Abstract
Growth arrest and DNA damage-inducible protein (GADD34/Ppp1r15a) is induced by various stimuli including DNA damage and ER stress. DNA damage and oncogene activation, accompanied by tumor-specific DNA repair defects and a failure to stall the cell cycle, are early markers of hepatocellular carcinoma (HCC). However, whether GADD34 accounts for regulating HCC tumorigenesis remains elusive. Here, we demonstrated that GADD34 expression was upregulated in the liver of mice after exposure to a carcinogen, diethylnitrosamine (DEN). In both acute and chronic DEN treatment models, GADD34 deficiency not only decreased oncogene expression, but also reduced hepatic damage. Moreover, loss of GADD34 attenuated immune cell infiltration, pro-inflammatory cytokine expression and hepatic compensatory proliferation. Finally, GADD34-deficient mice showed impaired hepatocarcinogenesis. Thus, the process of DEN-induced HCC proceeded as follows. First, DEN treatment induced DNA damage in hepatocytes, resulting in elevated expression of GADD34 in the liver. The increased expression of GADD34 augmented hepatic necrosis followed by elevated expression of interleukin (IL)-1β and monocyte chemoattractant protein 1. This process promoted immune cell infiltration and Kupffer cell/macrophage activation followed by production of reactive oxygen species and pro-tumorigenic cytokines such as IL-6 and tumor necrosis factor-α. The pro-tumorigenic cytokines stimulated compensatory proliferation of surviving and mutant hepatocytes. Together with oncogene c-Myc expression, these processes led to HCC. Our results suggest therapeutic opportunities for HCC by targeting GADD34-related pathways.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1690-8) contains supplementary material, which is available to authorized users.
Keywords: DEN, GADD34, Inflammation, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), which is a malignant tumor of liver parenchyma cells, is associated with hepatocellular dysfunction and mutant cellular pathways [1]. HCC is the sixth most common cancer worldwide, and it is the third leading cause of cancer-related death because of its poor prognosis [1, 2]. Annually, HCC causes 20–40 % of cancer deaths in China, Japan and sub-Saharan Africa and the incidence of this disease has been increasing dramatically in recent years [3]. Although several molecularly targeted therapies have been applied in the past 30 years, the prognosis of many patients remains dismal [4].
Chronic inflammation is associated with the initiation of cancer, indicating a strong link between inflammation and carcinogenesis [5]. In fact, more than 90 % of HCC progression stems from chronic liver damage and inflammation, suggesting that understanding the mechanisms of inflammation-mediated hepatocarcinogenesis is necessary for the treatment and prevention of HCC [5, 6]. In addition to viral-, alcohol- and diet-induced HCC, DNA adduct-forming agents or environmental carcinogens are also important risk factors for HCC [7]. Diethylnitrosamine (DEN) has been widely used as a potent hepatocarcinogenic initiator in animal models of carcinogenesis in which it induces DNA adduct formation, resulting in DNA mutations [8]. DNA adducts can trigger oncogenesis in liver cells, which further develop into neoplastic foci as a result of multiple rounds of apoptosis–inflammation–regeneration [9]. During this process, chronic inflammation participates in tumorigenesis through activation of various signals such as nuclear factor κB (NF-κB) and RAS–RAF pathways that induce inflammatory microenvironmental and genetic alterations [10]. Persistent liver injury induces immune cell migration into the injured tissue, a process that further suppresses cell death by releasing tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6 to promote cell proliferation [11]. Furthermore, the tumor-associated macrophages and myeloid-derived suppressor cells have been shown to suppress cytotoxic T cells, facilitating tumor cell invasion, extravasation and metastatic outgrowth [12, 13].
Growth arrest and DNA damage-inducible protein (GADD34/Ppp1r15a) was originally isolated from UV-inducible transcripts in Chinese hamster ovary cells [14]. The expression of GADD34 was induced by various stimuli including DNA damage and ER stress [15]. Increased expression of GADD34 is correlated with apoptosis [16]. Apoptosis-induced proliferation contributes to regeneration and cancer initiation [17]. Recently, our laboratory showed that GADD34 protein was highly expressed in myeloid-lineage cells [18]. It has been shown that myeloid-derived cells participate in the progression of tumor development and contribute to the promotion of tumor growth, angiogenesis and metastasis [19]. Based on these previous studies, we hypothesized that GADD34, induced by DNA-damaging stress, plays an important role in liver inflammation and tumorigenesis. However, it is not clear whether GADD34 involvement contributes to the pathogenesis of HCC.
Therefore, we suggest that understanding the mechanism(s) of GADD34 involvement in inflammation-mediated hepatocarcinogenesis is essential for the treatment and prevention of liver tumorigenesis. Here, we employed GADD34 KO mice to dissect the role of GADD34 and signal pathways in liver inflammation and tumorigenesis.
Materials and methods
Mice
C57BL/6 N mice were purchased from SLC Japan. GADD34 KO/GADD34−/− mice were generated as previously described [20] and backcrossed to C57BL/6N more than 11 generations [18]. These mice were maintained in the Animal Research Facility at Nagoya University Graduate School of Medicine under specific pathogen-free conditions and used according to institutional guidelines. All animal experiments were approved by the Animal Care and Use Committee of Nagoya University Graduate School of Medicine.
Tumorigenesis protocol
For acute liver injury, 6-week-old C57BL/6N wild-type (WT) mice and GADD34 KO mice were administrated 100 mg/kg of diethylnitrosamine (DEN, Sigma-Aldrich, MO, USA, #73861) by intraperitoneal (i.p.) injection. The mice were killed at the indicated time points. For chronic liver inflammation, 6-week-old mice of both genotypes received i.p. injections of low (25 mg/kg) or high (50 mg/kg) concentrations of DEN twice a week and were killed after 3 weeks of treatment. For hepatocarcinogenesis, 2-week-old mice of both genotypes were treated with a single i.p. injection of DEN at a dose of 25 mg/kg diluted in phosphate-buffered saline (PBS). Starting 2 weeks after DEN injection, mice were injected biweekly with 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (Sigma-Aldrich, St. Louis, MO, USA, #T1443) at a concentration of 3 µg/g diluted in corn oil as previously described [21]. These mice were used to observe the development of tumors and were killed at 5 months after initial DEN treatment.
Serum analysis
Alanine transaminase (ALT) activity in serum was measured using the ALT Reagent kit (Wako, Tokyo, Japan) with a microplate reader (Biotech, Tokyo, Japan).
