Abstract
Tumor immune escape has recently been shown to be related to the development of an immune tolerance state of the microenvironment. Cytokines activating the immune system such as IFN-γ can be used to reverse the immune escape and thus to potentiate the efficacy of immunotherapy. A clinical study was conducted in 18 stage IIIc/IV melanoma patients treated with tumor-infiltrating lymphocytes (TILs) in combination with intratumoral TG1042 injection (adenovirus expressing IFN-γ). The primary objective was to investigate the safety of treatment. Secondary objectives were to study the clinical response and translational research. The treatment was well tolerated. Among the 13 patients evaluable for tumor response, 38.5 % had an overall objective response (OOR = CR + PR) and disease control rate (DCR = CR + PR + S) of 46 %. The clinical response of the 37 targeted lesions led to an OOR of 51 % and a DCR of 75 %. Translational research on predictive markers did not significantly differ between responder and non-responder patients. However, specifically regarding injected lesions, the clinical response correlated with CD3−/CD56+ NK cells which could be activated by TG1042. Further larger studies of this combined immunotherapy are needed to confirm our findings. Intralesional TG1042 combined with antigen-selected TILs should be discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1691-7) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Adoptive T cell therapy, Interferon-γ, TG1042
Introduction
The ex vivo approach of tumor-infiltrating lymphocyte (TIL) amplification before re-administration to the patient has proven its protective activity against melanoma and other tumors. This technique is called adoptive cell therapy (ACT). The rationale is that after being infused back into a patient, expanded T cells can travel to tumor lesions and destroy cancer cells after specific recognition of their cognate antigen through major histocompatibility complex (MHC) class I presentation [1]. Clinically, the ACT strategy is associated with incomplete responses and tumor recurrence in melanoma. Many pathways through which melanoma cells can escape from a T cell response have been identified, and it is not surprising that T cell transfer alone may be insufficient for tumor eradication. Nonetheless, some patients achieve durable complete tumor clearance following ACT, suggesting its great potential. To consistently achieve optimal results, the ability to inhibit the escape mechanisms of melanoma cells must be improved and inhibitory approaches should be developed. Two main approaches may be used to improve ACT: first, changes to directly improve the efficacy of transferred T cells and second, changes to stimulate the innate immunity of melanoma cell microenvironment. To avoid local anergy, the intratumoral administration of an adenovirus expressing interferon-gamma (IFN-γ) leads to changes in the tumor microenvironment in different ways and is likely to optimize the balance toward the anti-tumor immune response. IFN-γ is known to exert locally different effects related to its biology. First, it is a potent activator of immune cell subtypes, including effector T cells, natural killer (NK) cells and macrophages which are usually already present within melanoma lesions, and it induces the expression of MHC class I and class II molecules in tumor cells, increasing their susceptibility to cytolysis by effector T cells. Second, IFN-γ has also a direct anti-proliferative effect on some tumor cells and anti-angiogenic properties [2–4]. The rationale for combining TILs with interleukin-2 (IL-2) is to enhance, as previously demonstrated, infused TIL maintenance over time. IL-2 by itself has also shown an anti-melanoma activity [5] mediated by several biological properties [6]. In addition, Donia et al. [7] have recently evidenced that in vitro anti-tumor reactivity of both CD8+ and CD4+ T cells could be enhanced by a pretreatment with low-dose IFN-γ, suggesting the interest of a combination strategy for the treatment of metastatic melanoma. The primary aim of this study was to assess the feasibility and safety of administrating subcutaneous IL-2 and intratumoral injections of an adenovirus expressing IFN-γ (TG1042) in combination with a TIL-based therapy in patients with unresectable relapsing melanoma. Secondary aims were to assess the clinical response and translational research to identify some predictive markers of response to help defining the best subpopulation which could benefit from this combined approach.
Materials and methods
Patients
Patients with unresectable stage III or stage IV M1a–c melanoma according to the American Joint Committee of Cancer (AJCC 2001) were included in an open-label, monocentric phase I/II clinical trial, promoted by Nantes University Hospital (France). The trial that met the tenets of the Declaration of Helsinki was approved by the local ethics committee, the French National Agency for Medicines and health products safety, and was registered with the regulatory authority (Clinicaltrials.gov identifier: NCT00720031). All patients gave written informed consent before enrollment and met the following inclusion criteria: metastatic stage III/IV melanoma with at least one non-resectable nodal or cutaneous lesion for IFN-γ injection and another one for TIL production, a performance status from 0 to 2, suitable hematology and biochemistry parameters and the absence of brain or bone metastases. The primary endpoint was the safety of the procedure according to the National Cancer Institute Common Terminology Criteria (NCI-CTC), and secondary endpoints included the clinical response according to the RECIST 1.1 [8] and translational research.
TIL production from cutaneous metastases
TILs were minimally cultured according to a procedure previously described [9, 10]. Briefly, short-term cultured TILs were isolated by culturing fragments of stage IV cutaneous metastases into 12-well tissue culture plates in X-VIVO 15 serum-free medium (BioWhittaker, Walkersville, MD, USA) containing 150 U/ml recombinant interleukin-2 (rIL-2) (Eurocetus, Rueil Malmaison, France) and glutamine (1 mM, BioWhittaker) for 10–14 days. Ex vivo expanded TILs were then amplified in vitro using irradiated feeder cells (allogeneic peripheral blood leukocytes (PBL) and Epstein–Barr virus infected B (B-EBV) cells) for 10 days and transferred into cell culture bags for ten additional days. A second TIL expansion was performed within 1 month after the first one.
