Abstract
Macrophage migration inhibitory factor (MIF) is known to be involved in oncogenic transformation, tumour progression, and immunosuppression and is overexpressed in many solid tumours, including paediatric rhabdomyosarcoma (RMS). We investigated the function of MIF in RMS during treatment with cytotoxic drugs. RMS cell lines were analysed by flow cytometry, immunofluorescence staining, and ELISA. We demonstrated the overexpression of MIF in RMS cells and the enhanced expression and secretion after treatment with cytotoxic agents. Migration assays of RMS cells revealed that inhibitors of MIF (ISO-1, Ant.III 4-IPP, Ant.V, sulforaphane (SF)) and blocking antibodies caused reduced migration, indicating a role for MIF in metastatic invasion. Additionally, we investigated the function of MIF in immune escape. The development of a population containing immunosuppressive myeloid-derived suppressor cells was promoted by incubation in conditioned medium of RMS cells comprising MIF and was reversed by MIF inhibitors but not by antibodies. Although most inhibitors may restore immune activity, Ant.III and 10 µM SF disturbed T cell proliferation in a CFSE assay, whereas T cell proliferation was not reduced by 3 µM SF, ISO-1 or antibodies. However, the inhibition of MIF by blocking antibodies did not increase the killing activity of allogenic PBMCs co-cultured with RMS cells. Our results reveal that MIF may be involved in an immune escape mechanism and demonstrate the involvement of MIF in immunogenic cell death during treatment with cytotoxic drugs. Targeting MIF may contribute to the restoration of immune sensitivity and the control of migration and metastatic invasion.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1896-4) contains supplementary material, which is available to authorized users.
Keywords: Rhabdomyosarcoma, Immunotherapy, Macrophage migration inhibitory factor (MIF), MDSCs, Immune escape and surveillance, MIF inhibitors
Introduction
Rhabdomyosarcoma (RMS) is the most common paediatric soft tissue sarcoma, accounting for 3–4 % of all paediatric malignancies [1] and 7–8 % of malignant solid paediatric tumours [2]. The latest system for classification of the heterogeneous RMS is based on the histological subtype together with molecular genetics [3]. The two main subtypes, alveolar RMS (RMA) and embryonal RMS (RME) [4, 5], differ not only in their histopathology and tumour biology but also in their responsiveness to treatment [6, 7]. The current multidisciplinary and multimodality treatment is based on risk stratification and includes surgery, chemotherapy, and radiotherapy [8, 9]. Although patients suffering from RMS show satisfactory responses to this treatment, especially the embryonal subtype [10], there are still several major problems, including metastatic invasion, multidrug resistance, and tumour recurrence [11]. Furthermore, the outcome of patients with tumours in advanced stages is poor.
One reason for this unsatisfactory outcome is the immune escape of tumours, which can occur due to different factors. These factors may include the accumulation or reduction of immunomodulatory factors or special immune cells such as myeloid-derived suppressor cells (MDSCs) [12, 13]. Hence, it is essential to develop novel treatment approaches, such as the activation of the individual’s immune system, to achieve increased anti-tumour activity.
With the aim of identifying novel treatment approaches, a differential gene expression analysis of 11 RMS samples was carried out, which revealed elevated expression of immunomodulatory proteins [14] such as macrophage migration inhibitory factor (MIF) [15]. MIF is a protein that is involved in oncogenic transformation, tumour progression and the disturbance of immune activation [15–17]. Moreover, MIF supports tumour cell growth, angiogenesis, and cell migration [18–20], potentially through its functional receptors CXCR2, CXCR4, and CD74 [20–22]. CXCR4 is also expressed in RMS [23]. Furthermore, in addition to its role in tumourigenesis, MIF has a strong influence on the immune system [17] by inhibiting the lysis of melanoma cells by natural killer cells [17], enhancing tumour-associated CD4+ regulatory T cells as well as CD8+ regulatory T cells (accompanied by decreased CD8+-induced tumour cytotoxicity) [24], and inducing MDSCs in the tumour microenvironment [25]. Moreover, tumour-derived MIF appears to participate in the immune escape of various malignant tumours [26, 27]. Taken together, tumour-derived MIF appears to have a strong impact on tumourigenesis by regulating the immune system.
In RMS, some proteins, such as calreticulin, have been shown to be upregulated during cellular stress, and this induction enhances susceptibility to macrophage attack [28]. However, during treatment with cytotoxic drugs, a shift in protein expression may alter treatment efficiency by inducing multidrug resistance or promoting immune escape. Therefore, the aim of this study was to investigate the expression profile of MIF during treatment with cytotoxic drugs. Additionally, we aimed to evaluate the immunomodulatory function of MIF in RMS after treatment with inhibitory molecules and MIF-blocking antibodies.
Materials and methods
Cells and culture conditions
We focused on the two major subtypes of RMS which are represented by RMA and RME [4, 5]. The RMA cell line Rh30 (fusion positive, PAX3-FOXO1, t(2;23) [29]; DSMZ, Braunschweig, Germany) as well as the RME cell lines RD (fusion negative [29]; ATCC, Manassas, VA, USA), A-204 (fusion negative [29]; DSMZ, Braunschweig, Germany), and RMS33-2 (unclassified fusion status, derived from an RME and adapted to the culture for 18 passages) were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % foetal calf serum (FCS), 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mML-glutamine (all from Biochrom, Berlin, Germany) at 37 °C in 5 % CO2 under humidified conditions of 95 % air. We were concentrating on the cytotoxic drug vincristine, an effective agent in the standard VAC protocol, and on doxorubicin and etoposid, which are incorporated in recent studies, especially for high-risk patients [8]. Influence of cytotoxic drugs on RMS cells was analysed in confluent cultures which were treated with 0.01, 0.03, and 0.1 µg/ml for doxorubicin and vincristine and 0.01, 0.1, and 1 µg/ml for etoposid for 48 h. Conditioned media were collected and passed through a 0.2 µM mash sterile filter from RMS cells cultured at confluence for 3 days in very low endotoxin Roswell Park Memorial Institute Medium (VLE RPMI-1640, Biochrom, Berlin, Germany), and were buffered by 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, 25 mM; Biochrom AG, Berlin, Germany). PBMCs were isolated from healthy voluntary donors out of whole-blood samples by density gradient centrifugation using Biocoll (Biochrom, Berlin, Germany) and were cultured in VLE RPMI-1640 with the same supplements except of heat-inactivated FCS. Generation of a population containing MDSCs was achieved by using 0.01 ng/µl granulocyte-macrophage colony-stimulating factor (GM-CSF, Becton–Dickinson, Heidelberg, Germany) in cultures of PBMC for 5 days, and MIF inhibitors were added in the provided samples. For detection of the population containing MDSCs, flow cytometry with PE-anti-human CD33 and VioBlue-anti-human CD14 (both Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) was performed according to the manufacturer’s instructions.