Flow cytometry
Before isolation of the liver, mice were perfused with ice-cold PBS via the left ventricle. Then the liver was explanted, the gall bladder removed, and the liver was rinsed with ice-cold 0.5 % bovine serum albumin (BSA) in PBS and chopped on ice into small 1-mm3 pieces and digested as previously reported [22]. Single-cell suspensions of liver were stained with the following antibodies: anti-CD11b-PE (#557397), anti-F4/80-APC (#17-4801), anti-LY6C-APC (#17-5932), anti-LY6G-PE (#551461), anti-CD4-FITC (#11-0042) and anti-CD8-PE (#555635) (eBioscience, BD Bioscience). For reactive oxygen species (ROS) detection, immune cells were isolated from WT and GADD34−/− mice after repeated treatments with 25 mg/kg DEN. Cells were loaded with CM-H2DCFDA (Molecular Probes, Invitrogen) for 30 min. The samples were analyzed using a FACSCanto II flow cytometer (BD Biosciences). All data analyses were performed using FlowJo software.
Histological analysis
Mouse livers and carcinomas were fixed in 4 % paraformaldehyde and embedded in paraffin. Four-micrometer sections were dewaxed and hydrated through graded ethanol dilutions and then used for hematoxylin and eosin (H&E) staining. Histology scores were calculated as follows: 0, no piecemeal necrosis; 1, focal piecemeal necrosis in few central vein areas; 2, focal piecemeal necrosis in most central vein areas; 3, continuous necrosis <50 % around central vein areas; and 4, continuous necrosis >50 % around central vein areas. For immunohistochemistry (IHC), the sections as described above were heated in citrate buffer (pH 6.0, 10 mM) in a pressure cooker at 121 °C for 15 min. Endogenous peroxidase activity was blocked with 3 % hydrogen peroxide followed by washing. Blocking was done in PBS containing 1.5 % nonfat dry milk and 0.3 % Triton X for 1 h. The sections were then incubated with the following antibodies: anti-rat F4/80 (1:100) (BMA, #T-2028) or anti-goat Ki67 (1:100) (Santa Cruz, sc7846) in blocking buffer overnight at 4 °C. After incubation with horseradish peroxidase (HRP)-conjugated anti-rat IgG (KPL) or HRP-conjugated anti-goat IgG (Santa Cruz, sc-2020) for 1 h, followed with 3,3′-diaminobenzidine tetrahydrochloride (Dojindo, Tokyo, Japan) staining, all sections were counterstained with hematoxylin and visualized using an Olympus light microscope.
RNA isolation and real-time PCR
Total RNA was isolated from liver tissue using Trizol reagent (Invitrogen). RNA was quantified by NanoDrop (Thermo Scientific). cDNA was synthesized from 1 µg total RNA with a high-capacity cDNA reverse transcription kit according to the manufacturer’s instructions (Applied BioSystems). PCR was performed by using Takara EX Taq kit (Takara Bio). Real-time PCR was performed on the MX3000P QPCR System (Agilent) using the following program: 10 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 20 s at 60 °C. The reactions were carried out using 0.5 µL cDNA with SYBR premix EX Taq II (Takara Bio). Values were normalized to β-actin mRNA. The primer set sequences are shown in supplementary Table 1.
Immunofluorescent staining
Liver tissues from mice were embedded in OCT compound (Sakura Finetek). Four-micrometer sections were fixed in 100 % cold acetone. After rinsing with PBS, sections were permeabilized and treated with blocking buffer (0.2 % Triton X-100, 0.2 % BSA, 0.1 % normal goat serum in PBS). Anti-F4/80-APC (eBioscience, #17-4801) and anti-IL-6-FITC (eBioscience, #11-7061) were used at 1:500 dilutions and incubated at 4 °C overnight, after which the sections were washed with PBS and incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. After rinsed with PBS, the sections were mounted with mounting fluid and visualized under A1Rsi inverted confocal microscopy (Nikon, Tokyo, Japan).
Immunoblotting
Freshly excised tissues were suspended in protein lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 5 mM Na2EDTA, 1 % Triton, 0.1 % sodium dodecyl sulfate, 0.5 % sodium deoxycholate, 10 mM sodium fluoride and 1 mM sodium orthovanadate. Tissues were subjected to sonication using a sonic dismembrator (TAITEC, Tokyo, Japan). The concentrations of protein were quantified by using DC protein assay method (Bio-Rad, 500-0120JA). Immunoblotting analysis was done as described with antibodies from Santa Cruz Biotechnology for detection of GADD34 (C-19, sc-825), caspase-1p10 (M-20, sc514), cMyc (sc-764) and alpha-fetoprotein (AFP) (sc-8108). Antibodies from Cell Signaling Technology were used to detect pH2AX (#2595), signal transducer and activator of transcription 3 (STAT3) (#9132) and phosphorylated STAT3 (#9145), phosphorylated Akt (Ser473, #9271) and Akt (#9272), phosphorylated NF-κB p65 (Ser468, #3039) and NF-Κb p65 (#3034), NF-κB p105/50 (#3035), p53 (#2524), phosphorylated p53 (Ser15, #9284), cleaved caspase-3 (Asp175, #9661). β-Actin (Cell Signaling Technology, #4967) or GAPDH (Cell Signaling Technology, #2118) were the loading controls.
Statistical analysis
All data are presented as mean ± SEM. For calculation of statistical probabilities, Student’s t test or analysis of variance (ANOVA) was used in this study. Statistical calculations were performed using GraphPad Prism 5 software. The animal numbers used for each experiment are indicated in the figure legends. The differences were considered statistically significant when the P value was <0.05.
Results
GADD34 deficiency alleviated acute liver injury
To investigate the role of GADD34 in liver inflammation and HCC, we evaluated three different models using male WT and GADD34−/− mice. The acute model used a single DEN treatment. Chronic liver injury was induced by repeated injections of DEN, and the hepatocarcinogenesis model utilized a single injection of DEN followed by repeated exposures to TCPOBOP (Fig. 1a).