TG1042 adenovirus IFN-γ
TG1042 consists of a suspension of recombinant type 5 adenoviral particles, carrying the gene coding for human IFN-γ. As it is a live virus expressing a transgene, it is considered as a genetically modified organism and has been classified from a regulatory standpoint at the biosafety level 2. An agreement to handle the virus in the different areas involved including pharmacy and clinical ward is needed for each center. Preparation was performed under a class II safety cabinet. TG1042 was supplied frozen at −80 °C in single-dose ampoules (Transgene SA, Illkirch, France), and it was thawed before use and injected by the physician into lesions selected for intratumoral injection.
Combined therapy
The sequences of TIL/IL-2 injections and TG1042 administration are shown in Fig. 1. All injections were performed at the investigational site (Department of Dermato-Oncology, Nantes). TILs were infused over a 30- to 60-min period on days 1 and 29 followed by subcutaneous IL-2 injections (6 M IU daily) for 10 days (Proleukine, Novartis Pharma SAS, France). TG1042 was injected into the tumor at a dose of 5 × 1010 viral particles per lesion (into up to six lesions) every 2 weeks from day −15 to month 2 and then every month up to month 11 or progression.
Fig. 1.
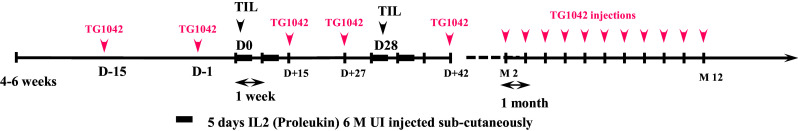
Sequences of TIL/IL-2 injections and TG1042 administration. D day, TG1042 adenovirus interferon-gamma
Tumor samples
Eleven tumor biopsy specimens from which TILs were obtained were used to determine different microenvironmental markers by immunohistochemistry (IHC) and the BRAF and NRAS mutational status.
Immunohistochemistry on cutaneous metastases
IHC was performed using the streptavidin/peroxidase technique as previously described [11]. Briefly, deep-frozen sections were incubated for 30 min at room temperature with the primary antibody. Seventeen different monoclonal antibodies were used to explore the expression of several markers (supplementary Table 1). Negative controls experiments were performed using a mouse monoclonal immunoglobulin G1 (IgG1) isotype control or a monoclonal immunoglobulin G2a (IgG2a) isotype control (DakoCytomation). Slides were read with a Leica microscope (magnification 25×). In each immunostained serial section, the entire tumor area was evaluated. Each score was evaluated on a five-point scale: absence of expression, weak expression (1–25 % of positive cells), moderate expression (26–50 %), intermediate expression (51–75 %) and strong expression (>75 %), corresponding, respectively, to 0, 1, 2, 3 and 4. To avoid the subjectivity of the reading, all slides were read by two independent blinded examiners.
Desoxyribonucleic acid (DNA) extraction
Serial sections were cut from each paraffin block and placed onto glass slides. The first 3-μm-thick section was stained with hematoxylin and eosin (H&E) for histopathological examination. The 2–5 following 10-μm-thick sections were processed for DNA extraction. To enrich the analyzed specimen with tumor cells, tumor areas evidenced by a pathologist on H&E preparation were macrodissected using single-use sterilized scalpels. DNA was extracted after paraffin removal and macrodissection using the Forensic kit and an iPrep system according to the manufacturer’s recommendations (Invitrogen, Life Technologies SAS, Villebon sur Yvette, France). DNA concentration was measured by spectrophotometry (NanoDrop ND-100 instrument, Thermo Fisher Scientific, Waltham, MA, USA) and normalized to 5 ng/μl.
Detection of BRAF V600 and NRAS Q61 mutations
The most frequent BRAF mutations were detected by allele-specific amplification as previously described [12] with minor modifications. NRAS exon 2 and c-KIT exon 11 and 13 mutations were analyzed by conventional Sanger DNA sequencing. Primers used for amplification and sequencing are listed in supplementary Table 2. These assays allowed detecting BRAF V600 or NRAS Q61 mutations when present in at least 10 % of cells.
Antibodies and flow cytometric analysis on injected TILs
TIL phenotype was determined in 13 patients. The following antibodies were used: phycoerythrin (PE) anti-CD2 (clone S5.2), PE anti-CD56 (clone MY31) and PE anti-CD25 (clone 2A3), all from BD Pharmingen, Le Pont de Claix, France. We also used PC5 anti-CD3 (clone UCHT1), PE anti-CD8 (clone B9.11), allophycocyanin (APC) anti-CD4 (clone 13B8.2), APC anti-CD19 (clone J3-119), PC7 anti-CD45 (clone J.33) and PE anti-CD16 (clone 3G8), all from Beckman Coulter, Marseille, France. For the evaluation of the markers associated with regulatory T cells, a five-color multiparametric analysis was used with the following antibodies: fluorescein isothiocyanate (FITC) anti-CD4 (clone RPA-T4), PE anti-CD127 (clone hIL-7R-M21), PE-Cy7 anti-CD25 (clone M-A251) and BD V450 Horizon anti-CD3 (clone UCHT1) all from Becton Dickinson and APC anti-Foxp3 (clone 236A/E7), from eBioscience, San Diego, CA, USA. Lymphocytes were gated according to their forward and size scatter characteristics, and Fluorescence-activated cell sorting (FACS) Canto analysis was performed using the BDFACS Diva software (BD Biosciences, San Jose, CA, USA).
