Abstract
Adaptive regulatory T cells (Tregs) contribute to an immunosuppressive microenvironment in colorectal cancer (CRC). Here, we examined whether the level of Treg-mediated inhibition of antitumor immune responses in patients with metastatic CRC (metCRC) selected for liver resection is associated with clinical outcome. Preoperatively and at follow-ups, we did flow-based phenotyping, examined antitumor immunity using peptides from carcinoembryonic antigen (CEA) protein in the presence or absence of CD4+CD25+CD127dim/− cells (Tregs) and determined cytokine and PGE2 levels in patient blood samples. At 18 months post-surgery, 8 patients were disease free (7 alive and 1 dead of unrelated cause) and 10 had experienced disease recurrence (7 alive and 3 dead of metCRC). Prior to surgery, the patients demonstrated Treg-mediated suppression of TNFα and IFNγ expression that could be perturbed through the PGE2/cAMP pathway and the immune suppression was significantly higher in the group that later developed disease recurrence (P = 0.046). Furthermore, the post-surgery plasma PGE2 levels were related to the clinical outcome (PGE2 levels of 280 ± 47 vs. 704 ± 153 pg/ml (mean ± SEM) for disease free and recurrent disease, respectively). T-cell phenotyping revealed higher frequencies of COX-2+ cells in the patients with recurrent disease. These findings support the notion that the level of Treg-mediated suppression of adaptive antitumor immune responses at the time of surgery may influence later clinical outcome of metCRC and provide valuable prognostic information.
Keywords: Colorectal cancer, Liver metastasis, Regulatory T cells, COX-2, PGE2
Introduction
Metastasis from colorectal cancer (CRC) to the liver is common [1]. About 20% of the patients present with synchronous metastases at the time of diagnosis, and 30–40% of the patients will later develop metachronous liver metastases [2]. Patients with untreated liver metastases have an expected median survival of 6 months [3]. However, surgical resection of colorectal metastases to the liver is now offered to an increasing number of patients and neoadjuvant chemotherapy further increases the number of patients with resectable disease [3]. Patients with resectable liver metastases have an estimated 5-year survival of 35–50% for selected cases [4]. However, in the recurring population, a significant proportion develops rapidly progressing disease and the mortality is 30% in the first year and 20% in the second year bringing the two-year survival to 50%. For the approximately 1/3 of the patients that die in the first year from disease recurrence, the surgical procedure offers little benefit [3]. On this background, tumor and immunological factors that predict or are associated with disease recurrence are important for patient selection and treatment strategy.
In CRC, cyclooxygenase-2 (COX-2) is over-expressed in the tumor tissue compared to the normal colonic mucosa, and the prostaglandin E2 (PGE2) level in peripheral blood is elevated [5]. PGE2 plays a crucial role in the neoplastic process by stimulating tumor cell proliferation, promoting angiogenesis, suppressing tumor cell apoptosis and stimulating tissue invasion by tumor cells [6–10]. In parallel, PGE2 contributes to an immunosuppressive microenvironment by suppression of T-, B- and NK-cell immune responses through the stimulation of EP-receptor signaling [11]. Suppression of adaptive antitumor immune responses in the tumor margin shields the tumor from the immune system and contributes to tumor immune tolerance [12]. In addition, intrinsic immunosuppressive mechanisms in the adaptive immune system, such as downregulation of co-stimulatory receptors and upregulation of inhibitory co-receptors, secretion of immunosuppressive cytokines and recruitment and induction of regulatory T cells (Tregs), contribute to the immunosuppressive microenvironment of solid malignant tumors [13].
Tumor infiltrating lymphocytes (TILs) are associated with improved prognosis in cancer [14], and the type, density and location of immune cells in CRC may have higher predictive power than the prognosis estimated by the conventional UICC-TNM histological classification [15]. Tregs are a T-cell subset with dominant immunosuppressive properties. Tumor-specific peripherally induced or adaptive Tregs play a significant role suppressing antitumor immunity, which may negatively affect the prognosis [16]. CRC patients have a higher proportion of circulating Tregs that suppress tumor immune responses [5] and tumor-associated antigen-specific Tregs control tumor-specific effector/memory T-cell responses [17], and inhibition of Treg activity may represent a future therapeutic avenue to improve antitumor immunity. To investigate possible prognostic and predictive parameters for use during the assessment and treatment of metastatic CRC (metCRC), we examined whether immune responses are associated with the clinical outcome. We found that Treg-mediated immune suppression at the time of surgery was more pronounced in patients with later recurring disease.
Materials and methods
Patient material
The study protocol was approved by the Regional Committee for Medical Research Ethics, Norway. Patients with liver metastasis from CRC at Oslo University Hospital, Ullevål, were enrolled in the study after obtaining the written informed consent. Patients (n = 18; 10 men and 8 women; mean age 61.5 years; range 40–81 years) were included and presented with 1–20 liver metastases with diameters of the largest tumor from 8 to 75 mm. CEA levels were routinely determined in serum preoperatively (n = 14). Blood samples from healthy blood donors at Blood Bank, Oslo University Hospital, Ullevål, were used as controls (n = 4). Patient data are presented in Table 1.
Table 1.
