Abstract
Most patients with thyroid cancer will evolve very well with current therapies. However, 10–30% of these patients will present recurrent disease and some of them will eventually die. IL-10 is an anti-inflammatory and immunosuppressive cytokine that can contribute to the immune escape of neoplastic cells. We aimed to investigate IL-10 as a molecular marker to improve the clinical management of patients with thyroid cancer. We retrospectively studied 162 patients with follicular cell-derived thyroid cancer who attended to our institution, including 63 classic papillary thyroid carcinomas, 46 follicular variant of papillary thyroid carcinomas, 11 poorly differentiated thyroid carcinomas and 42 follicular thyroid carcinomas. Patients were treated according to current guidelines and followed-up for 1–150 months. Additionally, we studied 96 samples of non-malignant tissues. We investigated the expression of IL-10 in tumor cells by semiquantitative and quantitative methods. Malignant tissues presented higher positivity (0.773 ± 0.140) than non-malignant samples (0.623 ± 0.190; p < 0.001). Tumors with extrathyroidal invasion at diagnosis presented higher levels of positivity for IL-10 (0.802 ± 0.125) than tumors without extrathyroidal invasion (0.731 ± 0.147; p = 0.004). We observed a positive correlation between tumor size and IL-10 positivity (correlation coefficient = 0.407; p < 0.001). Patients with IL-10 positivity above the median presented lower relapse-free survival rate compared to those patients whose tumors presented IL-10 positivity below the median. We suggest that a simple IL-10 IHC analysis could help selecting patients who would benefit from a more intensive approach.
Keywords: Thyroid neoplasm, Tumor immunology, Interleukin 10, Prognostic marker
Introduction
Most of the more than 62,000 new cases of thyroid cancer detected in the USA during 2015 will evolve very well with thyroidectomy and radioiodine therapy. However, 10–30% of these patients will present recurrent disease and some of them will eventually stop responding to treatment and metastasize, contributing to the 1950 deaths due to thyroid cancer estimated to occur in 2015 [1]. In fact, despite of the efficiency of current treatments, only a small decrease in death rate has been evidenced in historical series, and recent evidences suggest that we are overtreating a significant part of thyroid cancers, impairing these patients’ quality of life [2, 3]. Hence, new studies designed to ameliorate the clinical management of patients with thyroid cancer are of upmost need.
It has long been recognized that thyroid tumors are markedly infiltrated by a mixture of immune cells from both the innate and adaptive arms of the immune system, composing the huge complexity of the microenvironment in thyroid neoplasms [4, 5]. The production of both pro and anti-inflammatory cytokines by tumor cells is a key point to understand the molecular modulation of immune response, adding more complexity for the immune microenvironment of thyroid carcinomas [6–8].
IL-10 is an anti-inflammatory and immunosuppressive cytokine that may influence the clinical course of cancer by favoring immune escape through inhibition of the antitumor activity of immune cells [9–12]. IL-10 prevents antigen presentation by antigen-presenting cell (APC), reducing the expression of the class I major histocompatibility complex (MHC) on the cell surface [13]. IL-10 also inhibits monocyte class II MHC expression [14], inhibits T cell proliferation [15], regulates the differentiation of regulatory T cells [10], inhibits B7 upregulation in monocytes [16], prevents monocyte-associated production of nitric oxide (NO) [17] and induces the formation of a tumor cell phenotype resistant to the action of cytotoxic T lymphocytes [18].
We previously demonstrated that signs of immune escape of thyroid carcinoma cells may be related to tumor aggressiveness and advanced stages of disease [19, 20]. In addition, enrichment of CD8+ lymphocytes and expression of cyclooxygenase 2 (COX2) were associated with recurrence [21]. However, we still lack information on IL-10 role on the clinical presentation and the outcome of patients with thyroid cancer. The present investigation aimed to further investigate IL-10 role.
Materials and methods
Patients
The present study was approved by the Research Ethics Committee of the AC Camargo Cancer Center (São Paulo, Brazil). We retrospectively studied 162 patients with follicular cell-derived thyroid cancer who attended to our Head and Neck Unit, including 63 classic papillary thyroid carcinomas, 46 follicular variant of papillary thyroid carcinomas, 11 poor differentiated thyroid carcinomas and 42 follicular thyroid carcinomas. Additionally, we investigated 96 samples of non-malignant tissues (45 goiters, 38 follicular adenomas and 13 normal thyroid).