Establishment of melanoma cell lines
Melanoma cell lines were successfully established as previously described [13, 14] for six tumor samples. Briefly, fresh cutaneous metastases were minced into small tumor pieces and inoculated into a 24-well plate (NUNC), and 1.5 ml per well of RPMI (Roswell Park Memorial Institute) medium supplemented with 10 % fetal calf serum (FCS) was added. Plates were placed at 37 °C in a humidified incubator with 5 % CO2 and observed under a light microscope every week and subcultured if necessary.
Cytokine production assay for tumor-specific TIL quantification
The fraction of tumor-reactive TIL was determined from the measurement of the fraction of IFN-γ-secreting T cells among TIL stimulated with the autologous melanoma cell line, as described previously [10]. Briefly, about 1 × 105 lymphocytes were stimulated with 3 × 105 autologous melanoma cells in 200 µl of X-VIVO 15 medium in the presence of brefeldin A. Cultures were incubated for 6 h at 37 °C in 5 % CO2-humidified atmosphere. Cells were then stained for surface markers with anti-human CD4 APC and anti-human CD8 FITC (BD Biosciences, France) and then fixed and stained for cytokine production using the method described by Jung et al. [15]. Anti-IFN-γ-specific antibody was purchased from BD Biosciences. Cells were finally analyzed on a FACSCalibur flow cytometer using the BDFACS Cell Quest Pro software (BD Biosciences, San Jose, CA, USA). T cell responses were considered significant when the mean fluorescence labeling of TIL stimulated with the autologous tumor cell line exceeded, by at least half a log, the mean fluorescence of the background responses of non-stimulated TILs and/or TILs stimulated with a HLA-mismatched melanoma cell line. A value of 0.3 % was considered as the significance threshold.
Statistical analysis
Wilcoxon rank and Fisher exact tests were used to compare patients with or without progression. The Kaplan–Meier estimator was used for the survival analysis between the date of enrollment and the date of the last information known.
Results
Patients
Eighteen patients (seven women and 11 men), with an average age of 54 years, were included. At study inclusion, eight patients had AJCC stage III disease and ten had AJCC stage IV disease. Before their inclusion, 13 patients had previously been treated with at least one line of chemotherapy or immunotherapy. Five patients were excluded from data analysis: two because of insufficient tumor material for TIL production and three because they did not receive TIL infusions due to rapid disease progression with appearance of brain metastasis and development of major asthenia and anorexia. Finally, 13 patients whose characteristics are summarized in Table 1 were clinically assessed.
Table 1.
Patient characteristics and clinical responses
| Patient ID | Sex | Age | Disease stage at baseline | BRAF status | Previous treatment | Number of IFN-γ injections | Targeted lesions (n) IFN-γ injected/non-injected | Clinical response injected/non-injected lesions | Local cutaneous response | Overall clinical response |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 C-B | F | 32 | IV M1a | V600E | DTIC | 2 | (5) 4/1 | SD/P | P | P |
| 4 H-F | M | 38 | IV M1a | V600E | IFN | 11 | (1) 1/0 | SD/NA | SD | SD 6 M |
| 5 A-C | F | 40 | IIIc | Wt | DTIC, imiquimod | 16 | (4) 2/2 | CR/CR | CR | CR 15 M |
| 6 O-A | F | 62 | IV M1a | Wt | IFN, carb/DTIC | 7 | (6) 6/0 | SD/NA | SD | P |
| 7 G-M | F | 73 | IV M1a | Wt | IFN, DTIC | 8 | (5) 4/1 | CR/PR | CR | PR 2 M |
| 9 C-D | M | 60 | IIIc | Wt | IFN, MAGE A3 | 12 | (2) 1/1 | CR/CR | CR | CR 32 M+ |
| 11 L-M | F | 64 | IIIc | Wt | MAGE A3 | 6 | (4) 2/2 | P/P | P | P |
| 13 H-M | M | 64 | IIIc | V600E | NONE | 16 | (1) 1/0 | CR/NA | CR | CR 17+ M |
| 14 R-J | M | 62 | IV M1c | Wt | NONE | 2 | (6) 6/0 | P/NA | P | P |
| 15 T-J | M | 57 | IIIc | Wt | Roferon + DTIC/carbo | 9 | (5) 5/0 | PR/NA | PR | P |
| 16 L-A | F | 68 | IIIc | Wt | Roferon, melan-A, imiquimod | 15 | (4) 2/2 | PR/PR | PR | PR 5 M |
| 17 B-S | M | 31 | IIIc | V600E | IFN | 7 | (4) 4/0 | P/NA | SD | P |
| 18 G-J | M | 61 | IIIc | V600E | Roferon, vemurafenib | 7 | (1) 1/0 | P/NA | P | P |
F female, M male, NA not applicable, CR complete regression, PR partial regression, SD stable disease, P progression, M + ongoing complete regression, DTIC dacarbazine, IFN interferon
TIL production and administration
TIL could be produced for 16 out of the 18 patients included but only 13 patients received autologous TILs. Eleven patients received two TIL infusions and two patients received only the first cycle of TIL due to disease progression before protocol completion. All patients received between 1.66 × 109 and 22.2 × 109 TILs and between two and 16 cycles of IFN-γ injections. Autologous melanoma cell lines were obtained for eight patients allowing determining the proportion of specific reactive T cells producing IFN-γ (Table 2) which varied from 0.006 × 109 to 0.43 × 109 T lymphocytes.