Description of patient cohort
| Sex | Age (years) | Primary site | Time to liver surgery (months) | Synchronous cancer | Number of metastasis | Largest diameter (mm) | CEA at liver surgery (μg/l) | PGE2 at liver surgery (pg/ml) | HLA-A2 status | Disease-free survival (months) | Overall survival (months) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 40 | Colon | 8 | Yes | 7 | 75 | 1.426 | 205 | ND | 12 | 29 | CRD |
| F | 45 | Rectum | 6 | Yes | 5 | 50 | 0.9 | 487 | Neg | 8 | AWD | |
| F | 45 | Colon | 4 | Yes | 9 | 22 | 17.3 | 411 | Pos | 23 | AWD | |
| F | 61 | Colon | 1 | Yes | 2 | 22 | 5.1 | 42 | Neg | 4 | AWD | |
| F | 61 | Rectum | 5 | Yes | 1 | 19 | 0.9 | ND | Pos | FREE | ||
| F | 63 | Colon | 13 | No | 1 | 16 | 16.6 | 602 | Pos | FREE | ||
| F | 66 | Colon | 52 | No | 3 | 70 | 1.7 | 517 | Neg | 8 | FREE | |
| F | 67 | Rectum | 7 | No | 1 | 17 | 3.4 | 350 | ND | FREE | ||
| M | 44 | Colon | 6 | Yes | 5 | 10 | 1 | 353 | Pos | 6 | NCRD | |
| M | 60 | Colon | 7 | Yes | 20a | 13a | ND | 442 | Neg | AWD | ||
| M | 62 | Rectum | 2 | Yes | 1 | 25 | ND | 173 | Neg | 7 | AWD | |
| M | 64 | Rectum | 11 | Yes | 3 | 50 | 3.1 | 283 | Pos | 10 | 15 | CRD |
| M | 67 | Colon | 40 | No | 3 | 8 | 10.6 | 497 | Neg | 11 | 11 | CRD |
| M | 67 | Colon | 22 | No | 1 | 60 | 134 | 158 | Neg | 3 | AWD | |
| M | 68 | Colon | 41 | No | 1 | 45 | ND | 297 | Neg | 1 | AWD | |
| M | 72 | Colon | 25 | No | 1 | 18 | 2.4 | 397 | Pos | FREE | ||
| M | 74 | Rectum | 4 | Yes | 4 | 32 | 3.4 | 583 | Pos | FREE | ||
| M | 81 | Colon | 8 | No | 1 | 22 | ND | 345 | Pos | FREE |
Clinical, histopathological and laboratory data on the eighteen patients with liver metastasis from CRC that were included in the study. Observation time was 18 months before outcome was assessed and classified as follows: CRD cancer-related death, NRCD non-cancer-related death, AWD alive with disease, FREE disease free at follow-ups. ND: CEA, PGE2 and HLA-A2 not determined in given patient. aDenotes inoperable patient; size and number of metastases were estimated by radiography
Isolation of cells
Peripheral blood (50 ml) was drawn from available study subjects preoperatively and at 6 and 12 months postoperatively. Peripheral blood mononuclear cells (PBMC) were isolated by Isopaque-Ficoll (Lymphoprep, Nycomed Pharma AS, Oslo) gradient centrifugation. CD4+CD25+CD127dim/− T cells were isolated using regulatory T-cell isolation kit II according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). T cells were routinely analyzed by flow cytometry. The frequency of CD3+ and CD3+CD4+cells was typically 50–55 and 25–35% in PBMC. In the CD3+CD4+ subset in PBMC, the CD25+FOXP3+ double positive Tregs typically constituted 5–10%, whereas presence of Tregs was typically 2–5% after depletion of CD4+CD25+CD127dim/− and purity of Tregs pulled out by this method and used for add-back experiments typically 70–95% CD25+FOXP3+ double positive. Cells were cultured in RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% heat-treated FCS, 100 U/ml penicillin/streptomycin, 1 mM sodium pyruvate and 1:100 non-essential amino acids (in the following referred to as complete medium) in a humidified atmosphere with 5% CO2 at 37°C in the absence or presence of CEA peptides as indicated.
Phenotypic analysis of lymphocytes
Isolated cells were stained with antibodies against CD3 PerCP, CD38 FITC, CD69PE, HLA-A2 FITC, PD-1 FITC, CTLA-4 PE, ICOS PE, FOXP3 Alexa-647 (BD BioSciences, San Jose, CA), CD4 FITC, CD4 PE (Beckman Coulter, Brea, CA), CD25 PE (Miltenyi Biotec, Germany), COX-2 FITC (Cayman Chemical, Ann Arbor, MI) and CD4 APC (IQ Products, the Netherlands). Before staining intracellular targets, T cells were fixed and permeabilized with a FOXP3 buffer kit (BD BioSciences, San Jose, CA). Cells were washed once in PBS containing 1% BSA before acquiring data on a flow cytometer (FACSCalibur™; BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, San Carlos, CA).
Prostaglandin E2 and cytokine determination
Peripheral blood from patients and controls was collected in EDTA vacuum tubes and centrifuged, and plasma was isolated and stored for 6–24 months at −80°C, thawed and subsequently analyzed for PGE2 levels by ELISA (R&D, London, UK) and a panel of 17 cytokines (IL-1β, IL-2, IL-4, IL5, IL-6, IL-7, IL-8, IL-10, IL12(p70), IL-13, IL-17, G-CSF, GM-CSF, IFNγ, MCP-1, MIP-1β and TNFα) by multiplex assay (Bio-Rad, CA, USA) according to the manufacturer’s instructions.
CEA tumor antigen
Two sequences from the carcinoembryonic antigen (CEA), CEA61–69 [HLFGYSWYK] and CEA318–327 [TYACFVSNL], were selected based on responsiveness in our previous studies [5] and synthesized using an in-house Multipep automated peptide synthesizer (INTAVIS Bioanalytical Instruments AG) following a standard Fmoc-chemistry protocol. Scrambled peptides were generated in the same way with sequences LGSYHFWKY and NVLFSTCYA and used as controls as earlier shown [5]. The peptides were subjected to high-performance capillary electrophoresis (HPCE), isolated with >90% purity and quality assessed by mass spectrometry. They were dissolved in DMSO at a concentration of 5 mg/ml and further diluted in complete medium and used at a final concentration of 5 μg/ml. Patient CEA levels were measured routinely by ELISA (Roche, Germany) at the Department of Medical Biochemistry, Oslo University Hospital, Ullevaal. In healthy blood donors, CEA <5 μg/l was considered normal. We did not observe any correlation between high CEA levels at the time of liver metastasis and immune responses to CEA peptide stimulation r = 0.0052 (Linear regression).
