Abstract
The programmed death-1 (PD-1) pathway is important in the maintenance of peripheral tolerance and homeostasis through suppression of T cell receptor signaling. As such, it is employed by many tumors as a means of immune escape. We have investigated the role of this pathway in human ovarian cancer (OC) to assess its potential role as a diagnostic and/or prognostic marker and therapeutic target, following recent clinical trial success of antibody therapy directed at this pathway. We show programmed death ligand-1 (PD-L1) expression on monocytes in the ascites and blood of patients with malignant OC is strikingly higher than those with benign/borderline disease, with no overlap in the values between these groups. We characterize the regulation of this molecule and show a role of IL-10 present in ascitic fluid. Flow cytometric analysis of T cells present in the ascites and blood showed a correlation of PD-1 expression with malignant tumors versus benign/borderline, in a similar manner to PD-L1 expression on monocytes. Finally, we demonstrate functional links between PD-L1 expression on monocytes and OC tumor cells with suppression of T cell responses. Overall, we present data based on samples obtained from women with ovarian cancer, suggesting the PD-1 pathway may be used as a reliable diagnostic marker in OC, as well as a viable target for use with PD-1/PD-L1-directed antibody immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1503-x) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, PD-L1, Monocytes, B7-H1, Immune regulation, IL-10
Introduction
Ovarian cancer is the sixth most common cancer worldwide. Of all ovarian cancer, 90 % arises from the epithelium, and epithelial ovarian cancer (EOC) is the leading cause of death among gynecological malignancies, with 5-year survival rates of 30–40 % [1–4]. A lack of diagnostic markers contributes to this poor survival rate.
The immune system offers an attractive area to find diagnostic and prognostic markers. Zhang et al. [5] have shown that the 5-year survival rate is 38 % versus 4.5 % in the presence or absence of TILs (tumor-infiltrating lymphocytes), respectively, suggesting that the immune system plays a role in ovarian cancer and that cells and molecules involved in the regulation of T cell responses could be beneficial in the diagnosis and/or treatment for the disease.
The PD-1 pathway is an important co-inhibitory pathway involved in the suppression of T cell activation, regulating peripheral tolerance [6]. PD-L1/PD-L2 has been shown to interact with PD-1 on activated T cells, which results in an inhibitory signal being delivered to the T cell through SHP-1 signaling [7, 8]. PD-L1 exhibits a broad expression pattern on a range of hematopoietic and non-hematopoietic tissues, suggesting a role for this molecule in the maintenance of peripheral tolerance [6]. Studies have reported the expression of PD-L1 on a range of malignancies including renal, esophageal and colorectal cancers, suggesting that some tumors have evolved high levels of expression of this molecule, possibly suppressing anti-tumor T cell responses [9–11]. As a result, PD-L1 offers potential as a prognostic marker as well as being a target for therapy.
Hamanishi et al. studied expression of PD-L1 and PD-L2 on ovarian tumors of differing grades and found a significantly worse overall survival in patients whose tumor expressed one or both of these ligands. This study also showed an inverse correlation between PD-L1 expression and intraepithelial CD8 count, a known prognostic marker [12]. Recently, the same group has shown in murine models that PD-L1 on tumor cells reduces CTL activity directed against ovarian cancer [13]. In addition, expression of PD-L1 on monocyte-derived myeloid dendritic cells (MDCs) has been reported in patients with ovarian cancer. These cells appear to result in IL-10 (a regulatory cytokine) production by T cells [14].
There are currently six antibodies in clinical trials aimed at inhibiting the PD-1 pathway; three anti-PD-1, two anti-PD-L1 and one anti-PD-L2. Recently, Topalian et al. [16] and Brahmer et al. [15] reported results following treatment for solid tumors with anti-PD-1 and anti-PD-L1, respectively (the Brahmer study included 17 cases of ovarian cancer patients). Both studies report tumor response rates higher than 10–15 %, the highest rate of anti-tumor activity from immunotherapy tested in the clinic in the last 30 years [15–17]. Interestingly, the objective response observed in these trials correlated with PD-L1 expression on tumors (36 % vs 0 % response on PD-L1+ vs PD-L1− tumors), and not all tumors were found to respond. Colon and pancreatic cancer patients did not respond to either anti-PD-1 or PD-L1 treatment, raising the caveat that factors such as the tumor microenvironment will determine the outcome of the treatment to this pathway. It is particularly important, therefore, to characterize the tumor for PD-1 pathway involvement and tailor the treatment accordingly. We report the findings characterizing the involvement of PD-1 and PD-L1 in human ovarian cancer, which suggest that this is an important pathway for prognosis and an excellent target for antibody immunotherapy.
