Abstract
In recent years, the combination of cancer immunotherapy with standard therapeutic modality is gaining credibility due to a number of clinical trials demonstrating therapeutic success of such combination therapies. However, the mechanism of this phenomenon is poorly understood. Here, we will discuss recent findings that suggest novel mechanisms of synergistic effect of cancer immunotherapy and chemotherapy.
Keywords: Immunotherapy, Chemotherapy, Vaccines
Introduction
Despite the substantial progress made in recent years, the treatment of patients with advanced stage cancers faces many challenges. In many different types of cancers, conventional therapeutic modalities alone cannot provide satisfactory clinical results. It has become clear that future progress will depend on our ability to develop effective methods of combination therapy. Cancer immunotherapy has long been an attractive therapeutic approach due to its low toxicity and high specificity. However, it often failed to provide clear clinical benefits [1]. Chemotherapy, the conventional treatment option in patients with advanced stage cancer, can cause immunologically active tumor cell death; but it has immune suppressive effects rendering it rather detrimental to the immune system. Therefore, for many years, the combination of immunotherapy and chemotherapy was not considered as very promising approach. This paradigm has been revisited in recent years in light of unexpected clinical observations describing substantial clinical benefits for patients treated with cancer vaccines followed by chemotherapy [2–6]. The mechanism of this phenomenon remains unclear. We will review our attempts to address this question.
Antitumor immunity in solid tumors is mainly mediated by cytotoxic T cells (CTLs), natural killer (NK), and natural killer T (NKT) cells. Dendritic cells (DCs) play a central role in coordinating the activities of these cells. On the other end, tumor-associated macrophages (TAM), T regulatory cells (Treg), and myeloid-derived suppressor cells (MDSC) form an effective network capable of suppressing the immune system. The question is whether it is possible, with these myriad of factors functioning simultaneously, to induce effective anticancer T cell reactivity?
Effect of chemotherapy on immune system
Most of chemotherapeutic drugs target rapidly dividing tumor cells. Therefore, dividing cells of the immune system are also vulnerable to these drugs. Intensive chemotherapy causes lymphopenia with decreased percentage of circulating T cells [7]. There is ample evidence that conventional chemotherapy can ablate T cell function and thus blunt anti-tumor immune responses [8].
However, when used in non-cytotoxic doses, several drugs like paclitaxel, doxorubicin, mitomycin C, and methotrexate increased antigen presentation in an autocrine IL-12-dependent manner [9]. DCs, treated with vinblastine, underwent maturation and exhibited better ability in inducing CD8 T cell responses, when compared with untreated DCs [10]. Chemotherapy may also induce an antitumor response by influencing tumor cells directly, causing immunogenic cell death [11]. Spisek et al. showed that treatment of myeloma cells with the proteasome inhibitor bortezomib lead to surface expression of HSP90 on the cell surface [12]. Heat shock proteins (HSPs) act as danger signals, and are expressed on the cell surface as “eat me” signals to DCs [13]. Chemotherapy has also been shown to render cancer cells more susceptible to killing by CTLs. 5-Fluorouracil (5-FU), CPT-11, and cisplatin (CIS) were all shown to increase the sensitivity of the SW480 colon cancer cell line to killing by T cells [14]. Chemotherapy, even at conventional doses, can eliminate MDSC and Tregs, thus removing some of the immune suppressive factors present in cancer patients [15]. Cytotoxic drugs can modulate systemic mechanisms of active immune suppression or by amplifying expansion of antigen-specific T cell expansion via cytoreduction (review in [16]).
These observations suggested that chemotherapy can be immunogenic if used in low non-cytotoxic doses. However, in cancer patients treated with conventional doses of chemotherapy, this apparently was not the case [17] raising the question of how exactly chemotherapy may augment the effect of immunotherapy.