Table 2.
Overall characteristics of infused TILs with clinical response for the 13 patients
| Patients | Specific T cells (×109) | Injected TIL (×109) | CD3+ (×109) | CD3+/CD4+ (×109) | CD3+/CD8+ (×109) | CD3/CD56+ (×109) | CD127lowFoxp3+ among CD4+ CD25+ (×109) | Local cutaneous response | Overall clinical response |
|---|---|---|---|---|---|---|---|---|---|
| 3 C-B | 0.04 | 2.24 | 2.24 | 1.71 | 0.52 | 0 | 0.03 | P | P |
| 4 H-F | 0.102 | 1.66 | 1.41 | 0.35 | 0.81 | 0.24 | 0.01 | SD | SD |
| 5 A-C | NA | 9.87 | 9.40 | 1.8 | 7.5 | 0.43 | 0.02 | CR | CR |
| 6 O-A | 0.042 | 9.66 | 8.10 | 3.67 | 4.40 | 1.29 | 0.17 | SD | P |
| 7 G-M | NA | 20.64 | 20.47 | 8.28 | 12.40 | 0.17 | 0.09 | CR | PR |
| 9 C-D | NA | 22.2 | 22.14 | 7.73 | 13.80 | 0.09 | 0.06 | CR | CR |
| 11 L-M | NA | 8.01 | 8.01 | 3.20 | 4.84 | 0 | 0.01 | P | P |
| 13 H-M | NA | 2.4 | 2.39 | 1.94 | 0.44 | 0.01 | 0.03 | CR | CR |
| 14 R-J | 0.006 | 1.99 | 1.98 | 1.66 | 0.31 | 0.01 | 0.02 | P | P |
| 15 T-J | 0 | 8.37 | 8.29 | 7.41 | 0.89 | 0.06 | 0.19 | PR | P |
| 16 L-A | 0.43 | 9.73 | 9.65 | 7.79 | 1.62 | 0.07 | 0.17 | PR | PR |
| 17 B-S | 0.0075 | 8.22 | 8.19 | 4.71 | 3.43 | 0.01 | 0.37 | SD | P |
| 18 G-J | 0.109 | 5.78 | 5.77 | 1.95 | 4.13 | 0.01 | 0.23 | P | P |
Results are expressed as absolute number
Clinical efficacy
Overall clinical response (targeted cutaneous lesions and other visceral metastasis)
Among the 13 patients evaluable for tumor response, three patients experienced a complete regression (CR) (15, 32 and 17 months+), two patients presented a partial response (2 and 5 months), one patient presented a stable disease for 6 months, and seven patients progressed (Tables 1, 2). Thus, the objective response (CR + PR) was 38.5 %, and the disease control rate (CR + PR + SD) was 46 %.
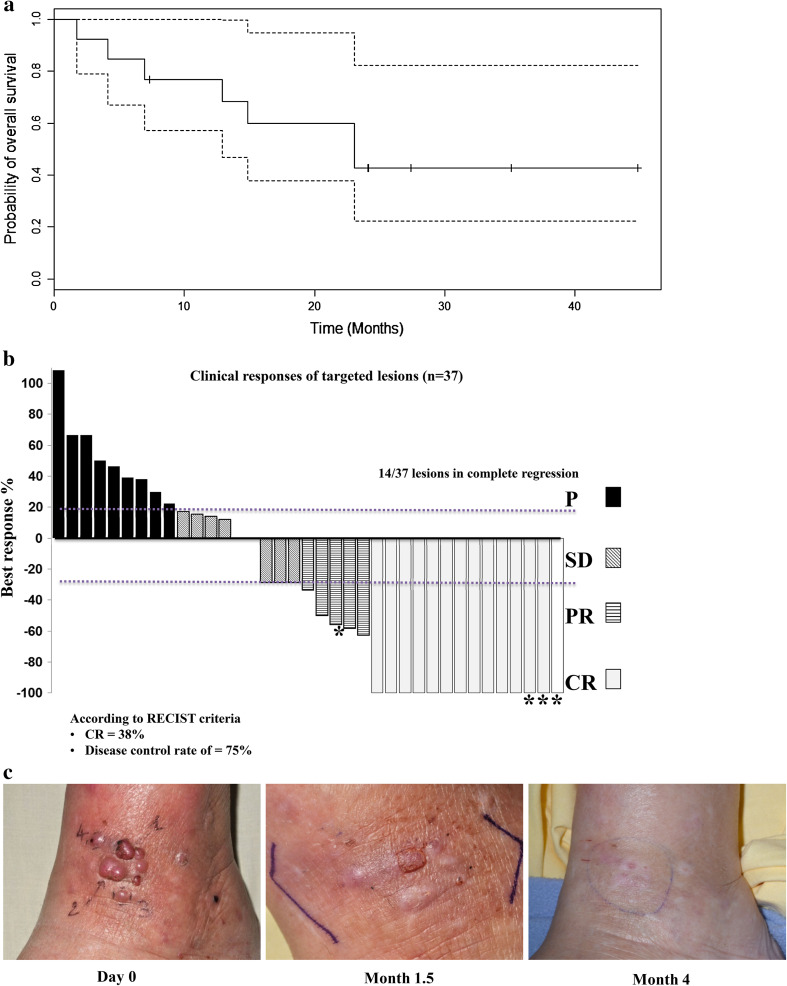
The median overall survival was of 21.1 months [95 % CI (12.1–NA)] with a 30-month follow-up (Fig. 2a).