Materials and methods
Patient sample collection
This study was approved by the Hammersmith and Queen Charlotte Hospitals Research Ethics Committee. Ascites and peripheral blood samples were collected over a period of 3 years from patients with ovarian tumors from Imperial College NHS Trust, London. Peripheral blood was also obtained from healthy volunteers. Written informed consent was obtained from participants prior to enrolling them in the study.
Separation of cells from ascites and blood
Peripheral blood mononuclear cells (PBMCs) and ascitic mononuclear cells (AMCs) were isolated from blood and ascites, respectively, by Histopaque (Axis Shield, Cambridge, UK) and used for subsequent assays. Cells were checked for viability before use by trypan blue staining and microscopic examination.
Staining and Flow Cytometry
Cells were incubated with Fc receptor blocking agent (Miletenyi Biotech, Surrey, UK). Cells were then double-stained by adding murine anti-human PD-L1 APC (BioLegend, San Diego, USA) and CD14 FITC (BD Bioscience, Oxford, UK) on ice for 30 min. Stained cells were washed and analyzed using a FACSCalibur flow cytometer (BD Biosciences). Matched isotype controls were used for each antibody to determine the gates. Propidium iodide (PI) staining was used for the exclusion of dead cells by flow cytometry. Flowjo (Treestar, Ashland, OR) software was used for the analysis of flow cytometry data.
Isolation of monocytes
Monocytes were isolated from fresh PBMCs and AMCs using anti-CD14 MACS microbeads (Miltenyi Biotech). Isolation was carried out according to the manufacturer’s protocol using MS column separation (Miltenyi Biotech) and eluting with R10 medium (RPMI medium (Invitrogen, Paisley, UK) containing 10 % v/v fetal calf serum (FCS) (PAA Laboratories, Somerset, UK), 100 U/ml penicillin/streptomycin (Lonza, Wokingham, UK) and 2 mM l-glutamine (Cambrex, Wokingham, UK)).
Isolation of T cells
T cells were separated from fresh PBMCs using negative magnetic selection. Up to 108 PBMCs were incubated with murine anti-human antibodies against CD56, CD14, CD16, CD33 and CD19 (all at 0.1 μg/ml, all sourced from Chemicon, Watford, UK) for 30 min at 4 °C. Cells were then washed and resuspended in 5 ml of Biomag goat anti-mouse magnetic beads (Qiagen, West Sussex, UK) and incubated for 30 min at 4 °C. Cells were then placed against a magnet, and the beads were allowed to settle on the sides of the tube and the supernatant was transferred to a fresh tube. T cells were activated by the addition of anti-CD3/CD28 beads (Invitrogen) (3 μl of beads per 2 × 106 cells) and cultured in RPMI medium (supplemented as above) at 37 °C and 5 % CO2. Ex vivo stimulation of T cells resulted in T cell proliferation as well as upregulation of surface PD-1.
Blocking antibodies
Goat anti-human PD-1 antibody was used at 10 μg/ml (R&D systems, Abingdon, UK). Mouse anti-human PD-L1 antibody (MIH1 clone) was used at 10 μg/ml (eBioscience, UK). Rat anti-human IL-10 antibody (JES3-9D7 clone) was used at 1 μg/ml (eBioscience) for the functional experiments. Mouse anti-human IFN-γ antibody (MD-1 clone) was used at 10 μg/ml (Biolegend, San Diego).
Cell culture
The ovarian cancer lines OVCAR-3, OVCAR-5, CaOV-3, SKOV-3, TOV-21G, OVISE, SMOV and IGROV-1 were kind donation of Professor Hani Gabra, Ovarian Cancer Action, Imperial College London, UK. The cell lines were maintained in R10 medium (RPMI medium) containing 10 % v/v fetal calf serum (FCS), 2 mM l-glutamine and 100 U/ml penicillin/streptomycin. Ovarian cancer lines were used to screen for PD-L1 cell-surface expression by flow cytometry, and cell lines were incubated for 48 h before flow cytometric staining. Monocytes were cultured in conditions of R10 medium alone, R10 medium containing 10 ng/ml IFN-γ (PreProtech, London, UK), 10 ng/ml IL-10 (PreProtech), 10 ng/ml TGF-β (PreProtech) or both IL-10 and TGF-β at 10 ng/ml or using multiples of these concentrations as noted. Cells were collected at various time points as indicated and stained for flow cytometric analysis as described above.