Immunofluorescence staining
RMS cells were seeded at a density of 3000 cells/100 µl on Poly-d-lysine (Poly-d-lysine hydrobromide, Sigma-Aldrich, Munich, Germany)-coated glass slides in double-chamber culture inserts (Ibidi, Munich, Germany) overnight followed by application of cytotoxic drugs for 48 h. MIF was detected by a monoclonal mouse anti-human MIF antibody (KloneAbcam, Cambridge, UK) at a 1:1250 dilution in PBST overnight and AlexaFlour546®-goat anti-mouse antibody (2 µg/ml in PBST, Invitrogen, Darmstadt, Germany) as described previously [28].
Flow cytometry analysis of RMS cells
RMS cells were treated with cytotoxic drugs for 48 h, trypsinized, fixed in 3.7 % formaldehyde solution for 5 min and permeabilized with FACS buffer (PBS with 2 % FCS, 2 mM EDTA, 0.005 % NaN3) containing 0.2 % Tween 20 for 10 min. Intracellular MIF was detected by a two-step incubation with primary antibody anti-human MIF (1:1250 in FACS buffer) and FITC goat anti-mouse antibody (1:2000; Sigma-Aldrich, Munich, Germany) for each 30 min. Flow cytometry was carried out with FACSCalibur (Becton–Dickinson, Heidelberg, Germany) and evaluated with FCS Express 3 Flow Cytometry (De Novo Software, Los Angeles, USA).
Elisa
Quantitative analysis of human MIF was performed by using an enzyme immunoassay kit (Hölzel Diagnostika, Köln, Germany). Supernatants from cultures of confluent RMS cells were collected after 24 and 48 h of incubation with cytotoxic drugs as described above. ELISA was conducted according to the manufacturer’s protocol.
Migration assay
RMS cells at a density of 3 × 104/100 µl/well were plated in double-chamber culture inserts (Ibidi, Munich, Germany). After incubation overnight to receive confluence cell monolayer, culture inserts were gently removed and wells were filled with media containing MIF inhibitors ISO-1 (25 µM), Ant.III 4-IPP (25 µM), Ant.V (25 µM; all Inhibitors Merck Millipore, Darmstadt, Germany), SF (3 µM; Enzo Life Sciences GmbH, Lörrach, Germany), and anti-human MIF antibody (2 µg/ml), respectively. The cell-free space between the double chambers was measured 0 h, 5 h, and 24 h after removing the chambers on images from Axiovert 40 equipped with AxioCamMRcand AxioVision 3.1 (Carl Zeiss, Oberkochen, Germany). Migration is expressed as relative quotient of measured space to those of the untreated control culture.
Cell viability
Cell viability was performed by seeding 3 × 103 A204, RD, and Rh30 cells and 1 × 104 RMS33-2 cells per well in a 96-well plate. After 24 h, cells were treated with MIF inhibitors ISO-1, Ant.III 4-IPP, Ant.V, SF or MIF antibodies in serial twofold dilutions of inhibitors ranging from 0 to 100 µM. In the case of combining the treatment with doxorubicin, its concentration ranged from 0.0003 to 3 µg/ml. After 96 h, cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously [30]. The percentage of cell viability was calculated by normalization between background of cultures without cells (only culture medium) and untreated cells as control.
CFSE analysis of PBMCs proliferation
For detection of T cell proliferation, PBMCs were stained with CFSE (Life Technologies GmbH, Invitrogen, Darmstadt, Germany) prior to plating. 5 µM CFSE solution in PBS containing 0.1 % heat-inactivated FCS was added warm to PBMCs (37 °C, 10 min). Reaction was stopped with ice-cold VLE RPMI-1640 and cells were centrifuged, resuspended, and plated at a density of 5 × 105/well/250 µl. 200 IU/ml interleukin 2 (IL-2, Novartis AG, Basel, Switzerland) and 1 ng/ml anti-human CD3 (Clone OKT3; eBioscience, Frankfurt, Germany) were used for stimulation. MIF inhibitors Ant.III 4-IPP (50 µM), ISO-1 (50 µM), and SF (10 and 3 µM) as well as anti-human MIF antibody (2 and 1 µg/ml) were added, respectively. In the provided samples, 200 µl of supernatants of RMS cell cultures was added to 50 µl of PBMC cultures. Media were changed with fresh additives after 2 days. PBMCs were analysed by BD LSR II (Becton–Dickinson GmbH, Heidelberg, Germany) to determine the different populations and T cell proliferation using BD FACSDiva (BD Bioscience, San Jose, USA) after 5 days of cultivation. CD3+ cells were detected by using PE/Cy7 anti-human CD3 Antibody (BioLegend, Fell, Germany).
Kill assay
Reporter Rh30 cells with stable integration of Gaussia Luciferase (Rh30-Gluc) [31] were seeded in 96-well plates (BectonDickinson GmbH, Heidelberg, Germany) at a density of 5 × 103 cells/well. After 24 h, 2 µg/ml, 1 µg/ml, or 0.5 µg/ml anti-MIF antibody was added to the provided wells and incubated for 30 min at 37 °C. Freshly isolated PBMCs were administrated in tumour cell: effector cell ratios of 1:10, 1:20, and 1:40 and stimulated with IL-2 (200 IU/ml). After 24 h at 37 °C, supernatants were discharged, followed by washing Rh30 Gluc cells with PBS, adding 200 µl culture medium and incubating for 2 h at 37 °C. RMS cells which are still alive are able to secret Gaussia Luciferase into the supernatants. 5 µl of these supernatants was transferred onto a white 96-well plate. The substrate Coelenterazine (Gaussia Glow Juice, P.J.K. GmbH, Kleinblittersdorf, Germany) was dissolved in buffer, and 50 µl of this solution was added to each well directly before measurement. Luminescence was detected for 1 s by using WALLAC Victor plate reader (PerkinElmer, San Diego, USA). All assays were conducted in triplicate. Cell viability in per cent was calculated by normalizing the background of cultures without cells and Rh30 Gluc cells without PBMCs.
Statistics
Data were analysed by ANOVA and Bonferroni’s post-test (GraphPad Prism 4.00; GraphPad Softwares Inc., San Diego, USA) and shown as mean ± standard error of the mean (SEM). p < 0.05 was considered to be significant. Additive effects of MIF inhibitors and cytotoxic drugs were evaluated by Isobolograms according to Lieber et al. 2013 [32].