Stimulation and cytokine production assays
PBMC, PBMC depleted of CD4+CD25+CD127dim/− T cells or PBMC co-cultured with autologous CD4+CD25+CD127dim/− T cells (300,000 cells total/well, where add-back 1:3 Tregs:CD3+ T cells) were stimulated with CEA peptides (5 μg/ml) or complete medium alone for 18–20 h. Brefeldin A (Sigma-Aldrich, Louis, MI) was added to a final concentration of 5 μM for the last 6 h of incubation. When used, COX inhibitor indomethacin (25 μM) (Sigma-Aldrich, Louis, MI) or PKA type I antagonist Rp-8-Br-cAMPS (1 mM) (Lauras AS, Oslo, Norway) was added 2 h prior to activation with CEA peptides. The cells were fixed (PFA 4%), permeabilized (Perm Buffer; BioSciences, San Jose, CA), stained for CD3, CD4, IFNγ (BD BioSciences, San Jose, CA) and TNFα (BD BioSciences, San Jose, CA) and analyzed by flow cytometry as described above. Staphylococcal enterotoxin B and anti-CD2/CD3/CD28-coated microbeads were used as positive controls for activation in the assay. The cells were washed once in PBS containing 1% BSA before acquiring data on a flow cytometer (FACSCalibur™; BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, San Carlos, CA).
Statistical analysis
The data were analyzed using SigmaPlot 11.0 (CA, USA). Paired data were compared using Wilcoxon Signed Rank test. Mean values were compared by Student’s t test, or when Shapiro–Wilk Normality test failed, median values between groups were compared with Mann–Whitney Rank Sum test. P < 0.05 was considered significant.
Results and discussion
Patient material and clinical outcome
Eighteen consecutive patients with metCRC selected for the resection of liver metastasis were included. The mean age at time of surgery was 61.6 years (range 40–81) and encompassed 8 female and 10 male patients (Table 1). One patient was intraoperative assessed as inoperable due to extensive disease. The mean time from intestinal surgery (primary tumor) to liver surgery (metastatic disease) was 14.6 months. Ten patients presented with synchronous cancer and 9 patients received neoadjuvant chemotherapy prior to surgery of the metastases. Laparoscopic surgery was performed in 4 patients. Wedge resections were performed alone or in combinations with right or left hemihepatectomy in 14 of the patients. The mean size of the largest tumor was 31.9 mm (range 8–75 mm), the number of tumors 3.8 (range 1–20) and mean CEA levels 116 (range 0.9–1,426.0 μg/l). Twelve patients had colon and 6 patients had rectum as primary tumor site. Of the 10 patients presenting with recurrent metastatic disease, five went through a second liver resection and one was subject to a third resection.
Patients were sampled for the assessment of antitumor immune activity in circulating T cells preoperatively (n = 18) and to the extent available at 6- and 12-month follow-ups (n = 9 and n = 3, respectively). At 18 months post liver resection, clinical outcome was assessed and revealed that 9 patients had experienced recurrent metCRC, 6 of which were alive at the time of preparation of this report (disease-free interval 1–12 months) and 3 of which were dead (survival time 11–29 months post liver resection). In addition, one patient presented with recurrent disease at 23 months while preparing this report and was included in the group with recurrent metCRC, bringing the average time to recurrence to 8.7 months. At 18 months or more post liver resection, 8 patients remained disease free, one of which died of a cause related to the adjuvant treatment and not of metCRC (Table 1).