We consecutively included patients with histopathological confirmation of thyroid cancer, whose clinical information was minimally evaluable in the charts and tumor sample was stored in our biobank. Exclusion criteria were: no histopathological confirmation of thyroid carcinoma by pathologists, limited or no clinical information on the charts and insufficient tissue sample in our biobank. Patients’ clinical and pathological information was obtained from their charts. Aggressiveness at diagnosis was defined by the TNM classification and stage classification system for thyroid cancer [22]. Follow-up standard protocol included periodic total body scans, serum thyroid-stimulating hormone (TSH) and thyroglobulin measurements, X-ray, ultrasonography, computed tomography scan and other procedures to detect distant metastasis for a period of 1–150 months (55.00 ± 34.16 months). Patients presenting high non-stimulated serum thyroglobulin levels (>2 mg/dl) were submitted to a thorough image search. The aforementioned parameters were used to define tumors as persistent/recurrent and/or presenting long distance metastasis. Patients were considered free-of-disease when they evolved with stable low (unstimulated serum thyroglobulin levels <2 ng/dl) or undetectable thyroglobulin levels for more than 2 years after tumor resection, without any suspicion of recurrence. Patients who could not be ascertained as free-of-disease or presenting persistent/recurrent disease were excluded from further survival analysis. Since we considered 99 patients free-of-disease and 39 presented recurrence/metastasis during the observation period, only 138 patients with complete data were included in the final relapse-free survival analysis. Seventy patients were followed for greater than or equal to 5 years.
Evaluation of concurrent chronic lymphocytic thyroiditis (CT)
Formalin-fixed paraffin-embedded tissues from all samples were reviewed for diagnostic confirmation and investigation of concurrent CT. Concurrent CT was investigated in non-malignant thyroid parenchyma of the tumor contralateral lobe. Concurrent CT was histologically characterized by lymphocytic infiltration with lymphoid follicles and follicular regenerative activity with numerous small follicles, lined by Hurthle cells and scaring [23]. Clinical diagnosis of Hashimoto’s thyroiditis was confirmed with presence of patient serum antithyroid antibodies (anti-thyroperoxidase and/or anti-thyroglobulin), as previously published [24].
IHC and immunohistochemical analysis
Samples from all tissues were reviewed in order to select the most representative areas designed to build a tissue microarray (Beecher Instruments®, Silver Springs, MD, USA) for immunohistochemical analysis.
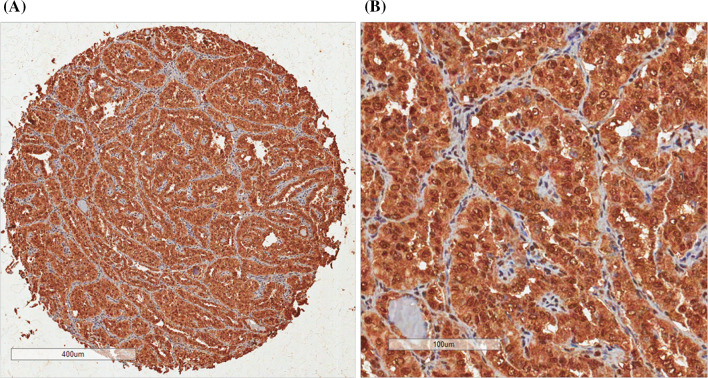
We obtained four tumor tissue cores from each case. Then, two spots were chosen from representative areas of the lesion presenting relevant leukocyte infiltration whereas two other spots were chosen from areas free of leukocytes. We investigated the expression of IL-10 in tumor cells (Fig. 1). Immunohistochemical procedure was held as previously described, always running positive and negative controls in the same batch of reactions [25].
Fig. 1.
a one tissue spot of classic papillary thyroid carcinoma, and b an expanded view of the same tissue. IL-10 presented a diffuse cytoplasmic pattern of expression. Note a weak IL-10 expression in stroma cells, suggesting that IL-10 is fairly related to cell transformation
Semiquantification was performed by two of the authors (Lucas Leite Cunha and/or Elaine Cristina Morari). Slides were further submitted to evaluation of two experienced pathologists (José Vassallo and Fernando Augusto Soares). They were blinded to tumor features. Cells were defined as positive for immunohistochemical markers when a clear cut brown staining was observed in the corresponding cellular localization. An individual evaluation of IL-10 was completed for each tissue spot. Visual evaluation of IL-10 was performed considering an approximate area of 0.79 mm2 per tissue microarray spot. Then, we estimated the percentage of positive tumor cells and the intensity of staining, as previously described [26].