Understanding the synergy between chemotherapy and immunotherapy
In our recent work [18], we used various murine tumor models and different methods to generate tumor-specific CTLs. The tumor models were established using MC38 colon carcinoma and TUBO mammary carcinoma cell lines in C57BL/6 and Balb/c mice. The DC vaccines used p53 and p66 (rat Neu) derived peptides as tumor antigens. The rationale for using p53 and p66 as tumor antigens is that the p53 mutation in colon cancer produces a non-functional, but stable p53 protein [19]. Amplification of the Her2/neu gene and the resultant protein over-expression is seen in a variety of cancers like prostate, breast, ovarian and gastric. The overexpression of HER-2/neu is implicated in the malignant transformation of breast cancer [20, 21]. We sought to exploit differential expression of these antigens between tumor cells and normal cells. We found that a combination of suboptimal doses of paclitaxel (TAX) and the p53 DC vaccine potently suppressed tumor growth in the MC38 model for at least 5 weeks after start of the treatment. Chemotherapy and immunotherapy as single agents had short-lived effects. In the breast tumor TUBO model, TAX alone had very little antitumor activity; but chemotherapy, combined with DC vaccines, resulted in a substantial delay in tumor progression [18].
The treatment of mice with TAX substantially increased the ability of CTLs to penetrate tumor parenchyma. This was probably the result of disruption of tumor stroma by chemotherapy. However, our in vivo and in vitro experiments demonstrated that antitumor effect was not due to upregulation of CTL activity. In vitro studies showed that suboptimal doses of chemotherapy sensitized tumor cells to cell-mediated killing by CTLs. This was true for all three chemotherapeutic drugs tested: TAX, doxorubicin (DOX) and cisplatin (CIS), despite the fact that these drugs have different mechanisms of action [22–24].
Cytotoxic T cells exert their cytotoxic effect via several mechanisms that include IFN-γ, Fas/FasL [25], and perforin/granzyme B [26]. Although it is known that IFN-γ can kill cells, the biological role of IFN-γ induced programmed cell death is still not well defined; and the precise correlation between IFN-γ mediated inflammatory changes and apoptosis has not been well established. In an in vivo tumor model, NK and NKT cells produced IFN-γ in response to a growing tumor; which, in turn, caused limited tumor death and promoted production of chemokines, such as CXCL10, that recruited immune cells at the tumor site. IFN-γ, along with IL-12 was also implicated in apoptosis, via production of reactive oxygen species and nitrogen intermediates [27]. IFN-γ up-regulated the expression of Fas and Fas ligand (FasL) on HT29 tumor cells [28], thus contributing to induction of apoptosis. Kiefer et al. [29] have proposed that IFN-γ modulates a p53-independent apoptotic pathway, by both directly and indirectly inducing select apoptosis-related genes. We investigated the possibility of IFN-γ induced apoptosis due to combination therapy, but found that the IFN-γ levels between the control and treated groups were not significantly different (data not shown).
The Fas pathway, involving FasL on the effector cell and engaging the Fas receptor (CD95) on the target cell, is a well-known pathway for inducing apoptosis. FasL binds and activates receptors via their trimerization. Activated receptors recruit adaptor molecules, such as Fas-associating protein with death domain (FADD), which in turn activates procaspase 8. Caspase 8 activates caspase 3 through either the BID or the cytochrome C pathways, which ultimately lead to DNA fragmentation [30]. We evaluated the expression of Fas on tumor cells and of FasL on splenocytes after treatment with three chemotherapeutic agents (TAX, CIS and DOX). None of the drugs altered the expression of Fas or FasL, but dramatically increased the permeability of the target cell membrane to granzyme B (GrzB) [18]. All tested chemotherapeutic drugs increased the intracellular levels of GrzB in murine and human cell lines. In addition, the inhibition of GrzB activity abrogated the effect of TAX on CTL-induced apoptosis, confirming the critical role of this mechanism in the synergistic effect of chemotherapy and CTLs [18].