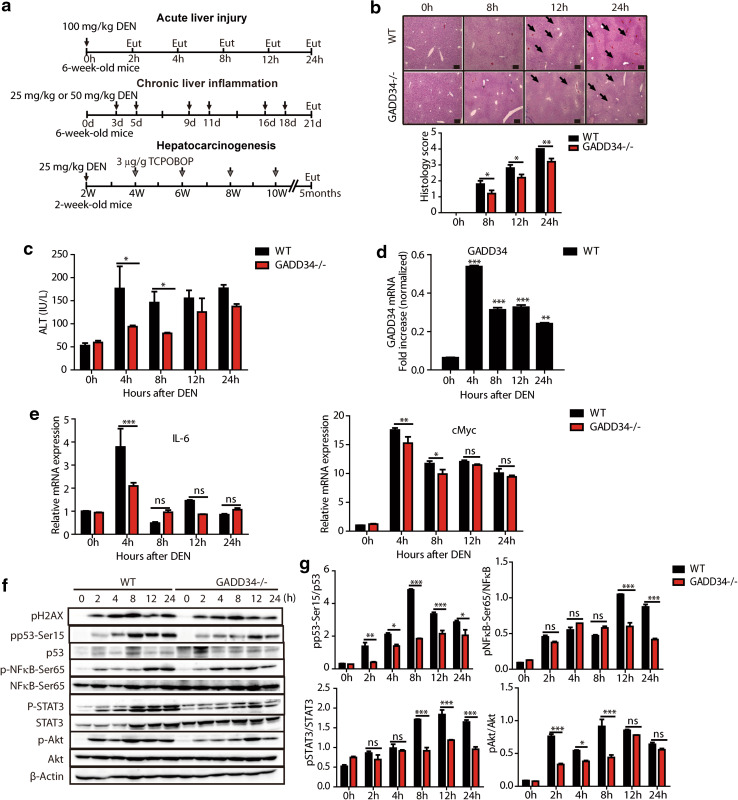
Fig. 1.
GADD34 deficiency alleviated acute liver injury after DEN treatment. a Representative time courses of three experiments in this study. Intraperitoneal injection (i.p.). Eut, euthanize. b H&E staining of liver sections in the indicated time points after 100 mg/kg DEN treatment (WT mice, n = 22; GADD34−/− mice, n = 22). Black arrows indicate central vein hypertension and necrosis, characterized by hepatocyte vacuolization. Scale bars 200 μm. *P < 0.05, **P < 0.01 versus WT. Histology score represented four samples in each time point and each group (six fields of view for each sample). c Measurement of mouse serum alanine aminotransferase (ALT) activity at the indicated time points after 100 mg/kg DEN treatment. Data from three independent trials are averaged for each time point. *P < 0.05 versus WT. d Representative RT-PCR result of GADD34 (Ppp1r15a) mRNA from WT mice at different times after 100 mg/kg DEN treatment. The statistical data for GADD34 mRNA are normalized to β-actin mRNA in three independent samples. ***P < 0.001, **P < 0.01 versus 0 h. e Real-time PCR analysis of pro-inflammatory cytokine gene Il-6 and oncogene cMyc from WT and GADD34−/− mice at the indicated time points after 100 mg/kg DEN treatment. Data represent mean ± SEM of triplicates for each sample (n = 3). ***P < 0.001 **P < 0.01, *P < 0.05 versus WT; ns no significance versus WT. f Representative analysis of protein expression levels by Western blotting using liver samples from WT and GADD34−/− mice after 100 mg/kg DEN treatment. g Densitometric analysis of band intensities of pp53, pNF-κB, pSTAT3 and pAkt relative to p53, NF-κB, STAT3 and Akt after treatment with DEN. Data represent mean ± SEM of triplicates for samples. ***P < 0.001, **P < 0.01, *P < 0.05 versus WT; ns no significance versus WT
We initially investigated the effects of GADD34 in the acute liver injury model, using 6-week-old male WT and GADD34−/− mice that were given a single intraperitoneal (i.p.) injection of high-dose DEN (100 mg/kg) and killed at 2, 4, 8, 12 and 24 h (Fig. 1a). H&E staining showed visible necrosis around the central vein area at 8, 12 and 24 h, these areas were characterized by hepatocyte vacuolization, and some parenchyma areas of the liver appeared darker than others (Fig. 1b). The H&E stained sections were quantified by histological scoring, and the scores revealed significant differences between WT and GADD34−/− mice (Fig. 1b). Similarly, the serum ALT activity was increased in WT mice from 4 to 24 h compared to those in GADD34−/− mice (Fig. 1c). These differences indicated that WT mice were subjected to greater liver injury and functional loss than GADD34−/− mice after DEN treatment. GADD34 (Ppp1r15a) mRNA expression was significantly increased from 4 to 24 h when compared to 0 h after DEN treatment (Fig. 1d). Moreover, the WT mice showed higher levels of cytokine IL-6 and oncogene c-Myc expression than did GADD34−/− mice at 4 h (Fig. 1e). These results suggested that GADD34 enhanced pro-inflammatory cytokine production and oncogene activation.
To understand the pathways involved in this process, protein expression levels were examined. We found that the expression of DNA damage marker pH2AX was increased after DEN treatment in both WT and GADD34−/− mice (Fig. 1f). These results indicated that double-strand DNA damage may recruit multiple proteins involved in the DNA damage response and repair. Phosphorylated p53, an apoptosis biomarker, showed significantly higher expression levels in WT mice than in GADD34−/− mice (Fig. 1f, g), which suggested that pp53 may be downstream from GADD34. Consistent with the production of pro-inflammatory cytokine IL-6, the phosphorylated NF-κB and STAT3 displayed significantly higher levels of expression in WT livers than livers in GADD34−/− mice from 12 to 24 h (Fig. 1f, g). The phosphorylation of Akt, which was related to cell proliferation, was highly expressed in WT compared to GADD34−/− from 2 to 8 h (Fig. 1f, 1 g). These results indicated higher compensatory proliferation ability of oncogene-activated hepatocytes in WT mice. Taken together, these results clearly showed that DEN induced more severe liver damage in WT mice than in GADD34−/− mice.
GADD34 deficiency attenuated oncogene expression, apoptotic protein expression, inflammatory cell infiltration and ROS production after chronic DEN treatment
Since we observed less liver damage in GADD34−/− mice upon treatment with a single high dose of DEN, we examined the function of GADD34 in chronic liver inflammation. To address this question, we subjected mice to chronic treatment with DEN (Fig. 1a). Similar to the acute liver injury induced by a single injection of DEN, we found that mRNA expression of GADD34 (Ppp1r15a) in WT mice was significantly increased after chronic DEN treatment (Fig. 2a and supplementary Fig. 1a). Moreover, oncogene c-Myc, phosphorylated p53, pyroptosis-related protein caspase-1p10 and apoptosis-related protein cleaved caspase-3 showed higher expression levels in the livers of WT mice than in GADD34−/− mice (Fig. 2b, c). These results showed that GADD34 enhanced DNA damage, oncogene expression and hepatic damage after chronic exposure to DEN.