Results
Treatment with cytotoxic drugs leads to enhanced expression of MIF in human RMS cell lines
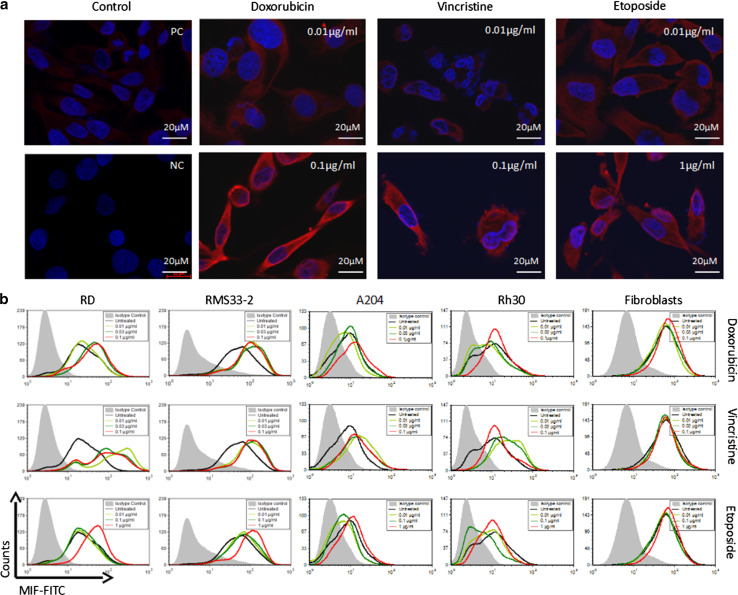
MIF expression was detected in A204 cells by immunofluorescence staining in the cytoplasm but not in the nucleus (Fig. 1a). Treatment with increasing concentrations of doxorubicin (0.01–0.1 µg/ml) led to enhanced expression of MIF without changes in the cellular distribution. However, MIF expression was strongly enhanced with increasing concentrations of doxorubicin, and this phenomenon was also observed with the drugs vincristine (0.01–0.1 µg/ml) and etoposide (0.01–1 µg/ml) (Fig. 1a). The same observations were noted in other RMS cell lines such as RD, Rh30, and RMS33-2 (data not shown).
Fig. 1.
Enhanced expression of MIF after incubation with cytotoxic drugs. RMS cell lines and human fibroblasts were incubated with three different cytotoxic drugs at various concentrations: doxorubicin, vincristine, and etoposide. a Immunofluorescence staining of A204 cells after 48 h of treatment. Enhanced expression of MIF was observed with increasing drug concentrations, as denoted by the red fluorescence. PC is a positive control without treatment, and NC is a negative control without primary antibody. Similar results were observed in RD, Rh30, and RMS33-2 cells. b At 48 h after administration, the cells were analysed by flow cytometry. In all RMS cell lines, increased MIF expression was observed compared to the untreated control. In fibroblasts, MIF expression did not change with treatment
To confirm these observations, treated and untreated RMS cells were analysed by flow cytometry (Fig. 1b). With all variables held constant aside from drug concentration, all four RMS cell lines showed increased MIF expression as the drug concentration increased, particularly with vincristine, which resulted in intense staining even at the lowest concentration (0.01 µg/ml). The greatest changes were observed in the cell lines RD and RMS33-2, followed by A204 and Rh30. However, in non-tumour cells such as fibroblasts, the intracellular concentration of MIF did not change as a result of incubation with cytotoxic drugs (Fig. 1b). These findings suggest that MIF expression is a general process in tumour cells but not in normal cells such as fibroblasts. Additionally, there are differences between tumour cell lines and cytotoxic drugs in their capacity to induce MIF. However, dependency of MIF induction on the cell type could be not ruled out.
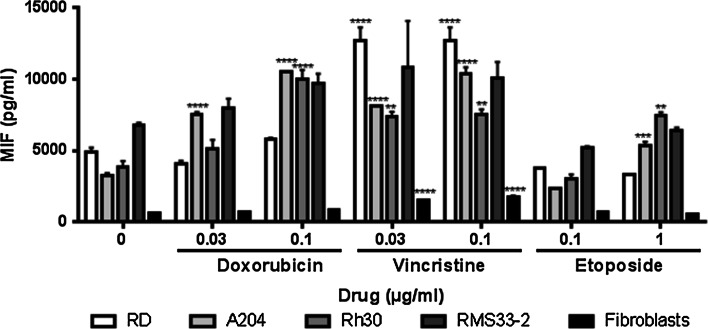
As MIF exists not only in the intracellular environment but also in a soluble form, we investigated whether the secretion of MIF by RMS cells is altered during treatment with cytotoxic drugs (Fig. 2). MIF was quantified in cell culture medium after 48 h of incubation with drugs at the same concentrations used in the previous assays. Depending on the cell line, enhanced MIF secretion was observed in a dose-dependent manner, which was significantly higher in nearly all the treated cultures compared to the untreated control (Fig. 2). Although the cell line RMS33-2 showed increased secretion of MIF compared with the negative control, especially in the presence of vincristine, no significant difference could be detected. However, in the cell lines Rh30 and A204, a significant (up to threefold) increase in secretion was identified for doxorubicin (0.1 µg/ml), etoposide (1 µg/ml), and both concentrations of vincristine (0.03 and 0.1 µg/ml). For cell line A204, a significant change was also observed at 0.03 µg/ml of doxorubicin (approximately twofold increase). All cell lines except for RMS33-2 showed significantly higher secretion in the presence of vincristine (Fig. 2).
Fig. 2.
Enhanced secretion of MIF after incubation with cytotoxic drugs. RMS cell lines and human fibroblasts were incubated with various concentrations of doxorubicin, vincristine, and etoposide for 48 h. The amount of secreted MIF in the cell culture supernatants from two independent replicates, as determined by ELISA, is represented as the mean and SEM. Significantly enhanced MIF secretion was detected in every RMS cell line in the presence of each drug (**P < 0.05, ****P < 0.0001, two-way ANOVA)
In contrast, cultured normal fibroblasts secreted a low amount of MIF (659 ± 3 pg/ml) compared to the RMS cells (3271 ± 161 – 6825 ± 132 pg/ml). Although the secretion of MIF increased (approximately 2-fold) during the treatment of fibroblasts with vincristine, the amount of MIF did not reach 15 % of that observed in the corresponding cultures of RMS cells (Fig. 2).
Taken together, our data indicate that RMS cells express and secrete a large amount of MIF, and this secretion can be further enhanced during treatment with cytotoxic drugs. This effect was less prominent in non-tumour cells such as fibroblasts.