We further proceeded with immunohistochemical quantification with Spectrum Plus© automated image analysis software (Aperio, Vista, CA). Since IL-10 presented cytoplasmic staining, Positive Pixels Count V9.0 algorithm was used, with the resulting variable being the positivity, which is the total number of positive pixels divided by total number of pixels.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS)® software, version 13.0. We define first relapse as endpoint. Kaplan–Meier method was used to calculate relapse-free survival rates, and survival curves were compared using log-rank test. Cox’s proportional hazards model was adopted for multivariate analysis. The recurrence risk was estimated by hazard ratios (HR). The modeling technique was the stepwise forward selection method. Chi-square or Fisher’s exact test were assessed to perform nonparametric analysis, as indicated. A multivariate logistic regression model was conducted considering clinical risk factors (i.e., gender, age) as explicative variables. Mann–Whitney tests were used to compare continuous measures between two independent groups whose variables did not fit to Gaussian distribution; Kruskal–Wallis test was performed to compare continuous measures between three or more groups whose variables did not fit Gaussian distribution. Quantitative results are expressed in mean ± standard deviation. All tests were conducted at a 0.05 significance level.
Results
This study included 127 female and 35 male patients. Mean age at diagnosis was 46 ± 16.29 years, and mean tumor size was 3.4 ± 2.38 cm. The majority of the patients (n = 117) presented no evidence of concurrent CT, but in 45 patients there were evidences of concurrent autoimmunity. Extrathyroidal invasion was detected in 50.8% of patients, and multifocality was noted in 50.4% of patients. According to the pTNM staging system, there were 73 cases classified as pTNM I at presentation; 23 cases pTNM II; 22 cases pTNM III; and 20 pTNM IV. LN metastasis at diagnosis was observed in 41.1% of patients.
IHC detected IL-10 expression in the great majority of samples (Fig. 1). Semiquantitive analysis showed that 83.6% of all samples presented intense expression in more than 75% of cells per spot. Only 25 samples presented a variation of intensity/positivity ranging from absolute absence of expression (n = 3; 1.9%) to a mix of weak and scarce IL-10 staining. The three patients whose tumor tissue presented no IL-10 expression included two patients with follicular variants of papillary thyroid carcinomas and one patient with the classic form of papillary thyroid carcinoma. These three patients were women diagnosed between 53 and 66 years old, with no extrathyroidal invasion, no concurrent CT and no LN metastasis at the diagnosis. We have no outcome information on the patient with the classic papillary thyroid carcinoma, whereas the two other patients remain with no evidence of recurrence after 18 and 55 months of follow-up, respectively.
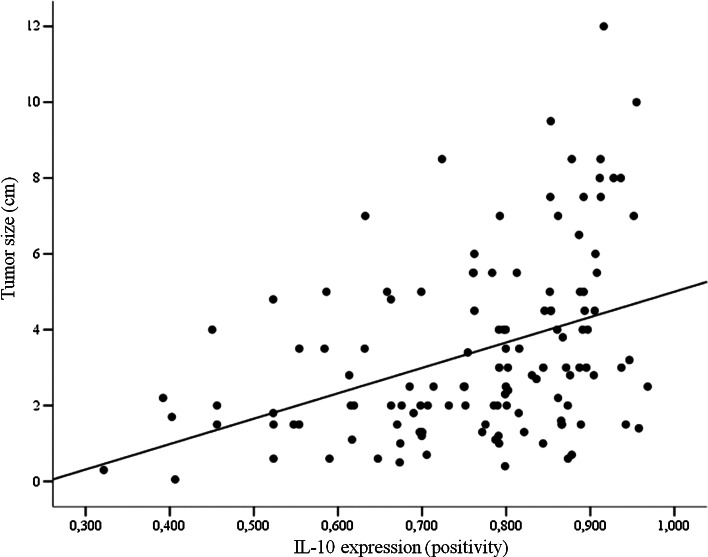
Since the semiquantitive analysis failed to identify minimal variations of IL-10 expression, we conducted a quantitative analysis. Table 1 summarizes the relationship among clinical and pathological signs of aggressiveness and quantitative parameters of IL-10 expression. Tumors with extrathyroidal invasion at diagnosis presented higher levels of positivity for IL-10 (p = 0.004). In addition, Spearman’s test showed a positive correlation between tumor size and IL-10 positivity (correlation coefficient = 0.407; p < 0.001; Fig. 2) but not between age at diagnosis and IL-10 positivity (p > 0.05). IL-10 positivity did not relate to other tumor aggressiveness features. Tumor infiltrating lymphocytes (TIL) were found in 81 samples of thyroid carcinomas. Tumors with TIL presented higher expression of IL-10 compared with tumors with no TIL (Table 1; p = 0.017). Also, tumors with concurrent CT presented similar IL-10 positivity to those samples in which concurrent CT was not found (Table 1; p > 0.05).