Increased frequency of COX-2 expressing regulatory T cells in patients with recurrent metCRC
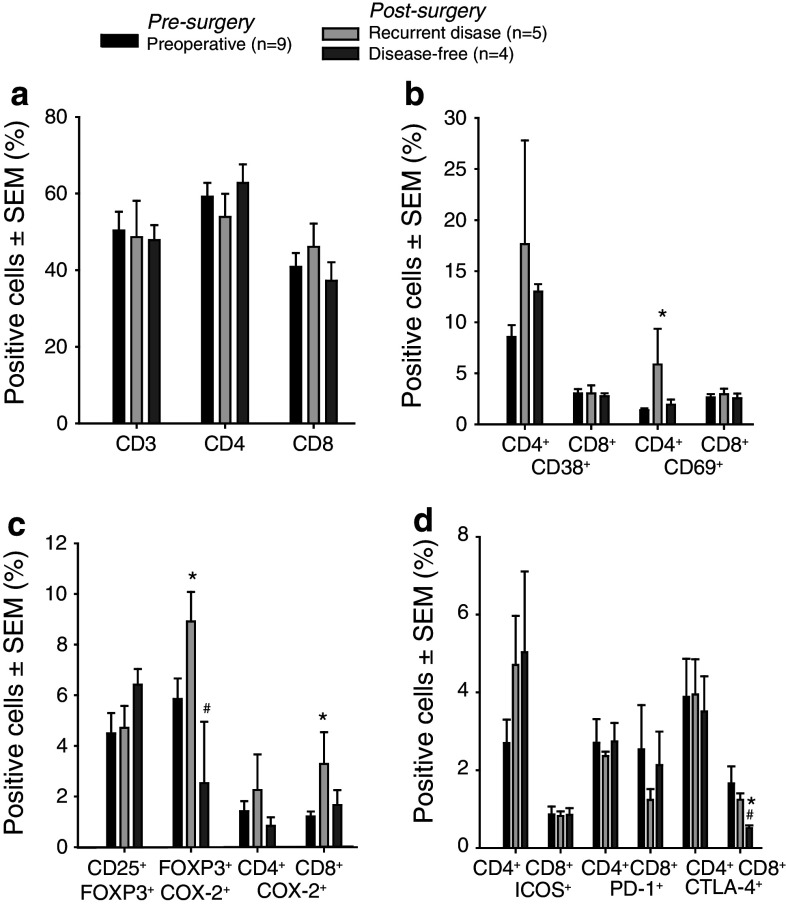
T cells from metCRC patient blood samples pre- and post-surgery were characterized with respect to CD4, CD8 and Treg distribution (Fig. 1a, c), activation status (Fig. 1b, c), and co-receptor expression (Fig. 1d) using flow cytometry. There were no major changes in CD3, CD4 or CD8 T-cell subsets or in Treg frequency in patients prior to surgery compared to 6-month postoperative controls. However, changes were observed in the frequency of CD69- and COX-2-expressing cells in patients presenting with recurrent disease (RD, n = 5) compared to patients that remained disease free (DF, n = 4) and also when preoperative and postoperative frequencies were compared in the same patients (n = 9). Specifically, the CD4+CD69+ T-cell population increased in RD patients from pre- to post-surgery indicating immune activation upon recurrence. The frequency of COX-2+ increased in the Treg and CD8 T-cell subsets in RD patients (in comparison FOXP3+COX2+ T cells was 1.34 ± 0.12 (mean ± SEM), in n = 3 normal blood donors). Frequencies of the co-receptors inducible T-cell co-stimulator (ICOS), programed death (PD)-1 and cytotoxic T-lymphocyte antigen (CTLA)-4 the two latter of which are inhibitory, were not altered.
Fig. 1.
Phenotypical characterization of T cells in patients with metastatic colorectal cancer. a–d Bars represent percent cells (mean ± SEM) in indicated subpopulations as determined by flow cytometry after staining with directly conjugated antibodies. Analysis done after gating on lymphocytes and CD3+ cells. Pre-surgery and post-surgery phenotypic analyses from patients with or without recurrent disease during follow-up are shown. Mean ± SEM is shown, and changes were considered significant when P < 0.05. Mean values were compared by Student’s t test, or when Shapiro–Wilk Normality test failed, median values were compared with Mann–Whitney Rank Sum test. Paired t test or Wilcoxon Signed Rank test was used to compare the pre-surgery and the post-surgery data. *Compared to pre-surgical data; #post-surgical data compared
Increased cytokine levels in metCRC patients
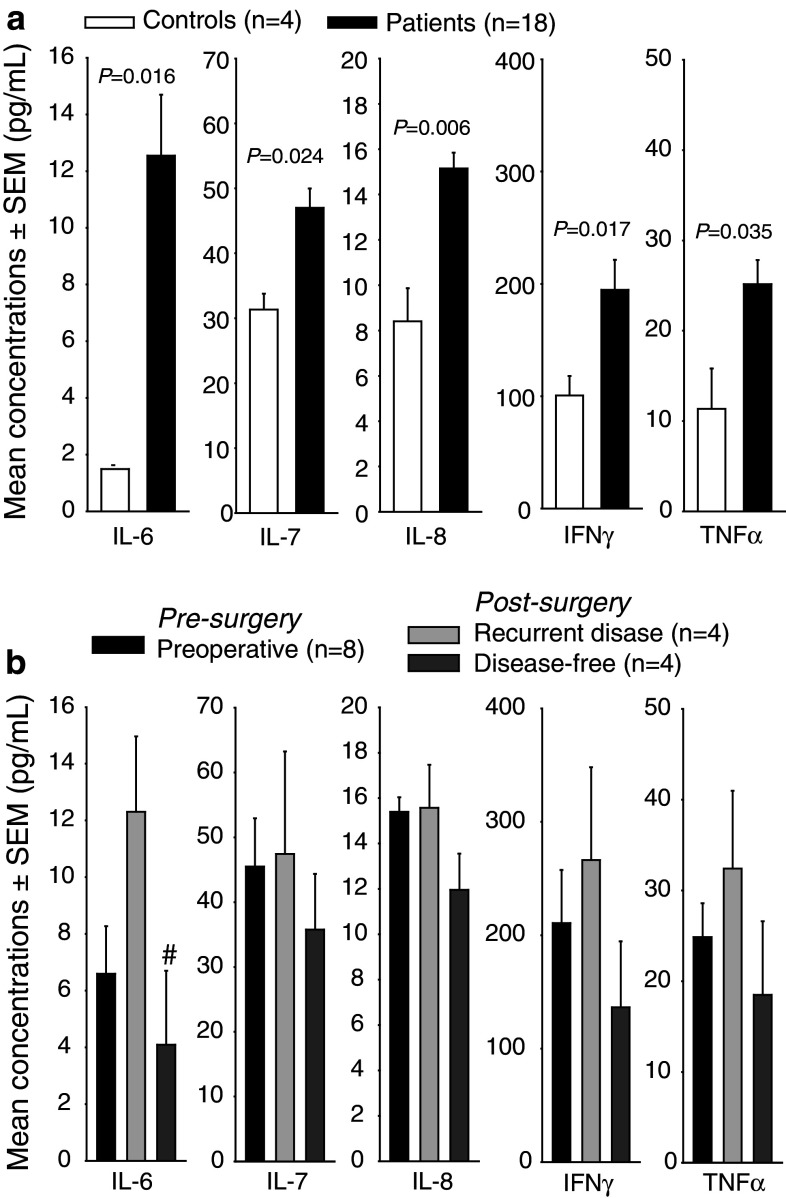
Multiplex analysis of a panel of cytokines on plasma samples from metCRC patients prior to (n = 18) and after surgery (n = 8- and 6-month follow-ups) and healthy blood donors (n = 4) revealed significantly increased levels of IL-6, IL-7, IL-8, IFNγ and TNFα in patients compared to healthy donors (Fig. 2a). Disease-free patients had significant lower concentrations of IL-6 compared to patients with recurrent disease at 6 months post-surgery, and a similar trend of immune activation in recurrent disease was observed also for IL-7, IL-8, IFNγ and TNFα (Fig. 2b).