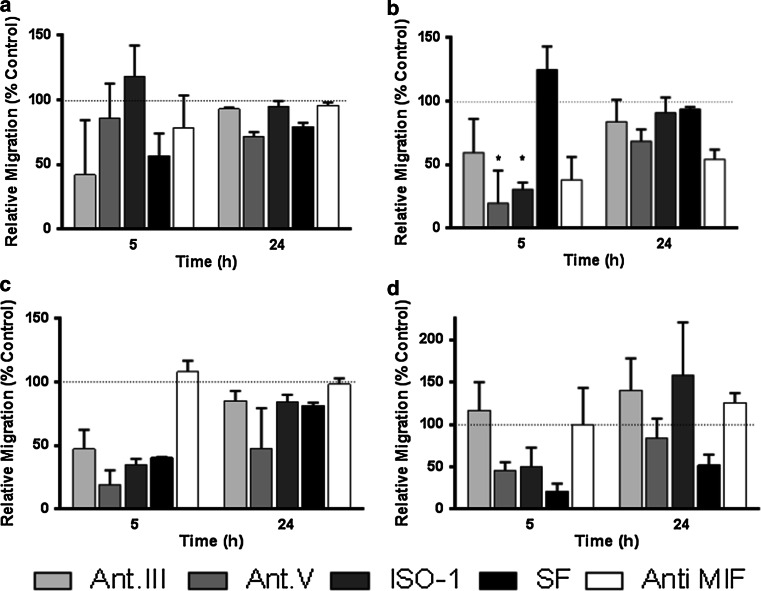
Impaired migration of RMS cells through MIF inhibition
RMS cell motility is linked to the metastatic capacity of RMS tumours, and tumour-derived MIF promotes motility in different tumour cells. Therefore, we analysed whether the inhibition of MIF leads to alterations in RMS cell migration (Fig. 3). RMS cells were found to migrate rapidly, as the surface of the cell-free gap (500 µm) within confluent cultures was overgrown within 24 h in a migration assay. Rh30 and RD cells migrated faster than A204 cells and especially RMS33-2 cells. Addition of the MIF inhibitors Ant.III 4-IPP (25 µM), Ant.V (25 µM), ISO-1 (25 µM), and SF (3 µM) as well as the blocking antibody (2 µg/ml) to the cultures led to a cell line- and inhibitor-dependent decrease in cell motility in migration assays (Fig. 3). The greatest impact on cell migration was observed after 5 h. With SF treatment, cell motility was reduced in nearly every cell cultures tested compared with the control cultures (RMS33-2: fivefold slower, Rh30: 40 % ± 0.6 % of the control, A204: 56.6 % ± 17.5 % of the control) after 5 h and even after 24 h (from 52 % ± 8.8 % (RMS33-2) to 93.8 % ± 1.7 (RD)). The MIF-blocking antibody had only a minimal impact on cell migration in most cases (RMS33-2: 62.0 ± 75.4 % and Rh30: 108.0 ± 8.9 % of the control after 5 h); however, this antibody had a greater effect on RD, which was 38.2 ± 17.9 % after 5 h and 54.3 ± 7.5 % of the control after 24 h. Nevertheless, the influence of MIF on RMS cell migration was confirmed via the use of the other inhibitors. Ant.V (19.1 ± 11.6 % of the control) and Ant.III (47.3 ± 15.3 % of the control) had the greatest ability to retard Rh30 cells, whereas ISO-1, at 30.7 ± 5.3 % of the control, showed the greatest ability to retard RD cells after 5 h.
Fig. 3.
Influence of MIF inhibitors on tumour cell migration. A204 (a), RD (b), Rh30 (c), and RMS33-2 (d) cells were treated with the MIF inhibitors Ant.III (25 µM), Ant.V (25 µM), ISO-1 (25 µM), and SF (3 µM) as well as anti-MIF antibodies (2 µg/ml). At the indicated time points, migration was evaluated in relation to untreated controls (100%). Impaired migration of tumour cells was obvious after 5 h in some treatments (*)
These observations suggest that MIF contributes to the motility of RMS cells; therefore, MIF may modulate the metastatic capacity of RMS tumours.
MIF and the development of a population containing MDSCs
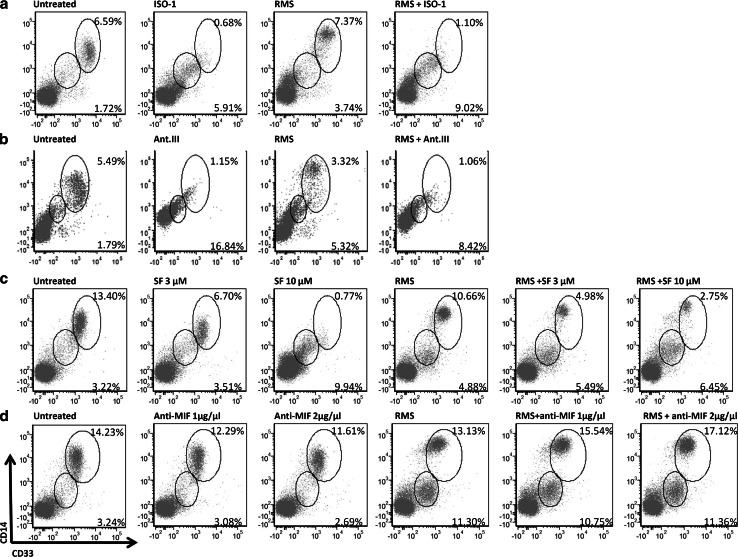
Tumour-derived MIF has been demonstrated to be involved in protecting tumours against the immune system via the induction of MDSCs, among other mechanisms [25]. Therefore, we analysed the development of a population containing human MDSCs (CD33+CD14+) in PBMCs after incubation with RMS culture medium, which resulted in the production of a different population of CD33+CD14+ cells (Fig. 4a–d). Although the cell count of the CD33+CD14+ population was nearly the same, the RMS-conditioned medium caused increased expression of CD14+ in the CD33+ population. In these cultures, the development of a new cell population showing low CD33 expression together with low CD14 was observed. The MIF inhibitor SF (3 µM) did not affect the CD33loCD14lo cell population compared with the control (no inhibitors and no conditioned medium). The expression of CD14 on CD33+ cells was not altered, although this population was reduced by twofold, indicating an off-target effect of SF which can influence the cells by HMGB1. Adding SF to PBMC cultures with RMS-conditioned medium did not impair the increased expression of CD14; however, the CD33hiCD14hi cell population comprised merely half of the same cell population compared to the cultures with conditioned medium alone. A stronger impact on the development of the population containing MDSCs was observed using a higher concentration of SF (10 µM), which induced a 3.9-fold reduction in the CD33hiCD14hi population. The CD33+CD14lo cell population increased as the concentration of SF increased.