Table 1.
Comparison of IL-10 positivity and clinical and pathological features of aggressiveness of 162 differentiated thyroid carcinomas
| Variable | IL-10 positivity | ||
|---|---|---|---|
| Mean | SD | p value | |
| Gender | |||
| Female | 0.77 | 0.142 | 0.619 |
| Male | 0.773 | 0.116 | |
| Histological type | |||
| Classic PTC | 0.763 | 0.141 | 0.208 |
| Follicular variant of PTC | 0.747 | 0.155 | |
| Poor differentiated PTC | 0.817 | 0.096 | |
| FTC | 0.801 | 0.129 | |
| Multifocality | |||
| Absent | 0.767 | 0.139 | 0.699 |
| Present | 0.758 | 0.142 | |
| Extrathyroidal invasion | |||
| Absent | 0.731 | 0.147 | 0.004 |
| Present | 0.802 | 0.125 | |
| Concurrent CT | |||
| Absent | 0.765 | 0.137 | 0.799 |
| Present | 0.772 | 0.146 | |
| TIL | |||
| Absent | 0.746 | 0.148 | 0.017 |
| Present | 0.798 | 0.127 | |
| LN metastasis at diagnosis | |||
| Absent | 0.752 | 0.144 | 0.223 |
| Present | 0.782 | 0.132 | |
| pTNM | |||
| I | 0.76 | 0.14 | 0.307 |
| II | 0.742 | 0.148 | |
| III | 0.757 | 0.149 | |
| IV | 0.805 | 0.153 | |
Bold values represent p-value < 0.05
Equality of means was assessed with t-test
SD standard deviation; PTC papillary thyroid carcinoma; FTC follicular thyroid carcinoma
Fig. 2.
Scatter plot of tumor size (cm) and IL-10 positivity show a positive correlation between these variables
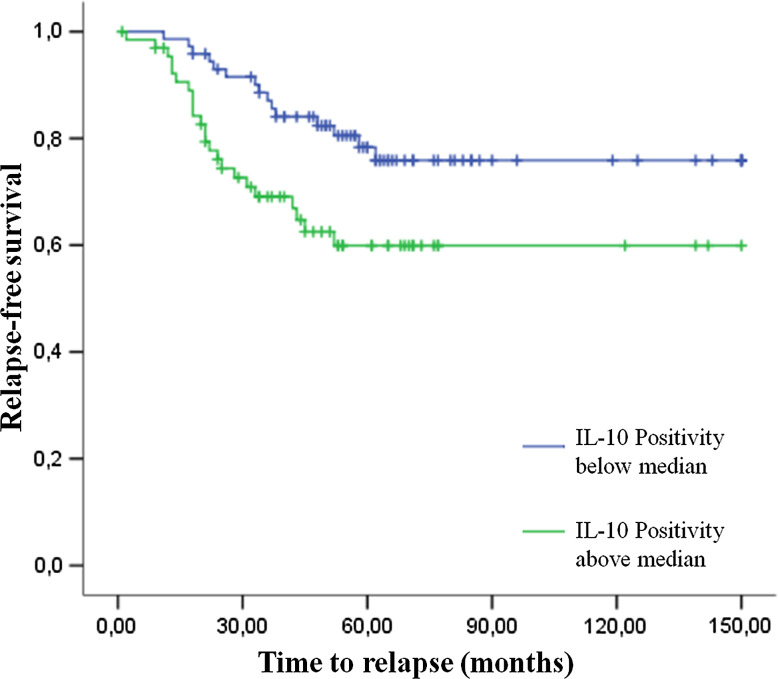
We conducted a relapse-free survival analysis by categorizing IL-10 positivity in two classes according to the median of the data. Patients with IL-10 positivity above the median presented lower relapse-free survival rate compared to those patients whose tumors presented IL-10 positivity below the median (p = 0.016, Fig. 3). Also, poor prognosis was predicted by poor histological class differentiation (p = 0.002), advanced stages of disease according to pTNM (p = 0.029), presence of metastasis at diagnosis (p = 0.048) and absence of concurrent CT (p = 0.029).