Fig. 2.
Clinical outcome. a Overall survival. The median overall survival was 21.1 months 95 % CI [12.1–NA] with a follow-up of 30 months. Dotted lines illustrate the 95 % confidence intervals. b Clinical responses of targeted cutaneous lesions (n = 37). CR complete regression, PR partial regression, SD stable disease, P progression. Asterisk indicates non-injected lesions. c Example of clinical response in patient 7 who experienced a global partial response and a complete local regression
Local cutaneous response (injected and non-injected lesions)
When focusing on the analysis of the 37 targeted cutaneous lesions, the combination of TIL and TG1042 led to a CR in 11 injected lesions and three non-injected lesions. Four injected lesions and one non-injected lesion presented a PR, and three injected lesions were stabilized. Thus, the local cutaneous response of targeted lesions resulted in 38 % of CR and a DCR of 75 % (Fig. 2b).
Patient 7, whose global response was a PR for 2 months, experienced a local CR in injected and non-injected lesions 4 months after treatment initiation (Fig. 2c), and the disease progressed outside the injected area. The local CR was maintained until death 18 months later.
Patient 5 also experienced a CR in two IFN-γ-injected lesions and two non-injected lesions for 15 months; then, progression was observed outside the targeted area, with visceral metastases.
Safety profile
TIL infusion in combination with IFN-γ injection was well tolerated in all patients. No grade 4 adverse events related to the study treatment were observed, and only six grade 3 adverse events were reported in five patients (one flu-like syndrome, three asthenias, one nausea–vomiting and one axillary pain) (Table 3). Most mild or moderate adverse events reported were asthenia (77 %), headache (23 %), flu-like syndrome (31 %), fever (46 %), nausea and vomiting (31 %), erythema and induration at injection site (31 %) and pain at injection site (54 %). Most of these adverse events, mainly experienced few hours after TG1042 injection, seemed to be related to a systemic immune activation induced by the combined therapy.
Table 3.
Attributable adverse events
| Event | Number of patients (N = 13) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1/2 N (%) |
Grade 3 N (%) |
|||||||
| IFN-γ | IL2 | TIL | % | IFN-γ | IL2 | TIL | % | |
| Flu-like syndrome | 3 | 1 | 0 | 31 | 1 | – | – | 8 |
| Asthenia | 9 | 1 | – | 77 | 3 | – | – | 23 |
| Fever | 4 | 1 | 1 | 46 | – | – | – | |
| Headache | 3 | 0 | 0 | 23 | – | – | – | |
| Nausea–vomiting | 3 | 1 | 0 | 31 | 1 | – | – | 8 |
| Erythema induration at injection site | 3 | 0 | 1 | 31 | – | – | – | |
| Pain at injection site | 6 | 0 | 1 | 54 | – | – | – | |
| Axillary pain | – | – | – | – | 1 | – | – | 8 |
Correlation between TIL expansion and clinical response
The overall clinical response did not correlate (Wilcoxon rank tests) with the number of injected TILs or the CD4/CD8 ratio and the number of CD4+/CD25+/Foxp3+ cells (Table 4). However, taking only into account the local cutaneous response, the clinical response correlated with the absolute CD3−/CD56+ NK cell number in TIL expansion infused to patients (Table 4).
Table 4.
Comparison of TIL characteristics between responder and non-responder patients for overall clinical response and local cutaneous response
| T cells number | Overall clinical response | Local cutaneous response | ||||
|---|---|---|---|---|---|---|
| CR or PR or SD [mean (min–max)] | P [mean (min–max)] | p value | CR or PR or SD [mean (min–max)] | P [mean (min–max)] | p value | |
| Specific T cells (×109) | 0.266 (0.102–0.43) | 0.047 (0–0.109) | 0.133 | 0.116 (0–0.43) | 0.0516 (0.006–0.109) | 1 |
| Injected TIL (×109) | 11.08 (1.66–22.2) | 6.32 (1.99–9.66) | 0.267 | 10.3 (1.66–22.2) | 5.248 (1.99–8.22) | 0.075 |
| CD3+ (×109) | 10.91 (1.41–22.14) | 6.08 (1.98–8.29) | 0.234 | 10 (1.41–22.14) | 5.23 (1.98–8.19) | 0.075 |
| CD3+/CD4+ (×109) | 4.64 (0.35–8.28) | 3.47 (1.66–7.41) | 0.628 | 4.85 (0.35–8.28) | 2.65 (1.66–4.71) | 0.148 |
| CD3+/CD8+ (×109) | 6.1 (0.44–13.8) | 2.65 (0.31–4.84 | 0.445 | 5.03 (0.44–13.8) | 2.64 (0.31–4.84) | 0.414 |
| CD3−/CD56+ (×109) | 0.168 (0.01–0.43) | 0.197 (0–1.29) | 0.081 | 0.263 (0.01–1.29) | 0.006 (0–0.01) | 0.015 |
| CD127lowFoxp3+ among CD4+ CD25+ (×109) | 0.063 (0.01–0.17) | 0.145 (0.01–0.37) | 0.350 | 0.1233 (0.01–0.37) | 0.132 (0.01–0.37) | 0.4379 |
Bold values are statistically significant
Immunochemistry of cutaneous melanoma biopsies
Regarding the expression of melanoma antigens and markers of innate immunity studied from the cutaneous metastasis used to obtain TILs before initiating any treatments, no immunological markers or antigens were statistically associated with a good overall clinical response or local cutaneous response (supplementary Table 3). Furthermore, no clinical correlation between the overall clinical response and the BRAF status was noted (p = 1, Fisher exact test).