Fig. 2.
Increased circulating levels of cytokines in patients with metastatic colorectal cancer. a A multiplex cytokine assay was used to detect the serum levels of 17 cytokines in patients with metastatic CRC (n = 18) and controls (n = 4), and cytokine levels where the patients with metastatic CRC were significantly different from controls are shown. b Postoperative changes in levels of cytokines that were elevated preoperatively stratified on clinical outcome. Mean ± SEM is shown, and changes were considered significant when P < 0.05. Mean values were compared by Student’s t test, or when Shapiro–Wilk Normality test failed, median values were compared with Mann–Whitney Rank Sum test. Paired t test or Wilcoxon Signed Rank test was used to compare the pre-surgery and the post-surgery data. #Post-surgical data compared
Elevated PGE2 levels in the presence of metastatic colorectal cancer
We have earlier reported that the plasma levels of PGE2 are elevated in patients with primary CRC compared to normal blood donors [5]. Examination of plasma PGE2 in patients with metCRC also revealed high levels (431 ± 52 pg/ml, mean ± SEM). As observed in studies of primary CRC, preoperative PGE2 levels appeared not to correlate with size or number of metastases, suggesting that tumor load is not the only determining factor (confer COX-2 expression also in immune cells, Fig. 1c). Furthermore, a time-dependent decline in circulating PGE2 levels was observed after surgery and followed by an increase upon recurrence of metCRC (Fig. 3a). Again, PGE2 levels did not decline as rapidly upon surgical debulking as would be expected in light of its short half-life, if PGE2 levels were determined only by tumor load. Indeed, post-surgery PGE2 levels appeared to relate to clinical outcome as 6-month follow-up levels went down in most disease-free patients (Fig. 3b) and up in most recurring patients (Fig. 3c), and as levels in the two groups differed significantly between disease-free and recurring patients ((280 ± 47 vs. 704 ± 153 pg/ml, respectively, P = 0.021; Fig. 3d, left panel). Finally, a similar tendency was observed with respect frequency of CD3+COX-2+ cells in pre- and postoperative samples from the same patients (Fig. 3d, right panel).
Fig. 3.