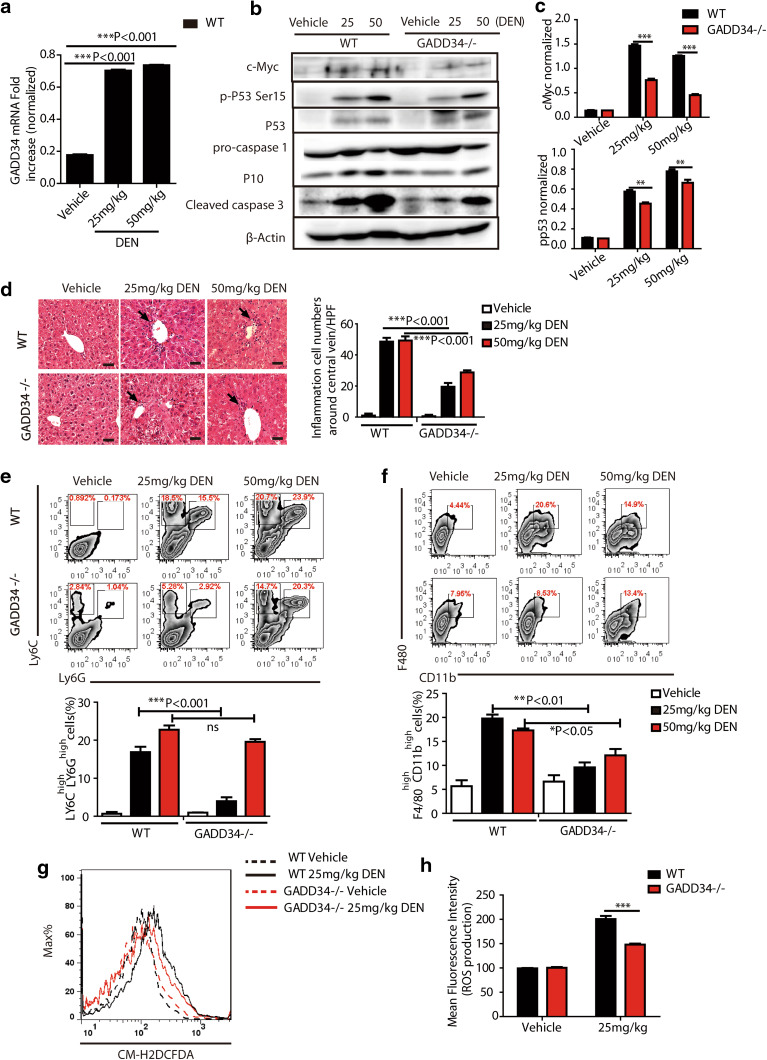
Fig. 2.
GADD34 deficiency reduced oncogene expression, apoptosis, inflammatory cell infiltration and ROS production after chronic DEN treatment. a Representative RT-PCR result of GADD34 (Ppp1r15a) mRNAs from WT mice (n = 15 for WT, n = 15 for GADD34−/−). GADD34 mRNAs are normalized to β-actin mRNAs in three independent samples. n = 5/group. b Analysis of oncogene and apoptotic protein expression levels by Western blotting using liver samples from WT and GADD34−/− mice after chronic DEN treatment. n = 5/group. c Densitometric analysis of band intensities of pp53 and cMyc relative to β-actin. Data represent mean ± SEM from three independent experiments. d Representative H&E staining of liver sections after chronic DEN treatment. Scale bars 50 μm. Statistical data indicated immune cells around central vein in each high-power field (HPF, six fields of view for each sample, n = 5) that are counted. ***P < 0.001 versus WT. e–f Flow cytometric analysis of immune cell infiltration in the liver after chronic DEN treatment. Single-cell suspensions from livers are stained and analyzed for the indicated cell surface markers. Statistical data indicated the percentage of LY6Chigh LY6Ghigh cells (e) and F4/80high CD11bhigh cells (f). Data represent mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT. ns no significance versus WT. g Immune cells isolated from mouse liver are stained with CM-H2DCFDA after chronic DEN treatment, and ROS production is analyzed by flow cytometry. h Histogram for mean fluorescence intensity in (g). N = 3 for each group, ***P < 0.001 versus WT, mean ± SEM
Histological analysis showed that WT mice were subjected to significantly higher hepatic inflammation than were GADD34−/− mice. The former were characterized by higher-level infiltration of immune cells around central veins (Fig. 2d). To determine the phenotype of involved immune cells, flow cytometric analyses were undertaken. Among the dissociated cells, the populations of neutrophils (Ly6Chigh Ly6Ghigh) and Kupffer cells/macrophages (F4/80highCD11bhigh) were significantly increased in WT mice after chronic DEN treatment. By contrast, fewer infiltrating immune cells were seen in GADD34−/− mice (Fig. 2e, f). In addition, the increased infiltration of macrophages into WT livers was confirmed by IHC staining with an anti-F4/80 antibody (Supplementary Fig. 1b), an interesting observation because macrophage activation and recruitment favored inflammatory cytokine production. CD4+ and CD8+ T cells were decreased in both WT and GADD34−/− livers, especially after high-dose DEN treatment (Supplementary Fig. 1c). This observation indicated an immune dysfunction in mice after DEN treatment.
To further investigate whether the enhanced hepatic inflammation with the presence of GADD34 was associated with excess production of oxidative stress species, we stained immune cells with CM-H2DCFDA and used flow cytometric analyses to evaluate ROS production. The results showed significantly higher levels of ROS production in WT than in GADD34−/− following repeated treatments with 25 mg/kg DEN (Fig. 2g, h). Those data indicated that ROS production by activated immune cells in WT mice may have contributed to higher levels of pro-tumorigenic cytokine production. Together, these results suggested that GADD34 played an important role in both acute injury and chronic liver inflammation, as loss of GADD34 could attenuate oncogene expression, hepatic inflammation, immune cell infiltration and ROS production after chronic DEN treatment.