T cell proliferation assays
Tritiated thymidine was used to measure proliferation of T cells. Following the incubation of T cells with beads and stimulator cells for a given period of time, 1 μCi/well of 3H thymidine was added to each well and incubated at 37 °C and 5 % CO2 for 18 h. Cells were harvested (Harvester 96 MACH III M, TOM TEC) (Receptor Technologies, Adderbury, UK) onto a filter mat and counted using a Wallac Trilux 1450 Microbeta liquid scintillation counter (PerkinElmer, UK).
Carboxyfluorescein succinimidyl ester (CFSE) labeling was also used to measure T cell proliferation. T cells were isolated as previously described by negative bead separation technique and then labeled with CFSE. CFSE-labeled T cells were then stimulated as described before. Stimulated, CFSE-labeled T cells were added for the desired period of incubation in 10:1 ratio with tumor cells and monocytes in co-culture experiments. CFSE-labeling efficiency was checked on a BD FACSCalibur flow cytometer on day 1. T cell proliferation was analyzed on day 5 by flow cytometry. When T cells isolated from ascites, they were CFSE-labeled and co-cultured without further stimulation for 48 h with monocytes as these T cells are already activated and do not survive in culture for long periods.
Cytokine analysis
Supernatant was collected from patients’ ascites following centrifugation and stored at −80 °C until used. IL-10, TGF-β, TNFα, IL-1β, IL-8, IL-6, VEGF, IL-4, IL-17, IFN-γ, IL-2 and GM-CSF were analyzed using Flurokine® Multianalyte Luminex profiling kits (R&D systems) following the manufacturer’s protocol. Ascitic supernatant was diluted 1:8 prior to analysis.
Statistical analysis
Statistical analysis was carried out using Prism GraphPad 5 (GraphPad, La Jolla, USA) software using Welch’s t-test.
Results
PD-L1 expression on monocytes from ascites and peripheral blood correlates with malignancy
Mononuclear cells were purified from blood and ascites and double-stained for CD14 and PD-L1. Monocytes from the peripheral blood of patients with malignant ovarian tumors showed a significantly higher percentage of PD-L1 expression compared to patients with benign/borderline ovarian tumors or healthy controls (mean percentage of monocytes expressing PD-L1: 64 % malignant and 1 % benign/borderline (combined data from both groups are shown in Fig. 1a and representative histograms are shown Fig. 1c)). Similarly, ascitic monocytes from patients with malignant ovarian tumors showed a significantly higher percentage of PD-L1 expression than their benign/borderline counterparts (mean percentage of monocytes expressing PD-L1: 67 % malignant and 2 % benign/borderline) (Fig. 1b). Interestingly, there is a localized effect of PD-L1 expression in ascites. Patients with malignant ovarian cancer show a significantly higher level of PD-L1 on ascitic monocytes compared to monocytes stained with peripheral blood (supplementary figure 1). These data suggest that the expression of PD-L1 on monocytes correlates with malignancy in human ovarian cancer.
Fig. 1.
PD-L1 expression on monocytes correlates with malignancy and is upregulated by ascitic supernatant (asc sn). a Percentage of PD-L1 positive cells in the CD14 gate of PBMCs from patients with benign/borderline (n = 29) or malignant ovarian cancer (n = 29). b % of PD-L1 positive cells in the monocyte gate of ascites from patients with benign/borderline (n = 10) or malignant ovarian cancer (n = 44). c Histogram from flow cytometry showing increased PD-L1 cell surface expression on monocytes (gated on CD14 positive cells) isolated from PBMCs from representative patients with a benign or malignant ovarian tumor compared to antibody isotype control samples. d Monocytes (CD14+) were purified from healthy donors and cultured in ascitic supernatant harvested from patients with either benign (n = 3) or malignant (n = 4) ovarian tumors for 0, 2, 6 and 8 h. The cells were then stained for PD-L1 and analyzed by flow cytometry. The experiments were carried out twice, and the data have been pooled. The figure shows each group’s mean value of MFIs for each time point ± SEM
Ascitic supernatant can upregulate PD-L1 on normal peripheral blood monocytes
In order to investigate the mechanism of PD-L1 upregulation on monocytes in ovarian cancer, normal monocytes from peripheral blood of healthy donors were isolated and cultured in ascitic supernatant from patients with either malignant or benign/borderline ovarian tumors. PD-L1 expression was measured at 0, 2, 6 and 8 h (Fig. 1d).