Fig. 4.
Development of MDSCs during incubation with RMS culture medium and MIF inhibitors. a–d The cultivation of PBMCs with RMS culture medium led to the development of CD33hiCD14hi as well as CD14loCD33lo populations. Addition of the MIF inhibitor SF (a), ISO-1 (b), or Ant.III (c) prevented the formation of CD33hiCD14hi cells and enhanced the formation of CD14loCD33lo cells; however, these effects were not observed after treatment with the anti-MIF antibodies (d)
Taken together, these data show that SF prevented the development of CD33+CD14hi cells and increased the CD14loCD33lo cell population.
When ISO-1 was used to block the activity of MIF, a 9.7-fold reduction in the CD33+CD14hi population of PBMCs was observed compared to the untreated control, whereas a 6.7-fold reduction in the same population was found when RMS culture medium, PBMCs, and ISO-1 were combined compared to RMS-conditioned medium alone. Similar to SF, treatment with ISO-1 led to an increase in the CD14loCD33lo population (3.4-fold with ISO-1 alone and 2.4-fold with the addition of RMS-conditioned medium). Similar changes were also observed when cells were incubated with Ant.III 4-IPP.
In contrast, the anti-MIF antibody did not produce the same results. Indeed, only a small decrease in the CD33+CD14hi population was identified, and there was an increase in this population with the addition of RMS-conditioned medium. The CD14loCD33lo population changed only slightly.
MIF and the proliferation of T cells
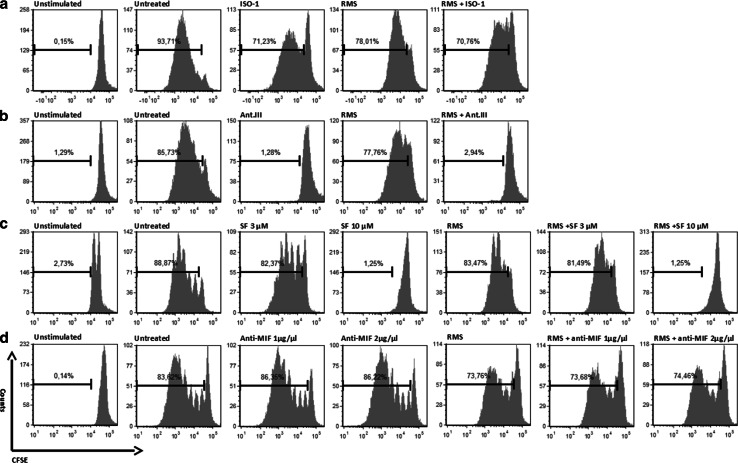
By incubating PBMCs with RMS culture medium, a slight decrease in the proliferation of T cells was observed in all CFSE assays (Fig. 5a–d). High concentrations of SF (10 µM) and Ant.III 4-IPP (50 µM) prevented T cell proliferation in both cultures with RMS-conditioned and non-RMS-conditioned samples (up to a 70-fold reduction). Only a slight disturbance in the proliferation of immune cells was observed after treatment with a low concentration of SF (3 µM), anti-MIF antibodies, or ISO-1 (50 µM) (Fig. 5).
Fig. 5.
Proliferation of T cells during incubation with RMS culture medium and MIF inhibitors. a–d PBMCs were stimulated with IL-2 and OKT3 and cultured as indicated for 5 days. Proliferation was detected by a CFSE assay. Incubation with RMS supernatant (A204) slightly decreased the proliferation rate of T cells in each culture. No defects in the proliferation of T cells were observed during treatment with low concentrations of SF (3 µM), ISO-1, or anti-MIF antibodies, whereas higher concentrations of SF and Ant.III prevented T cell proliferation
Role of MIF in the killing activity of PBMCs
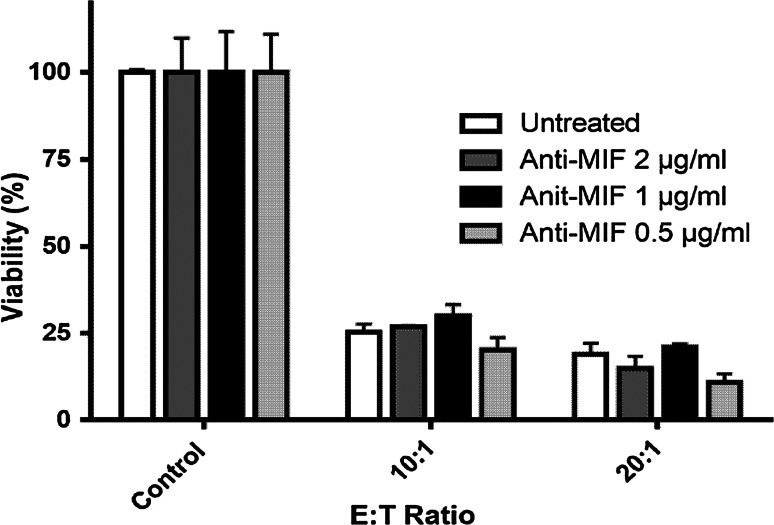
To investigate the ability of PBMCs to kill human RMS cells during MIF inhibition, we co-cultured Rh30-Gluc cells together with PBMCs at different ratios (Fig. 6). By pre-incubating Rh30 cells with the anti-MIF antibody, we aimed to stimulate PBMCs by disturbing the activity of tumour-derived MIF. We observed that PBMCs efficiently killed Rh30-Gluc cells. At a 10:1 ratio of effector to tumour cells, 25 % of the Rh30-Gluc cells remained viable after 24 h, and this was further reduced to 20 % at a 20:1 ratio. Blocking MIF with two different concentrations of anti-MIF antibody (1 µg/ml and 2 µg/ml) did not influence the killing efficiency.
Fig. 6.
Influence of the MIF antibody on tumour cell lysis. Rh30 Gluc cells were incubated with 2, 1, and 0.5 µg/ml anti-MIF antibodies for 30 min before the addition of effector cells. PBMCs were added at different ratios for a killing assay. After 24 h of co-culture, Gaussia luciferase activity was measured in the live tumour cells. The relative tumour cell viability of three replicates is shown as the mean and SEM. Treatment with the anti-MIF antibody did not significantly enhance the killing activity
Discussion
In recent years, the treatment of paediatric rhabdomyosarcoma has been improved for standard-risk patients [10]. However, the survival of high-risk patients is still poor [11], indicating the importance of the search for novel therapeutic options such as immunotherapeutic approaches [13]. Modulation of the immune system has shown to be promising in the treatment of RMS, which is demonstrated by bone marrow transplantation [33], antibody treatment [28], clinical trials investigating T cell or NK-cell transplantations (Clinicaltrials.gov) [34], or T cell-based immunotherapy approaches in RMS [35, 36]. Therefore, our aim was to investigate potential novel mechanisms that impair the immune response in RMS and to reverse their effects by using a therapeutic applicable way with small inhibitors or antibodies.