CTLs and NK cells utilize contact-dependent mechanisms to kill malignant cells. Within a few minutes after contact of effector cells with target cells, CTLs release pore-forming protein, perforin, and a family of serine proteases (granzymes) [31, 32]. GrzB is the most studied and arguably most important member of granzyme family. GrzB induces both caspase-mediated, as well as caspase-independent, cell death. GrzB mainly triggers caspase activation indirectly via pro-apoptotic BH3 members of the BCL-2 family, such as the BH3-interacting domain death agonist (BID); which results in the leakage of pro-apoptotic mitochondrial mediators, such as cytochrome C, into the cytosol [33, 34]. There have been reports that GrzB also induces apoptosis via BID-independent pathways [35]. It was initially assumed that perforin acted as a conduit, by inserting itself into the plasma membrane to create a channel, allowing GrzB to passively cross over [30, 36]. More recent studies suggested that perforin, although important as an enabler of granzyme-mediated apoptosis, was not the sole mechanism for uptake of GrzB into cells. It has been shown that the penetration of GrzB into cells can be arbitrated by receptor-mediated endocytosis [37, 38]. One of the major receptors, mediating GrzB uptake by cells, is the multi-functional cation-independent mannose 6-phosphate receptor (CI-MPR) [39].
The two mannose-6-phosphate receptors, the 46 kDa MW cation-dependent (CD-MPR) and the ~300 kDa MW cation-independent (CI-MPR), are the integral membrane proteins. On the cell surface CI-MPR, along with CD-MPR, is responsible for the binding and uptake of mannose-6-phosphate containing molecules [40]. Within the cell, CI-MPR delivers the ligand-receptor complex from the trans-golgi network (TGN) to endosomes, where the ligands are subsequently transferred to lysosomes [41, 42]. Among other things, CI-MPR is also known to facilitate activation of TGF-β1 and to modulate the level of the insulin growth factor II (IGF-II) [43]. Over-expression of the CI-MPR results in growth inhibition, both in vitro and in vivo [44, 45]. Some findings suggest that CI-M6PR may play an important role in GrzB mediated cell killing [46, 47]. Our data indicate that CI-MPR is a key player in the combination therapy induced apoptotic pathway.
All three chemotherapeutic agents caused up-regulation of CI-MPR expression on tumor cells and a concurrent increase in the uptake of GrzB in these treated cells. Blocking the CI-MPR expression, using MPR specific siRNA, resulted in a dramatic decrease in the uptake of GrzB in TAX treated tumor cells suggesting that CI-MPR could indeed be responsible for the sensitization of tumor cells to CTLs [18]. Using perforin-deficient CTLs, we observed that, while these CTLs could not kill non-treated target cells, they were effective against TAX-treated tumor cells. An adoptive transfer model using these cells in tumor-bearing mice gave the same results [18]. These data emphasize that chemotherapy may regulate GrzB uptake via up-regulation of CI-MPR and bypass the requirement for perforin. This led us to the conclusion that activated CTLs, releasing Grz B, were capable of killing the chemotherapy sensitized cells at the tumor site even in the absence of antigen expression.