Fig. 3.
Kaplan–Meier curve evidence of the two patterns of relapse-free survival. Patients whose tumors presented IL-10 positivity above the median (green line) presented a poor prognosis compared to those whose tumors showed IL-10 positivity below median (blue line)
Cox’s proportional hazards model was performed in order to identify putative misleading prognostic predictors. Relapse-free survival was adjusted by histological classification, IL-10 positivity and pTNM. Poor prognosis was, in fact, independently predicted by poor histological differentiation (p = 0.009; HR = 4.22). IL-10 positivity (p = 0.059; HR = 2.008) and advanced pTNM stage (p = 0.095; HR = 1.99) did not reach significance.
We proceeded with a new multivariate analysis considering only well-differentiated thyroid carcinomas. Advanced stage of disease predicted poor relapse-free outcome (p = 0.029; HR = 2.527), but IL-10 positivity did not achieve significance (p = 0.079; HR = 1.971).
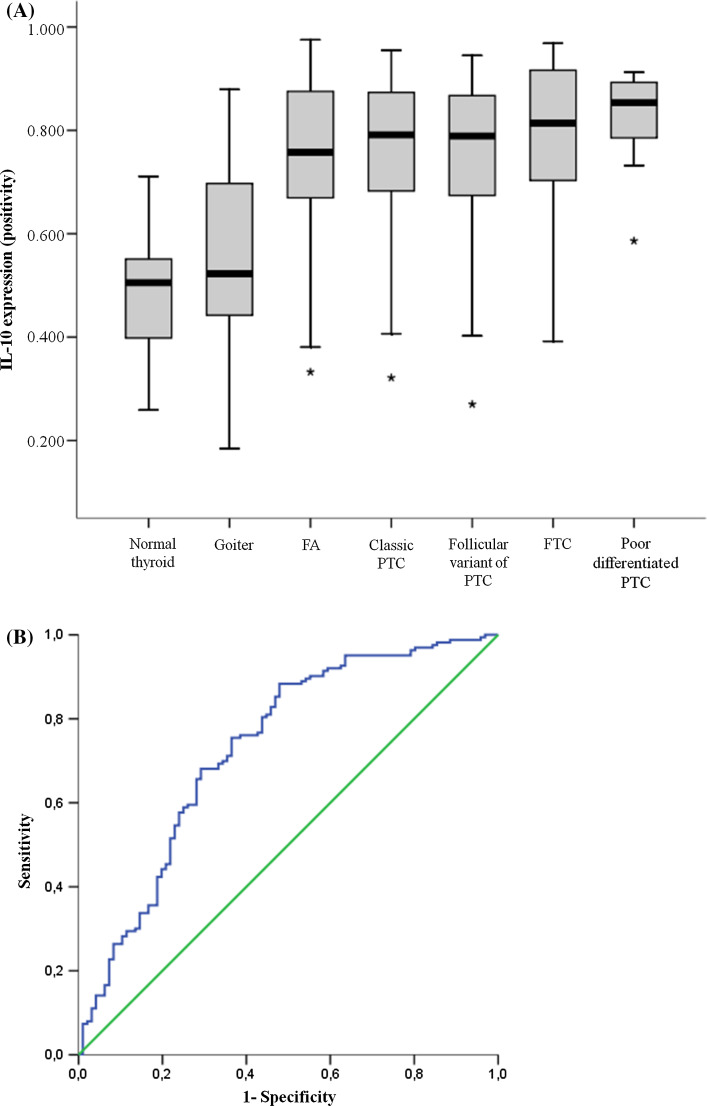
We further studied IL-10 expression considering non-malignant samples (normal thyroid, goiters and follicular adenomas) as a set of comparison. Absolute absence of IL-10 expression was more frequent among non-malignant samples (13/96) compared to malignant samples (3/162; p < 0.001). The non-malignant tissues presented a gradient of expression that ranged from faint and scarce staining to strong and widely stained. Quantitative analysis showed that malignant tissues presented higher positivity (0.773 ± 0.140) than non-malignant samples (0.623 ± 0.190; p < 0.001). One-way analysis of variance showed that variation among the means of all categories was significantly greater than expected by chance (Fig. 4a; p < 0.001). In fact, normal thyroid tissues presented lower IL-10 positivity than follicular adenomas (p < 0.001) and all follicular cell-derived thyroid carcinomas (all p < 0.001). Likewise, goiters presented lower IL-10 positivity than follicular adenomas (p < 0.001) and all other histological types of thyroid carcinomas (all p < 0.001).