The immunomodulatory protein MIF is overexpressed not only in RMS but also in many other tumours, such as neuroblastoma, melanoma, hepatocellular carcinoma, and colon cancer [17, 37–39], leading to tumour progression, oncogenic transformation, and disturbed immune activation [15–17]. Various studies have provided evidence for the involvement of tumour-derived MIF in immune escape by increasing tumour-associated CD4+ Tregs and CD8+ Tregs accompanied by decreased CD8+-induced tumour cytotoxicity in MIF+/+ mice [24] or by promoting the differentiation and increasing the proportion of MDSCs within the tumour [25]. By using inhibitors of the tautomerase activity of MIF, such as SF, the development of MDSCs can be reduced and tumour growth and metastasis can be repressed [19]. Furthermore, MIF appears to participate in immune escape by counteracting NK and CD8+ T cell-mediated immune surveillance in malignant gliomas [26]. MIF interacts with different receptors such as CD74, CXCR2, CXCR4, and CXCR7 [22, 40] which could serve in possible receptor-based targeted therapies. Various intracellular and extracellular signalling pathways in which MIF is involved have been linked to different cellular effects, including the inhibition of p53 [41], the activation of ERK pathways via the induction of N-Myc expression, and the enhanced expression of VEGF [42]. Furthermore, MIF has been shown to induce COX-2, leading to cell proliferation, enhanced motility, disturbed apoptosis, and suppression of the immune response [43].
The inhibitors used in this paper were characterized by blocking the enzymatic activity of MIF which are able to block both the intracellular and extracellular signalling pathways. On the contrary, MIF antibodies are only competitive for extracellular MIF. ISO-1, an isoxazoline substance, inhibits the tautomerase activity of MIF by binding to its catalytic site. Ant.III, an iodo-pyrimidine compound, is known to covalently modify MIF. Ant.V is able to antagonize MIF against receptor binding as a symmetrical bis-(amino, hydroxynaphthalenedisulfonate) compound. Finally, SF is a version of natural R-sulforaphane, an ingredient in broccoli which seems to inhibit tumour formation. All inhibitors can penetrate the cell membrane, on contrary to MIF antibodies.
Despite its effects on multiple pathways that influence the viability of tumour cells, it appears that MIF inhibitors do not disturb viability in RMS. We did not observe a general decrease in RMS cell viability in vitro by inhibiting MIF with its inhibitors ISO-1, Ant.III, Ant.V or MIF antibodies (Supplementary Fig. 1), and the same result was observed previously when investigators knocked down MIF expression in human RH18 [15]. We were able to show a decrease in RMS cell viability by treatment with SF and an intensification of this effect by combining doxorubicin with SF (Supplementary Figs. 1 and 2). Isothiocyanates such as SF are known to modulate broader cellular processes, including the potent inactivation of MIF tautomerase activity [44, 45]. Therefore, the observed inhibition of cell viability could be a result of an off-target effect. Though, no reduction in viability of fibroblasts was observed by SF treatment.
Various studies report the pro-metastatic and pro-migration capacity of MIF, indicating that the use of MIF inhibitors may influence cell migration [19]. Our data showed that MIF inhibitors disrupted RMS cell migration, and this inhibition was slightly correlated with the basal secretion of MIF in the different RMS cell lines. This correlation was not observed in experiments testing SF, suggesting an off-target effect of SF. In addition, the differences observed between the inhibitors indicate that the small molecules tested may have non-specific effects. As blocking antibodies, which act only extracellularly, did not influence migration, intracellular MIF appears to play a role in RMS cell migration through intracellular signalling.
In fact, we showed that the production of tumour-derived MIF is induced in RMS by three commonly used cytotoxic drugs. Usually, MIF is secreted by different pathways, e.g. through the mediation of p115 [46]. Furthermore, cytotoxic drugs can induce cell death, following by an enhanced release of intracellular proteins which may include MIF. The levels of intracellular and secreted MIF but not mRNA (data not shown) increased during treatment with different anticancer drug classes in different tumour cell types. To date, few data have been produced to support the drug-induced overexpression of MIF in non-tumour patients [47]. Further analysis of gene expression databases (GEO, http://www.ncbi.nlm.nih.gov/gds/) revealed an induction of MIF in breast cancer cells treated with doxorubicin (GDS2244/217871_s_at) and in endometrial cells treated with carboplatin (GPL570, 217871_s_at). The enhanced expression of MIF in response to cytotoxic drugs may result in a reduction of immunogenicity and a disturbance of the immune response against tumours. Although conventional chemotherapeutics are known to be immunosuppressive, there is evidence that these drugs are able to induce immunogenic cell death, especially for the anthracycline drug doxorubicin [48–51]. Thus, to assess the role of enhanced MIF expression in immunogenic cell death, we combined anti-MIF antibodies in cytotoxic assays. In these assays, MIF did not have an impact on the killing efficiency of leucocytes.
In agreement with the results obtained in a breast cancer model [25, 52], we were able to demonstrate the MIF-dependent induction of a population containing MDSCs in tumours and the reversal of this effect by SF in RMS cell cultures. Conditioned medium from MIF-expressing RMS cells enhanced the differentiation of the population containing MDSCs, and this enhanced differentiation was suppressed by the addition of MIF inhibitors. However, T cell proliferation was disturbed by these inhibitors, with the exception of low concentrations of SF, ISO-1, and MIF antibodies, in cultures containing PBMCs and conditioned medium. Therefore, although some MIF inhibitors, such as Ant.III, were able to impair the development of the population containing MDSCs, they also disturb T cell proliferation. Blocking antibodies did not have effects in this development and in T cell proliferation. This result indicates that a less active antibody was used in this work and in our functional assays, although we were able to show that this antibody binds to MIF using flow cytometry. To study the integrated influence of the complex populations of leucocytes on RMS cells during inhibition of MIF, we used primary PBMCs and not NK cells which were used by Korckenberg et al. [27]. Although we could not reveal enhanced tumour cell lysis by PBMCs during treatment with MIF antibodies, Krockenberg et al. showed enhancement of ovarian tumour lysis by NK cells with the use of an anti-MIF antibody, which restored the MIF-induced immune escape [27]. Therefore, antibodies appear to be promising for inhibiting tumour-derived MIF [53].