Unlike the ascites from patients with benign ovarian disease, supernatant from patients with malignant ovarian tumors upregulated PD-L1 on monocytes from 6 h and reaches statistical significance at 8 h.
IL-10 and TGF-β upregulate PD-L1 expression on monocytes
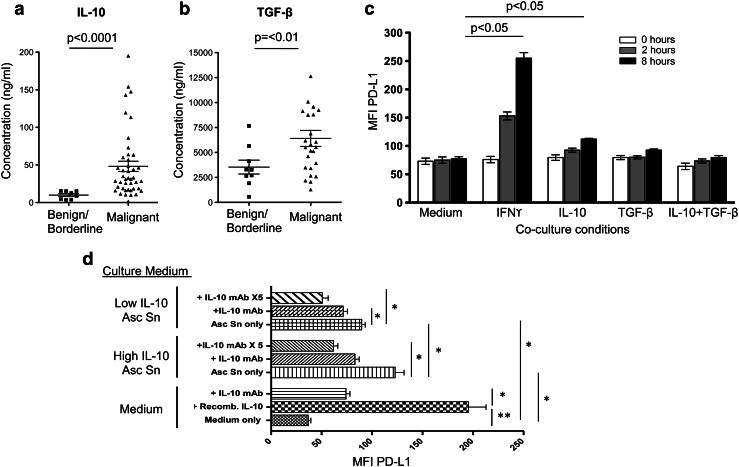
In order to investigate whether there was a difference in the cytokines found in ascites in patients with benign/borderline or malignant ovarian tumors, ascitic supernatant from these groups of patients were collected and analyzed using Luminex technology for IL-10, TGF-β TNFα, IL-1β, IL-8, IL-6, VEGF, IL-4, IL-17, IFN-γ, IL-2 and GM-CSF. Only IL-10 and TGF-β showed a significant difference in concentration between the two groups of patients, with higher concentrations of both cytokines in malignant ascites compared to benign/borderline ascites (Fig. 2a, b).
Fig. 2.
Cytokine regulation of PD-L1 expression on monocytes. Using a Luminex assay, IL-10 (a) and TGF-β (b) concentrations were measured in the ascitic supernatant of patients with either benign/borderline (IL-10, n = 8; TGF-β, n = 9) or malignant (IL-10, n = 42; TGF-β, n = 24) ovarian tumors. Each data point represents 1 patient sample and the bar represents the mean c IL-10 or TGF-β can upregulate PD-L1 on monocytes. Monocytes from PBMCs from healthy individuals were cultured in medium containing IFN-γ, IL-10, TGF-β or IL-10+, TGF-β for 0, 2 and 8 h. PD-L1 expression was analyzed by flow cytometry and expressed as mean ± SEM of triplicate cultures, and the figure shows a representative experiment of two. d Monocytes from healthy individuals were cultured in medium or ascitic supernatant (asc sn) from patients with either high IL-10 concentration (>100 ng/ml) or low IL-10 concentration (<35 ng/ml). IL-10 blocking antibody was added into the cultures as indicated (anti-IL-10 mAb was used at 1 μg/ml. “x5” = 5 μg/ml), and PD-L1 expression was measured after 8 h by flow cytometry. This experiment was performed in triplicate and repeated twice, and the figure shows the mean ± SEM. *p < 0.05, **p < 0.001
Consequently, we decided to investigate whether IL-10 or TGF-β is responsible for PD-L1 upregulation on monocytes in ovarian cancer. Monocytes from peripheral blood of healthy donors were isolated and cultured in medium containing IL-10, TGF-β or IFN-γ. IFN-γ was used as a positive control. PD-L1 expression was measured at 0, 2 and 8 h (Fig. 2c). Addition of IL-10 or IFN-γ significantly upregulated PD-L1. Combining TGF-β and IL-10 had no additive effect on PD-L1 expression. Addition of TGF-β alone to the culture showed a small increase in the percentage of cells expressing PD-L1, which was significantly different to medium alone at 2 h but not at 8 h (Supplementary Figure 2); however, the MFI of PD-L1 on these monocytes remained unchanged following the culture with TGF-β (Fig. 2c).