GADD34 deficiency reduced cytokine/chemokine production and compensatory proliferation after chronic DEN treatment
Infiltrating immune cells such as macrophages produce cytokines, chemokines and matrix proteases that are responsible for the inflammatory process and compensatory proliferation [23]. Thus, we used real-time PCR to examine the mRNA levels of cytokines and chemokines after chronic DEN treatment. The expression of IL-1β, matrix metalloproteinase-9 (MMP9), monocyte chemoattractant protein 1 (MCP1), IL-6 and TNF-α was significantly increased in the livers of WT mice, whereas these inflammatory molecules were comparatively lower in GADD34−/− mice (Fig. 3a). Furthermore, the immunofluorescent staining showed that IL-6 was expressed in F4/80-positive Kupffer cells/macrophages, and the expression of IL-6 in these cells presented significantly higher immunofluorescence intensity in WT than in GADD34−/− (Fig. 3b, c). These results suggested that GADD34 deletion led to reduced production of IL-6 by Kupffer cells. It was noteworthy that IL-1β, MMP9 and MCP1 showed much higher-level expression than IL-6 and TNF-α in both WT and GADD34−/− mice after DEN treatment. These results demonstrated that GADD34 rendered hepatocytes more susceptible to DEN-induced hepatic damage, leading to higher levels of IL-1β and MCP1 expression and subsequently enhanced activation of Kupffer cells to release higher levels of IL-6.
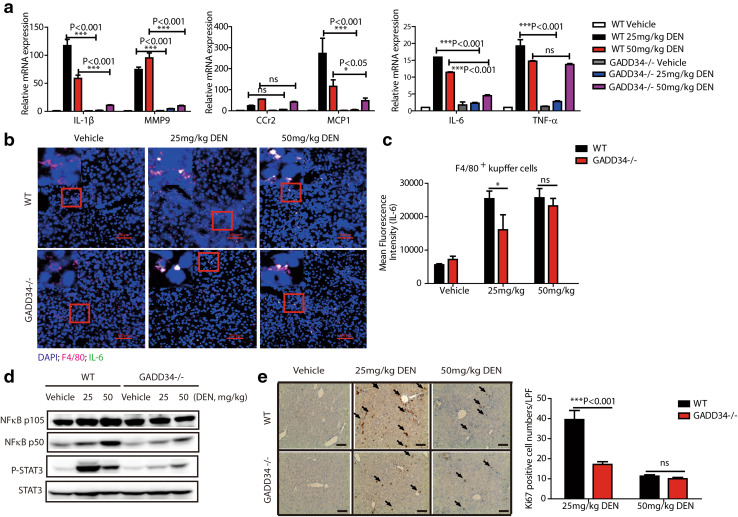
Fig. 3.
GADD34 deficiency exhibited lower cytokine expression and hepatic compensatory proliferation after chronic DEN treatment. a Real-time PCR analysis of mRNA expression levels of the indicated genes in liver from WT and GADD34−/− mice after chronic DEN treatment. Data represent mean ± SEM of three independent experiments, *P < 0.05, ***P < 0.001 versus WT; ns no significance versus WT. b Representative immunofluorescent confocal microscopic images of tissues co-stained for F4/80 and IL-6 in liver tissues after chronic DEN treatment. Nuclei (4′,6-diamidino-2-phenylindole, DAPI), blue; IL-6, green; F4/80, pink. The red outlined areas are enlarged in top left corners. Scale bars 100 μm. c Statistical analysis of mean fluorescence intensity of IL-6 in Kupffer cells/macrophages. N = 3 with >8 fields of view for each group, *P < 0.05 versus WT, mean ± SEM. no significance versus WT. d Representative protein expression levels of liver samples from WT and GADD34−/− mice after chronic DEN treatment. e Analysis of hepatic compensatory proliferation by staining for Ki67. Black arrows indicate Ki67-positive cells. Scale bars 100 μm. Data represent mean ± SEM of three independent samples with >6 fields of view for each sample (LPF, low-power field). ***P < 0.001 versus WT. ns no significance versus WT
The expression of pro-inflammatory and pro-tumorigenic genes is modulated by signal transduction pathways mediated by NF-κB and STAT proteins [6, 24]. To understand whether these pathways and molecules were downregulated in the absence of GADD34, we assessed the expression of signaling molecules, including NF-κB p50 subunit and phosphorylated-STAT3. Indeed, significantly higher activation levels of NF-κB and STAT3 were observed after chronic DEN treatment in the livers of WT mice compared to those in GADD34−/− mice (Fig. 3d). These results suggested that enhanced activation of Kupffer cells/macrophages may provide signals that contribute to hepatic compensatory proliferation in the presence of GADD34.
To confirm our hypothesis, Ki67 expression was analyzed in DEN-treated livers. We found that higher expression levels of Ki67 were present in the livers of WT mice than that in GADD34−/− mice following repeated treatments with 25 mg/kg DEN. In contrast, Ki67 expression was reduced after repeated treatments with 50 mg/kg DEN in both genotypes. These results indicated that repeated administration of 50 mg/kg DEN could be hepatotoxic, and there existed no dose-dependent effect of DEN on GADD34 over this concentration range (Fig. 3e). Based on these results, it appears that exposure to DEN upregulates GADD34, increasing hepatic damage and the compensatory proliferation of mutant hepatocytes. This process may well contribute to the triggering of HCC.