Fig. 4.
a Box-plot of IL-10 positivity of all histological sets of our samples. Note that normal thyroid presents the lowest IL-10 positivity, followed by goiters. These two tissues differ significant from all other pattern of thyroid lesions, including follicular adenomas and thyroid carcinomas. b ROC curve of IL-10 positivity, considering malignancy as positive. Abbreviations: FA follicular adenoma, FTC follicular thyroid carcinoma, PTC papillary thyroid carcinoma
We further investigated IL-10 utility in the diagnostic of thyroid malignancy. Although IL-10 expression was markedly increased in thyroid carcinomas, the parameter showed a sensitivity of 65.9% and specificity of 70.8% (area under the curve = 0.732; p < 0.001 considering the null hypothesis that true area = 0.5; Fig. 4b). In fact, 50.6% of non-malignant samples presented an intense brown staining in more than 75% of cells per spot and this fact may prevent IL-10 positivity to reach satisfactory qualities as a diagnostic marker.
Discussion
Our data demonstrate that IL-10 staining can be found in samples of normal thyroid, goiters and follicular adenomas. However, IL-10 expression was more frequently observed among thyroid carcinomas, suggesting that the progressively acquisition of IL-10 expression could represent a hallmark of malignant transformation of the follicular thyroid cell. Since the immune system has a fundamental role resisting or even eradicating formation and progression of incipient neoplasias [27], tumor promotion requires the immune microenvironment to provide conditions to avoid an effective immune response. In this context, IL-10 emerges as the single most potent immunosuppressive cytokine and may play a pivotal role in the tumor escaping from immune system [28].
According to this rationale, IL-10 may also influence thyroid carcinoma progression and aggressiveness. In fact, we found that tumors with extrathyroidal invasion at diagnosis had higher IL-10 positivity than tumors confined to the thyroid gland. In addition, a positive correlation was noted between IL-10 positivity and tumor size, suggesting that tumor aggressiveness could be associated to IL-10 expression. In fact, log-rank test showed that an unfavorable prognosis was predicted by higher IL-10 staining. Multivariate analysis showed that advanced stage of disease predicted poor relapse-free outcome but IL-10 expression failed to achieve significance. This suggests that even though IL-10 may be associated with aggressiveness and may even help to distinguish patients with unfavorable prognosis, an appropriated and personalized clinical approach remains the best watershed in the conduction of patients with thyroid cancer.
We observed that tumors with concurrent CT presented similar IL-10 positivity to those tumors in which concurrent CT was not found. We previously demonstrated that concurrent CT correlates with clinical and pathological features of favorable prognosis, including the absence of metastasis at diagnosis, female gender, no extrathyroidal tumor invasion and smaller sizes of tumors [29]. Concurrent CT is related to favorable prognosis while IL-10 is related to unfavorable prognosis. In fact, Stassi et al. [30] confirmed that cytokines provided by Hashimoto’s thyroiditis promote caspase upregulation and CD95-induced apoptosis in thyrocytes, whereas IL-10 protects thyrocytes by preventing CD95-induced apoptosis in sensitized thyrocytes, suggesting that concurrent CT and IL-10 may exert an opposite influence on thyrocyte survival. This influence on thyrocyte survival would explain our result that concurrent CT and IL-10 are opposite influencing prognosis of patients with thyroid cancer.
Our results corroborate the findings of Stassi and colleagues [31]. Similarly to our data, they observed that papillary thyroid carcinomas, follicular thyroid carcinomas and undifferentiated thyroid carcinomas produced IL-10. It was noted that IL-10 upregulated antiapoptotic molecules (Bcl-xL and Bcl-2), preventing thyrocytes from cell death induced by chemotherapeutic agents. When thyroid carcinoma cells where treated with IL-10-neutralizing antibody, the levels of antiapoptotic molecules decreased significantly and thyroid cancer cell apoptosis was dramatically increased by the exposure to chemotherapeutic drugs. Few years later, the same group demonstrated that the autocrine production of IL-10 neutralized CD95-generated signals and allowed growth and survival of thyroid cancer cells. This may help to explain why IL-10 positivity was associated to larger tumors, extrathyroidal invasion and poor prognosis.