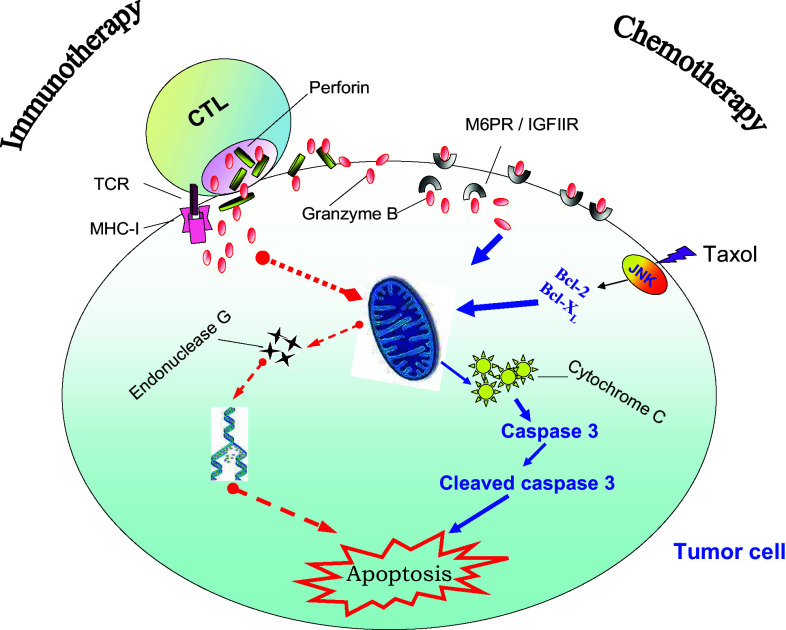
It appeared that chemotherapy (TAX) and immunotherapy affected different components of the apoptotic pathway (Fig. 1). TAX increased the levels of cleaved caspase-3, while CTLs up-regulated cytochrome C (cyt C) [18]. Caspase-3 is the main target for cyt C activity. However, CTLs can also induce apoptosis in a caspase-independent fashion by upregulating levels of endonuclease G, a protein that causes caspase-independent DNA degradation [18]. This can explain synergistic effect of CTLs and TAX on induction of tumor cell apoptosis in our experimental system.
Fig. 1.
Schematic of apoptosis induced by CTLs and chemotherapeutic drug (TAX). CTL cytotoxic T cells, TCR T cell receptor, MHC I MHC class I, M6PR mannose 6-phosphate receptor (see description in the text)
Conclusions
Based on these findings, we can suggest a working hypothesis describing the synergistic effect of CTLs and chemotherapy in cancer. In the case of monotherapy, a small number of low-affinity CTLs, generated by cancer vaccines or generated ex vivo and adoptively transferred to cancer patients, can penetrate tumor parenchyma. There they come in contact with a small number of antigen-expressing tumor cells. Interaction of CTLs with their target results in CTL activation, release of perforin and GrzB, and killing of tumor cells. However, only tumor cells that form synapse with CTLs will be killed. Thus, the effect of immunotherapy is limited by the number of CTLs able to penetrate tumor and by the number of tumor cells expressing specific antigen. This process is further compromised by different immune suppressive cells present in tumor site. As a result, only a limited proportion of patients treated with immunotherapy alone have detectable antitumor clinical responses. Chemotherapy causes the disruption of tumor stroma that allows more CTLs to penetrate into tumor site [18]. It also may inhibit negative regulatory network inside the tumor by eliminating MDSC and Treg and by decreasing production of immune suppressive cytokines by tumor cells. However, most importantly, chemotherapy up-regulates expression of CI-MPR on tumor cells. As a result GrzB released by activated CTLs can be picked up by a large number of neighboring tumor cells. Thus, a relatively small number of CTLs can cause apoptosis in large numbers of tumor cells manifesting in a clinically evident antitumor effect.