GADD34 deficiency reduced tumor burden in liver
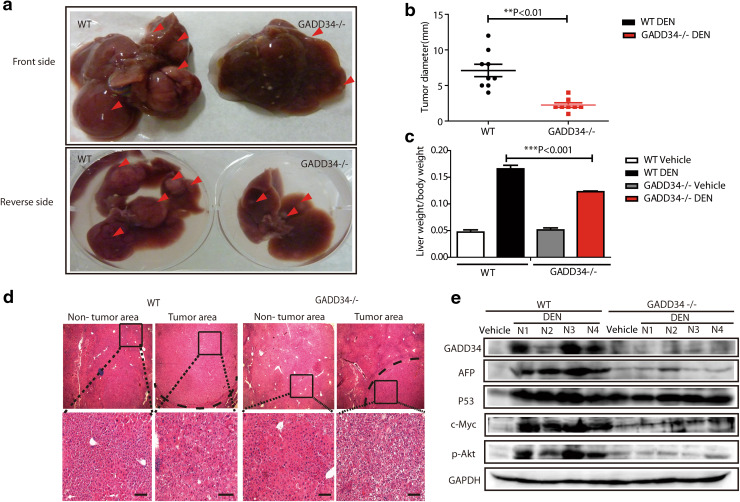
After confirming that GADD34 played an important role in chronic inflammation, we next aimed at investigating whether GADD34 was involved in hepatocarcinogenesis by using a previously described protocol to generate HCC [21]. After administration of one dose of DEN, TCPOBOP was administered once every 2 weeks. We killed the WT and GADD34−/− mice at 5 months after the initial DEN treatment and removed the livers to assess tumor formation macroscopically and histologically (Fig. 1a). The sizes of the HCC and the liver/body weight ratios in GADD34−/− mice were significantly lower than in WT mice (Fig. 4a–c). Histological analysis of liver tissue demonstrated that the non-tumor area in WT mice showed more dysplasia around central vein areas than in GADD34−/− mice (Fig. 4d). Moreover, the borders of localized tumor areas were larger in WT mice than in GADD34−/− mice (Fig. 4d). These results indicated that an increased tumor burden was associated with severe dysplasia in WT mice. Interestingly, we found that the expression of GADD34 protein level was strongly increased in WT mice after HCC development (Fig. 4e). The expression of AFP, c-Myc and phosphorylated Akt was significantly higher in WT mice than in GADD34−/− mice (Fig. 4e), which provided further evidence of higher tumor burden in WT mice than in GADD34−/− mice.
Fig. 4.
GADD34 deficiency reduced hepatocellular carcinoma (HCC) progression after DEN and TCPOBOP treatment. a Representative images with front and back side of HCC from WT and GADD34−/− mice. Red arrows indicate tumor nodules. b Statistical analysis of average tumor size. N = 9 for WT, n = 7 for GADD34−/−. **P < 0.01 versus WT. c Statistical analysis of liver/body weight ratios. ***P < 0.001 versus WT. d H&E staining of non-tumor and tumor liver sections obtained from WT and GADD34−/− mice. The outlined areas are enlarged in the lower panel. Scale bars 100 μm. e Analysis of indicated protein expression by Western blotting using whole-liver samples including tumor and non-tumor areas from WT and GADD34−/− mice
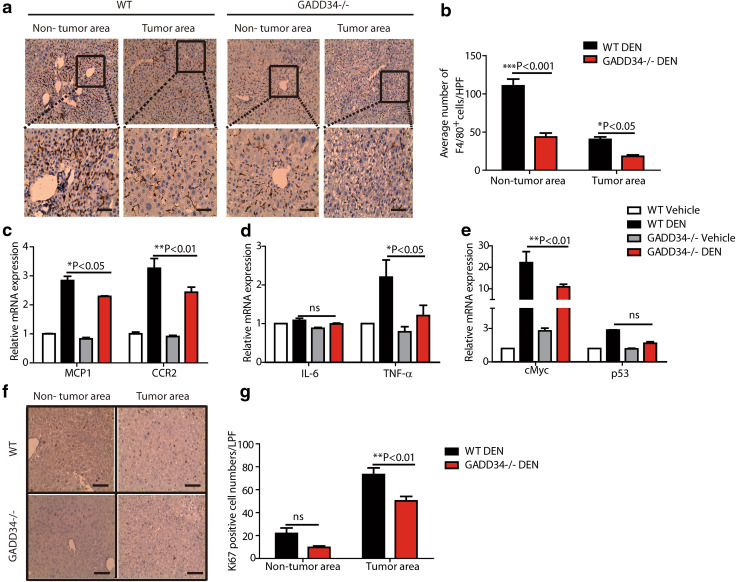
Next, we tried to delineate which factor played a dominant role in HCC progression. It has been reported that macrophages are a key component of the tumor microenvironment and orchestrate various aspects of cancer [25]. Accordingly, we used IHC staining and observed higher numbers of F4/80-positive Kupffer cells/macrophages in both non-tumor areas and tumor areas of WT livers than in GADD34−/− livers (Fig. 5a, b). Consistent with this result, the chemokine MCP1 and its receptor C-C chemokine receptor type 2 (CCR2) showed significantly higher expression levels in the livers of WT mice than in GADD34−/− mice (Fig. 5c). The expression levels of pro-tumorigenic mRNAs of c-Myc and TNF-α were significantly increased in WT livers relative to GADD34−/− livers (Fig. 5d, e). Since tumor size was related to cell proliferative capacity, we examined Ki67 expression by IHC. The results showed that the expression of Ki67 was significantly higher in the tumor area of WT mice than in GADD34−/− mice (Fig. 5f, g), indicating that GADD34 promoted hepatocarcinogenesis. Collectively, GADD34 deficiency reduced HCC progression through attenuating oncogene expression, ROS production, cytokine secretion, Kupffer cell activation and hepatic compensatory proliferation (Fig. 6).
Fig. 5.
GADD34 deficiency reduced HCC progression through attenuating macrophage infiltration, cytokine level, oncogene expression and hepatic proliferation. a Representative IHC images of non-tumor and tumor areas in liver samples from WT and GADD34−/− mice using anti-F4/80 antibody. The outlined areas are enlarged in the lower panel. Scale bars 50 μm. b Statistical analysis of F4/80-positive cells from different locations in different samples. N = 6 with >8 fields of view for each group, *P < 0.05, ***P < 0.001 versus WT, mean ± SEM. c–e Real-time PCR analysis of indicated genes in non-tumor and tumor areas from WT and GADD34−/− mice. N = 4 for each experiment. *P < 0.05, **P < 0.01 versus WT, mean ± SEM. f Representative IHC results of Ki67 expression in both non-tumor and tumor area. Scale bars 100 μm. g Statistical analysis of Ki67-positive cells in (f). N = 6 with >8 fields of view for each group, **P < 0.01 versus WT, mean ± SEM. no significance versus WT
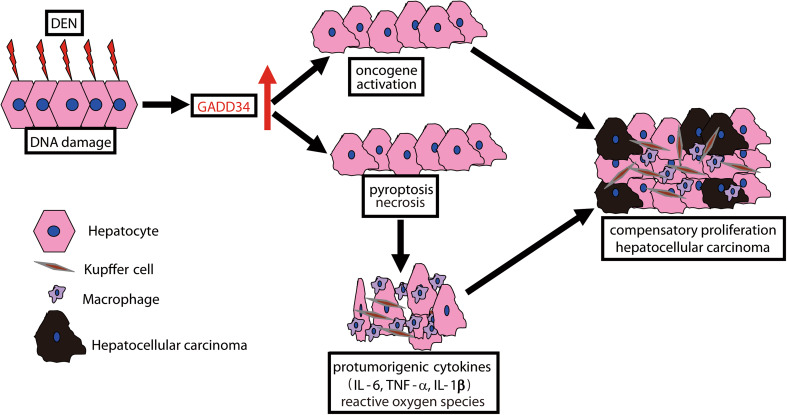
Fig. 6.