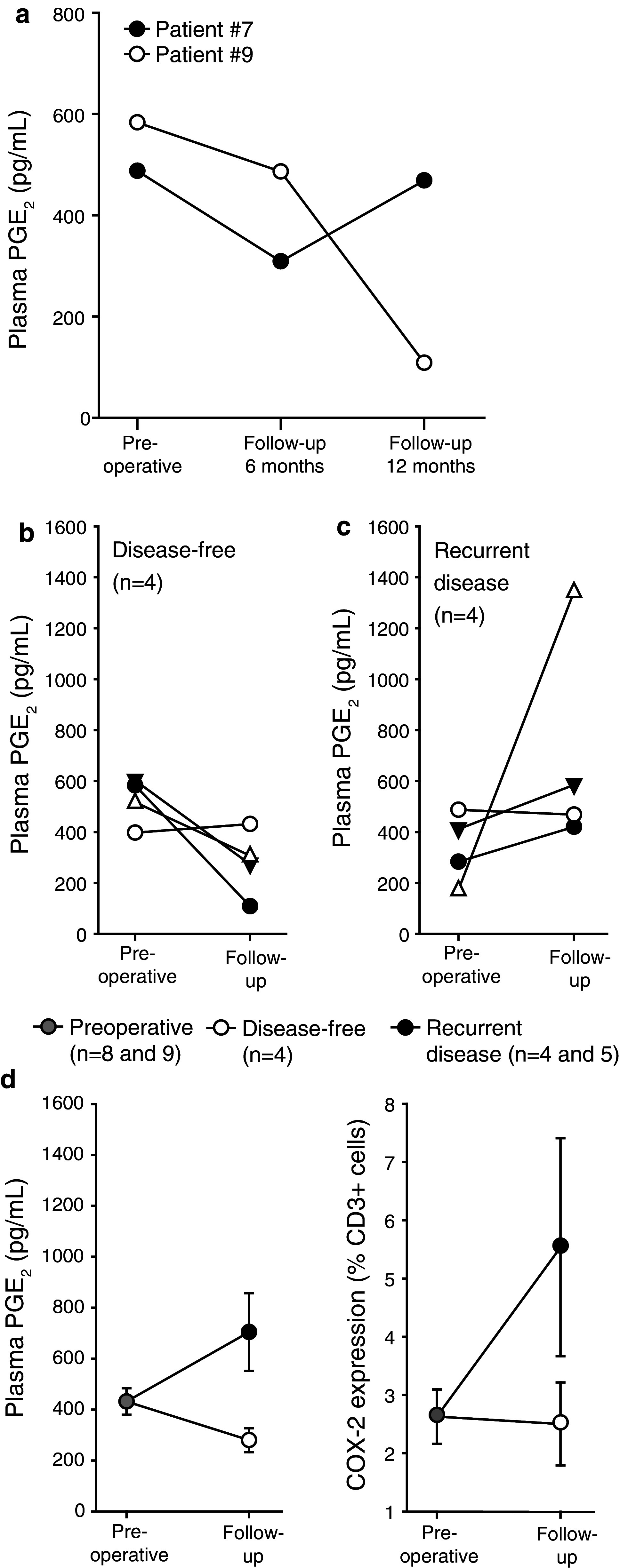
Development in plasma PGE2 concentration levels depending on clinical outcome in patients with metastatic colorectal cancer. a Time-dependent development of postoperative plasma PGE2 levels in two patients, both disease free at 6 months, one (patient #7) with recurrent metastatic disease at 12 months. b–d Concentrations of PGE2 preoperatively and at 6-month follow-up in patients that remained disease free (b, d; n = 4) or developed recurrent disease (c, d; n = 4). Mean ± SEM is shown. d Patients combined PGE2 concentrations P = 0.021 (left panel) and COX-2 expression in peripheral CD3+ cells measured by flow cytometry (right panel)
Regulatory T cells inhibit antitumor immunity in a COX-2-PGE2-dependent manner in patients with metastatic colorectal cancer
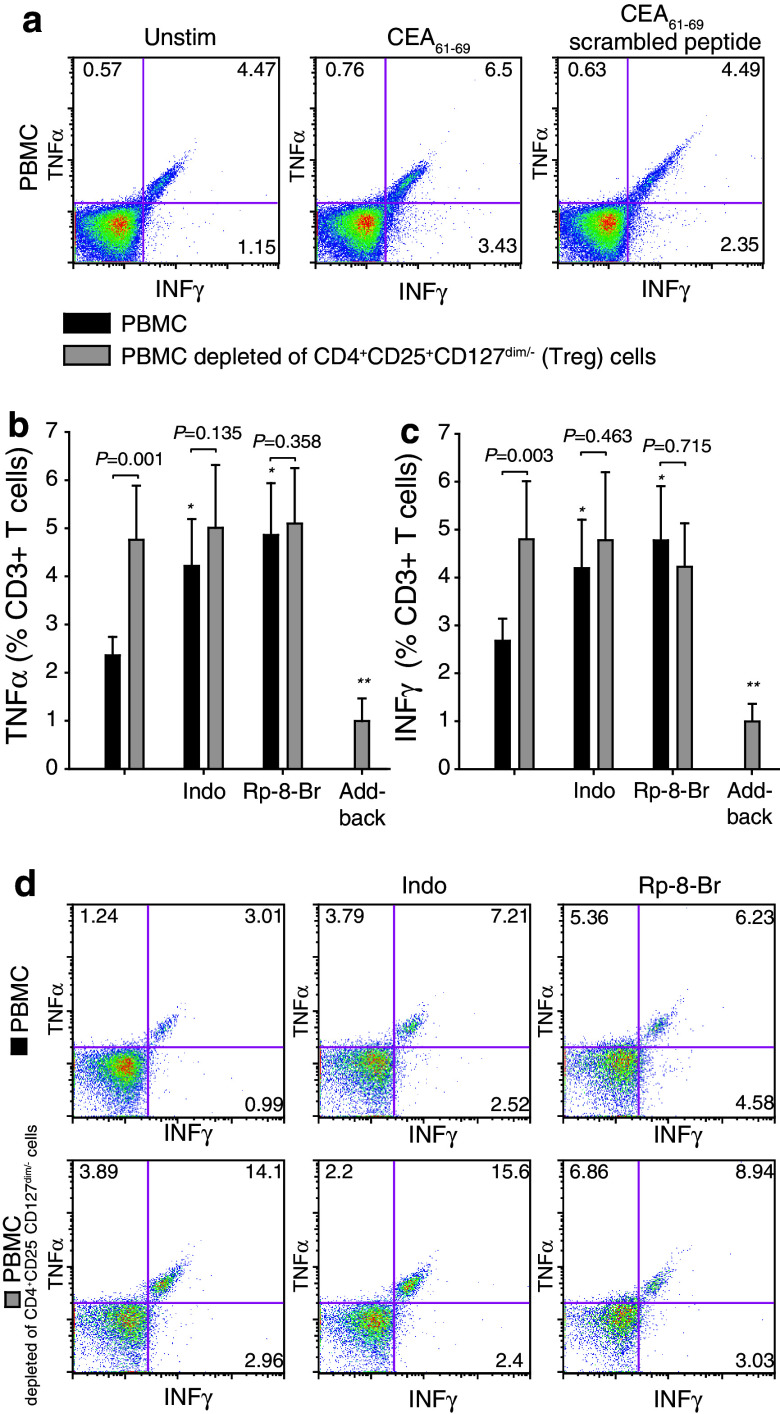
Carcinoembryonic antigen (CEA) is a tumor-associated antigen, expressed in normal fetal tissue, but in abnormal quantities and locations in CRC [18] and to which both antigen-specific Tregs and effector T cells have been show to developed [17]. T-cell immune responses to CEA (CEA61–69 and CEA318–327 peptides) were examined in PBMC and PBMC depleted of Treg (CD4+CD25+CD127dim/−) from metCRC patients pre-surgery (n = 18). Upon stimulation with CEA61–69 peptide, CD3+ T cells (as well as CEA318–327 peptide and CD4+ and CD8+ T cells examined separately with similar results, data not shown) displayed antitumor immune activity as evident from intracytoplasmic expression of TNFα and INFγ (Fig. 4a–c, first black bars, n = 18). The absence or presence of HLA-A2 (Table 1) did not correlate with CEA responsiveness. Treg depletion increased the fraction of CEA-induced TNFα and INFγ-positive CD3+ T cells (Fig. 4b–d, first gray bars, n = 18), and the increase in cytokine expression was reversed when Tregs were added back in some cases (Fig. 4b, c, last gray bars, n = 4). This indicates that antitumor immune responses in circulating T cells are suppressed by endogenously active Treg. Addition of indomethacin, a COX inhibitor, or Rp-8-Br-cAMPS, a PKA type I antagonist prior to activation by CEA61–69 peptide, reversed Treg-mediated suppression of TNFα and INFγ responses to levels comparable to those obtained by depletion of Tregs (Fig. 4b–d, n = 18).