Immunohistochemical evolution of melanoma tumor before and after treatment
Changes in innate immunity markers and melanoma antigens were studied in four patients (two CR, one PR and one SD) before and after 3 months of treatment with intralesional interferon. In situ IFN-γ expression was increased in three patients with initial low level (two CR and one PR), and CD8+ T cells were increased in these three same patients (supplementary Table 4).
Discussion
Tumor immune escape has recently been shown to be related to increases in immunosuppressive state of the melanoma metastasis microenvironment. In this clinical study, we report for the first time the safety profile and clinical efficacy of TIL adoptive transfer combined with TG1042, an adenovirus expressing IFN-γ, aimed to enhance the anti-tumor cytotoxicity of autologous infused lymphocytes.
The administration of TILs/IL-2 combined with intratumoral TG1042 injections was feasible in most patients (13/18), as previously reported in studies using TILs in metastatic stages or in adjuvant stage III situation [16, 17]. Clinically, this therapeutic approach combining TILs with TG1042 led to an objective overall response (CR + PR) of 38.5 % and an overall DCR (CR + PR + SD) of 46 %. Among the 13 patients evaluable, three long-lasting CR (15, 25 and 10 months+), two PR (2 and 5 months) and one SD for 6 months were obtained. The seven remaining patients experienced disease progression.
Thus, our overall objective responses were similar to that obtained with TIL transfer combined with previous lymphodepletion in metastatic stage [18, 19]. The main strength of our combined therapy was the duration of the clinical response (15 months and two responses lasting for 32 and 17 months are still ongoing) which is also similar to the results obtained with the use of previous lymphodepletion. Focusing on the local clinical response of targeted cutaneous lesions, the clinical benefit obtained from the TIL-TG1042 combination was particularly high with a response rate of 75 % in injected lesions. Furthermore, a bystander effect with regression of non-injected lesions was obtained in two patients, confirming that the therapeutic combination could act locoregionally.
Regarding the safety, TIL/IL-2 in combination with TG1042 was well tolerated and the safety profile was in line with the expected adverse events related to the individual administration of each treatment component as previously reported [5, 20, 21]. Fatigue, flu-like symptoms and injection site reactions were the most frequent adverse events, usually of minor-to-moderate intensity. Most of these adverse events, especially flu-like syndrome, asthenia and nausea, were related to IFN-γ, IL-2 or endogenous cytokines induced by the live virus TG1042. TILs induced no adverse event, and for TG1042 injections, the main local adverse event was pain at the injection site in metastases (grade 1 or grade 2 according to NCI-CTC). Only six patients experienced a grade 3 adverse event once, and no grade 4 adverse event and no patient withdrawal because of adverse event were noted. The different treatment components did not seem to potentiate the adverse events. No autoimmune adverse events were observed in this series; especially no vitiligo appeared after ACT. As the treatment was well tolerated, it could easily be repeated. In this regard, the gene therapy approach with TG1042 assessed in this study showed a good risk/benefit ratio with a high response rate and long response duration. The adverse events were less severe than with lymphodepletion combined with ACT, in particular when considering the risk of EBV infection which was absent in our trial.
At the translational level, a correlation was observed between the absolute number of CD3−/CD56+ cells in the T cell expansion and the local cutaneous response. CD3−/CD56+ cells or lymphokine-activated killer (LAK) cells are essentially activated by NK cells. A higher number of NK cells in the final T cell expansion transfused to patients are associated with a significantly higher local cutaneous response (CR, PR, SD). CD3−/CD56+ cells mediate important immunoregulatory “helper” functions in addition to their cytolytic activity. In particular, NK cells can prevent the maturation of dendritic cells related to exhaustion [22]. During in vitro TIL expansion, a significant number of TILs adhere to the bag surface several weeks after culture initiation and they contain more CD3−/CD56+ NK cells and exhibit higher cytotoxicity than non-adherent TILs. Adherent TILs, unlike non-adherent TILs, produce IFN-γ [23]. In addition to the low doses of IL-2 injected subcutaneously to the patient at the same time than TILs (10 days), the cytotoxicity of CD3−/CD56+ NK cells in the T cell expansion could be strongly stimulated by the high IFN-γ level at the site of cutaneous metastasis intralesionally injected with TG1042. Indeed, it has been shown that IFN-γ stimulates CD56+ cells [24, 25] which could play a critical role in regulating the effective anti-tumor T cell activity [26]. In accordance with this assumption, four patients experienced a good local clinical response and their cutaneous biopsies were taken before and after initiating the study treatment. The in situ IFN-γ level was increased in three injected lesions after 3 months of treatment, and in the remaining patient, the IFN-γ level was already high before treatment initiation and remained stable after 3 months. In these four patients, the absolute number of CD8+ T lymphocytes was stable (1) or increased (3). The expression of melanoma antigens was not significantly changed after 3 months.