This hypothesis needs to be tested in cancer patients and combination therapy of cancer faces many challenges. The optimal chemotherapeutic agents available to physicians most of the time are dictated by the nature of the type of cancer they are treating. Therefore, the time frame of the effect needs to be determined, as well as the possibility that different chemotherapeutics can cause the same effect on tumor cells. Cancer patients are currently enrolled in clinical trials for therapeutic vaccines at the late stages of their disease. As a result, patients enrolled in cancer immunotherapy clinical protocols have already received a variety of standard treatments that may have had negatively influenced for a long period of time their immune competence. Therefore, clinical effect of combination therapy can be really tested only in the settings of a frontline treatment. Given the fact that each patient may differently respond to the chemotherapeutic treatment most effective combination therapy probably needs to be developed separately for each chemotherapeutic drug. However, despite all these challenges, combination therapy is very promising approach to the treatment of patients with advanced stage cancers.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eighth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, 26–28th May, 2010.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gribben JG, Ryan DP, Boyajian R, Urban RG, Hedley ML, Beach K, Nealon P, Matulonis U, Campos S, Gilligan TD, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11(12):4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 3.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, Bepler G, Simon G, Janssen W, Lee JH, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 4.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12(4):1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13(13):3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10(16):5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 7.Behl D, Porrata LF, Markovic SN, Letendre L, Pruthi RK, Hook CC, Tefferi A, Elliot MA, Kaufmann SH, Mesa RA, et al. Absolute lymphocyte count recovery after induction chemotherapy predicts superior survival in acute myelogenous leukemia. Leukemia. 2006;20(1):29–34. doi: 10.1038/sj.leu.2404032. [DOI] [PubMed] [Google Scholar]
- 8.Liseth K, Ersvaer E, Hervig T, Bruserud O. Combination of intensive chemotherapy and anticancer vaccines in the treatment of human malignancies: the hematological experience. J Biomed Biotechnol. 2010;2010:692097. doi: 10.1155/2010/692097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183(1):137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69(17):6987–6994. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(11):4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen H, Andresen L, Hansen KA, Skov S. Cell-surface expression of Hsp70 on hematopoietic cancer cells after inhibition of HDAC activity. J Leukoc Biol. 2009;86(4):923–932. doi: 10.1189/jlb.0209056. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother. 2001;50(9):445–455. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herber DL, Nagaraj S, Djeu JY, Gabrilovich DI. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res. 2007;67(11):5067–5069. doi: 10.1158/0008-5472.CAN-07-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65(18):8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 17.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: a phase i trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chada S, Mhashilkar A, Roth JA, Gabrilovich D. Development of vaccines against self-antigens: the p53 paradigm. Curr Opin Drug Discov Devel. 2003;6(2):169–173. [PubMed] [Google Scholar]
- 20.Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE, Schell MJ, Melton LJ, 3rd, Liu ET, Conway K. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. J Clin Oncol. 2000;18(2):267–274. doi: 10.1200/JCO.2000.18.2.267. [DOI] [PubMed] [Google Scholar]
- 21.Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, Osborne CK, Tormey DC, McGuire WL. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23(9):974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–S6. [PubMed] [Google Scholar]
- 23.Sartiano GP, Lynch WE, Bullington WD. Mechanism of action of the anthracycline anti-tumor antibiotics, doxorubicin, daunomycin and rubidazone: preferential inhibition of DNA polymerase alpha. J Antibiot (Tokyo) 1979;32(10):1038–1045. doi: 10.7164/antibiotics.32.1038. [DOI] [PubMed] [Google Scholar]
- 24.Reedijk J, Lohman PH. Cisplatin: synthesis, antitumour activity and mechanism of action. Pharm Weekbl Sci. 1985;7(5):173–180. doi: 10.1007/BF02307573. [DOI] [PubMed] [Google Scholar]