Our results suggest that IL-10 positivity may help predict an aggressive behavior of thyroid cancer, helping select cases that deserve a more intensive clinical and surgical approach. Likewise, IL-10 production was associated to advanced stages of disease and poor prognosis in several solid tumors, such as oral and oropharyngeal squamous cell carcinoma [32, 33], non-small-cell lung cancer [34], hepatocellular carcinoma [35] and melanoma [12]. In addition, IL-10 can be considered an autocrine growth factor for immune cells and malignant cells [36, 37] helping to establish an unfavorable disease course.
The present study has some limitations. In fact, a retrospective design was adopted. It may have hindered an accurate detailing of clinical information of our cohort, although we thoroughly searched for the data records in charts. The fact that cases were gathered consecutively reduces the risk of a selection bias. Also, including a larger number of samples is necessary in order to assemble a satisfactory number of patients for statistical analysis.
In conclusion, we observed that thyroid carcinomas express IL-10. We found that IL-10 positivity was tightly associated to extrathyroidal invasion and larger tumor size, suggesting that IL-10 may influence disease presentation. Log-rank test confirmed that IL-10 positivity is associated to relapse-free survival, suggesting that IL-10 could help select cases that would benefit from a more intensive clinical approach.
Funding
The authors’ research grant was supported by the São Paulo State Research Foundation/FAPESP. First author was supported by a fellowship grant from FAPESP.
Abbreviations
- APC
Antigen-presenting cell(s)
- CD
Cluster of differentiation
- COX
Cyclooxygenase
- CT
Chronic lymphocytic thyroiditis
- HR
Hazard ratio
- IHC
Immunohistochemistry
- IL
Interleukin
- LN
Lymph node
- MHC
Major histocompatibility complex
- NO
Nitric oxide
- TIL
Tumor infiltrating lymphocyte(s)
- pTNM
Tumor lymph node metastasis stage
- TSH
Thyroid-stimulating hormone
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ahn HS, Welch HG. South Korea’s thyroid-cancer “epidemic”—turning the tide. N Engl J Med. 2015;373:2389–2390. doi: 10.1056/NEJMc1507622. [DOI] [PubMed] [Google Scholar]
- 3.Guimaraes RM, Muzi CD, Parreira VG, Santos RD, Sampaio JR. Evolution of thyroid cancer mortality in adults in Brazil. Arq Bras Endocrinol Metabol. 2013;57:538–544. doi: 10.1590/S0004-27302013000700007. [DOI] [PubMed] [Google Scholar]
- 4.Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. 2014;21:R85–R103. doi: 10.1530/ERC-13-0431. [DOI] [PubMed] [Google Scholar]
- 5.Marcello MA, Malandrino P, Almeida JF, Martins MB, Cunha LL, Bufalo NE, Pellegriti G, Ward LS. The influence of the environment on the development of thyroid tumors: a new appraisal. Endocr Relat Cancer. 2014;21:T235–T254. doi: 10.1530/ERC-14-0131. [DOI] [PubMed] [Google Scholar]
- 6.Coperchini F, Pignatti P, Carbone A, et al. TNF-alpha increases the membrane expression of the chemokine receptor CCR6 in thyroid tumor cells, but not in normal thyrocytes: potential role in the metastatic spread of thyroid cancer. Tumour Biol. 2015;37:5569–5575. doi: 10.1007/s13277-015-4418-7. [DOI] [PubMed] [Google Scholar]
- 7.Haabeth OA, Bogen B, Corthay A. A model for cancer-suppressive inflammation. Oncoimmunology. 2012;1:1146–1155. doi: 10.4161/onci.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zivancevic-Simonovic S, Mihaljevic O, Majstorovic I, et al. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol Immunother. 2015;64:1011–1019. doi: 10.1007/s00262-015-1705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 12.Nemunaitis J, Fong T, Shabe P, Martineau D, Ando D. Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Invest. 2001;19:239–247. doi: 10.1081/CNV-100102550. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci MG, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992;148:1143–1148. [PubMed] [Google Scholar]
- 16.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 18.Kurte M, Lopez M, Aguirre A, Escobar A, Aguillon JC, Charo J, Larsen CG, Kiessling R, Salazar-Onfray F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and transporter associated with antigen processing 1/2 in human melanoma cells. J Immunol. 2004;173:1731–1737. doi: 10.4049/jimmunol.173.3.1731. [DOI] [PubMed] [Google Scholar]