Fig. 4.
Treg-mediated suppression of cytokine expression in patients with metastatic CRC. a Representative FACSplots showing TNFα and IFNγ expression in CD3+ cells in unstimulated, CEA61–69-peptide stimulated and scrambled CEA-peptide-stimulated cultures. b, c Expression of TNFα (b) and IFNγ (c) in CD3+ T cells stimulated with CEA61–69 peptide before and after depletion of CD4+CD25+CD127dim/− cells (regulatory T cells; Tregs) or Tregs add-back in the absence or presence of indomethacin or Rp-8-Br-cAMPS. Mean ± SEM is shown. Suppression assay, n = 18; Treg add-back assay, n = 4 (*P < 0.025 compared to the black bars in the first group, **P < 0.05 compared to gray bars in the first group). Wilcoxon Signed Rank test used to compare the cytokine expression before and after Treg depletion. d FACS plots showing cytokine response (TNFα and IFNγ) in PBMC cultures (top panel) and Treg-depleted PBMC cultures (lower panel) stimulated with CEA61–69 peptide and treated with indomethacin, Rp-8-Br-cAMPS or not
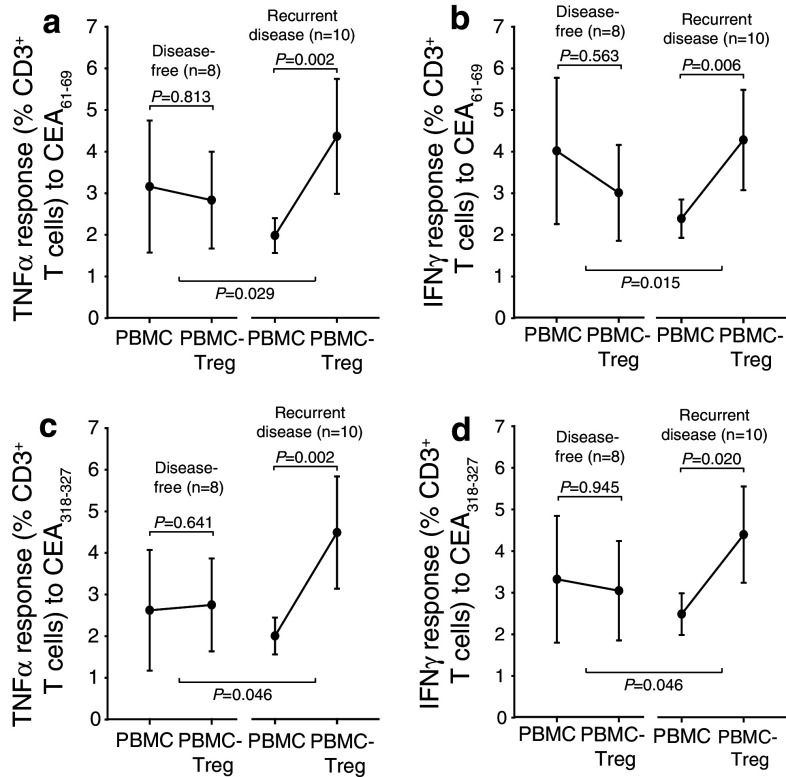
Level of Treg-mediated suppression of antitumor immunity predicts outcome in patients with metastatic colorectal cancer
Anti-CEA tumor immune responses in CD3+ T cells from pre-surgery metCRC patients determined by intracellular TNFα and INFγ expression as described in Fig. 4b, c in the absence and presence of Treg (CD4+CD25+CD127dim/−) were next stratified on outcome at 18 months (observation time up to 30 months for some patients). As evident from TNFα (Fig. 5a, c) and INFγ (Fig. 5b, d) expression in response to CEA61–69 and CEA318–327 peptide stimulation (Fig. 5a–d, respectively), the group of patients that later presented with recurrent disease (n = 10, right groups) had significant Treg-mediated suppression of antitumor immune responses at the time of surgery, while the group of patients who remained disease free (n = 8, left groups) had little or no Treg-mediated suppression based on paired comparisons inside the two groups. Lastly, the later disease-free patients also tended to have higher levels of antitumor immunity in PBMC prior to surgery compared to the patients that later developed recurrent disease, although this finding did not reach statistical significance. In conclusion, our findings suggest that Treg activity is important for outcome. The frequency of Tregs was not significantly different in PBMC or Treg-depleted PBMC between the two patient groups.
Fig. 5.
Treg-mediated suppression of CEA responses in preoperative samples correlate with clinical outcome in patients with metastatic CRC. a–d PBMC and PBMC depleted of regulatory T cells from patients with metastatic CRC were stimulated with different CEA peptides [CEA61–69 H L F G Y S W Y K (a, b) and CEA318-327 T Y A C F V S N L (c, d)] and TNFα (a, c) and IFNγ (b, d) expression determined by flow cytometry in both cell populations. Patients were classified as disease free (n = 8, 18 months) or with recurrent disease (n = 10). Mean ± SEM is shown. Wilcoxon Signed Rank test was used to analyze paired data and Mann–Whitney Rank Sum test to assess differences between groups
Concluding remarks
The presence of liver metastases indicates systemic malignant disease and breach of tumor immune surveillance. After complete surgical resection of metCRC, effective antitumor immunity is critical for the eradication of micrometastases and long-term survival. In the present report, we show that the level of Treg-mediated suppression at the time of surgery is associated with clinical outcome in metCRC.