- 25.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 26.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76(6):977–987. doi: 10.1016/0092-8674(94)90376-X. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998;58(13):2832–2837. [PubMed] [Google Scholar]
- 29.Ossina NK, Cannas A, Powers VC, Fitzpatrick PA, Knight JD, Gilbert JR, Shekhtman EM, Tomei LD, Umansky SR, Kiefer MC. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272(26):16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 30.Henkart PA, Sitkovsky MV. Cytotoxic lymphocytes. Two ways to kill target cells. Curr Biol. 1994;4(10):923–925. doi: 10.1016/S0960-9822(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 31.Raja SM, Wang B, Dantuluri M, Desai UR, Demeler B, Spiegel K, Metkar SS, Froelich CJ. Cytotoxic cell granule-mediated apoptosis. Characterization of the macromolecular complex of granzyme B with serglycin. J Biol Chem. 2002;277(51):49523–49530. doi: 10.1074/jbc.M209607200. [DOI] [PubMed] [Google Scholar]
- 32.Trapani JA, Davis J, Sutton VR, Smyth MJ. Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo. Curr Opin Immunol. 2000;12(3):323–329. doi: 10.1016/S0952-7915(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 33.Heibein JA, Goping IS, Barry M, Pinkoski MJ, Shore GC, Green DR, Bleackley RC. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members bid and Bax. J Exp Med. 2000;192(10):1391–1402. doi: 10.1084/jem.192.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton VR, Davis JE, Cancilla M, Johnstone RW, Ruefli AA, Sedelies K, Browne KA, Trapani JA. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J Exp Med. 2000;192(10):1403–1414. doi: 10.1084/jem.192.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DA, Scorrano L, Putcha GV, Korsmeyer SJ, Ley TJ. Granzyme B can cause mitochondrial depolarization and cell death in the absence of BID, BAX, and BAK. Proc Natl Acad Sci USA. 2001;98(26):14985–14990. doi: 10.1073/pnas.261581498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185(5):855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinkoski MJ, Hobman M, Heibein JA, Tomaselli K, Li F, Seth P, Froelich CJ, Bleackley RC. Entry and trafficking of granzyme B in target cells during granzyme B-perforin-mediated apoptosis. Blood. 1998;92(3):1044–1054. [PubMed] [Google Scholar]
- 38.Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah GM, Bleackley RC, Dixit VM, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271(46):29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 39.Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, Barry M, Shostak I, Sawchuk T, Holmes CF, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103(3):491–500. doi: 10.1016/S0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 40.Stein M, Braulke T, Krentler C, Hasilik A, von Figura K. 46-kDa mannose 6-phosphate-specific receptor: biosynthesis, processing, subcellular location and topology. Biol Chem Hoppe Seyler. 1987;368(8):937–947. doi: 10.1515/bchm3.1987.368.2.937. [DOI] [PubMed] [Google Scholar]
- 41.Stein M, Zijderhand-Bleekemolen JE, Geuze H, Hasilik A, von Figura K. Mr 46, 000 mannose 6-phosphate specific receptor: its role in targeting of lysosomal enzymes. EMBO J. 1987;6(9):2677–2681. doi: 10.1002/j.1460-2075.1987.tb02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52(3):329–341. doi: 10.1016/S0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 43.O’Gorman DB, Weiss J, Hettiaratchi A, Firth SM, Scott CD. Insulin-like growth factor-II/mannose 6-phosphate receptor overexpression reduces growth of choriocarcinoma cells in vitro and in vivo. Endocrinology. 2002;143(11):4287–4294. doi: 10.1210/en.2002-220548. [DOI] [PubMed] [Google Scholar]
- 44.Zaina S, Squire S. The soluble type 2 insulin-like growth factor (IGF-II) receptor reduces organ size by IGF-II-mediated and IGF-II-independent mechanisms. J Biol Chem. 1998;273(44):28610–28616. doi: 10.1074/jbc.273.44.28610. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Sahagian GG. Demonstration of tumor suppression by mannose 6-phosphate/insulin-like growth factor 2 receptor. Oncogene. 2004;23(58):9359–9368. doi: 10.1038/sj.onc.1208039. [DOI] [PubMed] [Google Scholar]
- 46.Dressel R, Raja SM, Honing S, Seidler T, Froelich CJ, von Figura K, Gunther E. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J Biol Chem. 2004;279(19):20200–20210. doi: 10.1074/jbc.M313108200. [DOI] [PubMed] [Google Scholar]
- 47.Trapani JA, Sutton VR, Thia KY, Li YQ, Froelich CJ, Jans DA, Sandrin MS, Browne KA. A clathrin/dynamin- and mannose-6-phosphate receptor-independent pathway for granzyme B-induced cell death. J Cell Biol. 2003;160(2):223–233. doi: 10.1083/jcb.200210150. [DOI] [PMC free article] [PubMed] [Google Scholar]