To test this in a more physiological setting, healthy monocytes were cultured in the supernatant from ascites. Ascitic supernatants were chosen which contained either high (>100 ng/ml) or low (<35 ng/ml) concentration of IL-10, and monocytes were cultured in these for 8 h. Compared to medium alone, monocytes cultured in ascitic supernatant significantly upregulated the level of PD-L1 on CD14+ cells. This was significantly higher in ascitic supernatant containing high IL-10 compared to low IL-10. Addition of an anti-IL-10 blocking antibody significantly reduced PD-L1 expression on monocytes (Fig. 2d).
When the same experiment was performed using anti-IFN-γ antibody, no effect of the blocking antibody was observed, though addition of the anti-IFN-γ antibody could block upregulation caused by recombinant IFN-γ (Fig. 3). This suggests that, in keeping with the observation of low levels of IFN-γ in ascites, this cytokine plays little or no role in PD-L1 upregulation on monocytes in the ascites in ovarian cancer.
Fig. 3.
IFN-γ does not influence PD-L1 expression in ascitic supernatant. Monocytes from healthy individuals were cultured in medium or ascitic supernatant (ascitic SN) from patients with either high IL-10 (>100 ng/ml) or low IL-10 (<35 ng/ml). Recombinant IFN-γ and IFN-γ blocking antibody (mAb) was added into the cultures as indicated, and PD-L1 expression was measured after 8 h by flow cytometry. Statistical analysis of indicated groups is compared to the medium only group. The bars show mean, error bars = SEM. **p < 0.001, ***p < 0.0001
Overall, these data suggest that IL-10 can control PD-L1 expression on monocytes and that the IL-10 present in ascites in patients with ovarian cancer is one of the factors responsible for PD-L1 upregulation on monocytes in these patients.
PD-1 expression is upregulated in T cells in ovarian cancer
As PD-L1 was increased on monocytes, we decided to investigate the expression of the receptor PD-1 on the T cells from different groups of patients with ovarian tumors. Lymphocytes were separated from blood and ascites fluid from patients with ovarian cancer, stained for CD3 and PD-1 and then analyzed by flow cytometry. These data show that there are significantly higher percentage of PD-1+ T cells in both PBMCs and ascites of patients with malignant ovarian tumors compared with benign/borderline tumors (Fig. 4a, b). Again, similar to monocytes, there is a localized effect of ascites on T cell expression of PD-1. T cells in ascites express significantly higher levels of PD-1 than T cells in peripheral blood of patients with malignant ovarian cancer (supplementary figure 3).
Fig. 4.
PD-1 is upregulated on T cells from patients with malignant ovarian cancer. PD-1 cell surface expression on T cells isolated from PBMCs (a) (B/B n = 23, malignant n = 29) and ascites (b) (B/B n = 12, malignant n = 44) from patients with malignant compared to benign/borderline ovarian tumors. Graphs represent individual cases of benign/borderline or malignant, while horizontal lines represent mean values. c CFSE-labeled, bead-stimulated T cells (ST) derived from healthy donors were co-cultured with ascitic monocytes (AM) or monocytes from a healthy female donor; with and without anti-PD-1, anti-PD-L1 and anti-IL-10 blocking antibodies alone or in combination. Each bar represents the percentage of stimulated T cells that underwent proliferation after 5 days of co-culture. Error bars = SEM. d CFSE-labeled T cells from the ascites of a patient were co-cultured with monocytes derived from either ascites or the PBMCs of healthy female donor. Each bar represents the mean percentage of stimulated T cells that underwent proliferation after 48 h of co-culture. Data shown are the mean ± SEM of triplicate cultures
Ascitic monocytes can suppress T cell proliferation in a partially PD-1/PD-L1-dependent manner
We have shown that ascitic monocytes from patients with malignant ovarian cancer have a significantly higher level of expression of PD-L1 than patients with benign/borderline tumors. To assess the functional relevance of this finding, we set up proliferation assays using ascitic monocytes to investigate their effect on T cell proliferation. Monocytes were cultured with T cells isolated from healthy donors’ PBMCs using negative magnetic bead selection as described in the methods. T cells were stimulated with anti-CD3/CD28 beads and cultured with monocytes for 5 days. The presence of ascitic monocytes significantly inhibited T cell proliferation on day 5, compared to stimulated T cells cultured alone or with monocytes from PBMCs from healthy donors (Fig. 4c). Addition of PD-1, PD-L1 or IL-10 blocking antibody alone partially but significantly reverses this suppression. However, the combination of blocking antibodies to PD-L1 and PD-1 or PD-L1 and IL-10 appear to result in almost complete rescue of T cell proliferation. Therefore, monocytes derived from the ascites of patients with ovarian cancer can suppress T cell responses in a manner that is at least partially dependent on IL-10 and/or PD-1 PD-L1 interactions.