In summary, we demonstrated the involvement of MIF in tumour cell migration in RMS. We also demonstrated that treatment with cytotoxic drugs leads to enhanced MIF expression, which may disrupt immunogenic cell death via the induction of a population containing MDSCs. To reactivate the immune system, targeting of MIF using blocking antibodies, low concentrations of SF, or even ISO-1 appears to be promising for reversing treatment-dependent MIF induction. Indirect procedure to inhibit MIF activity on immune and tumour cells may consist on blocking of MIF receptors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The work was supported in part by Else-Uebelmesser-Stiftung Tübingen. Sarah Maria Johler was a fellow of the Interdisciplinary Center for Clinical Research (IZKF) Tübingen.
Abbreviations
- CD
Cluster of differentiation
- CFSE
Carboxyfluorescein succinimidyl ester
- DMEM
Dulbecco’s modified Eagle medium
- FCS
Foetal calf serum
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IL
Interleukin
- MDSCs
Myeloid-derived suppressor cells
- MIF
Macrophage migration inhibitory factor
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NC
Negative control
- PE
Phycoerythrin
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PC
Positive control
- RMA
Alveolar rhabdomyosarcoma
- RME
Embryonal rhabdomyosarcoma
- RMS
Rhabdomyosarcoma
- RT
Room temperature
- SF
Sulforaphane
- VLE RPMI-1640
Very low endotoxin Roswell Park Memorial Institute Medium
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Guido Seitz and Sorin Armeanu-Ebinger have equally contributed to the authorship of the paper.
References
- 1.Pastore G, Peris-Bonet R, Carli M, Martinez-Garcia C, Sanchez de Toledo J, Steliarova-Foucher E. Childhood soft tissue sarcomas incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2136–2149. doi: 10.1016/j.ejca.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 2.McDowell HP. Update on childhood rhabdomyosarcoma. Arch Dis Child. 2003;88(4):354–357. doi: 10.1136/adc.88.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parham DM. Pathologic classification of rhabdomyosarcomas and correlations with molecular studies. Mod Pathol. 2001;14(5):506–514. doi: 10.1038/modpathol.3880339. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Guo W, Shen JK, Mankin HJ, Hornicek FJ, Duan Z. Rhabdomyosarcoma: advances in molecular and cellular biology. Sarcoma. 2015;2015:232010. doi: 10.1155/2015/232010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wachtel M, Runge T, Leuschner I, Stegmaier S, Koscielniak E, Treuner J, Odermatt B, Behnke S, Niggli FK, Schafer BW. Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry. J Clin Oncol. 2006;24(5):816–822. doi: 10.1200/JCO.2005.03.4934. [DOI] [PubMed] [Google Scholar]
- 6.Pappo AS, Shapiro DN, Crist WM, Maurer HM. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13(8):2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy N. Rhabdomyosarcoma: flexibility could be important. Nat Rev Cancer. 2014;14(3):156–157. doi: 10.1038/nrc3684. [DOI] [PubMed] [Google Scholar]
- 8.Huh WW, Skapek SX. Childhood rhabdomyosarcoma: new insight on biology and treatment. Curr Oncol Rep. 2010;12(6):402–410. doi: 10.1007/s11912-010-0130-3. [DOI] [PubMed] [Google Scholar]
- 9.Seitz G, Dantonello TM, Int-Veen C, Blumenstock G, Godzinski J, Klingebiel T, Schuck A, Leuschner I, Koscielniak E, Fuchs J, CWS-96 Study Group Treatment efficiency, outcome and surgical treatment problems in patients suffering from localized embryonal bladder/prostate rhabdomyosarcoma: a report from the Cooperative Soft Tissue Sarcoma trial CWS-96. Pediatr Blood Cancer. 2011;56(5):718–724. doi: 10.1002/pbc.22950. [DOI] [PubMed] [Google Scholar]
- 10.Wolden SL, Anderson JR, Crist WM, Breneman JC, Wharam MD, Jr, Wiener ES, Qualman SJ, Donaldson SS. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17(11):3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 11.Koscielniak E, Morgan M, Treuner J. Soft tissue sarcoma in children: prognosis and management. Paediatr Drugs. 2002;4(1):21–28. doi: 10.2165/00128072-200204010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10(1):41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 13.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armeanu-Ebinger S, Bonin M, Habig K, Poremba C, Koscielniak E, Godzinski J, Warmann SW, Fuchs J, Seitz G. Differential expression of invasion promoting genes in childhood rhabdomyosarcoma. Int J Oncol. 2011;38(4):993–1000. doi: 10.3892/ijo.2011.921. [DOI] [PubMed] [Google Scholar]
- 15.Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, Mitchell RA, Ratajczak MZ, Kucia M. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8(10):1328–1343. doi: 10.1158/1541-7786.MCR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repp AC, Mayhew ES, Apte S, Niederkorn JY. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol. 2000;165(2):710–715. doi: 10.4049/jimmunol.165.2.710. [DOI] [PubMed] [Google Scholar]
- 17.Bach JP, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75(3–4):127–133. doi: 10.1159/000155223. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264(3):751–758. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- 19.Rendon BE, Roger T, Teneng I, Zhao M, Al-Abed Y, Calandra T, Mitchell RA. Regulation of human lung adenocarcinoma cell migration and invasion by macrophage migration inhibitory factor. J Biol Chem. 2007;282(41):29910–29918. doi: 10.1074/jbc.M704898200. [DOI] [PubMed] [Google Scholar]
- 20.Dessein AF, Stechly L, Jonckheere N, Dumont P, Monte D, Leteurtre E, Truant S, Pruvot FR, Figeac M, Hebbar M, Lecellier CH, Lesuffleur T, Dessein R, Grard G, Dejonghe MJ, de Launoit Y, Furuichi Y, Prevost G, Porchet N, Gespach C, Huet G. Autocrine induction of invasive and metastatic phenotypes by the MIF-CXCR4 axis in drug-resistant human colon cancer cells. Cancer Res. 2010;70(11):4644–4654. doi: 10.1158/0008-5472.CAN-09-3828. [DOI] [PubMed] [Google Scholar]