In CRC, PGE2 stimulates tumor cell proliferation [8], angiogenesis [6, 10], induction and recruitment of Tregs [19] and local immune suppression [13]. In our study, elevated levels of PGE2 were observed in patients with recurrent metCRC compared to those who remained disease free during follow-up. Both epithelial cancer cells and Tregs express COX-2 and may contribute to PGE2 production. Clinically, COX-2 expression and PGE2 production correlates with CRC risk and metastasis [9, 20] and regular use of COX inhibitors including aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) reduce the incidence of CRC by 40–50% [21–23]. As PGE2 levels remained elevated after surgery in patients that later developed disease recurrence, it is possible that Tregs are a significant source of PGE2 production after tumor removal and may contribute to the immune suppression observed in these patients.
In healthy individuals, an intact immune system protects against malignant transformation through antigen-unspecific and antigen-specific immune mechanisms. Congenital and acquired immune dysfunction is associated with increased cancer risk [24]. In human malignant diseases, TILs are associated with improved prognosis [14], whereas the presence of tumor infiltrating immunosuppressive Tregs are associated with poor outcome in a variety of cancers including ovarian carcinoma [25]. In contrast, the role of infiltrating Tregs in CRC where high density of intratumoral FOXP3+ cells has been suggested to be both a positive and negative prognostic marker [26–29]. However, it is not always clear whether the FOXP3+ infiltrating cells are functionally suppressive [30]. Furthermore, two of the studies show a correlation between early tumor stage and FOXP3+ cell density, raising the question of whether Treg infiltration an early event in the tumorigenesis. In addition, a positive correlation is seen between tumor infiltration by CD3+ and FOXP3+ as well as CD8+ and FOXP3+ cells, thus Tregs may be a marker for increased intratumoral tumor immunity [26, 27]. Lastly, bacterial translocation from the microbial flora could trigger the production of proinflammatory cytokines with proangiogenic and tumor-enhancing effects [31] and producing TILs and Tregs with antigen specificity to the commensal microflora and not the tumor [32].
In CRC, circulating and tumor infiltrating Tregs correlate with Duke stage and prognosis [33]. Here, we assessed the level of Treg-mediated suppression of tumor-specific immune responses at the time of surgery and at follow-ups in a cohort of patients with poor expected outcome. We observed significant Treg-mediated suppression of antitumor immune responses at the time of surgery in the group of patients with later recurrent disease, while the group of patients who remained disease free had little or no Treg-mediated suppression. The disease-free patients also tended to have higher levels of antitumor immunity in PBMC prior to surgery compared to the patients that developed recurrent disease. These data indicate that the level of antitumor immune activity is important for the outcome and that Tregs are an important determinant for the immune reactivity in these patients. However, it is possible that the antitumor immune function is determined by the extent of the disease at the time of surgery, although we did not identify any clinical variables such as tumor size, number of metastases, chemotherapy or co-morbidities that could discriminate between the two groups. Furthermore, neither age nor sex appeared to differ between the groups. In contrast, HLA-A2 status differed (2/9 and 6/7 HLA-A2-positive patients in the recurrent and disease-free groups, respectively, P = 0.012), but MHC restriction did not appear to give bias as CEA responses were similar in HLA-A2-positive and HLA-A2-negative patients. Finally, when stratifying the population on increase in TNFα responses to CEA61–69 upon Treg depletion (delta more than 1%), we obtained a specificity of 88% and a sensitivity of 70% for the identification of disease recurrency. This, however, has to be validated in a larger and independent material. In summary, we show in a small metCRC patient population that the effect of Treg inhibition of immune responses to tumor antigens correlates with outcome and may provide useful prognostic information.
Acknowledgments
This work was supported by the Norwegian Cancer Society and the Research Council of Norway. KWB is a fellow of the Norwegian Cancer Society.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Kristoffer Watten Brudvik and Karen Henjum contributed equally.
References
- 1.Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381–396. doi: 10.1016/j.bpg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.LeGolvan MP, Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol. 2010;102:898–908. doi: 10.1002/jso.21817. [DOI] [PubMed] [Google Scholar]
- 3.Lewis AM, Martin RC. The treatment of hepatic metastases in colorectal carcinoma. Am Surg. 2006;72:466–473. [PubMed] [Google Scholar]
- 4.Dimitroulis D, Nikiteas N, Troupis T, Patsouras D, Skandalakis P, Kouraklis G. Role of surgery in colorectal liver metastases: too early or too late? World J Gastroenterol. 2010;16:3484–3490. doi: 10.3748/wjg.v16.i28.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, Tasken K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–821. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujino H, Toyomura K, Chen Xb, Regan JW, Murayama T. Prostaglandin E2 regulates cellular migration via induction of vascular endothelial growth factor receptor-1 in HCA-7 human colon cancer cells. Biochem Pharmacol. 2011;81:379–387. doi: 10.1016/j.bcp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 8.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 9.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 11.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation - overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Yaqub S, Tasken K. Role for the cAMP-protein kinase A signaling pathway in suppression of antitumor immune responses by regulatory T cells. Crit Rev Oncog. 2008;14:57–77. doi: 10.1615/critrevoncog.v14.i1.40. [DOI] [PubMed] [Google Scholar]
- 13.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 14.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 15.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 16.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29:6301–6313. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 19.Mahic M, Yaqub S, Johansson CC, Taskén K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 20.Cai Q, Gao YT, Chow WH, Shu XO, Yang G, Ji BT, Wen W, Rothman N, Li HL, Morrow JD, Zheng W. Prospective study of urinary prostaglandin E2 metabolite and colorectal cancer risk. J Clin Oncol. 2006;24:5010–5016. doi: 10.1200/JCO.2006.06.4931. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 23.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 28.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 29.Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB, Banham AH, Cerundolo V. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]
- 30.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 31.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 32.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB, Banham AH, Cerundolo V. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]