Schematic summary of this study. The chemical carcinogen diethylnitrosamine (DEN) led to DNA damage that in turn could enhance GADD34 upregulation. GADD34 augmented oncogene activation and the death of DEN-exposed hepatocytes through both pyroptosis and necrosis pathway. This process led to Kupffer cell/macrophage activation and immune cell infiltration that subsequently produced pro-tumorigenic cytokine and ROS, thereby stimulating the compensatory proliferation of surviving, mutant hepatocytes. Finally, abnormal proliferation enhanced HCC progression
Discussion
GADD34 (Ppp1r15a) was originally described as a growth arrest and DNA damage-inducible gene. GADD34 transcript levels tend to increase in response to genotoxic stress, nutrient deprivation and myeloid cell differentiation [16]. GADD34 expression regulates apoptosis through various signaling pathways and also enhances apoptotic responses to DNA damage [26].
To date, no direct evidence has identified a linking between GADD34 expression and hepatocellular tumorigenesis. In the current study, we evaluated the function of GADD34 in a well-characterized carcinogen-induced HCC model by using WT and GADD34 KO mice. Here, we have shown that GADD34 facilitates hepatocarcinogenesis by promoting liver inflammation and compensatory proliferation.
It has been demonstrated that GADD34 expression is correlated with the onset of apoptosis in certain cell lines following ionizing irradiation or treatment with an alkylating agent methyl-methanesulfonate [27]. Here, we have shown that GADD34 expression is increased by treatment with DEN, and this is correlated with the expression of phosphorylated p53 at Ser15. Previous experiments directly showed that the phosphorylation of p53 at Ser15 was enhanced by increased expression of GADD34 [28, 29].
We also confirmed that DEN treatment induced severe cell damage and death in WT animals through elevated levels of cleaved caspase-1 and cleaved caspase-3. Conversely, this phenomenon was aborted in GADD34 KO mice. Following DEN-induced GADD34 expression and the associated necrosis of hepatocytes, cellular components (ATP, high mobility group box1 and heat shock proteins) and extracellular matrix fragments may work as damage-associated molecular patterns (DAMPs). DAMPs are recognized by Kupffer cells or infiltrated macrophages through Toll-like receptors or inflammasomes to trigger inflammation–regeneration signaling pathways [30, 31]. Our results showed that repeated injection of DEN enhanced production of pro-inflammatory cytokines because of elevated GADD34 expression. These cytokines included IL-1β, MCP1 released from damaged hepatocytes and IL-6, and TNF-α released from Kupffer cells/macrophages, indicating that GADD34 promoted susceptibility to DEN-induced hepatocyte damage, leading to secretion of cytokines and chemokines and enhanced activation of Kupffer cells.
ROS production by activated immune cells contributes to pro-tumorigenic cytokine production and cancer promotion [32]. Release of biologically active molecules (cytokines and reactive oxygen intermediates) from activated Kupffer cells has been implicated in hepatocarcinogenic events [33]. Accordingly, we demonstrated that GADD34 deficiency led to reduced ROS production by immune cells. Higher-level activation of NF-κB and STAT3 suppresses apoptosis and promotes cell cycle progression in damaged liver cells [34]. The enhanced activation of Kupffer cells or macrophages provides signals that contribute to compensatory proliferation of hepatocytes in the presence of GADD34. Moreover, it has been shown that inflammation enhances cellular susceptibility to mutagenesis and genomic destabilization by altering cell cycle checkpoints and disruption of DNA repair pathways, and this results in the accumulation of random genetic alterations [35, 36]. Previous reports showed that inactivation of p53-mediated genomic surveillance and over-expression of Myc result in the suppression of DNA repair and acceleration of the mutation rate in cancer cells [10]. Once tumors become established, c-Myc is a key player in alternative macrophage activation and pro-tumorigenic gene expression [37]. Accordingly, we found that several oncogenic mediators such as IL-1β, IL-6, TNF-α and c-Myc were downregulated in the absence of GADD34.
Although GADD34 has been shown to induce apoptosis, our results further demonstrated that GADD34 promoted DEN-mediated liver tumorigenesis. GADD34 rendered hepatocytes more vulnerable to DEN-induced damage, leading to high levels of cytokine and chemokine expression. This is notable because chemokines, especially CCL2 (MCP1) and CCL5, are associated with macrophage infiltration and tumor progression [38]. Similarly, our results revealed that enhanced proliferation of Kupffer cells and migration of macrophages in WT mice following DEN-induced HCC. Kupffer cells/macrophages are common and fundamental components of cancer-related inflammation. For example, in response to persistent infections or chronic irritation, macrophages synthesize inflammatory cytokines such as TNF-α and IL-6 that could contribute to tumor initiation and promotion. These inflammatory molecules also promote genetic instability within the developing tumor [39]. Moreover, Kupffer cells provide essential mitogens to HCC. Specifically, NF-κB and STAT3 pathways promote mitogen synthesis, leading to non-autonomous proliferation of surrounding cells and enhancing tumor burden [19, 40]. Thus, GADD34 may work as a mediator by causing hepatic DNA damage and necrosis, followed by abnormal compensatory proliferation of surviving hepatocytes. In addition, mutations of crucial genes in the DNA damage-sensing pathway of undead cells also contribute to tumor growth and progression [41]. Because carcinogens such as DEN inflict DNA damage and production of DNA adducts, the presence of adducts accelerates the response to inflammation by releasing of tumor-promoting cytokines during the early stages of HCC development [42]. Furthermore, in response to persistent damage, macrophages produce inflammatory cytokines that recruit other immune cells to sustain the chronic inflammation [43]. Chronic liver injury may evolve into an inflammation-wound healing response round that promotes the development of hepatic fibrosis and eventually HCC [44]. Collectively, GADD34 acts as an important mediator during liver tumorigenesis. GADD34 renders the hepatocytes more susceptible to necrosis and pyroptosis after exposure to DEN. Furthermore, the enhanced damage to the liver promotes higher numbers of Kupffer cell activation and macrophage infiltration. The fact that tumor nodules are much bigger in WT than in GADD34−/− mice provides solid evidence that GADD34 enhances HCC development.