- 21.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13(5):587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 22.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197(11):1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarnowski M, Grymula K, Reca R, Jankowski K, Maksym R, Tarnowska J, Przybylski G, Barr FG, Kucia M, Ratajczak MZ. Regulation of expression of stromal-derived factor-1 receptors: CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Cancer Res. 2010;8(1):1–14. doi: 10.1158/1541-7786.MCR-09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S, Kim HR, Leng L, Kang I, Jorgensen WL, Cho CS, Bucala R, Kim WU. Role of macrophage migration inhibitory factor in the regulatory T cell response of tumor-bearing mice. J Immunol. 2012;189(8):3905–3913. doi: 10.4049/jimmunol.1102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189(12):5533–5540. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C, Harter PN, Weller M, Wischhusen J. Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta Neuropathol. 2011;122(3):353–365. doi: 10.1007/s00401-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 27.Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, Weller M, Bucala R, Dietl J, Wischhusen J. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. 2008;180(11):7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann D, Seitz G, Fuchs J, Armeanu-Ebinger S. Susceptibility of rhabdomyosarcoma cells to macrophage-mediated cytotoxicity. Oncoimmunology. 2012;1(3):279–286. doi: 10.4161/onci.18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinson AR, Jones R, Crose LE, Belyea BC, Barr FG, Linardic CM. Human rhabdomyosarcoma cell lines for rhabdomyosarcoma research: utility and pitfalls. Front Oncol. 2013;3:183. doi: 10.3389/fonc.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellerkamp V, Lieber J, Nagel C, Wenz J, Warmann SW, Fuchs J, Armeanu-Ebinger S. Pharmacological inhibition of beta-catenin in hepatoblastoma cells. Pediatr Surg Int. 2013;29(2):141–149. doi: 10.1007/s00383-012-3237-9. [DOI] [PubMed] [Google Scholar]
- 31.Armeanu-Ebinger S, Griessinger CM, Herrmann D, Fuchs J, Kneilling M, Pichler BJ, Seitz G. PET/MR imaging and optical imaging of metastatic rhabdomyosarcoma in mice. J Nucl Med. 2014;55(9):1545–1551. doi: 10.2967/jnumed.114.138578. [DOI] [PubMed] [Google Scholar]
- 32.Lieber J, Dewerth A, Wenz J, Kirchner B, Eicher C, Warmann SW, Fuchs J, Armeanu-Ebinger S. Increased efficacy of CDDP in a xenograft model of hepatoblastoma using the apoptosis sensitizer ABT-737. Oncol Rep. 2013;29(2):646–652. doi: 10.3892/or.2012.2150. [DOI] [PubMed] [Google Scholar]
- 33.Lang P, Pfeiffer M, Muller I, Schumm M, Ebinger M, Koscielniak E, Feuchtinger T, Foll J, Martin D, Handgretinger R. Haploidentical stem cell transplantation in patients with pediatric solid tumors: preliminary results of a pilot study and analysis of graft versus tumor effects. Klin Padiatr. 2006;218(6):321–326. doi: 10.1055/s-2006-942256. [DOI] [PubMed] [Google Scholar]
- 34.Mackall CL, Rhee EH, Read EJ, Khuu HM, Leitman SF, Bernstein D, Tesso M, Long LM, Grindler D, Merino M, Kopp W, Tsokos M, Berzofsky JA, Helman LJ. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res. 2008;14(15):4850–4858. doi: 10.1158/1078-0432.CCR-07-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meadors JL, Cui Y, Chen QR, Song YK, Khan J, Merlino G, Tsokos M, Orentas RJ, Mackall CL. Murine rhabdomyosarcoma is immunogenic and responsive to T-cell-based immunotherapy. Pediatr Blood Cancer. 2011;57(6):921–929. doi: 10.1002/pbc.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang E, Rubin BP, Keller C. The long road to immunotherapy for childhood rhabdomyosarcoma. Pediatr Blood Cancer. 2011;57(6):899–901. doi: 10.1002/pbc.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hira E, Ono T, Dhar DK, El-Assal ON, Hishikawa Y, Yamanoi A, Nagasue N. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer. 2005;103(3):588–598. doi: 10.1002/cncr.20818. [DOI] [PubMed] [Google Scholar]
- 38.Legendre H, Decaestecker C, Nagy N, Hendlisz A, Schuring MP, Salmon I, Gabius HJ, Pector JC, Kiss R. Prognostic values of galectin-3 and the macrophage migration inhibitory factor (MIF) in human colorectal cancers. Mod Pathol. 2003;16(5):491–504. doi: 10.1097/01.MP.0000068235.45178.C1. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Siegler KL, Bellino MA, Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. 2002;94(5):1449–1456. doi: 10.1002/cncr.10354. [DOI] [PubMed] [Google Scholar]
- 40.Alampour-Rajabi S, El Bounkari O, Rot A, Muller-Newen G, Bachelerie F, Gawaz M, Weber C, Schober A, Bernhagen J. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. 2015;29(11):4497–4511. doi: 10.1096/fj.15-273904. [DOI] [PubMed] [Google Scholar]
- 41.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190(10):1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren Y, Chan HM, Li Z, Lin C, Nicholls J, Chen CF, Lee PY, Lui V, Bacher M, Tam PK. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene. 2004;23(23):4146–4154. doi: 10.1038/sj.onc.1207490. [DOI] [PubMed] [Google Scholar]
- 43.Telliez A, Furman C, Pommery N, Henichart JP. Mechanisms leading to COX-2 expression and COX-2 induced tumorigenesis: topical therapeutic strategies targeting COX-2 expression and activity. Anticancer Agents Med Chem. 2006;6(3):187–208. doi: 10.2174/187152006776930891. [DOI] [PubMed] [Google Scholar]
- 44.Crichlow GV, Fan C, Keeler C, Hodsdon M, Lolis EJ. Structural interactions dictate the kinetics of macrophage migration inhibitory factor inhibition by different cancer-preventive isothiocyanates. Biochemistry. 2012;51(38):7506–7514. doi: 10.1021/bi3005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Healy ZR, Liu H, Holtzclaw WD, Talalay P. Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: a potential biomarker for anti-inflammatory intervention. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1516–1523. doi: 10.1158/1055-9965.EPI-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merk M, Baugh J, Zierow S, Leng L, Pal U, Lee SJ, Ebert AD, Mizue Y, Trent JO, Mitchell R, Nickel W, Kavathas PB, Bernhagen J, Bucala R. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J Immunol. 2009;182(11):6896–6906. doi: 10.4049/jimmunol.0803710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livni E, Halevy S, Stahl B, Joshua H. The appearance of macrophage migration-inhibition factor in drug reactions. J Allergy Clin Immunol. 1987;80(6):843–849. doi: 10.1016/S0091-6749(87)80275-0. [DOI] [PubMed] [Google Scholar]
- 48.Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71(14):4809–4820. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 49.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3(1):e27878. doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Simpson KD, Cross JV. MIF: metastasis/MDSC-inducing factor? Oncoimmunology. 2013;2(3):e23337. doi: 10.4161/onci.23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussain F, Freissmuth M, Volkel D, Thiele M, Douillard P, Antoine G, Thurner P, Ehrlich H, Schwarz HP, Scheiflinger F, Kerschbaumer RJ. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol Cancer Ther. 2013;12(7):1223–1234. doi: 10.1158/1535-7163.MCT-12-0988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.