A, Spriggs D, McGuire W, Reinartz S, Schneider S, Grande C, Lele S, Rodabaugh K, Kepner J, Ferrone S, Odunsi K. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clin Cancer Res. 2006;12:5503–5510. doi: 10.1158/1078-0432.CCR-05-2670. [DOI] [PubMed] [Google Scholar]
- 19.Sabbatini P, Harter P, Scambia G, Sehouli J, Meier W, Wimberger P, Baumann KH, Kurzeder C, Schmalfeldt B, Cibula D, Bidzinski M, Casado A, Martoni A, Colombo N, Holloway RW, Selvaggi L, Li A, del Campo J, Cwiertka K, Pinter T, Vermorken JB, Pujade-Lauraine E, Scartoni S, Bertolotti M, Simonelli C, Capriati A, Maggi CA, Berek JS, Pfisterer J. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a Phase III trial of the AGO OVAR, COGI, GINECO, and GEICO—the MIMOSA study. J Clin Oncol. 2013;31:1554–1561. doi: 10.1200/JCO.2012.46.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J Immunol. 1998;161:5284–5295. [PubMed] [Google Scholar]
- 21.Fattorossi A, Battaglia A, Ferrandina G, Coronetta F, Legge F, Salutari V, Scambia G. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer. 2004;100:1418–1428. doi: 10.1002/cncr.20130. [DOI] [PubMed] [Google Scholar]
- 22.Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen (TA)- specific monoclonal antibodies: does its therapeutic efficacy require cooperation with TA-specific CTL? Clin Cancer Res. 2010;16:11–20. doi: 10.1158/1078-0432.CCR-09-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.