Taken together, we conclude that the effects of GADD34 in DEN-induced HCC proceed as follows. First, the chemical carcinogen DEN damages hepatocyte DNA, which leads to elevated expression of GADD34 in the liver. The increased expression of GADD34 augments hepatic necrosis followed by release of IL-1β and MCP1. This process promotes Kupffer cell activation and macrophage infiltration followed by the production of ROS and pro-tumorigenic cytokines such as IL-6 and TNF-α. These pro-tumorigenic cytokines stimulate compensatory proliferation of surviving and mutant hepatocytes. Finally, the abnormal compensatory proliferation leads to HCC progression (Fig. 6).
In conclusion, GADD34 contributes to hepatic damage, inflammation and compensatory proliferation during HCC. However, it is still necessary to explore the detailed mechanisms underlying GADD34 regulation of signal pathways during liver tumorigenesis and to seek therapeutic opportunities for HCC by targeting associated pathways.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Mr. Tanaka for technical assistance with flow cytometry experiments and Mrs. Mizuguchi for confocal microscopic analysis (Laboratory of Division for Medical Research Engineering, Nagoya University Graduate School of Medicine) as well as N. Oiwa for administrative assistance. This research was supported by the Ministry of Education, Science, Technology, Sports and Culture, Japan, and China Scholarship Council, China (Project Number: 201408050020).
Conflict of interest
We declare that there are no conflicts of interest.
Abbreviations
- AFP
Alpha-fetoprotein
- ALT
Alanine transaminase
- BSA
Bovine serum albumin
- CCR2
C-C chemokine receptor type 2
- DAMPs
Damage-associated molecular patterns
- DEN
Diethylnitrosamine
- GADD34
Growth arrest and DNA damage-inducible protein
- H&E
Hematoxylin and eosin
- HCC
Hepatocellular carcinoma
- HRP
Horseradish peroxidase
- IHC
Immunohistochemistry
- IL
Interleukin
- KO
Knockout
- MCP1
Monocyte chemoattractant protein 1
- MMP9
Matrix metalloproteinase-9
- NF-κB
Nuclear factor κB
- PBS
Phosphate-buffered saline
- ROS
Reactive oxygen species
- STAT3
Signal transducer and activator of transcription 3
- TCPOBOP
1,4-Bis-[2-(3,5-dichloropyridyloxy)]benzene
- TNF-α
Tumor necrosis factor-α
- WT
Wild type
References
- 1.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Leong TY, Leong AS. Epidemiology and carcinogenesis of hepatocellular carcinoma. HPB (Oxford) 2005;7:5–15. doi: 10.1080/13651820410024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci. 2011;1:5. doi: 10.1186/2045-3701-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol. 2012;18:4071–4081. doi: 10.3748/wjg.v18.i31.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Biol. 2004;14:473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Poirier MC. Chemical-induced DNA damage and human cancer risk. Nat Rev Cancer. 2004;4:630–637. doi: 10.1038/nrc1410. [DOI] [PubMed] [Google Scholar]
- 9.Min L, Ji Y, Bakiri L, et al. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013;190:3815–3823. doi: 10.4049/jimmunol.1203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA. 1988;85:8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farook JM, Shields J, Tawfik A, Markand S, Sen T, Smith SB, Brann D, Dhandapani KM, Sen N. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013;4:e754. doi: 10.1038/cddis.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander MC, Poola-Kella S, Fornace AJ., Jr Gadd34 functional domains involved in growth suppression and apoptosis. Oncogene. 2003;22:3827–3832. doi: 10.1038/sj.onc.1206567. [DOI] [PubMed] [Google Scholar]
- 17.Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4:a008797. doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishio N, Ito S, Isobe K. Loss of GADD34 induces early age-dependent deviation to the myeloid lineage. Immunol Cell Biol. 2014;92:170–180. doi: 10.1038/icb.2013.78. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 20.Kojima E, Takeuchi A, Haneda M, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–1575. doi: 10.1096/fj.02-0462com. [DOI] [PubMed] [Google Scholar]
- 21.Scaiewicz V, Nahmias A, Chung RT, Mueller T, Tirosh B, Shibolet O. CCAAT/enhancer-binding protein homologous (CHOP) protein promotes carcinogenesis in the DEN-induced hepatocellular carcinoma model. PLoS One. 2013;8:e81065. doi: 10.1371/journal.pone.0081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang TW, Yevsa T, Woller N, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 23.Lanaya H, Natarajan A, Komposch K, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972–981. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 25.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 26.Adler HT, Chinery R, Wu DY, Kussick SJ, Payne JM, Fornace AJ, Jr, Tkachuk DC. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19:7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollander MC, Zhan Q, Bae I, Fornace AJ., Jr Mammalian GADD34, an apoptosis- and DNA damage-inducible gene. J Biol Chem. 1997;272:13731–13737. doi: 10.1074/jbc.272.21.13731. [DOI] [PubMed] [Google Scholar]
- 28.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/S0092-8674(00)80416-X. [DOI] [PubMed] [Google Scholar]
- 29.Yagi A, Hasegawa Y, Xiao H, Haneda M, Kojima E, Nishikimi A, Hasegawa T, Shimokata K, Isobe K. GADD34 induces p53 phosphorylation and p21/WAF1 transcription. J Cell Biochem. 2003;90:1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- 30.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 33.Rose ML, Rivera CA, Bradford BU, Graves LM, Cattley RC, Schoonhoven R, Swenberg JA, Thurman RG. Kupffer cell oxidant production is central to the mechanism of peroxisome proliferators. Carcinogenesis. 1999;20:27–33. doi: 10.1093/carcin/20.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 35.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 36.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pello OM, De Pizzol M, Mirolo M, et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–421. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 38.Bonecchi R, Locati M, Mantovani A. Chemokines and cancer: a fatal attraction. Cancer Cell. 2011;19:434–435. doi: 10.1016/j.ccr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Zaki MH, Vogel P, Malireddi R, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti T-D. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The cell is dead. long live the cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda S, Kamata H, Luo J-L, Leffert H, Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luedde T, Schwabe RF. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.