Interestingly, the immunochemical analysis of the innate immunity in the tumor tissue did not show any correlation between the local or overall clinical responses and the immunotolerance level of the microenvironment before initiating the combined therapy. Indeed, the tumor infiltrate profile (CD8+ and Tregs), the expression level of inhibitory molecules [programmed cell death protein 1 (PD-1), programmed death ligand 1 (PDL-1), anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) and indoleamine dioxygenase (IDO) and cytokines (IFN-γ, IL-10, transforming growth factor beta (TGF-β)] in the melanoma tissue before treatment initiation did not correlate with the overall clinical response and local cutaneous response. Regarding melanoma antigens, the expression of non-differentiated antigens [melanoma-associated antigen (MAGE) and New York esophageal squamous cell carcinoma (NY-ESO-1)] did not correlate with the responding lesions although Donia et al. have shown that IFN-γ selectively enhanced responses to tumor-associated antigens (such as MAGE-A1 in cancer testis) and thus could increase the reactivity of CD8+ T lymphocyte-infiltrating tumor cells. No link with the BRAF status and the response to the combined treatment was noted. The identification of a correlation between the number of CD3−/CD56+ cells injected and the local cutaneous response but without any association with a tissue marker of the innate immunity suggests that the activity of CD3−/CD56+ NK cells could be independent of the local site of immunosuppression. Thus, adoptive T cell therapy in combination with intralesional TG1042 administrations could be a way to bypass the inhibition of the microenvironment-induced TIL activation.
Obviously, one of the limitations of our study was the small number of patients, in particular for translational research, but it was a pilot study, and our results encourage conducting a phase II study with a larger patient series to confirm that this combined therapy could target NK cells.
In conclusion, we report the first clinical study of treatment with “TILs in combination with intralesional adenovirus 5 expressing IFN-γ” in metastatic melanoma. We showed both the feasibility and safety of this approach with a high clinical response rate. In addition, the duration of the complete clinical responses lasted long (longer than 1 year in some patients) with disappearance of all adverse events. Thus, our results pave the way for the use of ACT in further combination immunotherapies. As an example, combining ACT with oncolytic viruses could take benefit from both approaches since in the field of melanoma, oncolytic viruses have proven efficacy and their mechanism of action involves an immune component [27, 28]. A study conducted in melanoma patients using the same adenoviral vector combined with IL-2 instead of IFN-γ has shown the regression of metastatic melanoma lesions in six out of the 21 patients [29]. Finally, the effect of intralesional adenovirus 5 expressing IFN-γ could be improved in the future by combining it with a selected expansion of TILs directed against specific antigens [30].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank M. Yviquel, G. Hatton and J. David from the laboratory of immunodermatology and also the laboratory of immunology from Nantes Hospital for their technical support. This study was partially funded by Transgene and Nantes Hospital.
Conflict of interest
V. Bataille and J.M. Limacher are employees of Transgene SA. Other authors have no conflict of interest to disclose.
Abbreviations
- ACT
Adoptive cell therapy
- AJCC
American Joint Committee of Cancer
- APC
Allophycocyanin
- CR
Complete regression
- FACS
Fluorescence-activated cell sorting
- FITC
Fluorescein isothiocyanate
- H&E
Hematoxylin and eosin
- IFN-γ
Interferon-gamma
- IL
Interleukin
- MAGE
Melanoma-associated antigen
- NCI-CTC
National Cancer Institute Common Terminology Criteria
- NK
Natural killer
- NY-ESO-1
New York esophageal squamous cell carcinoma
- P
Progression
- PE
Phycoerythrin
- PR
Partial regression
- SD
Stable disease
- TIL
Tumor-infiltrating lymphocytes
References
- 1.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Ernstoff MS. Role of interferons in the therapy of melanoma. J Invest Dermatol. 1990;95:180S–184S. doi: 10.1111/1523-1747.ep12875497. [DOI] [PubMed] [Google Scholar]
- 3.Nemunaitis J, Fong T, Robbins JM, Edelman G, Edwards W, Paulson RS, Bruce J, Ognoskie N, Wynne D, Pike M, Kowal K, Merritt J, Ando D. Phase I trial of interferon-gamma (IFN-gamma) retroviral vector administered intratumorally to patients with metastatic melanoma. Cancer Gene Ther. 1999;6:322–330. doi: 10.1038/sj.cgt.7700019. [DOI] [PubMed] [Google Scholar]
- 4.Khorana AA, Rosenblatt JD, Sahasrabudhe DM, Evans T, Landrigan M, Marquis D, Rosell K, Whiteside T, Phillippe S, Acres B, Slos P, Squiban P, Ross M, Kendra K. A phase I trial of immunotherapy with intratumoral adenovirus-interferon-gamma (TG1041) in patients with malignant melanoma. Cancer Gene Ther. 2003;10:251–259. doi: 10.1038/sj.cgt.7700568. [DOI] [PubMed] [Google Scholar]