- 19.Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, Soares FA, Vassallo J, Ward LS. Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer. 2013;20:103–110. doi: 10.1530/ERC-12-0313. [DOI] [PubMed] [Google Scholar]
- 20.Cunha LL, Marcello MA, Vassallo J, Ward LS. Differentiated thyroid carcinomas and their B7H1 shield. Future Oncol. 2013;9:1417–1419. doi: 10.2217/fon.13.89. [DOI] [PubMed] [Google Scholar]
- 21.Cunha LL, Marcello MA, Nonogaki S, Morari EC, Soares FA, Vassallo J, Ward LS. CD8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clin Endocrinol (Oxf). 2015;83:246–253. doi: 10.1111/cen.12586. [DOI] [PubMed] [Google Scholar]
- 22.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 23.Kasagi K, Kousaka T, Higuchi K, Iida Y, Misaki T, Alam MS, Miyamoto S, Yamabe H, Konishi J. Clinical significance of measurements of antithyroid antibodies in the diagnosis of Hashimoto’s thyroiditis: comparison with histological findings. Thyroid. 1996;6:445–450. doi: 10.1089/thy.1996.6.445. [DOI] [PubMed] [Google Scholar]
- 24.Souza SL, Montalli Da Assumpcao LV, Ward LS. Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer. Thyroid. 2003;13:491–495. doi: 10.1089/105072503322021160. [DOI] [PubMed] [Google Scholar]
- 25.Cunha LL, Morari EC, Nonogaki S, Soares FA, Vassallo J, Ward LS. Foxp3 expression is associated with aggressiveness in differentiated thyroid carcinomas. Clinics (Sao Paulo) 2012;67:483–488. doi: 10.6061/clinics/2012(05)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morari EC, Silva JR, Guilhen AC, Cunha LL, Marcello MA, Soares FA, Vassallo J, Ward LS. Muc-1 expression may help characterize thyroid nodules but does not predict patients’ outcome. Endocr Pathol. 2010;21:242–249. doi: 10.1007/s12022-010-9137-4. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Syrjanen S, Naud P, Sarian L, et al. Immunosuppressive cytokine Interleukin-10 (IL-10) is up-regulated in high-grade CIN but not associated with high-risk human papillomavirus (HPV) at baseline, outcomes of HR-HPV infections or incident CIN in the LAMS cohort. Virchows Arch. 2009;455:505–515. doi: 10.1007/s00428-009-0850-7. [DOI] [PubMed] [Google Scholar]
- 29.Cunha LL, Ward LS. Comments on “well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence”. Endocr Pathol. 2011;22:172–173. doi: 10.1007/s12022-011-9171-x. [DOI] [PubMed] [Google Scholar]
- 30.Stassi G, Di Liberto D, Todaro M, et al. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol. 2000;1:483–488. doi: 10.1038/82725. [DOI] [PubMed] [Google Scholar]
- 31.Stassi G, Todaro M, Zerilli M, et al. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res. 2003;63:6784–6790. [PubMed] [Google Scholar]
- 32.Arantes DA, Costa NL, Mendonca EF, Silva TA, Batista AC. Overexpression of immunosuppressive cytokines is associated with poorer clinical stage of oral squamous cell carcinoma. Arch Oral Biol. 2016;61:28–35. doi: 10.1016/j.archoralbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Fujieda S, Sunaga H, Tsuzuki H, Fan GK, Saito H. IL-10 expression is associated with the expression of platelet-derived endothelial cell growth factor and prognosis in oral and oropharyngeal carcinoma. Cancer Lett. 1999;136:1–9. doi: 10.1016/S0304-3835(98)00281-X. [DOI] [PubMed] [Google Scholar]
- 34.Hatanaka H, Abe Y, Kamiya T, et al. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann Oncol. 2000;11:815–819. doi: 10.1023/A:1008375208574. [DOI] [PubMed] [Google Scholar]
- 35.Chan SL, Mo FK, Wong CS, et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012;118:3984–3992. doi: 10.1002/cncr.26726. [DOI] [PubMed] [Google Scholar]
- 36.Sung WW, Lee H. The role of interleukin-10 in the progression of human papillomavirus-associated lung carcinoma. Oncoimmunology. 2013;2:e25854. doi: 10.4161/onci.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todaro M, Zerilli M, Ricci-Vitiani L, et al. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res. 2006;66:1491–1499. doi: 10.1158/0008-5472.CAN-05-2514. [DOI] [PubMed] [Google Scholar]