In order to test this using ascitic monocytes and T cells from ascites of the same patient, we co-cultured these two cell types under the same conditions as the previous experiment. T cells and monocytes were separated from ascites. T cells were labeled with CFSE and cultured with monocytes from the same ascites or from PBMCs of a healthy donor for 5 days. Ascitic monocytes significantly suppressed ascitic T cell proliferation, but monocytes from healthy PBMCs did not show any suppressive effect (Fig. 4d).
Ascitic supernatant suppresses T cell proliferation, which is partially mediated by IL-10
As demonstrated in Fig. 2, IL-10 was found to be present in the ascitic supernatant in significantly high concentrations and appeared to significantly upregulate PD-L1 expression on monocytes. We hypothesized that this supernatant may also have a direct effect on T cells. To test this, we cultured CD3-/CD28-activated T cells in either benign/borderline or malignant ascitic supernatant (Supplementary Figure 4a). Ascitic supernatant from malignant ovarian tumors could significantly inhibit the proliferation of T cells compared to the supernatant from benign/borderline ovarian tumors. To test whether this was IL-10 dependent, we chose ascitic supernatant from a range of donors, which we defined as having high (>50 ng/ml) or low IL-10 (<50 ng/ml), and repeated the experiment (Supplementary Figure 4b). Supernatant containing high IL-10 could suppress T cell proliferation more than that containing low IL-10. The addition of a blocking IL-10 antibody partially reversed this suppression, but not significantly, and not to levels of proliferation observed in the control group.
These data suggest that ascitic supernatant can directly suppress T cell proliferation, which may be in part mediated by immunosuppressive cytokines found to be present in the tumor environment.
Ovarian cancer cells express PD-L1
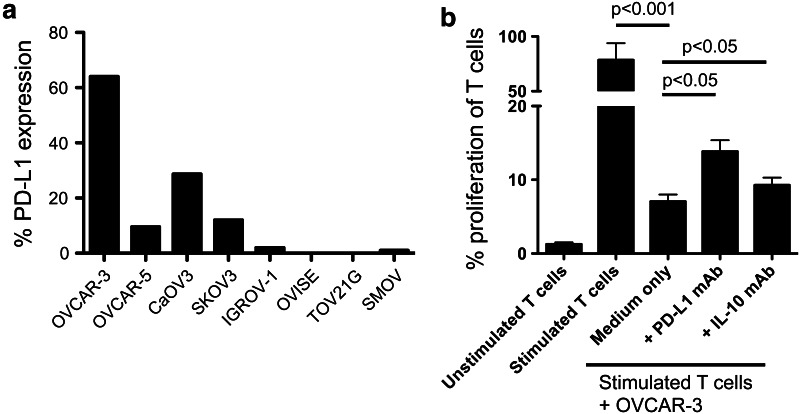
We investigated PD-L1 expression on ovarian cancer cells in several tumor cell lines. Cells that were confluent in culture were harvested, and using flow cytometry, expression of PD-L1 on the cell surface was analyzed. Our hypothesis was that PD-L1 expression on the tumor cells may be contributing to PD-1-mediated T cell suppression. Initially, we demonstrated that PD-L1 is upregulated in some ovarian cancer cell lines, in particular those that originated from serous (SKOV3, SMOV1, CaOV3, OVCAR3 and OVCAR5) histological type but not clear cells (OVISE and TOV21G clear cell, IGROV1 mixed) (Fig. 5a). These data confirm recent reports of constitutive expression of PD-L1 on human ovarian cancer cell lines [13].
Fig. 5.