- 5.Whittington R, Faulds D. Interleukin-2. A review of its pharmacological properties and therapeutic use in patients with cancer. Drugs. 1993;46:446–514. doi: 10.2165/00003495-199346030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann MF, Oxenius A. Interleukin 2: from immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8:1142–1148. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donia M, Hansen M, Sendrup SL, Iversen TZ, Ellebæk E, Andersen MH, Straten PT, Svane IM. Methods to improve adoptive T-cell therapy for melanoma: IFN-γ enhances anticancer responses of cell products for infusion. J Invest Dermatol. 2013;133:545–552. doi: 10.1038/jid.2012.336. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Jotereau F, Pandolfino MC, Boudart D, Diez E, Dreno B, Douillard JY, Muller JY, LeMevel B. High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J Immunother. 1991;10:405–411. doi: 10.1097/00002371-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfino MC, Labarriere N, Tessier MH, Cassidanius A, Bercegeay S, Lemarre P, Dehaut F, Dreno B, Jotereau F. High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: predictability and relation with disease advancement. Cancer Immunol Immunother. 2001;50:134–140. doi: 10.1007/PL00006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chebassier N, El Houssein O, Viegas I, Dreno B. In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol. 2004;13:484–490. doi: 10.1111/j.0906-6705.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarry A, Masson D, Cassagnau E, Parois S, Laboisse C, Denis MG. Real-time allele-specific amplification for sensitive detection of the BRAF mutation V600E. Mol Cell Probes. 2004;18:349–352. doi: 10.1016/j.mcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino MC, Saiagh S, Knol AC, Dreno B. Comparison of three culture media for the establishment of melanoma cell lines. Cytotechnology. 2010;62:403–412. doi: 10.1007/s10616-010-9286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Yang JC. The adoptive transfer of cultured T cells for patients with metastatic melanoma. Clin Dermatol. 2013;31:209–219. doi: 10.1016/j.clindermatol.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Khammari A, Knol AC, Nguyen JM, Bossard C, Denis MG, Pandolfino MC, Quéreux G, Bercegeay S, Dréno B. Adoptive TIL transfer in the adjuvant setting for melanoma: long-term patient survival. J Immunol Res. 2014;2014:186212. doi: 10.1155/2014/186212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pt Straten, Becker JC. Adoptive cell transfer in the treatment of metastatic melanoma. J Invest Dermatol. 2009;129:2743–2745. doi: 10.1038/jid.2009.204. [DOI] [PubMed] [Google Scholar]
- 19.Dudley ME. Adoptive cell therapy for patients with melanoma. J Cancer. 2011;2:360–362. doi: 10.7150/jca.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, Jotereau F. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dummer R, Hassel JC, Fellenberg F, Eichmuller S, Maier T, Slos P, Acres B, Bleuzen P, Bataille V, Squiban P, Burg G, Urosevic M. Adenovirus-mediated intralesional interferon-gamma gene transfer induces tumor regressions in cutaneous lymphomas. Blood. 2004;104:1631–1638. doi: 10.1182/blood-2004-01-0360. [DOI] [PubMed] [Google Scholar]
- 22.Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, Mailliard RB. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa K, Salmeron MA, Parkinson DR, Markowitz AB, von Eschenbach AC, Legha SS, Balch CM, Ross MI, Augustus LB, Itoh K. Study of tumor infiltrating lymphocytes for adoptive therapy of renal cell carcinoma (RCC) and metastatic melanoma: sequential proliferation of cytotoxic natural killer and noncytotoxic T cells in RCC. J Immunother. 1991;10:313–325. doi: 10.1097/00002371-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JM, Bryant J, Schiller JH, Oken MM, Borden EC, Whiteside TL. Immunomodulatory function of interferon-gamma in patients with metastatic melanoma: results of a phase II-B trial in subjects with metastatic melanoma, ECOG study E 4987. Eastern Cooperative Oncology Group. J Immunother. 1997;20:146–157. doi: 10.1097/00002371-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Gritzapis AD, Dimitroulopoulos D, Paraskevas E, Baxevanis CN, Papamichail M. Large-scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cytokine-rich supernatants. Cancer Immunol Immunother. 2002;51:440–448. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarrondo FJ, Yang OO, Chodon T, Avramis E, Lee Y, Sazegar H, Jalil J, Chmielowski B, Koya RC, Schmid I, Gomez-Navarro J, Jamieson BD, Ribas A, Comin-Anduix B. Natural killer T cells in advanced melanoma patients treated with tremelimumab. PLoS One. 2013;8:e76829. doi: 10.1371/journal.pone.0076829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastrangelo MJ, Maguire HC, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, Kovatich AJ, Lattime EC. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–422. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 28.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, Goldsweig H, Marshall T, Love C, Coffin R, Nemunaitis JJ. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 29.Dummer R, Rochlitz C, Velu T, Acres B, Limacher JM, Bleuzen P, Lacoste G, Slos P, Romero P, Urosevic M. Intralesional adenovirus-mediated interleukin-2 gene transfer for advanced solid cancers and melanoma. Mol Ther. 2008;16:985–994. doi: 10.1038/mt.2008.32. [DOI] [PubMed] [Google Scholar]
- 30.Labarriere N, Fortun A, Bellec A, Khammari A, Dreno B, Saïagh S, Lang F. A full GMP process to select and amplify epitope-specific T lymphocytes for adoptive immunotherapy of metastatic melanoma. Clin Dev Immunol. 2013;2013:932318. doi: 10.1155/2013/932318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.