PD-L1 is expressed on ovarian cancer cell lines and contributes to T cell suppression. a Ovarian cancer cell lines from either serous or clear cell histological types were screened for PD-L1 expression by flow cytometry and presented as percentage of cells expressing PD-L1. b Proliferation of CFSE-labeled CD3/CD28-bead-stimulated T cells cultured alone and unstimulated T cells were used as controls. Stimulated T cells were then co-cultured with OVCAR-3 cancer cells alone or with PD-L1 blocking mAb or IL-10 blocking mAb. Proliferation was measured using CFSE dilution and flow cytometry on day 5. Bars represent mean ± SEM of values obtained from experiments repeated 3 times
Ovarian cancer cells that express PD-L1 are able to suppress T cell proliferation, which is partially reversed by blocking PD-L1 or IL-10
We stimulated CFSE-labeled T cells with anti-CD3/CD28 beads and co-cultured with OVCAR3 ovarian cancer cells alone or with antibodies against IL-10 or PD-L1. T cell proliferation was measured using CFSE dilution. OVCAR3 cells express PD-L1 on their surface as seen in Fig. 5a. Figure 5b shows that OVCAR3 cells can significantly suppress T cell proliferation. This suppression was partially but significantly reversed by addition of anti-PD-L1 or IL-10 blocking antibodies.
Discussion
PD-1 on T cells can interact with PD-L1 expressed on a wide range of cell types and result in inhibition of T cell proliferation and effector function [8]. Due to its inhibitory effect, the pathway may be a potential immunomodulatory mechanism employed by tumors to subvert the host immune response. A number of antibodies have entered clinical trials targeting this pathway and have shown efficacy and objective responses in solid tumors (including ovarian cancer) [15, 16]. However, this treatment is likely to rely on expression of PD-L1 or PD-1 (probably mainly on immune cells), and as such, it is important to characterize each tumor type for PD-1 pathway involvement to validate its potential as a therapeutic target.
We set out to investigate the role of the PD-1 pathway in human ovarian cancer as a potential diagnostic biomarker and immunotherapeutic target. Published work on the PD-1 pathway in human ovarian cancer is very limited. In this paper, we have presented data that lead to better understanding of PD-1/PD-L1 pathway and its importance in ovarian cancer.
Distinguishing benign/borderline from malignant tumors of the ovary is of vital importance in the clinical management of patients with an ovarian mass, particularly in younger women who wish to preserve their fertility. None of the currently available tests provide a definitive diagnosis until the mass is excised. This study has shown that PD-L1 expression on monocytes from the ascites of patients with malignant ovarian tumors was significantly higher than those from patients with benign/borderline tumors. What is even more striking is that this observation is also true for monocytes from peripheral blood. PD-L1 expression on monocytes in patients with an ovarian lesion shows a clear association with malignancy, with no overlap in the percentage of monocytes expressing PD-L1 in benign/borderline and malignant cases. This is an important finding, and PD-L1 expression on monocytes may be further exploited as a diagnostic test in the management of ovarian masses.
The ascitic supernatant from patients with malignant ovarian tumors is capable of upregulating PD-L1 expression on monocytes, unlike ascites from patients with benign/borderline ovarian tumors. Cytokine analysis of the ascitic supernatant revealed that TGF-β and IL-10 were both expressed at significantly higher levels in malignant ascites compared to benign/borderline ascites. Both IL-10 and TGF-β were able to increase PD-L1 expression on monocytes from healthy donors though the effect of IL-10 was more striking than TGF-β. Blocking IL-10 with a neutralizing antibody reduced PD-L1 expression. Our data suggest that IL-10 potentiates PD-L1 expression on monocytes as well as having a direct suppressive effect on T cells.
IL-10 has previously been shown to upregulate PD-L1 on dendritic cells in patients with HIV and hepatitis-B infections [18, 19]. In both cases, there has been a correlation with increased IL-10 and PD-L1 expression on dendritic cells; however, it remains to be seen whether this IL-10 increase results in the upregulation of PD-L1 or whether PD-L1 signaling results in production of IL-10 by the DCs.
In keeping with our findings, a study by Curiel et al. in ovarian cancer showed that dendritic cells removed from tumor-draining lymph nodes (TDLN) and ascites had high expression of PD-L1 compared to control lymph node dendritic cells. These dendritic cells could also upregulate PD-L1 in response to recombinant IL-10 and VEGF in vitro. The authors also found that blockade of PD-L1 on these cells with an anti-PD-L1 antibody resulted in increased T cell expression of IFN-γ, IL-2 and IL-12 and a downregulation of IL-10 [14]. Our study differs from this study in showing PD-L1 upregulation on monocytes in peripheral blood, which is more accessible in the clinical setting. We have also shown that blockade of both PD-L1 and PD-1 has a profound effect in the reversal of monocyte mediated T cell suppression. Our study using human samples also supports the findings of Krempski et al. [20] who have shown upregulation of PD-L1 and PD-1 on dendritic cells in a mouse model of ovarian cancer, suggesting that PD-L1/PD-1 may be responsible for T cell anergy.
The importance of PD-1 in ovarian cancers has also been reported in various mouse models. Recently, a report by Abiko et al. [13] has shown a direct effect between PD-L1 expression on murine ovarian carcinoma cells and suppression of the CD8 T cell response. Similarly, another recent report shows that PD-1+ (and a population of CTLA-4+ PD-1+) CD8 T cells benefit from PD-1 blockade in mouse models of colon cancer and ovarian cancer. PD-1 blocking resulted in a gain of CD8 function as well as attenuation of Treg-mediated suppression [21].
Our results in human ovarian cancer add further relevance to data published in mouse models on the role of PD-1/PD-L1 in this malignancy. Findings presented in our study confirm a suppressive nature of tumor-associated monocytes on T cells in a human disease setting. We have shown that over-expression of PD-L1 on the monocytes is accompanied by over-expression of PD-1 on T cells. In addition, the presence of ascitic monocytes can suppress proliferation of T cells (including ascitic T cells from patients with ovarian cancer). This suppression could be significantly reversed with anti-PD-1 or anti-PD-L1 blocking antibodies and suggests that the PD-1/PD-L1 pathway significantly contributes to the suppression observed. We have demonstrated that PD-L1 is expressed on serous ovarian cancer cell lines, in agreement with previous reports of PD-L1/2 expression on primary ovarian tumors and tumor cell lines [12, 13]. Tumor cell lines expressing PD-L1 could suppress T cell proliferation in vitro, in a partially PD-L1- and IL-10-dependent manner, suggesting a degree of redundancy in the suppressive mechanism. Based on these results, it is likely that tumor PD-L1 expression in vivo can suppress tumor-infiltrating T cells.
We also demonstrate that the ascitic supernatant is directly suppressive to T cell proliferation, supporting the idea that there may be multiple suppressive mechanisms present in the tumor environment. Future experiments would investigate other potential suppressive pathways and molecules, including ones employed by these ascitic monocytes, as well as other immunosuppressive mechanisms in the tumor microenvironment. This will allow us to identify and distinguish between redundant pathways and those that may be of benefit as a therapeutic target.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We wish to thank the clinical staff at Queen Charlotte’s hospital to their contribution to patient recruitment. Sadaf Ghaem-Maghami received funding from Rosie Williams Charitable Trust and Mann-Hodgson Trust for this work. Nor Haslinda Abdul Aziz was supported by a studentship from Malaysian Ministry of Education. Christian J. Maine was supported by an MRC studentship. Claudia Hayford was supported by Ovarian Cancer Action. Support was also provided by the Experimental Cancer Medicine Centre at Imperial College London and by the National Institute for Health Research (NIHR) Biomedical Research Centre and Ovarian Cancer Action Centre based at Imperial College London.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Christian J. Maine and Nor Haslinda Abdul Aziz have contributed equally to this work.
Andrew J.T. George and Sadaf Ghaem-Maghami are senior authors with equal contribution.
Nor Haslinda Abdul Aziz is currently a member of Institute for Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, Sungai Buloh, Selangor, Malaysia.
References
- 1.du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander ML. Prognostic factors in ovarian cancer. Semin Oncol. 1998;25(3):305–314. [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, Kosaka K, Konishi I. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19(6):1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 18.Geng L, Jiang G, Fang Y, Dong S, Xie H, Chen Y, Shen M, Zheng S. B7-H1 expression is upregulated in peripheral blood CD14 + monocytes of patients with chronic hepatitis B virus infection, which correlates with higher serum IL-10 levels. J Viral Hepat. 2006;13(11):725–733. doi: 10.1111/j.1365-2893.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 19.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, Dong H, Maserati R, Shearer GM, Chen L, Clerici M. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101(7):2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 20.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1 + dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186(12):6905–6913. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.